Abstract
Cervical carcinosarcoma is an extremely rare type of neoplasm that lacks standard of care. Preclinical and clinical evidence has suggested that cryoablation in combination with immunotherapy may result in a synergistic effect, generating a more robust immune response to distant lesions. A few clinical trials have evaluated the efficacy of such combination treatment in a variety of solid tumors, but with conflicting results. This report describes the first clinical efficacy of cryoablation followed by pembrolizumab observed in a patient with tumor mutational burden (TMB)‐high metastatic cervical carcinosarcoma that was negative for programmed cell death protein 1 expression, microsatellite instability stable, and had mutations in DNA polymerase epsilon (POLE). She had achieved complete response (CR) after 3 months of pembrolizumab treatment and had maintained CR as of the time of submission of this manuscript, with a progression‐free survival of 11 months and counting. The case exhibited an exceptional response to cryoablation followed by pembrolizumab, potentially attributed to mutations in POLE, which lead to an extremely high TMB. This report paves the avenue for establishing treatment regimens for patients with TMB‐high cervical carcinosarcoma.
Key Points
Owing to its rarity, cervical carcinosarcoma has not been well characterized, and currently, there is no standard of care for this disease.
This report describes the first case of clinical efficacy of cryoablation followed by pembrolizumab observed in a patient with tumor mutational burden‐high metastatic cervical carcinosarcoma.
The case exhibited an exceptional response (maintained CR as of the time of submission of this article: 11 months) to cryoablation followed by pembrolizumab.
This is the first POLE‐mutated cervical carcinosarcoma case.
Short abstract
This article presents the first case showing the clinical efficacy of cryoablation followed by pembrolizumab observed in a patient with tumor mutational burden‐high metastatic cervical carcinosarcoma.
Introduction
Carcinosarcomas are considered as mixed tumors with both malignant epithelial and malignant mesenchymal components, although at first they were referred to as sarcomas 1. They occur relatively commonly in the corpus of the uterus but are extremely rare in the cervix, constituting less than 1% of all cervical malignancies 2. Approximately only 80 cases have been reported in the English literature so far 3, 4, 5, and 128 cases have been documented according to the National Cancer Institute's Surveillance, Epidemiology, and End Results database 2. Owing to their rarity, cervical carcinosarcomas have not been well characterized, and currently there is no standard of care for this disease 5, 6. A recent meta‐analysis revealed that the 2‐year disease‐free survival and overall survival were 49% and 60%, respectively 5. Better prognosis was observed in the subgroup of patients with surgery followed by radiotherapy with or without chemotherapy.
It is hypothesized that after cryoablation, the intracellular contents of the damaged cancer cells are preserved and can be recognized by the immune system, thus allowing the stimulation of an immune‐specific reaction. Therefore, combination of cryoablation with immunotherapy is believed to enhance the efficacy of both therapies 7, 8. Both in vivo and clinical studies have shown favorable efficacies in cryoablation combined with immunotherapies. Currently, numerous clinical trials of cryoablation in combination with immunotherapy are ongoing in a variety of solid tumors including breast cancers, renal cell carcinoma, prostate cancer, and lung cancer (reviewed in 7). Here, we present the first case of clinical efficacy of cryoablation followed by pembrolizumab observed in a patient with TMB‐high metastatic cervical carcinosarcoma who had achieved complete response (CR) after 3 months of pembrolizumab treatment and had maintained CR as of the time of the submission of this manuscript, with a PFS of 11 months and counting.
Patient Story
A 58‐year‐old woman with metastatic cervical carcinosarcoma was referred to our clinic on September 10, 2018. The diagnostic and treatment history of the patient is shown in Figure 1. The patient had been initially diagnosed with stage IIb cervical carcinosarcoma in 2014 and treated with paclitaxel plus cisplatin for two cycles. Subsequently, conformal radiation therapy (50Gy in 25 fractions) with concurrent chemotherapy of cisplatin and afterloading brachytherapy (30Gy/5F) were given beginning November 2014, and complete response was achieved. The patient had regular visits from 2015 to May 2017, and no recurrence was detected. The patient started to experience left lumbar pain, progressive aggravation, and radiating pain on left lower limb from January, 2018. In May 2018, a positron emission tomography–computed tomography (CT) scan revealed a heterogeneous mass with a high metabolic activity in the left retroperitoneum accompanied with invasion in the upper left ureter, indicating a retroperitoneal metastasis. Subsequently, the patient was administered with paclitaxel plus lobaplatin for three cycles and developed progressive disease in August 2018.
Figure 1.
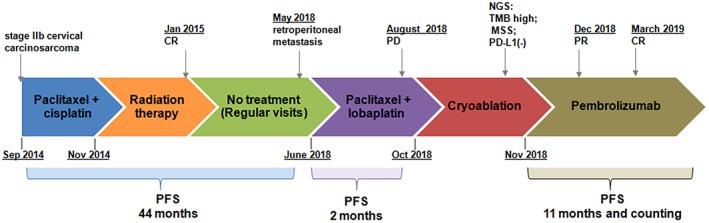
A summary of patient's treatment history.Abbreviations used: CR, complete response; PD, progressive disease; PFS, progression‐free survival; NGS, next‐generation sequencing; TMB, tumor mutation burden MSS, microsatellite stable.
When referred to our clinic, the patient had a Karnofsky performance score (KPS) of 70 and tumor invasion in lumbar vertebra and psoas major. She presented with left lumbar pain and radiating pain on the left lower limb. Her blood test indicated normal hepatorenal and coagulation function. Among the cancer serum biomarkers tested, only human epididymis protein 4 (HE4) was abnormal, measuring 291.1pmol/L, and others (carcinoembryonic antigen, alpha‐fetoprotein, cancer antigen 125, and cancer antigen 153) were all with the normal range. The metastatic lesion was examined by an enhanced CT scan measuring 7.2cm × 6.8cm × 8.5cm, which was heavily surrounded by blood vessels (Fig. 2A). Given prior intolerance to chemotherapy and radiotherapy, the patient received cryoablation. An argon‐helium cryosurgery was performed on October 18, 2018. An enhanced CT scan was performed 2 weeks postsurgery and showed an ablation rate of 80% (Fig. 2B). Both tumor and plasma samples obtained at cryosurgery were subjected to NGS‐based mutation profiling, TMB, and microsatellite instability (MSI) evaluation using a panel consisting of 520 cancer‐related genes. The sequencing results of her tumor tissue sample showed a total of 590 somatic mutations, including two POLE mutations (p.Pro286Arg and p.Ala724Val) and a TMB of 691.3 mutations per Mb; however, her MSI status was stable. In contrast, the result of plasma sample revealed a total of 645 somatic mutations including one POLE mutation (p.Pro286Arg) and a TMB of 731.7 mutations per Mb. Sixteen mutations were detected from both the tissue and the plasma, achieving a concordance of 55%. POLE p.Ala724Val was only detected from the tissue sample. Despite the large number of mutations detected, none of them has targeted therapy. Furthermore, her tissue sample was subjected to an immunohistochemical test and showed negative for the programmed cell death protein 1 protein expression.
Figure 2.
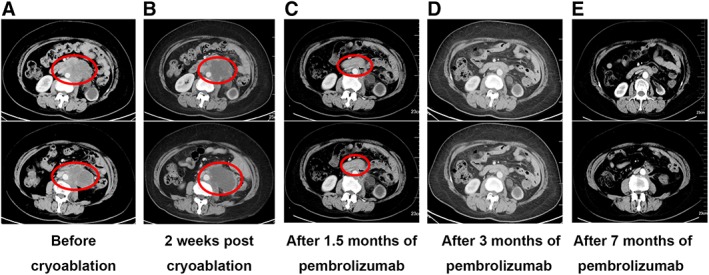
Clinical responses to cryoablation followed by pembrolizumab. The largest cross‐section area of lesion was 7.2 cm × 6.8 cm before cryoablation (A); 2 weeks after the cryoablation 7.3 cm × 6.7 cm (B). After 1.5 months of pembrolizumab treatment, the patient achieved partial response with a significant reduction in the size of lesion, measuring 2.9 cm × 1.5 cm (C). The lesion was undetectable after 3 months of pembrolizumab treatment (D). The patient maintained complete response after 7 months of pembrolizumab treatment (E).
Based on the extremely high TMB, the patient was administered pembrolizumab (200 mg, every 3 weeks) after cryoablation starting from November 2018. After two cycles of treatment, the patient achieved a partial response with a significant decrease in the target lesion (Fig. 2C). On March 5, 2019, a repeated CT scan showed a complete response (CR; Fig. 2D). Furthermore, the changes in HE4 level also reflected the efficacy of the treatment (Fig. 3). No treatment‐related adverse event was observed. The life quality of the patient was improved significantly with a KPS of 90. This patient remained as CR as of the submission of this manuscript, with a PFS of 11 months and counting (Fig. 2E).
Figure 3.
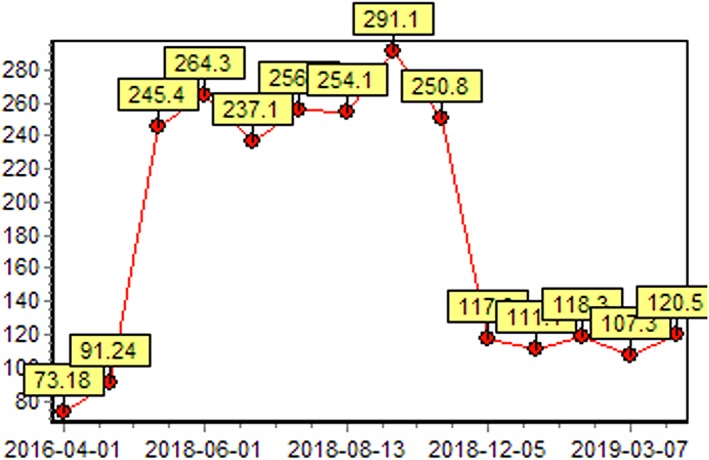
The change of human epididymis protein 4 (HE4) level (pmol/L). The HE4 level decreased and remained within the normal range (<120 pmol/L) after the initiation of pembrolizumab.
Discussion
Here, we present the first case of clinical efficacy of cryoablation followed by pembrolizumab observed in a patient with TMB‐high metastatic cervical carcinosarcoma who had achieved complete response after 3 months of pembrolizumab treatment and remained as CR as the submission of this manuscript, with a PFS of 11 months and counting.
Cryoablation of a solid tumor is mechanistically similar to a vaccination where hundreds of unique tumor‐derived self‐antigens are released into circulation 9. The immune‐specific reaction is believed to affect cancer cells outside of the primary ablation zone, known as the abscopal effect; however, it is rarely observed. Preclinical and clinical evidence has suggested that cryoablation combined with immunotherapy may result in a synergistic effect, generating a more robust immune response to distant lesions 7, 9. A few clinical trials of cryoablation combined with immunotherapies, including but not limited to immune checkpoint inhibitors (ICIs), dendritic cell therapy, NK cell therapy cytokine‐induced killer cells, have evaluated its efficacy in a variety of solid tumors but with conflicting results. A pilot study showed cryoablation in combination with ipilimumab before mastectomy is feasible and safe in early‐stage breast cancer. A single‐arm phase II trial of 12 patients with stage IV androgen‐ablated prostate cancer received pembrolizumab and whole gland cryoablation of the prostate showed that prostate‐specific antigen of five patients dropped below 0.6 ng/mL 1 year after the combination treatment (NCT02489357). However, in a phase II study in non‐small cell lung cancer, only 1 of 11 patients showed response to a combination of ablation and nivolumab (NCT02469701). In this case, the patient exhibited an exceptional response to cryoablation followed by pembrolizumab, potentially attributed to mutations in POLE, which lead to an extreme high TMB. It has been reported that mutation in POLE can result in a hypermutated tumor type, leading to an exceptional response to ICIs in a number of cancers (Fig. 4), including colorectal cancer, endometrial cancer, and glioblastoma 10. To the best of our knowledge, this is the first POLE‐mutated cervical carcinosarcomas case.
Figure 4.
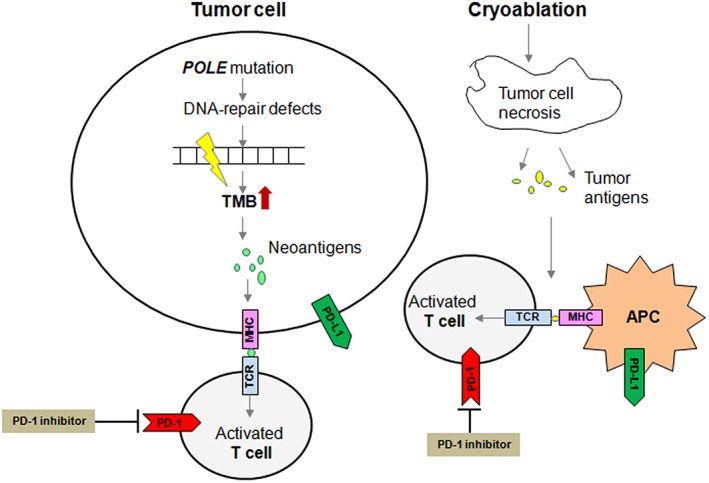
Schematic diagram of the impact of tumor cell with POLE mutation on enhanced immune cell recognition in response to cryoablation and anti‐PD‐1 antibody.Abbreviations: APC, antigen‐presenting cell; TMB, tumor mutational burden.
Author Contributions
Conception/design: Baorang Zhu, Wuwei Yang
Provision of study material or patients: Baorang Zhu, Ying Liu, Jing Li, Liyan Diao, Wuwei Yang
Collection and/or assembly of data: Baorang Zhu, Ying Liu, Jing Li, Liyan Diao, Qiaolin Kang, Wuwei Yang
Data analysis and interpretation: Baorang Zhu, Shao Lin, Han Han‐Zhang, Lu Zhang, Qiaolin Kang
Manuscript writing: Baorang Zhu, Ying Liu, Jing Li, Liyan Diao, Lin Shao, Han Han‐Zhang, Lu Zhang, Wuwei Yang
Final approval of manuscript: Baorang Zhu, Ying Liu, Jing Li, Liyan Diao, Lin Shao, Han Han‐Zhang, Lu Zhang, Qiaolin Kang, Wuwei Yang
Disclosures
Lin Shao: Burning Rock Biotech (E); Qiaolin Kang: Burning Rock Biotech (E). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Lu Z and Chen J. Introduction of who classification of tumours of female reproductive organs, fourth edition [in Chinese]. Zhonghua Bing Li Xue Za Zhi 2014;43:649–650. [PubMed] [Google Scholar]
- 2. Bansal S, Lewin SN, Burke WM et al. Sarcoma of the cervix: Natural history and outcomes. Gynecol Oncol 2010;118:134–138. [DOI] [PubMed] [Google Scholar]
- 3. Kim M, Lee C, Choi H et al. Carcinosarcoma of the uterine cervix arising from mullerian ducts. Obstet Gynecol Sci 2015;58:251–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ribeiro B, Silva R, Dias R et al. Carcinosarcoma of the uterine cervix: A rare pathological finding originating from mesonephric remnants. BMJ Case Rep 2019;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kimyon Comert G, Turkmen O, Karalok A et al. Therapy modalities, prognostic factors, and outcome of the primary cervical carcinosarcoma: Meta‐analysis of extremely rare tumor of cervix. Int J Gynecol Cancer 2017;27:1957–1969. [DOI] [PubMed] [Google Scholar]
- 6. Khosla D, Patel FD, Kumar R. Sarcomas of the uterine cervix: A united and multidisciplinary approach is required. Womens Health (Lond) 2013;9:501–504. [DOI] [PubMed] [Google Scholar]
- 7. Aarts BM, Klompenhouwer EG, Rice SL et al. Cryoablation and immunotherapy: An overview of evidence on its synergy. Insights Imaging 2019;10:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sidana A. Cancer immunotherapy using tumor cryoablation. Immunotherapy 2014;6:85–93. [DOI] [PubMed] [Google Scholar]
- 9. Abdo J, Cornell DL, Mittal SK et al. Immunotherapy plus cryotherapy: Potential augmented abscopal effect for advanced cancers. Front Oncol 2018;8:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang F, Zhao Q, Wang YN et al. Evaluation of pole and pold1 mutations as biomarkers for immunotherapy outcomes across multiple cancer types. JAMA Oncol 2019. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]


