Abstract
Purpose
Lung cancer is one of the most common types of cancer, resulting in approximately 1.8 million deaths worldwide. Immunotherapy using checkpoint inhibitors has become standard of care in advanced non‐small cell lung cancer (NSCLC), and there is increasing interest in further improving outcomes through combination with other therapeutics. This systematic review evaluates emerging phase III data on the efficacy and safety of checkpoint inhibitor combinations as first‐line treatment for advanced NSCLC.
Materials and Methods
Published and presented literature was searched using the key search terms “non‐small cell lung cancer” AND “checkpoint‐inhibitors” (OR respective aliases) AND phase III trials. Seven randomized phase III clinical trials reporting outcomes on checkpoint inhibitor combinations in first‐line advanced NSCLC were identified.
Results
Four first‐line trials reported outcomes for checkpoint inhibitor combinations in nonsquamous NSCLC. Pembrolizumab‐chemotherapy, atezolizumab‐chemotherapy, and atezolizumab‐bevacizumab‐chemotherapy showed significantly improved overall survival compared with controls in patients with advanced nonsquamous epidermal growth factor receptor‐negative (EGFR−)/ anaplastic lymphoma kinase gene (ALK)− NSCLC. Two trials reported outcomes for squamous NSCLC, with pembrolizumab‐chemotherapy reporting significantly improved overall survival (OS) compared with chemotherapy. The combination of nivolumab‐ipilimumab in all‐comer histology failed to improve OS compared with histology appropriate chemotherapy in patients regardless of their tumor mutational burden status. Based on improved survival and safety, either pembrolizumab monotherapy or pembrolizumab‐chemotherapy administered based on PD‐L1 status and histology is a preferred treatment option. Outcomes for atezolizumab‐bevacizumab‐chemotherapy in EGFR+/ALK+ patients are promising and require further exploration.
Conclusion
First‐line checkpoint inhibitors added to standard therapies improve overall survival for nonsquamous EGFR−/ALK− and squamous advanced NSCLC.
Implications for Practice
Single‐agent immune checkpoint inhibitors are now standard of care for advanced non‐small cell lung cancer (NSCLC), and emerging data show that combining these agents with established chemotherapy further improves outcomes. The phase III KEYNOTE‐189 and IMPower‐130 trials showed significantly improved survival using this strategy for nonsquamous NSCLC, and the phase III KEYNOTE‐407 trial showed similar results in squamous disease. Checkpoint inhibitor combinations are therefore an important new treatment option for first‐line NSCLC. Programmed death ligand‐1 expression may inform the use of checkpoint inhibitor combination therapy, and overall tumor mutation burden is also an emerging biomarker for this new treatment strategy.
Keywords: Advanced NSCLC, Immunotherapy, Checkpoint inhibitors, Combination therapy, First‐line
Short abstract
This review evaluated emerging phase III clinical trial data on the efficacy and safety of checkpoint‐inhibitor combinations as first‐line treatment for advanced non‐small cell lung cancer.
Introduction
Lung cancer is one of the most common types of cancer, with an estimated nearly 2.1 million new cases diagnosed resulting in an estimated 1.8 million deaths worldwide in 2018 1. Non‐small cell cancer (NSCLC) accounts for approximately 85% of lung malignancies, and about 70% are diagnosed with a nonsquamous histology such as adenocarcinoma or large cell carcinoma 2, 3, 4. Over half of newly diagnosed patients are considered incurable because of the presence of metastases at the time of initial presentation 5. Additionally, many patients with NSCLC have driver mutations such as epidermal growth factor receptor mutations (EGFR+; 10%–50% of patients) or anaplastic lymphoma kinase gene rearrangements (ALK+; 3%–5% of patients) 6, 7, 8, 9, and approximately 25%–45% of NSCLCs have a programmed death ligand‐1 (PD‐L1) tumor proportional score (TPS) ≥50 10, 11.
Tumor cells evade immune responses which normally function to prevent immune‐mediated damage to healthy tissues 12. The expression of checkpoint regulator molecules such as PD‐L1 can downregulate cytotoxic T lymphocytes (cluster of differentiation protein 8 [CD8] T cells) in the tumor microenvironment through programmed cell death protein 1 (PD‐1)/PD‐L1 interactions 13, 14, and the cytotoxic T‐lymphocyte associated protein 4 (CTLA‐4) displaces interactions between CD28 and its ligands, CD80 and CD86, preventing CD8 T‐cell priming in lymphatic tissue (Fig. 1) 12, 14, 15. Monoclonal antibody (mAb) checkpoint inhibitors have been developed, however, that disrupt both PD‐1/PD‐L1 and CTLA‐4/CD80(86) interactions, re‐engaging the effector and activation phases of T‐cell activity and enhancing immune‐mediated cytotoxic antitumor responses 14, 15. Although agents against both CTLA‐4 and PD‐1/PD‐L1 are considered checkpoint inhibitors, single‐agent anti‐PD‐1/PD‐L1 agents have had more success to date in the treatment of NSCLC, significantly improving overall survival (OS) in patients with advanced NSCLC 11, 16, 17, 18, 19, 20, 21, 22, often with durable responses. Despite the success of single‐agent checkpoint inhibitors in advanced disease, new regimens continue to be developed to improve efficacy and expand checkpoint inhibitor use.
Figure 1.
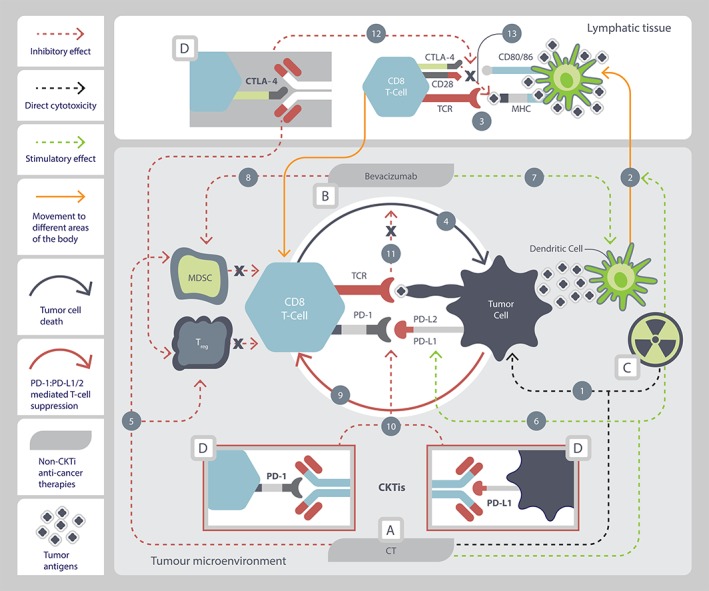
Rationale for checkpoint inhibitor combinations with chemo/targeted therapy in the antitumor response. (A): Cytotoxic chemotherapy. Chemotherapy kills tumor cells through cytotoxicity (1), releasing tumor antigens that are carried to lymphatic tissue by dendritic cells (2). Antigen is presented to naive CD8 T cells to prime them for the antitumor response (3). Primed CD8 T cells travel back to the tumor microenvironment, where they recognize the tumor cell and initiate T‐cell mediated immunogenic tumor cell death (4) 80, 81, 82. Chemotherapy inhibits immune‐suppressive cells (myeloid‐derived suppressor cells [MDSCs] and regulatory T cells) (5), which relieves inhibition of CD8 T cells to initiate T‐cell mediated immunogenic tumor cell death (4) 80, 82. Chemotherapy may increase expression of PD‐L1 on tumor cells, enhancing immunotherapy efficacy (6) 83. (B): Targeted therapy. Bevacizumab stimulates dendritic cell maturation (7) to increase CD8 T‐cell priming (2, 3) and T‐cell mediated immunogenic tumor cell death (4) in the tumor microenvironment 81. Bevacizumab inhibits MDSC immune‐suppressive cells (8), which relieves inhibition of CD8 T‐cells to initiate T‐cell mediated immunogenic tumor cell death (4) 81. (C): Radiation therapy. Ionizing radiation leads to direct cytotoxicity (1) and enhances both antigen uptake (2) and cross presentation of antigen by dendritic cells to prime CD8 T cells (3) for T‐cell mediated immunogenic tumor cell death (4) 82. Ionizing radiation may increase PD‐L1 expression on tumor cells 28, 29, 31, 84, 85, 86, 87, enhancing immunotherapy efficacy (6). (D): Immune checkpoint inhibitors. Interaction between PD‐L1/(PD‐L2) ↔ PD‐1 suppresses CD8 T‐cell activity (9) 13, 14, reducing T‐cell mediated immunogenic tumor cell death (4). Anti‐PD‐1/PD‐L1 agents disrupt the interaction between PD‐L1/(PD‐L2) ↔ PD‐1 (10), relieving tumor‐mediated PD‐L1/(PD‐L2) inhibition of T‐cell mediated immunogenic tumor cell death (11) 12, 14, 88. Anti‐CTLA‐4 agents disrupt the interaction between CTLA‐4 ↔ CD80/86 (12), blocking CTLA‐4 ↔ CD80/86 mediated inhibition (13) of CD8 T‐cell priming (3), and have also been reported to inhibit Tregs 34, 89, 90, 91. Primed CD8 T cells travel back to the tumor microenvironment, where they recognize the tumor cell and initiate T‐cell mediated immunogenic tumor cell death (4) 92, 93.
Abbreviations: CD28, cluster of differentiation 28; CD8 T‐Cell, cluster of differentiation 8 T lymphocyte; CD80/86, cluster of differentiation 80/86; CKTi, checkpoint‐inhibitor; CT, chemotherapy; CTLA‐4, cytotoxic T‐lymphocyte associated protein 4; MHC, major histocompatibility complex; MDSC, myeloid‐derived suppressor cell; PD‐1, programmed cell death protein 1; PD‐L1, programmed death ligand‐1; PD‐L2, programmed death ligand 2; TCR, T cell receptor; Treg, regulatory T cell.
Current standards of care for first‐line advanced NSCLC vary depending on the presence of absence of driver mutations, histology, and PD‐L1 expression 23, 24, 25. Patients with driver mutations (EGFR+/ALK+) should receive targeted therapy based on substantial survival benefit compared with chemotherapy 26, until resistance to available lines of targeted therapies has developed. For patients with EGFR/ALK wild‐type (EGFR−/ALK−) tumors and patients with unknown, no, or low levels of PD‐L1 expression (TPS 0%–49%), 4–6 cycles of platinum‐based chemotherapy (CT) for squamous disease and the same with or without bevacizumab followed with possible maintenance therapy with pemetrexed has been standard treatment for nonsquamous disease. For patients with higher levels of PD‐L1 expression (≥50% PD‐L1), single‐agent pembrolizumab is indicated 27. Second‐line therapy has consisted of either chemotherapy or a checkpoint inhibitor, depending on prior treatments.
Increasing interest has developed in combining checkpoint inhibitors with other therapeutics in order to improve outcomes. A number of biological rationales suggest potential additive or synergistic benefit for combining checkpoint inhibitors with other established therapies, including enhanced tumor antigen uptake and presentation for T‐cell priming, reducing the activity of immunosuppressive cells, and potentially increasing PD‐L1 expression on NSCLC tumor cells (Fig. 1) 11, 20, 22, 28, 29, 30, 31, 32, 33, 34. Early clinical trial data on checkpoint inhibitor combinations showed promising activity 35, 36, 37, 38, and rapidly emerging phase III data have led to the approval of some checkpoint inhibitor combinations for the first‐line treatment of metastatic nonsquamous 39, 40 and squamous 41 NSCLC. There is therefore a great need for a systematic analysis to guide the clinical use of checkpoint inhibitor combinations. The purpose of this review is to evaluate emerging phase III data on the efficacy and safety of checkpoint inhibitor combinations for first‐line advanced NSCLC and to assess whether additional changes in clinical practice are warranted.
Materials and Methods
PubMed (to October 17, 2018), the proceedings of the American Society of Clinical Oncology (2016–2018), the Annual Congress of the European Society for Medical Oncology (ESMO; 2016–2018), ESMO Immuno‐Oncology Congress (2016–2017), and the World Conference on Lung Cancer of the International Association for the Study of Lung Cancer (2016–2018) meetings were searched using the key search terms “non‐small cell lung cancer” AND “checkpoint inhibitors” (OR respective aliases) AND phase III trials aliases and/or filters. A supplemental bibliographic search of recent review articles and directed searches for updated reports of specific studies was also conducted. Records were vetted at abstract and confirmed at full text level as needed, and only phase III trials with published or presented efficacy results evaluating checkpoint inhibitor combinations for first‐line advanced NSCLC were eligible. Line of therapy was assigned based on the majority of the study population.
Findings
The literature search produced a total of 543 records representing seven randomized phase III clinical trials reporting outcomes of checkpoint inhibitor combinations in first‐line advanced NSCLC (PRISMA Diagram, Fig. 2) 42, 43, 44, 45, 46, 47, 48.
Figure 2.
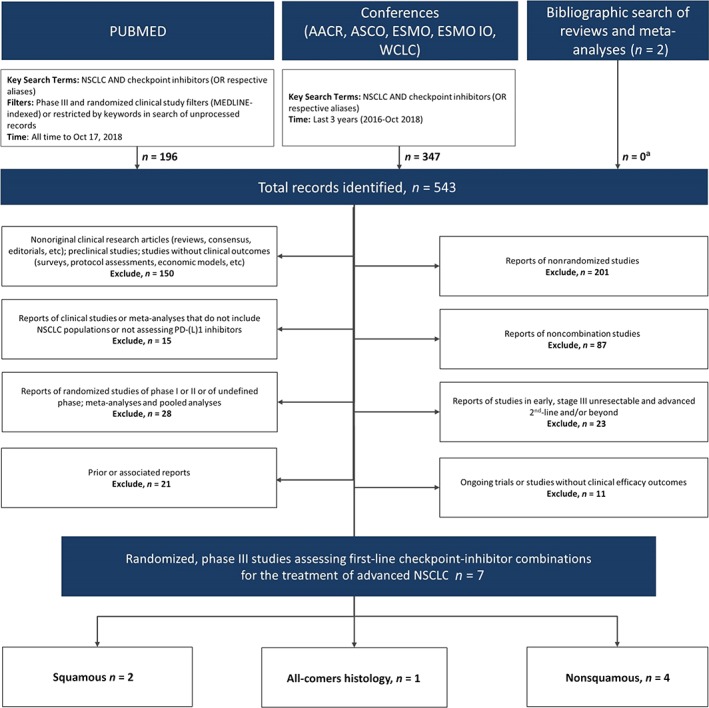
PRISMA diagram of eligible first‐line checkpoint inhibitor combination phase III trials.
aPrimary reports of eligible studies that were not identified through database.
Abbreviations: AACR, American Association for Cancer Research; ASCO, American Society of Clinical Oncology; ESMO, European Society for Medical Oncology; ESMO IO, European Society for Medical Oncology Immuno‐Oncology; NSCLC, non‐small cell lung cancer; PD‐(L)1, programmed cell death protein (ligand) 1; WCLC, World Conference on Lung Cancer
First‐Line Advanced
Nonsquamous
First‐line atezolizumab plus platinum CT showed promising efficacy with a tolerable safety profile in an early phase trial 35. The phase III IMpower‐150 trial randomized PD‐L1 unselected patients with stage IV or recurrent metastatic nonsquamous NSCLC to receive atezolizumab plus carboplatin and paclitaxel followed by atezolizumab maintenance (Atez‐CP, n = 402), atezolizumab plus bevacizumab and carboplatin and paclitaxel followed by atezolizumab and bevacizumab maintenance (Atez‐Bev‐CP, n = 400), or bevacizumab and carboplatin and paclitaxel followed by bevacizumab maintenance (Bev‐CP, n = 400). EGFR+/ALK+ patients were eligible if they progressed on or were intolerant to one or more approved targeted therapies. These patients were included in the intention‐to‐treat (ITT) analysis; however, they were excluded from the primary endpoint analyses, which included only EGFR−/ALK− patients (ITT EGFR−/ALK−; Atez‐Bev‐CP, n = 359; Atez‐CP, n = 349; Bev‐CP, n = 337 for the OS analysis). At a median follow‐up of approximately 15.5 months, significant improvements for Atez‐Bev‐CP versus Bev‐CP were seen in the coprimary endpoints of investigator‐assessed progression‐free survival (PFS) in ITT EGFR−/ALK− patients (median, 8.3 vs. 6.8 months; hazard ratio [HR], 0.62; 95% confidence interval [CI] 0.52–0.74, p < .001; Table 1), and in patients with high expression of the T‐effector (Teff) gene signature (Teff‐high)/ EGFR−/ALK− patients (median, 11.3 vs. 6.8 months; HR, 0.51; 95% CI, 0.38–0.68, p < .001) 48. At a median follow‐up of approximately 20 months, OS in the ITT EGFR−/ALK− population was also significantly improved (median, 19.2 vs. 14.7 months; HR, 0.78; 95% CI, 0.64–0.96; p = .02). Although not yet mature, median OS was not significantly improved for Atez‐CP versus Bev‐CP (19.4 vs. 14.7 months; HR, 0.88; 95% CI, 0.72–1.08; p = .20) 49. Discontinuation of any treatment because of any‐grade adverse events (AEs) was 33.8% for Atez‐Bev‐CP, 13.3% for Atez‐CP, and 24.9% for Bev‐CP (Table 2) 49. Grade 3/4 treatment‐related AEs (TRAEs) occurred in 56.7%, 43.0%, and 48.5% in patients receiving Atez‐Bev‐CP, Atez‐CP, and Bev‐CP, respectively, with the most common grade 3/4 immune‐related AEs (irAEs) reported in the Atez‐Bev‐CP arm being hepatitis/laboratory abnormalities (5.1%/4.6%), rash (2.3%), colitis (1.8%), and pneumonitis (1.5%).
Table 1.
Randomized first‐line phase III trials assessing efficacy of checkpoint inhibitor combinations for the treatment of advanced NSCLC in ITT populations
| Trial | Setting; line of treatment | Regimen(s) | Maintenance therapy | n | Median age [range], yr | Median follow‐up, mo | Overall response rate (95% CI), % | Median duration of response (95% CI) [range], mo | Median progression‐free survival, (95% CI), mo | Median overall survival, (95% CI), mo |
|---|---|---|---|---|---|---|---|---|---|---|
| Nonsquamous | ||||||||||
| IMpower‐150 44, 45 | Stage IV; (including EGFR+/ALK+)a PD‐L1 unselected | Atezolizumab 1,200 mg + carboplatin AUC 6 + paclitaxel 200 mg/m2 q3w for 4 or 6 cycles | Atezolizumab 1,200 mg q3w until PD | 349b | 63c [32–85] | ~20 | 40c | 8.3c [1.9–26.0] | NR |
Atez‐CP vs. Bev‐CP: HR 0.88 (0.72–1.08) p = .20 |
| Atezolizumab 1,200 mg + carboplatin AUC 6 + paclitaxel 200 mg/m2 q3w for 4 or 6 cycles + bevacizumab 15 mg/kg q3w | Atezolizumab 1,200 mg + bevacizumab 15 mg/kg IV q3w until PD | 359b | 63c [31–89] | 15.4b | 63.5b (58.2–68.5) | 9.0b [0.4–24.9] |
8.3b Atez‐Bev‐CP vs. Bev‐CP: HR 0.62 (0.52–0.74) p < .001 |
19.2 Atez‐Bev‐CP vs. Bev‐CPb , d: HR 0.78 (0.64–0.96) p = .02 |
||
| Carboplatin AUC 6 + paclitaxel 200 mg/m2 q3w for 4 or 6 cycles + bevacizumab 15 mg/kg q3w | Bevacizumab 15 mg/kg q3w until PD | 337b | 63c [31–90] | 15.5b | 48.0b (42.5–53.6) | 5.7b [0.0–22.1] | 6.8b | 14.7 | ||
| KEYNOTE‐189 40 | Metastatic EGFR−/ALK−; PD‐L1 unselected | Pembrolizumab 200 mg + pemetrexed 500 mg/m2 + (cisplatin 75 mg/m2 or carboplatin AUC 6) q3w × 4 | Pembrolizumab 200 mg q3w ≤x31 + pemetrexed 500 mg/m2 q3w | 410 | 65.0 [34.0–84.0] | 10.5 |
47.6 (42.6–52.5), p < .001 |
11.2 [1.1+ to 18.0+] |
8.8 HR 0.52 (0.43–0.64) p < .001 |
NYR HR 0.49 (0.38–0.64) p < .001 |
| Placebo + pemetrexed 500 mg/m2 + (cisplatin 75 mg/m2 or carboplatin AUC 5) q3w × 4 | Placebo + pemetrexed 500 mg/m2 q3wn | 206 | 63.5 [34.0–84.0] | 18.9 (13.8–25.0) | 7.8 [2.1+ to 16.4+] | 4.9 | 11.3 | |||
| IMpower‐132 47 | Stage IV EGFR−/ALK−; PD‐L1 unselected | Atezolizumab 1,200 mg + pemetrexed 500 mg/m2 + (cisplatin 75 mg/m2 or carboplatin AUC 6) q3w × 4 or 6 | Atezolizumab 1,200 mg + pemetrexed 500 mg/m2 q3w | 292 | 64.0 [31.0–85.0] | 14.8 | 47 | 10.1 |
7.6 HR 0.60 (0.49–0.72) p < .0001 |
18.1 HR 0.81 (0.64–1.03) p = .08 |
| Pemetrexed 500 mg/m2 + (cisplatin 75 mg/m2 or carboplatin AUC 5) q3w × 4 or 6 | Pemetrexed 500 mg/m2 q3w | 286 | 63.0 [33.0–83.0] | 32 | 7.2 | 5.2 | 13.6 | |||
| IMpower‐130 46 | Stage IV (including EGFR+/ALK+)b , e; PD‐L1 unselected | Atezolizumab 1,200 mg + carboplatin AUC 6 + nab‐paclitaxel 100 mg/m2 q3w for 4 or 6 cycles | Atezolizumab 1,200 mg q3w until PD | 451 | NR | ~19.0 | 49.2 |
8.4 (6.9–11.8) p = .0004 |
7.0 HR 0.64 (0.54–0.77) p < .0001 |
18.6 HR 0.79 (0.64–0.98) p = .033 |
| Carboplatin AUC 6 + nab‐paclitaxel 100 mg/m2 q3w for 4 or 6 cycles | BSC or pemetrexed 500 mg/m2 q3w | 228 | NR | 31.9 | 6.1 (5.5–7.9) | 5.5 | 13.9 | |||
| Squamous | ||||||||||
| KEYNOTE‐407 48 | Stage IV; PD‐L1 unselected | Pembrolizumab 200 mg + carboplatin AUC 6 q3w + paclitaxel 200 mg/m2 q3w or nab‐paclitaxel 100 mg/m2 q1w for 4 cycles | Pembrolizumab 200 mg q3w ≤x31 | 278 | 65.0 [29–87] | 7.8 |
57.9 (51.9–63.8) p = .0004 |
7.7 [1.1+to 14.7+] |
6.4 HR 0.56 (0.45–0.70) p < .001 |
15.9 HR 0.64 (0.49–0.85) p < .001 |
| Placebo + carboplatin AUC 6 q3w + paclitaxel 200 mg/m2 q3w or nab‐paclitaxel 100 mg/m2 q1w for 4 cycles | Placebo q3w ≤x31f | 281 | 65.0 [36–88] | 38.4 (32.7–44.4) | 4.8 [1.3+ to 15.8+] | 4.8 | 11.3 | |||
| IMPower‐131 42, 51 | Stage IV (including EGFR+/ALK+)j . k; PD‐L1 unselected | Atezolizumab 1,200 mg + carboplatin AUC 6 q3w + nab‐paclitaxel 100 mg/m2 q1w for 4 or 6 cycles | Atezolizumab 1,200 mg q3w until PD | 343 | 65 [23–83] | 17.1 | 49 | 7.2 [1.7–28.1] |
6.3 HR 0.71 (0.60–0.85) p = .0001 |
14.6 HR 0.92 (0.76–1.12) p = .41 |
| Carboplatin AUC 6 q3w + nab‐paclitaxel 100 mg/m2 q1w for 4 or 6 cycles | Best Supportive Care until PD | 340 | 65 [38–86] | 41 | 5.2 [2.1–27.6] | 5.6 | 14.3 | |||
| All‐comers histology | ||||||||||
| CHECKMATE‐227 41 | Stage IV or recurrent EGFR−/ALK−; PD‐L1 unselectedg | Nivolumab 3 mg/kg q2w + ipilimumab 1 mg/kg q6w | None | 583 | 64 [NR] | 11.2h | NR | NR |
4.9 HR 0.83 (0.72–0.96) p = NR |
NR |
| Histology‐based CTi |
Pemetrexed 500 mg/m2 q3w (nonsquamous only) |
583 | 64 [NR] | NR | NR | 5.5 | NR |
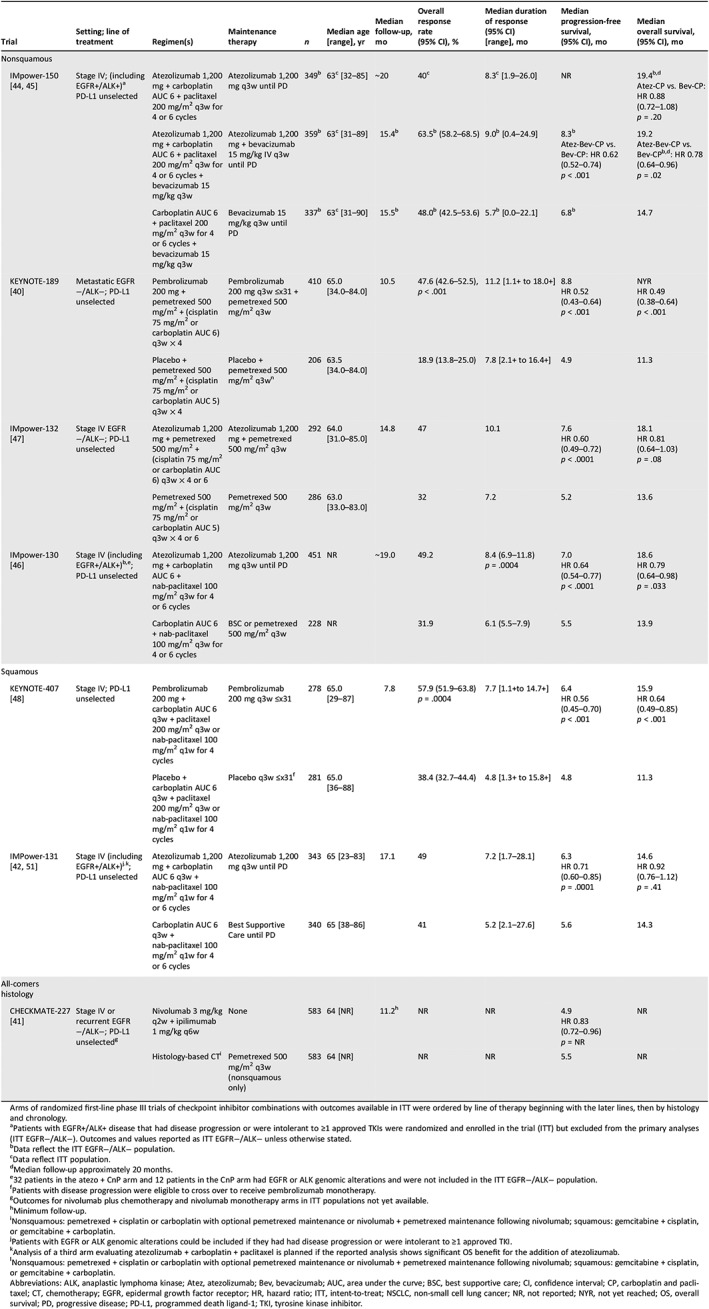
Arms of randomized first‐line phase III trials of checkpoint inhibitor combinations with outcomes available in ITT were ordered by line of therapy beginning with the later lines, then by histology and chronology.
Patients with EGFR+/ALK+ disease that had disease progression or were intolerant to ≥1 approved TKIs were randomized and enrolled in the trial (ITT) but excluded from the primary analyses (ITT EGFR−/ALK−). Outcomes and values reported as ITT EGFR−/ALK− unless otherwise stated.
Data reflect the ITT EGFR−/ALK− population.
Data reflect ITT population.
Median follow‐up approximately 20 months.
32 patients in the atezo + CnP arm and 12 patients in the CnP arm had EGFR or ALK genomic alterations and were not included in the ITT EGFR−/ALK− population.
Patients with disease progression were eligible to cross over to receive pembrolizumab monotherapy.
Outcomes for nivolumab plus chemotherapy and nivolumab monotherapy arms in ITT populations not yet available.
Minimum follow‐up.
Nonsquamous: pemetrexed + cisplatin or carboplatin with optional pemetrexed maintenance or nivolumab + pemetrexed maintenance following nivolumab; squamous: gemcitabine + cisplatin, or gemcitabine + carboplatin.
Patients with EGFR or ALK genomic alterations could be included if they had had disease progression or were intolerant to ≥1 approved TKI.
Analysis of a third arm evaluating atezolizumab + carboplatin + paclitaxel is planned if the reported analysis shows significant OS benefit for the addition of atezolizumab.
Nonsquamous: pemetrexed + cisplatin or carboplatin with optional pemetrexed maintenance or nivolumab + pemetrexed maintenance following nivolumab; squamous: gemcitabine + cisplatin, or gemcitabine + carboplatin.
Abbreviations: ALK, anaplastic lymphoma kinase; Atez, atezolizumab; Bev, bevacizumab; AUC, area under the curve; BSC, best supportive care; CI, confidence interval; CP, carboplatin and paclitaxel; CT, chemotherapy; EGFR, epidermal growth factor receptor; HR, hazard ratio; ITT, intent‐to‐treat; NSCLC, non‐small cell lung cancer; NR, not reported; NYR, not yet reached; OS, overall survival; PD, progressive disease; PD‐L1, programmed death ligand‐1; TKI, tyrosine kinase inhibitor.
Table 2.
Safety outcomes from phase III trials evaluating checkpoint inhibitor combinations for the treatment of advanced or stage III NSCLC
| Trial | IMpower‐150 Socinski ASCO 2018 Socinski NEJM 2018 | KEYNOTE‐189 Gandhi 2018 | IMpower 132 Papadimitrakopoulou WCLC 2018 | IMpower‐130 Cappuzzo ESMO 2018 | KEYNOTE‐407 Paz‐Ares 2018 | IMpower‐131 Jotte 2018 | CHECKMATE‐227 Hellmann 2018 Hellmann AACR 2018 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histology | Nonsquamous | Squamous | All‐comers histology | ||||||||||||
| Disease stage | IV | IIIb or IV | IV | IV | IV | IV | IV (or recurrent) | ||||||||
| Regimen(s) | Atez‐Bev‐CP | Atez‐CP | Bev‐CP | Pembro‐pemCT | Pb‐pemCT | Atez‐pemCT | pemCT | Atez‐CnP | CnP | Pembro‐C(n)P | Pb‐C(n)P | Atez‐CnP | CnP | Niv‐Ipi | HCT |
| Safety Population (n) | 393 | 400 | 394 | 405 | 202 | 291 | 274 | 473 | 232 | 278 | 280 | 334 | 334 | 576 | 570 |
| Overall | |||||||||||||||
| Treatment‐related AEs, n (%) | |||||||||||||||
| Any Gr | 370 (94.1) | 377 (94.3) | 377 (95.7) | 404 (99.8)a | 200 (99.0)a | 267 (91.8) | 239 (87.2) | 455 (96.2) | 215 (92.7) | 273 (98.2)a | 274 (97.9)a | 316 (94.6) | 303 (90.7) | 433 (75.2) | 460 (80.7) |
| Gr 3/4 | 223 (56.7) | 172 (43.0) | 191 (48.5) | 272 (67.2)a | 133 (65.8)a | 156 (53.6) | 107 (39.1) | 346 (73.2) | 140 (60.3) | 194 (69.8)a | 191 (68.2)a | 227 (68.0) | 190 (56.9) | 180 (31.2) | 206 (36.1) |
| Treatment‐related Serious AEs, n (%) | 100 (25.4) | NR | 76 (19.3) | NR | NR | 96 (33.0) | 43 (15.7) | 112 (23.7) | 30 (12.9) | NR | NR | 68 (20.4) | 35 (10.5) | 138 (24.0) | 79 (13.9) |
| AEs leading to discontinuation of any treatment, n (%) | 133 (33.8) | 53 (13.3) | 98 (24.9) | 112 (27.7) | 30 (14.9) | 69 (23.7) | 48 (17.5) | 125 (26.4) | 51 (22.0) | 65 (23.4) | 33 (11.8) | 97 (29.0) | 58 (17.4) | 100 (17.4)b | 51 (8.9) |
| AE‐ or treatment‐associated deaths, n (%) | 11 (2.8) | 4 (1.0) | 9 (2.3) | 27 (6.7)c | 12 (5.9)c | 11 (3.8) | 7 (2.6) | 25 (5.3) | 13 (5.6) | 10 (3.6) | 6 (2.1) | 4 (1.2) | 3 (0.9) | 7 (1.2) | 6 (1.1) |
| Select immune‐related AEs | |||||||||||||||
| Pneumonitis, n (%) | |||||||||||||||
| Any Gr | 13 (3.3) | 23 (5.8) | 5 (1.3) | 18 (4.4)d | 5 (2.5)d | 16 (5.5) | 6 (2.2) | 31 (6.6) | 3 (1.3) | 18 (6.5)d | 6 (2.1)d | 23 (6.9) | 5 (1.5) | NR (8)e | NR |
| Gr 3/4 | 6 (1.5) | 8 (2.0) | 2 (0.5) | 11 (2.7)d | 4 (2.0)d | 6 (2.1) | 3 (1.1) | 0 (0.0) | 1 (0.4) | 7 (2.5)d | 3 (1.1)d | 4 (1.2) | 3 (0.9) | NR (3)e | NR |
| Hypothyroidism, n (%) | |||||||||||||||
| Any Gr | 56 (14.2) | 34 (8.5) | 18 (4.6) | 27 (6.7)d | 5 (2.5)d | 23 (7.9) | 6 (2.2) | 70 (14.8) | 1 (0.4) | 22 (7.9)d | 5 (1.8)d | 34 (10.2) | 3 (0.9) |
Any grade: NR (23)e Grade 3/4: NR (~4)e |
NR |
| Gr 3/4 | 1 (0.3) | 1 (0.3) | 0 (0.0) | 2 (0.5)d | 0 (0.0)d | 1 (0.3) | 0 (0.0) | 3 (0.6) | 0 (0.0) | 1 (0.4)d | 0 (0.0)d | 2 (0.6) | 0 (0.0) | ||
| Hyperthyroidism, n (%) | |||||||||||||||
| Any Gr | 16 (4.1) | 11 (2.8) | 5 (1.3) | 16 (4.0)d | 6 (3.0)d | 6 (2.1) | 3 (1.1) | 23 (4.9) | 1 (0.4) | 20 (7.2)d | 2 (0.7)d | 11 (3.3) | 1 (0.3) | ||
| Gr 3/4 | 1 (0.3) | 0 (0.0) | 0 (0.0) | 0 (0.0)d | 0 (0.0)d | 1 (0.3) | 0 (0.0) | 1 (0.2) | 0 (0.0) | 1 (0.4)d | 0 (0.0)d | 1 (0.3) | 0 (0.0) | ||
| Rash/severe skin reaction, n (%) | |||||||||||||||
| Any Gr | 117/4 (29.8/1.0) | 119/NR (29.8/NR) | 53/1 (13.5/0.3) | 82f/8d (20.2f/2.0) | 23f/5d (11.4f/2.5)d | 71/4 (24.4/1.4) | 58/2 (21.2/0.7) | NR | NR | NR/5 (1.8)d | NR/1 (0.4)d | 74/NR (22.2/NR) | 39/NR (11.7/NR) | 96 (16.7)/NR (34)e | 29 (5.1)/NR |
| Gr 3/4 | 9/NR (2.3/NR) | 14/NR (3.5/NR) | 2/NR (0.5/NR) | 7f/8d (1.7f/2.0d) | 3f/4d (1.5i/2.0d) | 9/2 (3.1/0.7) | 5/0 (1.8/0.0) | NR | NR | NR/3 (1.1)d | NR/1 (0.4)d | 6/NR (1.8/NR) | 1/NR (0.3/NR) | 9 (1.6) /NR (4)e | 0 (0.0)/NR |
| Hepatitis, n (%) | |||||||||||||||
| Any Gr | 54 (13.7) | 42 (10.5) | 29 (7.4) | 5 (1.2)d | 0 (0.0)d | 13 (4.5) | 2 (0.7) | 8 (1.7) | 3 (1.3) | 5 (1.8)d | 0 (0.0)d | 58 (17.4) | 29 (8.7) | NR (15)e | NR |
| Gr 3/4 | 20 (5.1) | 12 (3.0) | 3 (0.8) | 4 (1.0)d | 0 (0.0)d | 7 (2.4) | 0 (0.0) | 2 (0.4) | 1 (0.4) | 5 (1.8)d | 0 (0.0)d | 18 (5.4) | 4 (1.2) | NR (8)e | NR |
| Colitis, n (%) | |||||||||||||||
| Any Gr | 11 (2.8) | 3 (0.8) | 2 (0.5) | 9 (2.2)d | 0 (0.0)d | 5 (1.7) | 0 (0.0) | 5 (1.1) | 1 (0.4) | 7 (2.5)d | 4 (1.4)d | 6 (1.8) | 0 (0.0) | NR (18)e | NR |
| Gr 3/4 | 7 (1.8) | 2 (0.5) | 2 (0.5) | 3 (0.7)d | 0 (0.0)d | 2 (0.7) | 0 (0.0) | 5 (1.1) | 0 (0.0) | 6 (2.2)d | 3 (1.1)d | 4 (1.2) | 0 (0.0) | NR (2)e | NR |
| Nephritis, n (%) | |||||||||||||||
| Any Gr | 3 (0.8) | NR | 0 (0.0) | 7 (1.7)d | 0 (0.0)d | NR | NR | NR | NR | 2 (0.7)d | 2 (0.7)d | NR | NR | NR (4)e | NR |
| Gr 3/4 | 1 (0.3) | NR | 0 (0.0) | 6 (1.5)d | 0 (0.0)d | NR | NR | NR | NR | 2 (0.7)d | 2 (0.7)d | NR | NR | NR (1)e | NR |
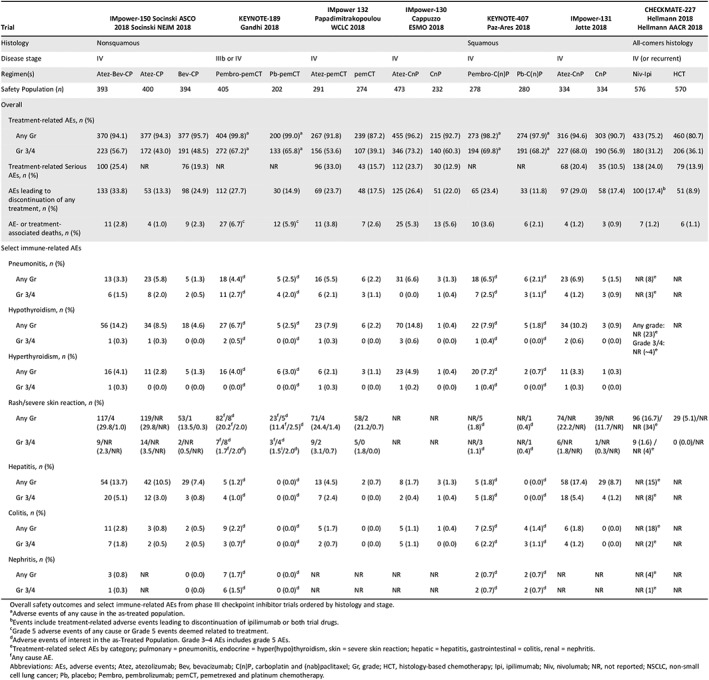
Overall safety outcomes and select immune‐related AEs from phase III checkpoint inhibitor trials ordered by histology and stage.
Adverse events of any cause in the as‐treated population.
Events include treatment‐related adverse events leading to discontinuation of ipilimumab or both trial drugs.
Grade 5 adverse events of any cause or Grade 5 events deemed related to treatment.
Adverse events of interest in the as‐Treated Population. Grade 3–4 AEs includes grade 5 AEs.
Treatment‐related select AEs by category; pulmonary = pneumonitis, endocrine = hyper(hypo)thyroidism, skin = severe skin reaction; hepatic = hepatitis, gastrointestinal = colitis, renal = nephritis.
Any cause AE.
Abbreviations: AEs, adverse events; Atez, atezolizumab; Bev, bevacizumab; C(n)P, carboplatin and (nab)paclitaxel; Gr, grade; HCT, histology‐based chemotherapy; Ipi, ipilimumab; Niv, nivolumab; NR, not reported; NSCLC, non‐small cell lung cancer; Pb, placebo; Pembro, pembrolizumab; pemCT, pemetrexed and platinum chemotherapy.
Early data from KEYNOTE‐021 (cohort G) in patients with untreated stage IIIb/IV EGFR−/ALK− nonsquamous NSCLC showed significantly improved PFS and OS for pembrolizumab plus pemetrexed and carboplatin versus pemetrexed and carboplatin followed by optional pemetrexed maintenance in both arms 37, 50, 51. The KEYNOTE‐189 phase III trial randomized PD‐L1‐unselected patients with stage IV nonsquamous EGFR−/ALK− NSCLC 2:1 to receive pembrolizumab plus pemetrexed and platinum chemotherapy followed by pembrolizumab plus pemetrexed maintenance (Pembro‐pemCT, n = 410) or placebo plus pemetrexed and platinum chemotherapy followed by pemetrexed maintenance CT (Pb‐pemCT, n = 206). Crossover to pembrolizumab was allowed in the Pb‐pemCT arm following disease progression. With a median follow‐up of 10.5 months, significant improvements were seen for the coprimary endpoints of median PFS (8.8 vs. 4.9 months; HR, 0.52; 95% CI, 0.43–0.64; p < .001) as assessed by blinded independent central review (BICR) and median OS (not yet reached vs. 11.3 months; HR, 0.49; 95% CI, 0.38–0.64; p < .001) for the Pembro‐pemCT versus Pb‐pemCT arms, respectively (Table 1) 43. Discontinuation of any treatment component because of AEs occurred in 27.7% versus 14.9% of patients in the Pembro‐pemCT versus Pb‐pemCT arms (Table 2). Although TRAEs were not reported, any‐cause grade 3–5 AEs were similar between arms (67.2% vs. 65.8%), and the most common grade 3–5 irAEs of interest were pneumonitis (2.7%), severe skin reactions (2.0%), nephritis (1.5%), and hepatitis (1.0%).
The phase III IMpower‐132 trial randomized PD‐L1‐unselected patients with stage IV nonsquamous EGFR−/ALK− NSCLC to receive atezolizumab plus pemetrexed and platinum chemotherapy followed by atezolizumab plus pemetrexed maintenance (Atez‐pemCT, n = 292) or pemetrexed and platinum chemotherapy followed by pemetrexed maintenance (pemCT, n = 286). At a median follow‐up of 14.8 months, significant improvements for Atez‐pemCT versus pemCT were seen in the coprimary endpoint of investigator‐assessed PFS (median, 7.6 vs. 5.2 months; HR, 0.60; 95% CI, 0.49–0.72; p < .0001; Table 1), with no improvement in OS (median, 18.1 vs. 13.6 months; HR, 0.81; 95% CI, 0.64–1.03; p = .08) 46. Discontinuation of any treatment because of any‐grade AEs occurred in 23.7% of patients receiving Atez‐pemCT and 17.5% of patients in the pemCT arms (Table 2). Grade 3/4 TRAEs occurred in 53.6% and 39.1% of patients receiving Atez‐pemCT and pemCT, respectively, with the most common grade 3/4 AEs of special interest in the Atez‐pemCT arm being rash (3.1%), hepatitis (2.4%), and pneumonitis (2.1%).
The phase III IMpower‐130 trial randomized PD‐L1‐unselected patients with stage IV nonsquamous NSCLC to receive atezolizumab plus carboplatin and nab‐paclitaxel followed by atezolizumab maintenance (Atez‐CnP) or carboplatin and nab‐paclitaxel followed by best supportive care (CnP). EGFR+/ALK+ patients were eligible for the trial but excluded from the primary endpoint analyses, which included only ITT EGFR−/ALK− patients (Atez‐CnP, n = 451; CnP, n = 228). At a median follow‐up of approximately 19 months, significant improvements for Atez‐CnP versus CnP were seen in the coprimary endpoints of investigator‐assessed PFS in ITT EGFR−/ALK− patients (median, 7.0 vs. 5.5 months; HR, 0.64; 95% CI, 0.54–0.77; p < .0001; Table 1) and OS in ITT EGFR−/ALK− patients (median, 18.6 vs. 13.9 months; HR, 0.79; 95% CI, 0.64–0.98; p = .033) 42. Discontinuation of any treatment because of AEs in ITT occurred in 26.4% of patients receiving Atez‐CnP and 22.0% of patients in the CnP arm (Table 2). Grade 3/4 TRAEs occurred in 73.2% and 60.3% of patients receiving Atez‐CnP and CnP, respectively, with the most common grade 3/4 AEs of special interest in the Atez‐CnP arm being colitis (1.1%), hypothyroidism (0.6%), hepatitis (0.4%), and diabetes melitus (0.4%).
Squamous
The phase III KEYNOTE‐407 study randomized PD‐L1‐unselected patients with stage IV untreated squamous NSCLC to receive pembrolizumab plus carboplatin and paclitaxel or nab‐paclitaxel followed by pembrolizumab maintenance [Pembro‐C(n)P, n = 278] or placebo plus carboplatin and paclitaxel or nab‐paclitaxel followed by placebo maintenance [Pb‐C(n)P, n = 281]. Crossover to pembrolizumab was permitted in the Pb‐C(n)P arm upon progressive disease. At a median follow‐up of 7.8 months, significant improvements were seen for Pembro‐C(n)P versus Pb‐C(n)P in the coprimary endpoints of BICR‐assessed PFS (median, 6.4 vs. 4.8 months; HR, 0.56; 95% CI, 0.45–0.70; p < .001) and OS (median, 15.9 vs. 11.3 months; HR, 0.64; 95% CI, 0.49–0.85; p < .001; Table 1) 47. Discontinuation of any treatment because of any‐cause AEs occurred in 23.4% and 11.8% of patients in the Pembro‐C(n)P and Pb‐C(n)P arms, respectively (Table 2). TRAEs were not reported, although any‐cause grade 3–5 AEs were similar between arms (69.8% vs. 68.2%). The most common grade 3–5 irAEs in the Pembro‐C(n)P arm were pneumonitis (2.5%), colitis (2.2%), and hepatitis (1.8%).
The phase III IMpower‐131 study randomized PD‐L1‐unselected patients with stage IV squamous NSCLC to receive atezolizumab plus carboplatin and paclitaxel followed by atezolizumab maintenance (Atez‐CP, n = 338), atezolizumab plus carboplatin and carboplatin and nab‐paclitaxel followed by atezolizumab maintenance (Atez‐CnP, n = 343), or carboplatin and nab‐paclitaxel followed by best supportive care (CnP, n = 340). Crossover during maintenance was not permitted. Outcomes for the Atez‐CnP and CnP arms were available at a median follow‐up of 17.1 months. A significant benefit in the coprimary endpoint of investigator‐assessed PFS was seen for Atez‐CnP versus CnP (median, 6.3 vs. 5.6 months; HR, 0.71; 95% CI, 0.60–0.85; p = .0001; Table 1) 45. The coprimary endpoint of OS was comparable between arms (median, 14.0 vs. 13.9 months; HR, 0.96; 95% CI, 0.78–1.18; p = .69) and did not improve with further follow‐up (HR, 0.92; p = .41) 52. Discontinuation of any treatment because of AEs (29.0% vs. 17.4%) and grade 3/4 TRAEs (68.0% vs. 56.9%) were moderately higher for Atez‐CnP versus CnP (Table 2) 45. The most common grade 3/4 irAEs in the Atez‐CnP arm were hepatitis (5.4%), rash (1.8%), pneumonitis (1.2%), and colitis (1.2%).
All‐Comers Histology
High durable responses with manageable tolerability were seen for nivolumab plus the CTLA‐4 inhibitor ipilimumab in CHECKMATE‐012 53. Additional data indicated greater responses in patients with high tumor mutation burden (TMB), established as ≥10 mutations per Mb in CHECKMATE‐568 54. The phase III CHECKMATE‐227 trial randomized PD‐L1‐unselected patients with stage IV or recurrent squamous or nonsquamous EGFR−/ALK− NSCLC to receive nivolumab plus ipilimumab (Niv‐Ipi; n = 583) or histology‐based chemotherapy (HCT; n = 583), or nivolumab with HCT (Niv‐HCT; n = 177) or without HCT (Niv; n = 396) depending on PD‐L1 expression. Optional maintenance pemetrexed or pemetrexed plus nivolumab was administered to nonsquamous patients in the HCT and Niv‐HCT arms, respectively. Crossover was not permitted. At a minimum follow‐up of 11.2 months, a BICR analysis of all randomized patients showed a comparable median PFS for Niv‐Ipi versus HCT (4.9 vs. 5.5 months; HR, 0.83; 95% CI, 0.72–0.96; p value not reported, Table 1) 44. An amended coprimary analysis among high‐TMB patients regardless of PD‐L1 expression, however, showed significantly improved median PFS for Niv‐Ipi versus HCT (7.2 vs. 5.5 months; HR, 0.58; 97.5% CI, 0.41–0.81; p < .001), although a recent press release showed no differences in OS between high and low TMB groups 55. Outcomes for the coprimary endpoint of OS in PD‐L1 selected patients have yet to be reported. Among patients receiving Niv‐Ipi and HCT, discontinuation of any treatment because of TRAEs occurred in 17.4% and 8.9%, respectively (Table 2). Grade 3/4 TRAEs were lower in patients receiving Niv‐Ipi versus HCT (31.2% vs. 36.1%). The most common grade 3/4 treatment‐related select AEs in the Niv‐Ipi arm were hepatic (8%), skin (4%), endocrine (4%), and pulmonary (3%) in origin.
Discussion
Results from seven phase III trials comparing checkpoint inhibitor combinations with previous standards of care have now been reported 42, 43, 44, 45, 46, 47, 48. Four trials evaluated checkpoint inhibitor combinations in nonsquamous NSCLC, with all meeting coprimary endpoints 42, 43, 46, 48, although only three showed OS benefit to date 42, 43, 48. Two trials evaluated checkpoint inhibitor combinations in squamous disease 45, 47, 52, with both meeting coprimary endpoints, although only one showing OS benefit 47. One trial evaluated checkpoint inhibitor combinations in patients with all‐comer histology, showing improvements in PFS but not OS for this strategy in patients with high‐TMB 44.
Do Checkpoint Inhibitor Combinations Improve Clinical Benefit Compared with Standard Therapy in First‐Line Biomarker Unselected EGFR−/ALK− Advanced NSCLC?
Four phase III trials evaluated checkpoint inhibitor combinations in patients with PD‐L1 unselected advanced nonsquamous EGFR−/ALK− NSCLC 42, 43, 46, 48, and three (KEYNOTE‐189, IMPower‐150, and IMPower‐130) demonstrated statistically significant improvements in OS 42, 43, 48. At a median follow‐up of 10.5 months, KEYNOTE‐189 showed an early (log‐rank curves separating after the first month) and statistically significant 51% reduction in risk of death (HR, 0.49; 95% CI, 0.38–0.64; p < .001) 43 and a higher proportion of patients with improved global health‐related quality of life (HRQoL) at week 21 (30.1% vs. 22.5%; p = .05) with Pembro‐pemCT compared with Pb‐pemCT 56. At a median follow‐up of 14.8 months, IMPower‐130 showed an early significant 21% reduction in the risk of death (HR, 0.79; 95% CI, 0.64–0.98; p = .033) for Atez‐CnP versus CnP 42. With a much longer follow‐up (median, ∼20 months), IMPower‐150 showed a late (log‐rank curves separating after 8 months) yet significant 22% reduction in risk of death for Atez‐Bev‐CP versus Bev‐CP (HR, 0.78; 95% CI, 0.64–0.96; p = .02) 48 with comparable HRQoL 57. The rates of discontinuation of any treatment because of AEs were highest for Atez‐Bev‐CP (33.8%) 49 followed by Pembro‐pemCT (27.7%) 43 and then Atez‐CnP (26.4%) 42. Both Pembro‐pemCT (August 20, 2018) 39 and Atez‐Bev‐CP (December 6, 2018) 40 were approved by the U.S. Food and Drug Administration (FDA), and Atez‐CnP is under review (decision expected September 2, 2019) 58. Although all three regimens are reasonable choices for the first‐line treatment of PD‐L1‐unselected NSCLC, the early and robust OS benefits and favorable toxicity profile, including the reduced risk of neuropathy, make Pembro‐pemCT a preferred option.
Two trials, KEYNOTE‐189 and IMPower‐132, assessed the benefits of adding checkpoint inhibitors, pembrolizumab and atezolizumab, to a pemetrexed‐platinum backbone 43, 46. Pembro‐pemCT showed an early, robust, and significant OS benefit (HR, 0.49; 95% CI, 0.38–0.64; p < .001) at 10.5 months follow‐up 43, whereas a preliminary analysis showed that Atez‐pemCT failed to show an OS benefit at a median follow‐up of 19.0 months (HR, 0.81; 95% CI, 0.64–1.03; p = .08) 46. As the two regimens were not directly compared, differences in outcomes might be explained by differences in trial design, patient populations, or the fact that OS data for the IMPower‐132 are not yet mature. However, outcomes may also point to differences in activity between the PD‐1 inhibitors pembrolizumab and the PD‐L1 inhibitor atezolizumab in this setting, a hypothesis that would need to be confirmed in a randomized setting. At this point, Pembro‐pemCT is recommended and we await mature OS data for IMPower‐132.
Two phase III trials, KEYNOTE‐407 and IMPower‐131, showed significant improvements in PFS with the addition of a checkpoint inhibitor to chemotherapy in patients with PD‐L1 unselected squamous NSCLC 45, 47, 52; however, only one demonstrated improved survival 47. At a median follow‐up of 7.8 months, KEYNOTE‐407 showed a significant 36% reduction in risk of death (HR, 0.64; 95% CI, 0.49–0.85; p < .001) for Pembro‐C(n)P versus Pb‐C(n)P, despite 31.7% of patients receiving chemotherapy alone, effectively crossing over to receive checkpoint inhibitors 47, and HRQoL was maintained or improved with the addition of pembrolizumab 59. At a minimum follow‐up of 12.8 months, IMPower‐131 survival data were not mature and did not show an OS benefit (HR, 0.92; 95% CI, 0.76–1.12; p = .41) for Atez‐CnP versus CnP 52. Given the current trajectory of the data and that 43% of patients in the CnP arm have received subsequent immunotherapy, it is unlikely that significant differences in OS will emerge with longer follow‐up. The early and significant OS benefit seen for Pembro‐C(n)P and not Atez‐CnP may be explained by differences in trial design and patient populations, differences in the activity of pembrolizumab and atezolizumab, or differences in the type of taxanes used 45, 47. Pembro‐C(n)P is the preferred regimen for squamous NSCLC and was approved by the FDA for use in this setting on October 30, 2018 41.
Should Biomarkers Be Used to Guide Checkpoint Inhibitor Combination Therapy in Advanced NSCLC?
PD‐L1 is an established biomarker for single‐agent checkpoint inhibitors in NSCLC 60. Cross trial comparisons of PD‐L1 subgroup outcomes are particularly complex, complicated by the fact that checkpoint inhibitor trials use various assays and scoring methods to ascertain PD‐L1 status. Atezolizumab trials evaluate both tumor cell and tumor microenvironment PD‐L1 immune cell expression, and other checkpoint inhibitor trials evaluate only tumor cell PD‐L1 levels. Moreover, checkpoint inhibitor trials are rarely stratified or powered for this level of analysis.
Five studies reported PD‐L1 OS subgroup outcomes for checkpoint‐ inhibitors compared with chemotherapy in advanced EGFR−/ALK− NSCLC (Table 3) 42, 43, 45, 47, 49. In the nonsquamous KEYNOTE‐189 trial, a strong relationship between higher levels of PD‐L1 expression and OS was evident, with PD‐L1‐negative patients experiencing a 41% reduction in risk of death (median OS, 15.2 months), and high PD‐L1‐expressing patients experiencing a 58% reduction in the risk of death (median OS not yet reached) for Pembro‐pemCT compared with Pb‐pemCT 43, 61. Differences were less evident in the nonsquamous atezolizumab combination trials, with reductions in the risk of death ranging from 18–19% in PD‐L1‐negative patients (median OS, 15.2–17.1 months) and 16–30% in patients with high levels of PD‐L1 expression (median OS, 17.3–25.2 months) 45, 47. As in the squamous CHECKMATE‐017 trial evaluating single‐agent nivolumab in second‐line patients 18, the relationship between OS and PD‐L1 expression in the squamous KEYNOTE‐407 and IMPower‐131 trials was even less apparent 45, 47, which may be due to the higher mutational burden from smoking in patients with squamous disease 62, 63.
Table 3.
OS outcomes by PD‐L1 expression for ITT EGFR−/ALK− trial populations
| Trial (checkpoint inhibitor) | ITT EGFR−/ALK−median OS HR (95% CI) | PD‐L1 Negative TC0 and ICO TC <1% % population, median OS HR (95% CI) | Low PD‐L1 TC1/2 or IC1/2 TC 1–49% % population, median OS HR (95% CI) | High PD‐L1 TC3 or IC3 TC ≥50% % population, median OS HR (95% CI) |
|---|---|---|---|---|
| Nonsquamous | ||||
| KEYNOTE‐18961 (Pembro) |
NYR 0.49 (0.38–0.64) p < .00001 |
31%, 15.2 mo 0.59 (0.38– 0.92) p = . 0095 |
30%, NYR 0.55 (0.34–0.90) p = .0081 |
33%, NYR 0.42 (0.26–0.68) p = .0001 |
| IMPower‐15045 (Atez)a |
19.2 mo 0.78 (0.64–0.96) p = .016 |
49%, 17.1 mo 0.82 (0.62–1.08) |
32%, 20.3 mo 0.80 (0.55–1.15) |
20%, 25.2 mo 0.70 (0.43–1.13) |
| IMPower‐13247 (Atez)b | Not reported | Not reported | Not reported | Not reported |
| IMPower‐13046 (Atez) |
18.6 mo 0.79 (0.64 to 0.98) p = .033 |
52%, 15.2 mo 0.81 (0.61–1.08) |
28%, 23.7 mo 0.70 (0.45–1.08) |
20%, 17.3 mo 0.84 (0.51–1.39) |
| Squamous | ||||
| KEYNOTE‐40748 (Pembro) |
15.9 mo 0.64 (0.49 to 0.85) p < .001 |
35%, 15.9 mo 0.61 (0.38–0.98) |
37%, 14.0 mo 0.57 (0.36–0.90) |
26%, NYR 0.64 (0.37–1.10) |
| IMPower‐13142 (Atez)c |
14.0 mo 0.96 (0.78 to 1.18) p = .69 |
48%, 13.8 mo 0.86 (0.65–1.15) |
37%, 12.4 mo 1.34 (0.95–1.90) |
15%, 23.6 mo 0.56 (0.32–0.99) |
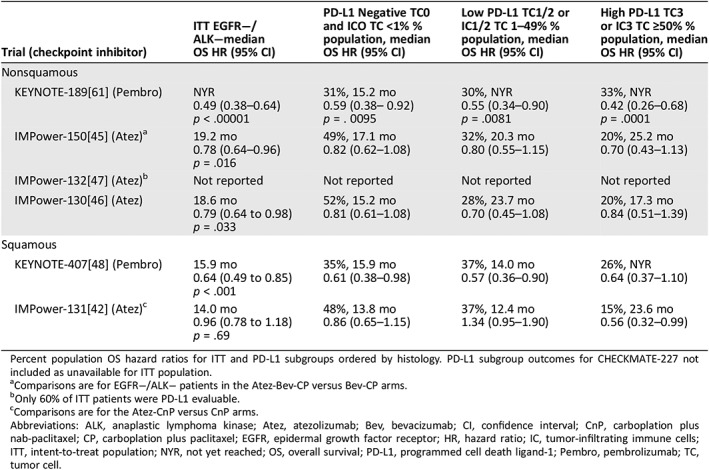
Percent population OS hazard ratios for ITT and PD‐L1 subgroups ordered by histology. PD‐L1 subgroup outcomes for CHECKMATE‐227 not included as unavailable for ITT population.
Comparisons are for EGFR−/ALK− patients in the Atez‐Bev‐CP versus Bev‐CP arms.
Only 60% of ITT patients were PD‐L1 evaluable.
Comparisons are for the Atez‐CnP versus CnP arms.
Abbreviations: ALK, anaplastic lymphoma kinase; Atez, atezolizumab; Bev, bevacizumab; CI, confidence interval; CnP, carboplation plus nab‐paclitaxel; CP, carboplation plus paclitaxel; EGFR, epidermal growth factor receptor; HR, hazard ratio; IC, tumor‐infiltrating immune cells; ITT, intent‐to‐treat population; NYR, not yet reached; OS, overall survival; PD‐L1, programmed cell death ligand‐1; Pembro, pembrolizumab; TC, tumor cell.
The Teff gene signature is defined by mRNA expression of three genes (PD‐L1, CXCL9, and IFNγ), may reflect pre‐existing immunity, and was hypothesized to be a more sensitive biomarker for PFS compared with PD‐L1 48, 64, 65. In the EGFR−/ALK− patients in IMPower‐150, PFS benefit for Atez‐Bev‐CT was seen regardless of Teff status, with outcomes showing greater benefit in Teff‐high (43% of patients, HR, 0.51; p < .0001) compared with Teff‐low (57% of patients, HR, 0.76; p = not reported) patients 48. As the Teff gene signature is not significantly more predictive than PD‐L1, development of this biomarker may not be pursued, although research into predictive gene signatures is ongoing 66.
TMB is an emerging biomarker for checkpoint inhibitor selection 67. CHECKMATE‐227 assessed outcomes based on TMB and included an amended coprimary endpoint of PFS in high‐TMB (<10 mutations per Mb) patients. An initial analysis showed a significantly improved median PFS (HR, 0.58; 97.5% CI, 0.41–0.81; p < .001) for Niv‐Ipi over HCT in patients with high‐TMB 44. However, a recent press release showed no significant OS benefit regardless of TMB status 55.Clinically meaningful improvements in HRQoL and time to deterioration in disease‐related symptoms were seen for Niv‐Ipi compared with HCT 68.
Should Patients with a Known Mutation Be Treated with Checkpoint Inhibitor Combinations?
Checkpoint inhibitor outcomes for EGFR+/ALK+ patients were only available for the IMPower‐150 and IMPower‐130 trials 42, 48, 64. In these studies, EGFR+/ALK+ patients were eligible for enrollment then later excluded from the primary analysis when signals from other trials indicated a lack of activity for checkpoint inhibitors in this population. An unstratified exploratory subgroup analysis of EGFR+/ALK+ patients of IMPower‐150 (13.5% of patients) indicated improved median PFS (9.7 vs. 6.1 months; HR, 0.59; 95% CI, 0.37–0.94) and median OS (17.5 vs. not estimable; HR, 0.54; 95% CI, 0.29–1.03) for Atez‐Bev‐CP compared with Bev‐CP 48, 49. However, there was no apparent PFS (HR, 0.75; 95% CI, 0.36–1.54) or OS (HR, 0.98; 95% CI, 0.41–2.31) benefit for Atez‐CnP versus CnP among EGFR+/ALK+ patients (6.1% of patients) in IMPower‐130 42. The combination of checkpoint inhibitors with bevacizumab and chemotherapy in patients with EGFR+/ALK+ disease is encouraging and requires further exploration.
What Is the Place in Therapy of Various Checkpoint Inhibitor Combinations?
There is considerable phase III evidence informing first‐line checkpoint inhibitor combination use in patients with advanced NSCLC and Eastern Cooperative Oncology Group performance status (PS) 0 and 1 42, 43, 44, 45, 46, 47, 48 and less evidence supporting their use in PS 2 patients or in those with untreated or symptomatic central nervous system metastases 43, 48, 69. For nonsquamous patients with PD‐L1 expression <50%, Pembro‐pemCT, Atez‐CnP, and Atez‐Bev‐CP are reasonable treatment options given the improved OS associated with these regimens compared with chemotherapy alone. However, given the early and robust OS benefits and favorable safety profile associated with Pembro‐pemCT, it should receive preferential consideration. For patients with squamous NSCLC with PD‐L1 expression <50%, Pembro‐C(n)P is the regimen of choice (Fig. 3).
Figure 3.
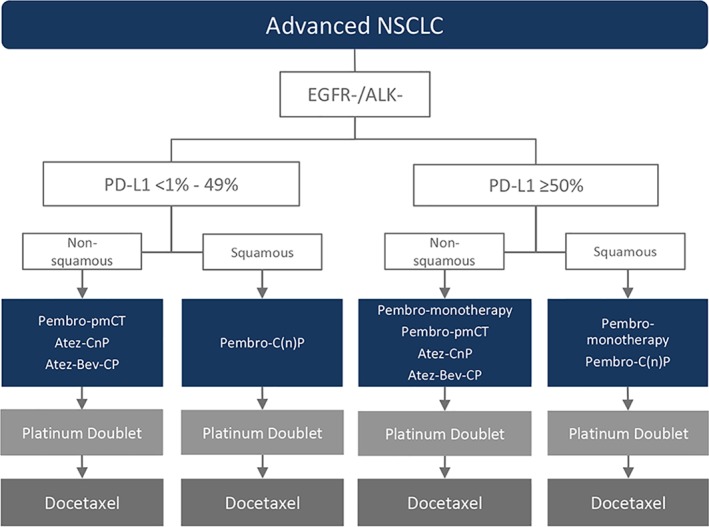
Proposed NSCLC treatment algorithm based on histology and biomarker status.
Abbreviations: ALK, anaplastic lymphoma kinase; Atez‐Bev‐CP, atezolizumab plus bevacizumab, carboplatin and paclitaxel; Atez‐CnP, atezolizumab plus carboplatin and nab‐paclitaxel followed by atezolizumab maintenance; EGFR, epidermal growth factor receptor; NSCLC, non‐small cell lung cancer; PD‐L1, programmed cell death ligand 1; Pembro‐C(n)P, pembrolizumab plus carboplatin and paclitaxel or nab‐paclitaxel followed by pembrolizumab maintenance; Pembro‐monotherapy, pembrolizumab monotherapy; Pembro‐pemCT, pembrolizumab plus pemetrexed and platinum therapy followed by pembrolizumab plus pemetrexed maintenance.
For patients expressing PD‐L1 ≥50%, pembrolizumab monotherapy has been the standard of care for patients regardless of histology since its FDA approval in May 2017 27. As both pembrolizumab monotherapy (40% reduction in risk of death) 16 and pembrolizumab‐chemotherapy have demonstrated improved OS compared with platinum‐based chemotherapy (nonsquamous, Pembro‐pemCT, 51% reduction in the risk of death 43 and squamous, Pembro‐C(n)P, 36% reduction in risk of death 47), it is difficult to make definitive statements about the relative activities of monotherapy and chemotherapy combination options. However, given that pembrolizumab monotherapy is active with a preferred safety profile (discontinuation due to AEs, 7.1% vs 23.4%–27.7%, monotherapy vs. chemotherapy combination, respectively), it should receive primary consideration, with pembrolizumab‐chemotherapy combinations reserved for higher‐risk patients or for those with a high tumor burden (overall response rate, all‐comer histology, 47.6%–57.9% vs. 44.8% for pembrolizumab‐chemotherapy vs. pembrolizumab alone) 16, 43, 47 (Fig. 3).
In later lines of therapy, treatment selection should consider efficacy and safety as well as clinical and disease characteristics, prior therapy, histology, administration schedules, and cost. PD‐L1 status may be helpful to guide treatment sequencing in patient subgroups in which benefit is less clear.
What Is the Direction of Ongoing Checkpoint Inhibitor Combination Research?
The landscape of checkpoint inhibitor therapy for NSCLC is rapidly evolving, with phase III trials evaluating checkpoint inhibitor combinations underway in both advanced and early disease (Table 4). In advanced disease, trials exploring optimal administration of both established combinations of checkpoint inhibitors plus chemotherapy, immunotherapy, and targeted therapy are underway. Trial data confirming the activity of checkpoint inhibitor combinations in PD‐L1‐negative, high‐TMB expressing, and EGFR+/ALK+ subpopulations in advanced NSCLC are pending, although the potential for minimal benefit and increased toxicity may prevent the combination of checkpoint inhibitors with TKIs in patients with EGFR+/ALK+ disease 70, 71, 72, 73, 74. In stage III unresectable disease, durvalumab added as consolidation following chemoradiotherapy has demonstrated improved OS in the phase III PACIFIC trial 75, leading to its approval in this setting on February 16, 2018 76, and additional trials evaluating checkpoint inhibitors in conjunction with chemoradiotherapy are underway 77, 78, 79. Finally, trials assessing the benefits of adding checkpoint inhibitor combinations to established neoadjuvant and adjuvant regimens in early disease are ongoing. Research into biomarkers for patient selection continues.
Table 4.
Ongoing phase III clinical trials checkpoint inhibitor combinations in NSCLC
| PD‐L1 inhibitor (target) | Trial ID (NCT no.) | Patient population | Experimental regimen | Comparator | Primary endpoint(s) | Estimated PCD |
|---|---|---|---|---|---|---|
| Neoadjuvant/adjuvant | ||||||
| Pembrolizumab (PD‐1) | PEARLS (NCT02504372) | Stage IB–IIIA, PD‐L1 unselected | Pembro following surgery ± adjuvant CT | Placebo following surgery ± adjuvant CT | DFS | August 19, 2021 |
| Nivolumab (PD‐1) | CheckMate‐816 (NCT02998528) | Stage IB–IIIA, PD‐L1 unselected | Neoadjuvant Niv + ipilimumab or Niv + Pt doublet | Neoadjuvant Pt doublet | EFS/pCR | May 8, 2023 |
| Pembrolizumab (PD‐1) | KEYNOTE‐671 (NCT03425643) | Stage IIB or IIIA, PD‐L1 unselected | Neoadjuvant Pembro + Pt doublet then adjuvant Pembro | Neoadjuvant placebo + Pt doublet then adjuvant placebo | EFS/OS | January 20, 2024 |
| Nivolumab (PD‐1) | ANVIL (NCT02595944) | Stage IB–IIIA, PD‐L1 unselected | Niv following surgery ± adjuvant CT ± RT | Observation following surgery ± adjuvant CT ± RT | OS/DFS | July 1, 2024 |
| Atezolizumab (PD‐L1) | IMPower010 (NCT02486718) | Stage IB–IIIA, PD‐L1 unselected | Atez following adjuvant Pt doublet | BSC following adjuvant Pt doublet | DFS | September 25, 2026 |
| Atezolizumab (PD‐L1) | IMPower030 (NCT03456063) | Stage II, IIIA, or select IIIB, PD‐L1 unselected | Neoadjuvant Atez + Pt doublet then adjuvant Atez | Neoadjuvant placebo + Pt doublet then BSC | MPR | March 31, 2020 |
| Stage III | ||||||
| Durvalumab (PD‐L1) | D933KC00001 (NCT03519971) | Stage III, unresectable | Durv + Pt doublet + RT | Placebo + Pt doublet + RT | PFS/ORR | September 30, 2020 |
| Nivolumab (PD‐1) | RTOG 3,505 (NCT02768558) | Stage III, unresectable | Niv following Pt doublet + RT | Placebo following Pt doublet + RT | OS/PFS | October 2022 |
| First‐line advanced | ||||||
| Checkpoint inhibitor and chemotherapy combinations | ||||||
| Camrelizumab (PD‐1) | SHR‐1210‐III‐303‐NSCLC (NCT03134872) | Nonsquamous, EGFR−/ALK−, PD‐L1 unselected | Cam + Pt + Pem then optional maintenance Cam and Pem | Pt + Pem then optional maintenance Pem | PFS | December 31, 2018 |
| Nivolumab (PD‐1) | EDEN (NCT03542461) | Squamous, PD‐L1 unselected | Pt doublet then maintenance Niv | Pt doublet followed by BSC until PD then Niv (2nd‐line) | OS | September 30, 2022 |
| Checkpoint inhibitor and immunotherapy combinations | ||||||
| Durvalumab (PD‐L1) | NEPTUNE (NCT02542293) | EGFR−/ALK−,PD‐L1 unselected | Durv + tremelimumab | Pt doublet | OS | October 4, 2018 |
| Durvalumab (PD‐L1) | MYSTIC (NCT02453282) | EGFR−/ALK−, PD‐L1 unselected | Durv ± tremelimumab | Pt doublet | OS/PFS | December 31, 2018 |
| Nivolumab (PD‐1) | CheckMate 9LA (NCT03215706) | PD‐L1 unselected | Niv + ipilimumab + Pt doublet | Pt doublet | OS | August 25, 2019 |
| Durvalumab (PD‐L1) | POSEIDON (NCT03164616) | EGFR−/ALK−, PD‐L1 unselected | Durv + Pt doublet ± tremelimumab | Pt doublet | PFS | September 30, 2019 |
| Nivolumab (PD‐1) | eNERGY (NCT03351361) | PS 2 or >75 years, EGFR−/ALK−, PD‐L1 unselected | Niv + ipilimumab | Pt doublet then maintenance Pem | OS | June 2021 |
| Cemiplimab (PD‐1) | R2810‐ONC‐16113 (NCT03409614) | EGFR−/ALK−, PD‐L1 <50% | Cem + Pt doublet or Cem + abbreviated Pt doublet + ipilimumab | Pt doublet | PFS | July 18, 2022 |
| Nivolumab (PD‐1) | LONESTAR (NCT03391869) | Immunotherapy‐naive, EGFR−/ALK−, PD‐L1 unselected | Niv + ipilimumab then LCT (surgery ± RT) | Niv + ipilimumab | OS | December 2022 |
| Cemiplimab (PD‐1) | R2810‐ONC‐16111 (NCT03515629) | EGFR−/ALK−, PD‐L1 ≥50% | Cem + ipilimumab ± Pt doublet | Pembro | PFS | December 22, 2022 |
| Pembrolizumab (PD‐1) | KEYNOTE‐598 (NCT03302234) | EGFR−/ALK−, PD‐L1 ≥50% | Pembro + ipilimumab | Pembro + placebo | OS/PFS | February 28, 2023 |
| Nivolumab (PD‐1) | DICIPLE (NCT03469960) | EGFR−/ALK−, PD‐L1 1–49% | Niv + ipilimumab (6 months) then observation until PD then Niv + ipilimumab until PD then Pt doublet | Niv + ipilimumab until PD then Pt doublet | PFS | May 2023 |
| Checkpoint inhibitor and targeted therapy combinations | ||||||
| Nivolumab (PD‐1) | ONO‐4538‐52 (NCT03117049) | Nonsquamous, EGFR−/ALK−, PD‐L1 unselected | Niv + bevacizumab + Pt + Pac | Placebo + bevacizumab + Pt + Pac | PFS | April 2020 |
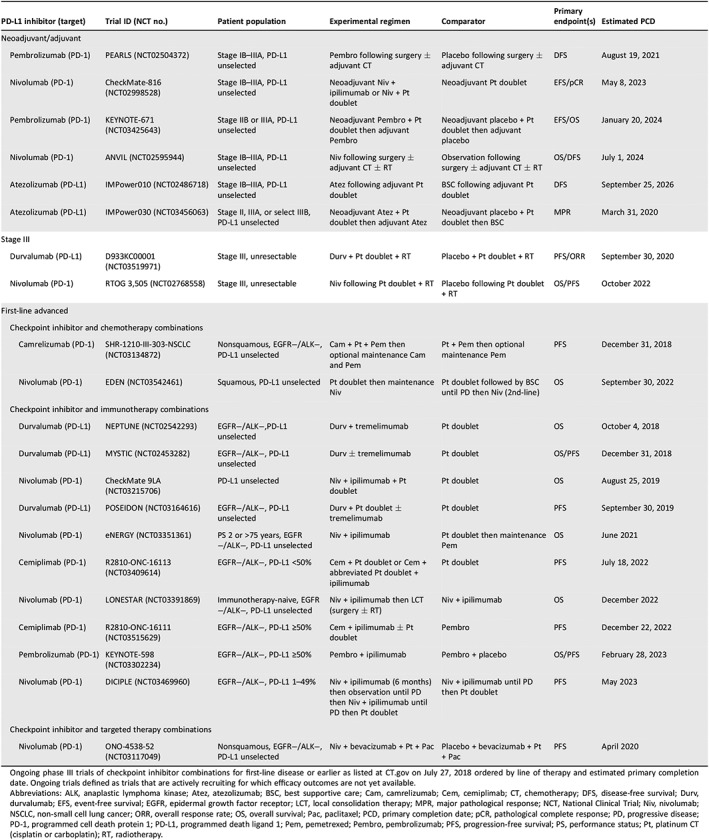
Ongoing phase III trials of checkpoint inhibitor combinations for first‐line disease or earlier as listed at http://ct.gov on July 27, 2018 ordered by line of therapy and estimated primary completion date. Ongoing trials defined as trials that are actively recruiting for which efficacy outcomes are not yet available.
Abbreviations: ALK, anaplastic lymphoma kinase; Atez, atezolizumab; BSC, best supportive care; Cam, camrelizumab; Cem, cemiplimab; CT, chemotherapy; DFS, disease‐free survival; Durv, durvalumab; EFS, event‐free survival; EGFR, epidermal growth factor receptor; LCT, local consolidation therapy; MPR, major pathological response; NCT, National Clinical Trial; Niv, nivolumab; NSCLC, non‐small cell lung cancer; ORR, overall response rate; OS, overall survival; Pac, paclitaxel; PCD, primary completion date; pCR, pathological complete response; PD, progressive disease; PD‐1, programmed cell death protein 1; PD‐L1, programmed death ligand 1; Pem, pemetrexed; Pembro, pembrolizumab; PFS, progression‐free survival; PS, performance status; Pt, platinum CT (cisplatin or carboplatin); RT, radiotherapy.
Summary
First‐line Pembro‐pemCT, Atez‐CnP, and Atez‐Bev‐CP showed significantly improved overall survival compared with controls in patients with advanced, nonsquamous, EGFR−/ALK− NSCLC, and Pembro‐C(n)P significantly improved OS compared with chemotherapy in advanced squamous NSCLC. Either pembrolizumab monotherapy or pembrolizumab plus chemotherapy, administered based on PD‐L1 status and histology, are preferred treatment options for first‐line patients, and the use of Atez‐Bev‐CP in patients with EGFR+/ALK+ disease is encouraging and requires further exploration.
Author Contributions
Conception/design: Barbara Melosky, Deanna McLeod, Quincy Chu
Collection and/or assembly of data: Barbara Melosky, Deanna McLeod, Paul B. Card
Data analysis and interpretation: Barbara Melosky, Rosalyn Juergens, Vera Hirsh, Deanna McLeod, Natasha Leighl, Ming‐Sound Tsao, Paul B. Card, Quincy Chu
Manuscript writing: Barbara Melosky, Rosalyn Juergens, Vera Hirsh, Deanna McLeod, Natasha Leighl, Ming‐Sound Tsao, Paul B. Card, Quincy Chu
Final approval of manuscript: Barbara Melosky, Rosalyn Juergens, Vera Hirsh, Deanna McLeod, Natasha Leighl, Ming‐Sound Tsao, Paul B. Card, Quincy Chu
Disclosures
Barbara Melosky: Merck, Bristol‐Myers Squibb, Boehringer Ingelheim, Eli Lilly & Co, Novartis, AstraZeneca (C/A, H), Roche (RF); Rosalyn Juergens: Amgen, AstraZeneca, Boehringer Ingelheim, Bristol‐Myers Squibb, EMD Serono, Lilly, Merck Sharp & Dohme, Novartis, Pfizer, Roche, Takeda (C/A, H), AstraZeneca, Bristol‐Myers Squibb, Merck Sharp & Dohme, Fusion Pharmaceuticals, Turnstone Biologics, Debio Pharmaceuticals (RF); Natasha Leighl: Novartis Canada (RF); Deanna McLeod: Merck, Roche, AstraZeneca, Bristol‐Myers Squibb (RF); Ming‐Sound Tsao: Merck, Bristol‐Myers Squibb, AstraZeneca, Abbvie, Celgene, Bayer, Pfizer (C/A, H), Merck (RF), Merck, Bristol‐Myers Squibb (H); Quincy Chu: Abbvie, AstraZeneca, Bayer, Bristol‐Myers Squibb, Boehringer Ingelheim, Eisai, Merck, Novartis, Eli Lilly & Co, Genentech/Roche, Takeda (C/A, H), AstraZeneca (RF), Merck (H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
We thank Ilidio Martins from Kaleidoscope Strategic Inc. for his research support and AstraZeneca Canada, Roche Canada, Bristol‐Myers Squibb Canada, and Merck Canada for their financial support. Funding for this review was provided through unrestricted educational grants from AstraZeneca Canada, Roche Canada, Bristol‐Myers Squibb Canada, and Merck Canada. No discussion or viewing of review content was permitted with sponsors at any stage of review development. This review was prepared according to ICMJE standards with editorial assistance from Kaleidoscope Strategic Inc.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Bray F, Ferlay J, Soerjomataram I et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2. Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med 2008;359:1367–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Travis WD, Travis LB, Devesa SS. Lung cancer. Cancer 1995;75:191–202. [DOI] [PubMed] [Google Scholar]
- 4. McKeage MJ, Jameson MB; AS1404‐201 Study Group Investigators . Comparative outcomes of squamous and non‐squamous non‐small cell lung cancer (NSCLC) patients in phase II studies of ASA404 (DMXAA) ‐ retrospective analysis of pooled data. J Thorac Dis 2010;2:199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cheng T‐YD, Cramb SM, Baade PD et al. The international epidemiology of lung cancer: Latest trends, disparities, and tumor characteristics. J Thorac Oncol 2016;11:1653–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hirsh V. Turning EGFR mutation‐positive non‐small‐cell lung cancer into a chronic disease: Optimal sequential therapy with EGFR tyrosine kinase inhibitors. Ther Adv Med Oncol 2018;10:1758834017753338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Williams AS, Greer W, Bethune D et al. ALK+ lung adenocarcinoma in never smokers and long‐term ex‐smokers: Prevalence and detection by immunohistochemistry and fluorescence in situ hybridization. Virchows Arch 2016;469:533–540. [DOI] [PubMed] [Google Scholar]
- 8. Salido M, Pijuan L, Martínez‐Avilés L et al. Increased ALK gene copy number and amplification are frequent in non‐small cell lung cancer. J Thorac Oncol 2011;6:21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chan BA and Hughes BG. Targeted therapy for non‐small cell lung cancer: Current standards and the promise of the future. Transl Lung Cancer Res 2015;4:36–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garon EB, Rizvi NA, Hui R et al. Pembrolizumab for the treatment of non‐small‐cell lung cancer. N Engl J Med 2015;372:2018–2028. [DOI] [PubMed] [Google Scholar]
- 11. Herbst RS, Baas P, Kim DW et al. Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): A randomised controlled trial. Lancet 2016;387:1540–1550. [DOI] [PubMed] [Google Scholar]
- 12. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Teixidó C, Vilariño N, Reyes R et al. PD‐L1 expression testing in non‐small cell lung cancer. Ther Adv Med Oncol 2018;10:1758835918763493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harvey RD. Immunologic and clinical effects of targeting PD‐1 in lung cancer. Clin Pharmacol Ther 2014;96:214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol 2015;33:1974–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reck M, Rodriguez‐Abreu D, Robinson AG et al. Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. N Engl J Med 2016;375:1823‐1833. [DOI] [PubMed] [Google Scholar]
- 17. Brahmer J, Rodríguez‐Abreu D, Robinson A, et al. Updated analysis of KEYNOTE‐024: Pembrolizumab vs platinum‐based chemotherapy for advanced NSCLC with PD‐L1 TPS ≥50%. J Thorac Oncol 2017;12(suppl 2):OA17.06. [Google Scholar]
- 18. Brahmer J, Reckamp KL, Baas P et al. Nivolumab versus docetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N Engl J Med 2015;373:123–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Felip Font E, Gettinger SN, Burgio M et al. Three‐year follow‐up from CHECKMATE 017/057: Nivolumab versus docetaxel in patients with previously treated advanced non‐small cell lung cancer (NSCLC). Ann Oncol 2017;28:1301PDa. [DOI] [PubMed] [Google Scholar]
- 20. Borghaei H, Paz‐Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med 2015;373:1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Herbst R, Garon E, Kim D‐W et al. KEYNOTE‐010: Durable clinical benefit in patients with previously treated, PD‐L1‐expressing NSCLC who completed pembrolizumab. J Thorac Oncol 2016;12: OA03.07 [Google Scholar]
- 22. Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non‐small‐cell lung cancer (OAK): A phase 3, open‐label, multicentre randomised controlled trial. Lancet 2017;389:255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ettinger DS, Wood DE, Aisner DL et al. Non‐small cell lung cancer, version 5.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2017;15:504‐535. [DOI] [PubMed] [Google Scholar]
- 24. Hanna N, Johnson D, Temin S et al. Systemic therapy for stage IV non‐small‐cell lung cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2017;35:3484–3515. [DOI] [PubMed] [Google Scholar]
- 25. Novello S, Barlesi F, Califano R et al. Metastatic non‐small‐cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2016;27(suppl 5):v1–v27. [DOI] [PubMed] [Google Scholar]
- 26. Recondo G, Facchinetti F, Olaussen KA et al. Making the first move in EGFR‐driven or ALK‐driven NSCLC: First‐generation or next‐generation TKI? Nat Rev Clin Oncol 2018;15:694–708. [DOI] [PubMed] [Google Scholar]
- 27. Pembrolizumab (KEYTRUDA) . Updated March, 2017. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125514s014lbl.Pdf. Accessed May 29, 2018.
- 28. Zhang P, Su DM, Liang M et al. Chemopreventive agents induce programmed death‐1‐ligand 1 (PD‐L1) surface expression in breast cancer cells and promote PD‐L1‐mediated T cell apoptosis. Mol Immunol 2008;45:1470–1476. [DOI] [PubMed] [Google Scholar]
- 29. Dovedi SJ, Adlard AL, Lipowska‐Bhalla G et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD‐L1 blockade. Cancer Res 2014;74:5458–5468. [DOI] [PubMed] [Google Scholar]
- 30. Shrimali RK, Yu Z, Theoret MR et al. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res 2010;70:6171–6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Reits EA, Hodge JW, Herberts CA et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med 2006;203:1259–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van der Most RG, Currie AJ, Mahendran S et al. Tumor eradication after cyclophosphamide depends on concurrent depletion of regulatory T cells: A role for cycling TNFR2‐expressing effector‐suppressor T cells in limiting effective chemotherapy. Cancer Immunol Immunother 2009;58:1219–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zitvogel L, Galluzzi L, Smyth MJ et al. Mechanism of action of conventional and targeted anticancer therapies: Reinstating immunosurveillance. Immunity 2013;39:74–88. [DOI] [PubMed] [Google Scholar]
- 34. Simpson TR, Li F, Montalvo‐Ortiz W et al. Fc‐dependent depletion of tumor‐infiltrating regulatory T cells co‐defines the efficacy of anti‐CTLA‐4 therapy against melanoma. J Exp Med 2013;210:1695–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu SV, Camidge DR, Gettinger SN et al. Atezolizumab (atezo) plus platinum‐based chemotherapy (chemo) in non‐small cell lung cancer (NSCLC): Update from a phase Ib study. J Clin Oncol 2017;35:9092. [Google Scholar]
- 36. Antonia S, Goldberg SB, Balmanoukian A et al. Safety and antitumour activity of durvalumab plus tremelimumab in non‐small cell lung cancer: A multicentre, phase 1b study. Lancet Oncol 2016;17:299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Borghaei H, Langer C, Gadgeel S et al. Pemetrexed‐carboplatin plus pembrolizumab as first‐line therapy for advanced nonsquamous NSCLC: KEYNOTE‐021 cohort G update. J Thorac Oncol 2017;12(suppl 2):OA17.01a. [DOI] [PubMed] [Google Scholar]
- 38. Juergens R, Hellmann M, Brahmer J et al. First‐line nivolumab plus platinum‐based doublet chemotherapy for advanced NSCLC: CHECKMATE 012 3‐year update. J Thorac Oncol 2017;12(suppl 2):OA17.03a. [Google Scholar]
- 39.FDA grants regular approval for pembrolizumab in combination with chemotherapy for first‐line treatment of metastatic nonsquamous NSCLC. U.S. Food and Drug Administration website. Available at https://www.Fda.Gov/drugs/informationondrugs/approveddrugs/ucm617471.Htm. August 20, 2018. Accessed October 22, 2018.
- 40.FDA approves atezolizumab with chemotherapy and bevacizumab for first‐line treatment of metastatic non‐squamous NSCLC. U.S. food and Drug Administration website. Available at https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm627874.Htm. December 12, 2018. Accessed February 28, 2019.
- 41.FDA approves pembrolizumab in combination with chemotherapy for first‐line treatment of metastatic squamous NSCLC. Available at https://www.Fda.Gov/drugs/informationondrugs/approveddrugs/ucm624659.Htm. December 14, 2018. Accessed February 22, 2019.
- 42. Cappuzzo F, McCleod M, Hussein M et al. IMpower130: Efficacy and safety from a randomised phase 3 study of carboplatin and nab‐paclitaxel with or without atezolizumab in 1L advanced non‐squamous NSCLC. Abstract presented at: the European Society for Medical Oncology Congress; October 19–23, 2018; Munich, Germany; LBA53.
- 43. Gandhi L, Rodríguez‐Abreu D, Gadgeel S et al. Pembrolizumab plus chemotherapy in metastatic non‐small‐cell lung cancer. New Engl J Med 2018;378:2078–2092. [DOI] [PubMed] [Google Scholar]
- 44. Hellmann MD, Ciuleanu TE, Pluzanski A et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. New Engl J Med 2018;378:2093–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jotte RM, Cappuzzo F, Vynnychenko I et al. IMpower131: Primary PFS and safety analysis of a randomized phase III study of atezolizumab + carboplatin + paclitaxel or nab‐paclitaxel vs carboplatin + nab‐paclitaxel as 1L therapy in advanced squamous NSCLC. J Clin Oncol 2018;36:LBA9000a. [Google Scholar]
- 46. Papadimitrakopoulou VA, Cobo M, Bordoni R et al. IMpower132: PFS and safety results with 1L atezolizumab + carboplatin/cisplatin + pemetrexed in stage IV non‐squamous NSCLC. Abstract presented at: World Conference on Lung Cancer 2018; September 23–26, 2018; Toronto, Canada; OA05.07
- 47. Paz‐Ares L, Luft A, Vicente D et al. Pembrolizumab plus chemotherapy for squamous non‐small‐cell lung cancer. N Engl J Med 2018;379:2040–2051. [DOI] [PubMed] [Google Scholar]
- 48. Socinski MA, Jotte RM, Cappuzzo F et al. Atezolizumab for first‐line treatment of metastatic nonsquamous NSCLC. N Engl J Med 2018;378:2288–2301. [DOI] [PubMed] [Google Scholar]
- 49. Socinski MA, Jotte RM, Cappuzzo F, et al. Overall survival (OS) analysis of IMpower150, a randomized Ph 3 study of atezolizumab (atezo) + chemotherapy (chemo) ± bevacizumab (bev) vs chemo + bev in 1l nonsquamous (NSQ) NSCLC. J Clin Oncol 2018;36:9002. [Google Scholar]
- 50. Langer CJ, Gadgeel SM, Borghaei H et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non‐squamous non‐small‐cell lung cancer: A randomised, phase 2 cohort of the open‐label KEYNOTE‐021 study. Lancet Oncol 2016;17:1497–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Papadimitrakopoulou V, Gadgeel SM, Borghaei H et al. First‐line carboplatin and pemetrexed (CP) with or without pembrolizumab (pembro) for advanced nonsquamous NSCLC: Updated results of KEYNOTE‐021 cohort G. J Clin Oncol 2017;35:9094a. [Google Scholar]
- 52. Socinski MA, Rittmeyer A, Shapovalov D et al. IMpower131: Progression‐free survival (PFS) and overall survival (OS) analysis of a randomised phase III study of atezolizumab 1 carboplatin 1 paclitaxel or nab‐paclitaxel vs carboplatin 1 nabpaclitaxel in 1L advanced squamous NSCLC. Abstract presented at: the European Society for Medical Oncology Congress; October 19–23, 2018; Munich, Germany; LBA65.
- 53. Hellmann MD, Rizvi NA, Goldman JW et al. Nivolumab plus ipilimumab as first‐line treatment for advanced non‐small‐cell lung cancer (CHECKMATE 012): Results of an open‐label, phase 1, multicohort study. Lancet Oncol 2017;18:31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ramalingam SS, Hellmann M, Awad M et al. Tumor mutational burden (TMB) as a biomarker for clinical benefit from dual immune checkpoint blockade with nivolumab (NIVO) + ipilimumab (IPI) in first‐line (1L) non‐small cell lung cancer (NSCLC): Identification of TMB cutoff from CHECKMATE 568. Presented at: American Association for Cancer Research Annual Meeting; April 16, 2018; Chicago, Illinois.
- 55.Press release: Bristol‐Myers Squibb provides update on the ongoing regulatory review of opdivo plus low‐dose yervoy in first‐line lung cancer patients with tumor mutational burden ≥10 mut/Mb. Bristol‐Myers Squibb website. Available at https://news.Bms.Com/press-release/corporatefinancial-news/bristol-myers-squibb-provides-update-ongoing-regulatory-review. October 19, 2018. Accessed February 22, 2019.
- 56. Garassino MC, Rodriguez‐Abreu D, Gadgeel SM et al. Health‐related quality of life (HRQoL) in the KEYNOTE‐189 study of pembrolizumab (pembro) or placebo (pbo)+ pemetrexed (pem)+ platinum (plt) for metastatic NSCLC. Presented at: the American Society of Clinical Oncology Annual Meeting; June 1–5, 2018; Chicago, IL.
- 57. Reck M, Karagiannis T, Wehler T et al. Patient‐reported outcomes (PROs) in the randomized, phase III iMpower150 study of atezolizumab (atezo)+ chemotherapy (chemo)±bevacizumab (bev) vs chemo+ bev in 1L nonsquamous metastatic NSCLC (mnsclc): Presented at: the American Society of Clinical Oncology Annual Meeting; June 1–5, 2018; Chicago, IL.
- 58.FDA accepts Genentech's supplemental biologics license application for Tecentriq plus chemotherapy (abraxane and carboplatin) for the initial treatment of metastatic nonsquamous non‐small cell lung cancer. Genentech (roche). Available at https://www.gene.com/media/press-releases/14773/2019%E2%80%9301%E2%80%9316/fda-accepts-genentechs-supplemental-biol. Januay 16, 2019. Accessed February 28, 2019.
- 59. Mazie'res J, Kowalski D, Luft A et al. Health‐related quality of life (HRQoL) for pembrolizumab or placebo plus carboplatin and paclitaxel or nab‐paclitaxel in patients with metastatic squamous NSCLC: Data from KEYNOTE‐407. Presented at: the European Society for Medical Oncology Congress; October 19–23, 2018; Munich, Germany; LBA62.
- 60. Pirker R. Biomarkers for immune checkpoint inhibitors in advanced nonsmall cell lung cancer. Curr Opin Oncol 2019;31:24–28. [DOI] [PubMed] [Google Scholar]
- 61. Gandhi L, Rodgríguez‐Abreu D, Gadgeel S et al. KEYNOTE‐189: Randomized, double‐blind, phase 3 study of pembrolizumab (pembro) or placebo plus pemetrexed (pem) and platinum as first‐line therapy for metastatic NSCLC. Presented at: the American Association for Cancer Research Annual Meeting; April 14–18, 2018; Chicago, IL.
- 62. Rizvi NA, Hellmann MD, Snyder A et al. Cancer immunology. Mutational landscape determines sensitivity to PD‐1 blockade in non‐small cell lung cancer. Science 2015;348:124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hellmann M, Rizvi N, Wolchok JD et al. Genomic profile, smoking, and response to anti‐PD‐1 therapy in non‐small cell lung carcinoma. Mol Cell Oncol 2016;3:e1048929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kowantz M, Socinski MA, Zou W et al. IMpower150: Efficacy of atezolizumab (atezo) plus bevacizumab (bev) and chemotherapy (chemo) in 1l metastatic nonsquamous NSCLC (mNSCLC) across key subgroups. Presented at: American Association for Cancer Research Annual Meeting; April 16, 2018; Chicago, IL.
- 65. Kowanetz M, Zou W, McCleland M et al. Pre‐existing immunity measured by teff gene expression in tumor tissue is associated with atezolizumad efficacy in NSCLC. J Thorac Oncol 2017;12(suppl 2):MA05.09a. [Google Scholar]
- 66. Cristescu R, Mogg R, Ayers M et al. Pan‐tumor genomic biomarkers for PD‐1 checkpoint blockade‐based immunotherapy. Science 2018;362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Peters S, Creelan B, Hellmann MD et al. Impact of tumor mutation burden on the efficacy of first‐line nivolumab in stage IV or recurrent non‐small cell lung cancer: An exploratory analysis of CheckMate 026. Cancer Res 2017;77(suppl):CT082a. [Google Scholar]
- 68. Reck M, Hellmann MD, Paz‐Ares LG et al. Nivolumab (Nivo)+ ipilimumab (Ipi) vs platinum‐doublet chemotherapy (Chemo) as first‐line (1L) treatment (Tx) for advanced non‐small cell lung cancer (NSCLC): Safety analysis and patient‐reported outcomes (PROs) from checkmate 227. Presented at: the American Society of Clinical Oncology Annual Meeting; June 1–5, 2018; Chicago, IL.
- 69. Paz‐Ares LG, Luft A, Tafreshi A et al. Phase 3 study of carboplatin‐paclitaxel/nab‐paclitaxel (Chemo) with or without pembrolizumab (Pembro) for patients (Pts) with metastatic squamous (Sq) non‐small cell lung cancer (NSCLC). J Clin Oncol 2018;36:105. [Google Scholar]
- 70. Felip E, De Braud FG, Maur M et al. Ceritinib plus nivolumab (nivo) in patients (pts) with anaplastic lymphoma kinase positive (alk+) advanced non‐small cell lung cancer (NSCLC). J Clin Oncol 2017;35(suppl):2502a. [Google Scholar]
- 71. Kim DW, Gadgeel SM, Gettinger SN et al. Safety and clinical activity results from a phase Ib study of alectinib plus atezolizumab in ALK+ advanced NSCLC (aNSCLC). J Clin Oncol 2018;36(suppl):9009a. [Google Scholar]
- 72. Ma B, Rudin C, Cervantes A et al. Preliminary safety and clinical activity of erlotinib plus atezolizumab from a phase Ib study in advanced NSCLC. Ann Oncol 2016;27:4410a. [Google Scholar]
- 73. Shaw AT, Lee SH, Ramalingam SS et al. Avelumab (anti–PD‐L1) in combination with crizotinib or lorlatinib in patients with previously treated advanced NSCLC: Phase 1b results from JAVELIN Lung 101. J Clin Oncol 2018;36(suppl):9008a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Spigel D, Reynolds C, Waterhouse D et al. Phase 1/2 study of the safety and tolerability of nivolumab plus crizotinib for the first‐line treatment of ALK translocation‐positive advanced non‐small cell lung cancer (CheckMate 370). J Thorac Oncol 2018;13:682–688. [DOI] [PubMed] [Google Scholar]
- 75. Antonia SJ, Villegas A, Daniel D et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 2018;379:2342–2350. [DOI] [PubMed] [Google Scholar]
- 76.FDA approves durvalumab after chemoradiation for unresectable stage III NSCLC. U.S. Food and Drug Administration website. Available at https://www.Fda.Gov/drugs/informationondrugs/approveddrugs/ucm597248.Htm. February 20, 2018. Accessed November 10, 2018.
- 77. Durm GA, Althouse SK, Sadiq AA et al. Phase II trial of concurrent chemoradiation with consolidation pembrolizumab in patients with unresectable stage III non‐small cell lung cancer: Hoosier Cancer Research Network LUN 14–179. J Clin Oncol 2018;36(suppl):8500a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lin S, Lin X, Clay D et al. DETERRED: Phase II trial combining atezolizumab concurrently with chemoradiation therapy in locally advanced non‐small cell lung cancer. J Thorac Oncol 2018;13(suppl):OA01.06a. [Google Scholar]
- 79. Peters S, Ruysscher DD, Dafni U et al. Safety evaluation of nivolumab added concurrently to radiotherapy in a standard first line chemo‐RT regimen in unresectable locally advanced NSCLC: The ETOP NICOLAS phase II trial. J Clin Oncol 2018;36(suppl):8510. [Google Scholar]
- 80. Fournier C, Rivera Vargas T, Martin T et al. Immunotherapeutic properties of chemotherapy. Curr Opin Pharmacol 2017;35:83–88. [DOI] [PubMed] [Google Scholar]
- 81. Heist RS. PD‐(L) 1 inhibitors and CTLA‐4 inhibitors: Rationale for combinations and recent data in non‐small cell lung cancer. Am J Hematol Oncol 2015;11:21–25. [Google Scholar]
- 82. Harris SJ, Brown J, Lopez J et al. Immuno‐oncology combinations: Raising the tail of the survival curve. Cancer Biol Med 2016;13:171–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lin EPY, Yang CY, Lin CW, et al. Priming PD‐L1 expression by chemotherapeutic agents in non‐small cell lung cancers. J Clin Oncol 2017;35:e20087. [Google Scholar]
- 84. Stewart R, Morrow M, Hammond SA et al. Identification and characterization of MEDI4736, an antagonistic anti‐PD‐L1 monoclonal antibody. Cancer Immunol Res 2015;3:1052–1062. [DOI] [PubMed] [Google Scholar]
- 85. Chakraborty M, Abrams SI, Coleman CN et al. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine‐mediated T‐cell killing. Cancer Res 2004;64:4328–4337. [DOI] [PubMed] [Google Scholar]
- 86. Deng L, Liang H, Burnette B et al. Irradiation and anti‐PD‐L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest 2014;124:687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Lugade AA, Moran JP, Gerber SA et al. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen‐specific effector cells that traffic to the tumor. J Immunol 2005;174:7516–7523. [DOI] [PubMed] [Google Scholar]
- 88. Momtaz P, Postow MA. Immunologic checkpoints in cancer therapy: Focus on the programmed death‐1 (PD‐1) receptor pathway. Pharmgenomics Pers Med 2014;7:357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Bulliard Y, Jolicoeur R, Windman M et al. Activating Fc gamma receptors contribute to the antitumor activities of immunoregulatory receptor‐targeting antibodies. J Exp Med 2013;210:1685–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Selby MJ, Engelhardt JJ, Quigley M et al. Anti‐CTLA‐4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol Res 2013;1:32–42. [DOI] [PubMed] [Google Scholar]
- 91. Tang F, Du X, Liu M et al. Anti‐CTLA‐4 antibodies in cancer immunotherapy: Selective depletion of intratumoral regulatory T cells or checkpoint blockade? Cell Biosci 2018;8:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Grosso JF, Jure‐Kunkel MN. CTLA‐4 blockade in tumor models: An overview of preclinical and translational research. Cancer Immun 2013;13:5. [PMC free article] [PubMed] [Google Scholar]
- 93. Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA‐4 blockade. Science 1996;271:1734–1736. [DOI] [PubMed] [Google Scholar]


