Abstract
Background
Academic physicians, such as those affiliated with National Cancer Institute (NCI)–designated Comprehensive Cancer Centers, may have different practice patterns regarding the use of high‐cost cancer drugs than nonacademic physicians.
Materials and Methods
For this cohort study, we linked cancer registry, administrative, and demographic data for patients with newly diagnosed cancer in North Carolina from 2004 to 2011. We selected cancer types with multiple U.S. Food and Drug Administration–approved, National Comprehensive Cancer Network–recommended treatment options and large differences in reimbursement between higher‐priced and lower‐priced options (stage IV colorectal, stage IV lung, and stage II–IV head‐and‐neck cancers). We assessed whether provider's practice setting—NCI‐designated Comprehensive Cancer Center (“NCI”) versus other location (“non‐NCI”)—was associated with use of higher‐cost treatment options. We used inverse probability of exposure weighting to control for patient characteristics.
Results
Of 800 eligible patients, 79.6% were treated in non‐NCI settings. Patients treated in non‐NCI settings were more likely to receive high‐cost treatment than patients treated in NCI settings (36.0% vs. 23.2%), with an unadjusted prevalence difference of 12.7% (95% confidence interval [CI], 5.1%–20.0%). After controlling for potential confounding factors, non‐NCI patients remained more likely to receive high‐cost treatment, although the strength of association was attenuated (adjusted prevalence difference, 9.6%; 95% CI −0.1%–18.7%). Exploratory analyses suggested potential heterogeneity across cancer type and insurance status.
Conclusion
Use of higher‐cost cancer treatments may be more common in non‐NCI than NCI settings. This may reflect differential implementation of clinical evidence, local practice variation, or possibly a response to the reimbursement incentives presented by chemotherapy billing.
Implications for Practice
Oncology care delivery and practice patterns may vary between care settings. By comparing otherwise similar patients treated in National Cancer Institute (NCI)–designated Comprehensive Cancer Centers with those treated elsewhere, this study suggests that patients may be more likely to receive treatment with certain expensive cancer drugs if treated in the non‐NCI setting. These practice differences may result in differences in patient costs and outcomes as a result of where they receive treatment.
Keywords: Antineoplastic agents; Drug therapy; Practice pattern, clinical; Reimbursement, incentive; Fee‐for‐service plans; National Cancer Institute; Medical overuse; Health services research
Short abstract
This article focuses on whether provider practice setting is associated with the use of higher‐cost cancer treatments that include biologic drugs.
Introduction
Health care spending in the U.S. continues to rise; the U.S. spends more on health care than any other country, projected to reach 20% of the gross domestic product by 2025 1. Cancer care is a significant contributor to this trend, with overall treatment costs expected to increase from $125 billion in 2010 to $173 billion by 2020 2. These trends may have negative consequences for the fiscal sustainability of U.S. safety net health care programs and the unmanageable out‐of‐pocket costs faced by many patients with cancer.
Cancer drug prices are a significant contributor to these high overall costs, accounting for approximately 20% of cancer treatment costs in 2014 3. In recent years, many cancers have seen the development of biologic drugs as a new therapeutic option, often in combination with traditional cytotoxic agents. These drugs have significantly higher prices than older ones; for example, bevacizumab, indicated for colon and lung cancer, is most commonly billed between $6,812 and $11,291 per infusion 4. Overall per‐patient cancer care spending increased by 36% for Medicare and 62% for privately insured patients from 2004 to 2014, whereas spending on biologic drugs increased 335% and 485%, respectively, during the same time period 3. Though some biologic drugs have produced substantial improvements in patient outcomes, the benefits from others have been smaller. With high prices and marginal benefits, the cost‐effectiveness of many targeted therapies has been questioned 5, 6, 7.
Because of the high costs of these drugs, it is important to understand provider factors associated with their use. Academic centers may be faster to implement newer therapies, including many targeted agents 8; on the other hand, emphasis on providing cost‐effective care may be more prominent in the academic setting 9, 10, 11, 12, 13. The high acquisition costs and relative difficulty negotiating discounts may be barriers to the use of expensive drugs in smaller, community practices 14. Additionally, the profitability of these drugs may also vary significantly across settings, particularly with respect to facility fees and participation in the 340B discount program 14, 15, resulting in different financial incentives to providers. If utilization of biologic drugs were found to be significantly variable across academic and nonacademic practice settings, this would raise questions about both patient access to newer therapies, as well as potentially unnecessary costs. Therefore, the goal of this study was to examine whether provider practice setting was associated with use of high‐cost cancer treatments that included biologic drugs.
Materials and Methods
Patient Population and Physician Assignment
We used state Medicaid and commercial insurance claims data with linked cancer case data from the North Carolina Central Cancer Registry for this study. We focused on adult patients aged 18 to 64 years, with newly diagnosed stage IV colorectal cancer, stage IV lung adenocarcinoma, or stage II–IV head‐and‐neck cancer. Medicare‐eligible patients and those with prior cancers within 5 years of diagnosis were excluded. Patients were also excluded if they did not begin a treatment regimen of interest within 120 days of diagnosis or did not receive all components of a defined treatment regimen within 60 days of starting treatment (supplemental online Appendix 1). We further required patients to have continuous insurance enrollment in either commercial insurance or Medicaid from 180 days prior to starting treatment through 60 days afterward. Patients with missing data for cancer type, insurance, or year of diagnosis were excluded.
Outcome Definition
We studied cancer types for which there were multiple treatment options, one or more of which included a high‐cost biologic drug not present in the other treatment options. We included only treatment options that would be considered standard‐of‐care, as defined by U.S. Food and Drug Administration approval and National Comprehensive Cancer Network recommendation for the given cancer type during the study period.
We identified three such cancer types during our study period. For each cancer, we defined treatment regimens as either “low‐cost” or “high‐cost.” The three cancer types and corresponding treatments, were as follows: stage IV colorectal cancer (low‐cost = FOLFOX or FOLFIRI, high‐cost = [FOLFOX or FOLFIRI] + [bevacizumab or cetuximab or panitumumab]), stage II–IV head‐and‐neck cancer (low‐cost = cytotoxic chemotherapy, high‐cost = cetuximab ± cytotoxic chemotherapy), and stage IV lung adenocarcinoma (low‐cost = [cisplatin or carboplatin] + [paclitaxel, nab‐paclitaxel, or pemetrexed], high‐cost = [cisplatin or carboplatin] + [paclitaxel, nab‐paclitaxel, or pemetrexed] + bevacizumab). A detailed description of cancer types and treatment definitions are provided in supplemental online Appendix 1.
The primary, patient‐level outcome was the receipt of a high‐cost treatment instead of a low‐cost treatment, as defined by agents received within the 60 days after the first observed cancer drug claim. We chose a 60‐day period to define treatment received in order to appropriately classify patients who were intended to receive a targeted agent (e.g., bevacizumab) as part of their first line therapy but could not do so immediately because of recent surgery, while avoiding inclusion of potential second‐line therapies after cancer progression.
Exposure Definition
The primary, patient‐level exposure was the practice setting of the treating provider. First, we used claims to determine the number of “treatment days” (those on which anticancer drugs were billed) during the 60‐day outcome period. Patients were then assigned to the provider who billed for drug administration on the plurality of treatment days (and to the provider billing on the first treatment day, in cases of ties), similar to previous approaches 16, 17.
As a proxy for identifying academic versus nonacademic practice, we classified providers as affiliated or not affiliated with the National Cancer Institute (NCI) based on the billing ZIP code in payer claims. First, we used provider network lists from all payers (private insurers and North Carolina Medicaid) to assemble a list of North Carolina ZIP codes that contained one or more oncology practices. Each ZIP code was then categorized as either containing or not containing an NCI‐designated Comprehensive Cancer Center, including cases in which “main campus” sites spanned multiple ZIP codes or providers had additional billing ZIP codes; there were eight such ZIP codes. Providers located within an NCI‐containing ZIP code were designated as NCI‐affiliated or “NCI providers.” We categorized providers based on ZIP codes because there was minimal overlap between NCI‐designated Comprehensive Cancer Centers and non‐NCI oncology practices within North Carolina; of eight NCI‐containing ZIP codes, only one of these also contained a non‐NCI oncology practice. Finally, patients were designated as “NCI patients” if their assigned provider was an NCI provider and as “non‐NCI patients” if their assigned provider was not an NCI provider.
Potential Confounders
We identified patient characteristics that we hypothesized would be likely to influence a physician's treatment choice. These characteristics included chemotherapy contraindications and frailty indicators. For chemotherapy contraindications, we used diagnosis codes to define common health conditions related to a contraindication or black box warning on the manufacturer label for any of the drugs in our defined treatment regimens. For frailty indicators, we also used a claims‐based algorithm to generate a list of conditions associated with frailty (supplemental online Appendix 2) 18, 19. All characteristics were assessed over the 180‐day period prior to the first cancer drug claim and were assessed for balance after weighting (see below).
We also controlled for other potential confounders, including cancer type (colorectal, head‐and‐neck, lung), treatment billing location (physician office vs. hospital outpatient 20, 21), and the following demographic factors: gender (female vs. male), race (white vs. nonwhite), age at cancer diagnosis, year of cancer diagnosis, insurance type (Medicaid vs. private), and county‐level poverty from U.S. Census data.
Statistical Analysis
To control for potential confounding by the characteristics mentioned above, we used stabilized inverse probability of exposure weights 22, 23. We assessed covariate balance across NCI versus non‐NCI patients by calculating the standardized mean difference for each covariate 24.
In the crude and weighted data, we fit logistic regression models to estimate the predicted prevalence of high‐cost treatment in NCI and non‐NCI patients, as well as the prevalence difference between groups. In the weighted analysis, we excluded patients in the nonoverlapping tails of the propensity score distribution 25. For each estimate, we obtained percentile‐based bootstrap 95% confidence intervals (CIs) using 2,000 replicates 26. To explore potential heterogeneity in the prevalence of high‐cost treatment, we conducted stratified analyses by cancer type. Because of differences in reimbursement between insurance types (North Carolina Medicaid reimburses for physician‐administered drugs at the federal rate, allowing a modest markup over average sales price [ASP] 27, whereas private insurers reimburse at higher rates 3, 4, 28), we also stratified by insurance type (private vs. Medicaid).
Data management and statistical analyses were conducted using SAS version 9.4 (SAS Institute, Inc., Cary, NC).
Sensitivity Analysis
We performed several sensitivity analyses to assess whether the results were affected by our patient‐provider assignment method and our approach for controlling patient‐level confounding. First, we excluded patients who had drug contraindications or frailty indicators that were infrequent (<2%) in either exposure group or were sparsely distributed with respect to our prespecified subgroups of cancer type and insurance type (acute kidney injury, chronic renal failure, end‐stage renal disease); this restriction of the cohort should increase the fundamental comparability between NCI and non‐NCI patients with regard to potential confounders (analysis 1). Second, we applied an alternative assignment method in which patients were assigned to the provider who billed on the first treatment day (and to the provider who billed on more treatment days, in cases in which multiple providers billed on the first day) (analysis 2). Finally, the changes described for the two sensitivity analyses above were applied jointly (analysis 3).
Separately, we tabulated patient distribution among unique physicians to assess whether our observations may have been disproportionately affected by a small number of physicians with high patient volume.
Results
After applying our eligibility criteria, 800 patients were included in the study population (Fig. 1). Among these patients, 31.9% had stage IV colorectal cancer, 38.8% had stage II–IV head‐and‐neck cancer, and 29.4% had lung cancer. NCI patients made up 20.4% of the total, and the remaining 79.6% were non‐NCI patients. Nonwhite race was more common among non‐NCI patients (24.5% vs. 16.6%), as was Medicaid insurance (28.4% vs. 16.6%). The receipt of >50% of treatment days in the hospital outpatient setting (as opposed to the office setting) was more common among NCI patients (50.9% vs. 19.0%). Non‐NCI patients were more likely to have ≥2 frailty indicators (16.0% vs. 10.4%; Table 1). After weighting, the magnitude of the standardized mean differences of the patient characteristics between exposure groups was significantly reduced, indicating improved confounding control (Table 1).
Figure 1.
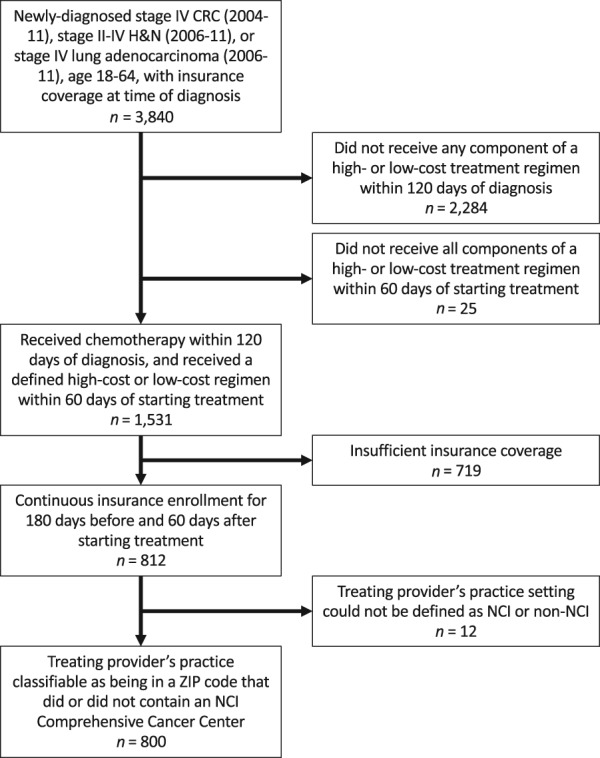
Study population.
Abbreviations: CRC, colorectal cancer; H&N, head‐and‐neck cancer; NCI, National Cancer Institute–designated Comprehensive Cancer Center.
Table 1.
Characteristics of patients with cancer during 2004–2011, by treating providera practice location (NCI ZIP code vs. non‐NCI ZIP code), in crude data and inverse probability of exposure weighted data (n = 800)
| Characteristics | Crude data | Weighted data | ||||||
|---|---|---|---|---|---|---|---|---|
| All patients (n = 800), n (%) | Treating provider's practice location | SMD | All patients (n = 746), n (%) | Treating provider's practice location | SMD | |||
| NCI ZIP code (n = 163, 20.4%), n (%) | Non‐NCI ZIP code (n = 637, 79.6%), n (%) | NCI ZIP code (n = 155, 20.8%), n (%) | Non‐NCI ZIP code (n = 591, 79.2%), n (%) | |||||
| Female gender | 307 (38.4) | 63 (38.6) | 244 (38.3) | 0.007 | 301 (40.3) | 66 (42.8) | 234 (39.7) | 0.065 |
| Age, median (IQR), years | 54 (49–59) | 54 (49–59) | 54 (49–59) | 0.112 | 55 (49–59) | 55 (49–60) | 55 (49–59) | −0.030 |
| Nonwhite race | 183 (22.9) | 27 (16.6) | 156 (24.5) | 0.197 | 142 (19.0) | 34 (21.8) | 108 (18.3) | −0.088 |
| Poverty prevalence in patient's county, median (IQR), % | 14 (12–17) | 14 (12–15) | 14 (12–17) | 0.289 | 14 (12–16) | 14 (13–16) | 14 (12–17) | −0.015 |
| Year of diagnosis | −0.394 | −0.011 | ||||||
| 2004–2007 | 233 (29.1) | 26 (16.0) | 207 (32.5) | 212 (28.4) | 44 (28.1) | 169 (28.5) | ||
| 2008–2011 | 567 (70.9) | 137 (84.0) | 430 (67.5) | 534 (71.6) | 112 (71.9) | 422 (71.5) | ||
| Type of insurance | −0.286 | −0.055 | ||||||
| Medicaid | 208 (26.0) | 27 (16.6) | 181 (28.4) | 186 (24.9) | 36 (23.1) | 150 (25.4) | ||
| Private | 592 (74.0) | 136 (83.4) | 456 (71.6) | 560 (75.1) | 119 (76.9) | 441 (74.6) | ||
| Cancer type | 0.199 | 0.067 | ||||||
| Colorectal | 255 (31.9) | 52 (31.9) | 203 (31.9) | 249 (33.4) | 50 (32.3) | 199 (33.7) | ||
| Head‐and‐neck | 310 (38.8) | 73 (44.8) | 237 (37.2) | 280 (37.5) | 57 (36.8) | 222 (37.7) | ||
| Lung | 235 (29.4) | 38 (23.3) | 197 (30.9) | 217 (29.1) | 48 (30.9) | 169 (28.6) | ||
| Percentage of treatment days occurring in hospital outpatient setting | 0.744 | 0.070 | ||||||
| 0% | 502 (62.8) | 61 (37.4) | 441 (69.2) | 452 (60.7) | 97 (62.3) | 356 (60.2) | ||
| >0%–50% | 94 (11.8) | 19 (11.7) | 75 (11.8) | 89 (11.9) | 19 (12.5) | 70 (11.8) | ||
| >50% | 204 (25.5) | 83 (50.9) | 121 (19.0) | 204 (27.4) | 39 (25.2) | 165 (28.0) | ||
| Number of drug contraindicationsb | 0.066 | 0.023 | ||||||
| 0 | 286 (35.8) | 57 (35.0) | 229 (35.9) | 269 (36.1) | 55 (35.4) | 214 (36.2) | ||
| 1 | 350 (43.8) | 75 (46.0) | 275 (43.2) | 322 (43.2) | 68 (44.0) | 254 (43.0) | ||
| ≥2 | 164 (20.5) | 31 (19.0) | 133 (20.9) | 155 (20.8) | 32 (20.7) | 123 (20.8) | ||
| Selected contraindications | ||||||||
| Recent surgery | 353 (44.1) | 66 (40.5) | 287 (45.1) | 0.092 | 324 (43.5) | 68 (43.6) | 257 (43.5) | −0.002 |
| Gastrointestinal bleeding or hemoptysis | 155 (19.4) | 35 (21.5) | 120 (18.8) | −0.066 | 146 (19.6) | 33 (21.6) | 113 (19.1) | −0.062 |
| Brain metastasis | 73 (9.1) | 17 (10.4) | 56 (8.8) | −0.056 | 77 (10.3) | 19 (12.2) | 58 (9.8) | −0.078 |
| Number of frailty indicators presentb | 0.179 | 0.112 | ||||||
| 0 | 531 (66.4) | 114 (69.9) | 417 (65.5) | 513 (68.7) | 101 (64.9) | 412 (69.7) | ||
| 1 | 150 (18.8) | 32 (19.6) | 118 (18.5) | 134 (18.0) | 31 (19.9) | 104 (17.5) | ||
| ≥2 | 119 (14.9) | 17 (10.4) | 102 (16.0) | 99 (13.2) | 24 (15.2) | 75 (12.7) | ||
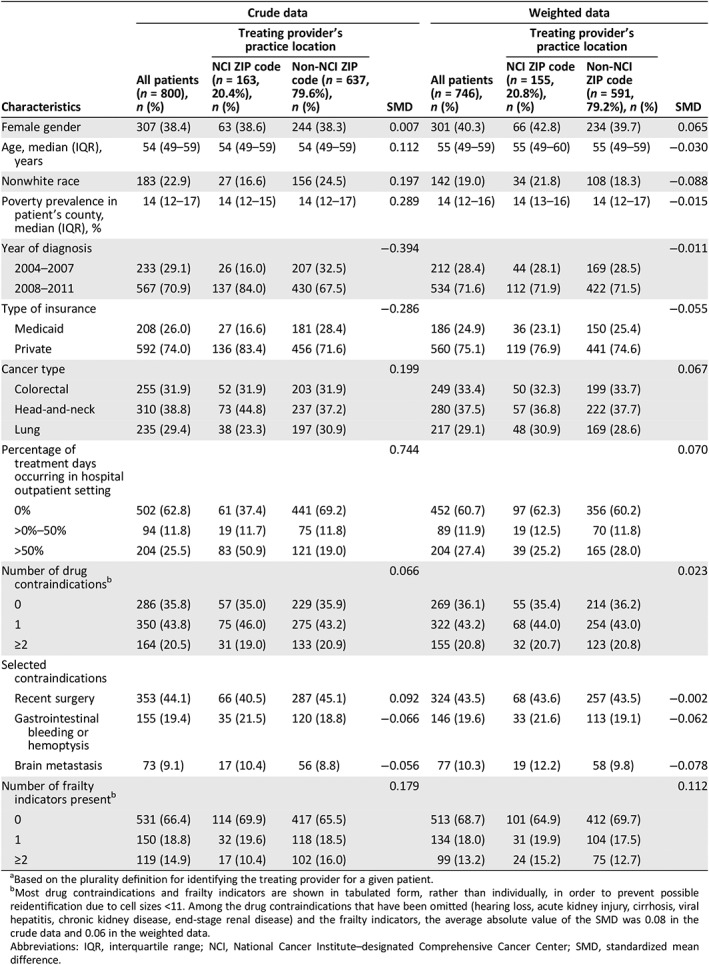
Based on the plurality definition for identifying the treating provider for a given patient.
Most drug contraindications and frailty indicators are shown in tabulated form, rather than individually, in order to prevent possible reidentification due to cell sizes <11. Among the drug contraindications that have been omitted (hearing loss, acute kidney injury, cirrhosis, viral hepatitis, chronic kidney disease, end‐stage renal disease) and the frailty indicators, the average absolute value of the SMD was 0.08 in the crude data and 0.06 in the weighted data.
Abbreviations: IQR, interquartile range; NCI, National Cancer Institute–designated Comprehensive Cancer Center; SMD, standardized mean difference.
Within the cohort, a unique physician was identified as the primary treating physician for 607 patients. These patients were assigned to 314 unique physicians; the mean number of patients per physician was 1.9 in both the NCI and non‐NCI groups (supplemental online Appendix 3). Only one NCI physician and one non‐NCI physician had more than ten assigned patients.
The prevalence of high‐cost treatment was 12.7% higher among non‐NCI patients versus NCI patients in the crude (unweighted) analysis. After applying inverse probability of exposure weights to balance potential confounders, 26.2% of NCI patients and 35.8% of non‐NCI patients received high‐cost treatment (prevalence difference 9.6%; 95% CI, −0.1%–18.7%; Fig. 2, Table 2).
Figure 2.
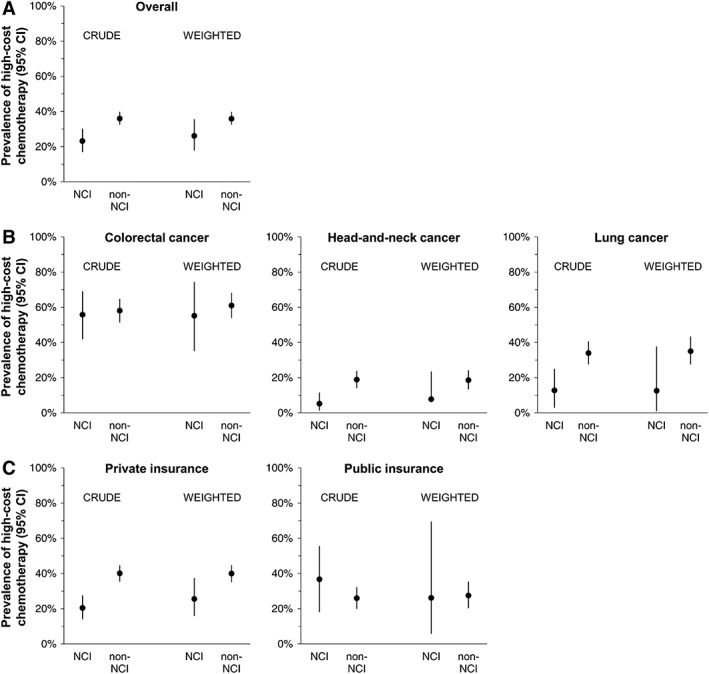
Crude and weighted prevalence of high‐cost treatment by NCI classification. (A): Overall study population. (B): Stratified by cancer type. (C): Stratified by insurance type.
Abbreviations: CI, confidence interval; NCI, National Cancer Institute–designated Comprehensive Cancer Center.
Table 2.
Crude and weighted prevalence and prevalence differences for high‐ versus low‐cost treatment comparing patients treated by NCI versus non‐NCI providers
| Crude data | Weighted data | |||||||
|---|---|---|---|---|---|---|---|---|
| Patient characteristics | n | Prevalence of HC, NCI, % | Prevalence of HC, non‐NCI, % | Prevalence difference, non‐NCI vs. NCI, % (95% CI) | Sum of weights | Prevalence of HC, NCI, % | Prevalence of HC, non‐NCI, % | Prevalence difference, non‐NCI vs. NCI, % (95% CI) |
| Overall | 800 | 23.2 | 35.9 | 12.7 (4.9–19.9) | 746 | 26.2 | 35.8 | 9.6 (−0.1–18.7) |
| Cancer type | ||||||||
| Colorectal | 255 | 55.8 | 57.9 | 2.1 (−12.7–17.8) | 238 | 55.2 | 60.8 | 5.6 (−14.8–26.6) |
| Head‐and‐neck | 310 | 5.3 | 18.8 | 13.5 (5.8–20.9) | 283 | 7.8 | 18.3 | 10.5 (−5.8–20.0) |
| Lung | 235 | 12.8 | 33.8 | 21.0 (7.2–33.2) | 221 | 12.6 | 34.8 | 22.2 (−3.5–37.0) |
| Type of insurance | ||||||||
| Private | 592 | 20.5 | 40.1 | 19.6 (11.2–27.8) | 575 | 25.6 | 40.0 | 14.4 (1.8–25.4) |
| Medicaid | 208 | 36.7 | 26.2 | −10.5 (−30.8–8.8) | 170 | 26.2 | 27.2 | 1.0 (−42.5–23.6) |
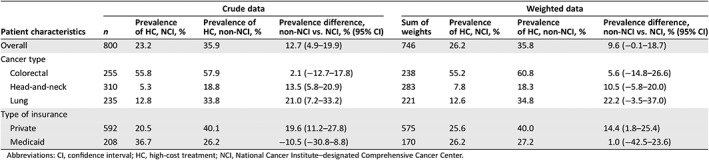
Abbreviations: CI, confidence interval; HC, high‐cost treatment; NCI, National Cancer Institute–designated Comprehensive Cancer Center.
In sensitivity analyses, the kappa for agreement between the two exposure classification methods (based on the provider on the plurality of treatment days, or the provider on the first treatment day) was 0.89 (95% CI, 0.85–0.93). The prevalence difference in high‐cost treatment for non‐NCI versus NCI patients was similar across sensitivity analyses: in analysis 1, the prevalence difference was 12.4% (95% CI, 2.6%–21.6%; supplemental online Appendices 4 and 5); in analysis 2, the prevalence difference was 7.2% (95% CI, −2.7%–16.3%; supplemental online Appendices 4 and 6); and in analysis 3, the prevalence difference was 10.6% (95% CI, 1.2%–19.9%; supplemental online Appendices 4 and 7).
The overall prevalence of high‐cost treatment varied across the three cancer types.
For both NCI and non‐NCI patients, high‐cost treatment was more common for colorectal cancer than head‐and‐neck or lung cancer (Fig. 2). The prevalence of high‐cost treatment was higher in non‐NCI than NCI patients across all three cancer types, although differences were not statistically significant within these subgroups (Table 2). Notably, the prevalence of high‐cost treatment was most similar between NCI and non‐NCI providers with respect to colorectal cancer, with greater differences for head‐and‐neck and lung cancer. We also observed variation with respect to patient insurance type; the adjusted prevalence of high‐cost treatment was similar between non‐NCI and NCI patients with Medicaid (prevalence difference, non‐NCI vs. NCI, 1.0% [95% CI, −42.5%–23.6%]), but was greater among non‐NCI than NCI patients with private insurance (prevalence difference, non‐NCI vs. NCI, 14.4% [95% CI, 1.8%–25.4%]; Table 2).
Discussion
After adjustment for patient characteristics, we observed higher utilization of high‐cost treatment in the non‐NCI setting compared with the NCI setting, among patients with colorectal, head‐and‐neck, and lung cancer in North Carolina. This suggests that higher‐cost treatment regimens may be used more commonly in nonacademic settings than in academic settings. The magnitude of this association was similar in sensitivity analyses modifying the patient‐provider assignment method and/or the patient cohort to achieve better covariate balance.
If the observed association between non‐NCI practice setting and more frequent use of high‐cost treatment represents a true difference in practice, there are several potential explanations. One possibility is that there are differences between NCI and non‐NCI practices with respect to the interpretation and application of clinical evidence. For example, concerns about the limited added benefit from biologic agents may be more common in the NCI setting. Concerns about the high financial cost of these agents, their cost‐effectiveness, and their contribution to patient out‐of‐pocket spending may also be more prevalent in academic settings such as NCI centers, although much of the research and awareness regarding drug costs and financial toxicity has occurred after our study period 5, 29, 30. Another possibility is that physicians in the non‐NCI setting receive relatively more information regarding new drugs through pharmaceutical detailing, which may be more favorable toward the benefits of newer, higher‐cost drugs. Another contributing factor may be that the financial incentive to use more lucrative drugs is greater in the non‐NCI setting compared with the NCI setting.
The Centers for Medicare and Medicaid Services (CMS) “buy and bill” reimbursement model may contribute to high cancer treatment costs. Under the current “buy and bill” reimbursement model, CMS reimburses providers for physician‐administered, “Part B” drugs at the ASP +4.3% (formerly 6% prior to sequestration, applicable to the current study period) 14, with providers keeping the margin between ASP +4.3% and the drug acquisition cost. In North Carolina, Medicaid also follows this formula 27; private insurers typically reimburse at higher rates 3, 4, 28. Because reimbursement is therefore tied to drug price, higher‐priced drugs are expected to result in greater revenue (though the margin can vary substantially across practices, depending on the prices negotiated with wholesalers) 31. The relationship between revenue and physician compensation is highly variable across practices and institutions. However, in general, large academic centers tend to compensate physicians on fixed salaries or with formulas to incentivize productivity, whereas physicians in nonacademic practices, particularly those characterized by physician ownership, are more likely to see their personal income affected by practice revenue 32. Physicians in the non‐NCI setting may therefore have a more direct financial incentive to use higher‐priced drugs 33.
That financial incentives may influence cancer treatment decisions would not be a new finding 34, 35, 36, 37, 38. However, few studies have analyzed the use of cancer drugs with respect to the financial incentives in place since the significant changes made by the Medicare Modernization Act during 2005 and 2006. Recent work has identified significant differences in practice with respect to usage of high‐price drugs between the physician office and hospital outpatient settings 9. Our results suggest that similar differences may be present between academic cancer centers and nonacademic practice. The high‐cost drugs we studied appear to be more prevalent in non‐NCI settings, and reimbursement policies for these drugs may be a contributing factor. Our finding of effect measure modification by patient insurance type (e.g., privately insured patients, but not Medicaid patients, were more likely to receive high‐cost treatment in the non‐NCI setting) may suggest an additional source of variation in the use of high‐cost drugs, warranting further study.
This study should be interpreted with respect to several limitations. We studied an adult, nonelderly population in a single state, and our results may not be generalizable to other geographic regions or to older patients. Our results are unlikely to be driven by the practice patterns of a small number of oncologists, given the large number of physicians involved in the treatment of our patient sample; however, it is possible that our results reflect institutional practice patterns, given the relatively small number (three) of NCI‐designated Comprehensive Cancer Centers in North Carolina. The eligibility requirement of continuous insurance enrollment removed a significant number of patients, particularly Medicaid patients, and therefore our sample may not fully reflect the population of patients with cancer across the socioeconomic spectrum. We used treatment outside of NCI‐designated Comprehensive Cancer Centers as our proxy for nonacademic practice; however, this categorization groups together many different practice types—including tertiary care hospitals, group practices, and solo practices—with different drug purchasing and physician compensation arrangements. We categorized providers as NCI or non‐NCI on the basis of practice ZIP code, rather than direct records of employment or institutional affiliation. Resulting misclassification should be minimal, however, because of minimal presence of non‐NCI providers within ZIP codes containing NCI‐designated Comprehensive Cancer Centers within North Carolina. If NCI patients received our high‐cost–defining agents (bevacizumab, cetuximab, and panitumumab), or other high‐cost experimental agents, through clinical trials, then it is possible that only the cytotoxic components of the treatment regimen would appear in claims; this could result in differential misclassification of high‐cost patients as low‐cost patients, which would bias results away from the null. If molecular diagnostic testing was differentially available in the NCI versus non‐NCI setting, this may have affected the portion of patients judged to be candidates for treatment with a biologic drug. As with all nonrandomized studies, confounding by unmeasured variables—such as patient‐level income or distance to care—remains possible.
Conclusion
These findings may have implications for patient care and for reimbursement policy. The biologic agents that we classified as belonging to “high‐cost” treatments in this study are all supported by randomized, phase III clinical trials demonstrating improvement in overall survival for certain patients with colorectal, head‐and‐neck, and lung cancer 39, 40, 41, 42, 43, 44, 45. From this perspective, increased utilization of these high‐cost treatments, which we observed in the non‐NCI setting, may indicate the delivery of higher‐quality care. However, there are also concerns regarding the costs of these drugs with respect to the magnitude of clinical benefit compared with lower‐cost alternatives. Bevacizumab is not cost‐effective for colorectal cancer within the U.S. health care system 5, and cetuximab or panitumumab are unlikely to be as well 29. Cetuximab in combination with radiotherapy has not been compared with cisplatin‐based chemoradiotherapy for head‐and‐neck cancer 46 and may be inferior 47 if given to patients who could tolerate cisplatin‐based chemoradiotherapy. The Dana Farber Cancer Center recently removed bevacizumab from its treatment pathway for stage IV non‐small cell lung cancer, leading to significant cost savings without an appreciable decrement in patient survival 48. The “high‐cost” treatment regimens examined in this study may offer clinical benefit over lower‐cost alternatives, but because of their high prices their value (defined as benefit per unit cost) may still be low. From this perspective, lower use of these agents in NCI‐approved Comprehensive Cancer Centers may also be appropriate and consistent with a prioritization of high‐value care. To the extent that the revenue‐generating capability of these drugs contributes to their use, our findings indicate an opportunity to reduce unnecessary spending by decoupling provider reimbursement from drug price.
Author Contributions
Conception/design: Aaron P. Mitchell, Alan C. Kinlaw, Sharon Peacock‐Hinton, Stacie B. Dusetzina, Hanna K. Sanoff, Jennifer L. Lund
Collection and/or assembly of data: Aaron P. Mitchell, Alan C. Kinlaw, Sharon Peacock‐Hinton
Data analysis and interpretation: Aaron P. Mitchell, Alan C. Kinlaw, Sharon Peacock‐Hinton
Manuscript writing: Aaron P. Mitchell, Alan C. Kinlaw, Sharon Peacock‐Hinton, Stacie B. Dusetzina, Hanna K. Sanoff, Jennifer L. Lund
Final approval of manuscript: Aaron P. Mitchell, Alan C. Kinlaw, Sharon Peacock‐Hinton, Stacie B. Dusetzina, Hanna K. Sanoff, Jennifer L. Lund
Disclosures
Aaron P. Mitchell: Conquer Cancer Foundation (H); Hanna K. Sanoff: Bayer, Merck (RF); Jennifer L. Lund: GlaxoSmithKline (E—spouse). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1: Supporting Information
Acknowledgments
This study was approved by the UNC‐Chapel Hill institutional review board, study number 17‐0177. All analysis was performed by the listed authors. There was no involvement of a medical writer or editor in the creation of this manuscript. Results of this study were first presented in abstract form at the Academy Health 2018 Annual Research Meeting. This work was partially supported by a National Research Service Award Post‐Doctoral Traineeship from the Agency for Healthcare Research and Quality (Grant No. 5T32 HS000032‐28). This work was partially supported by a Conquer Cancer Foundation Young Investigator Award.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Keehan SP, Stone DA, Poisal JA et al. National health expenditure projections, 2016‐25: Price increases, aging push sector to 20 percent of economy. Health Aff (Millwood) 2017;36:553–563. 10.1377/hlthaff.2016.1627 [DOI] [PubMed] [Google Scholar]
- 2. Cox C. How much does the US spend to treat different diseases? Peterson‐Kaiser Health System Tracker Web site. Available at https://www.healthsystemtracker.org/chart-collection/much-u-s-spend-treat-different-diseases/?_sft_category=spending#item-start. Published May 22, 2017. Accessed November 25, 2017.
- 3. Fitch K, Pelizzari PM, Pyenson B. Cost Drivers of Cancer Care: A Retrospective Analysis of Medicare and Commercially Insured Population Claim Data 2004‐2014. Seattle, WA: Milliman; April 2016. Available at http://www.milliman.com/uploadedFiles/insight/2016/trends-in-cancer-care.pdf. Accessed November 23, 2017. [Google Scholar]
- 4. Dusetzina SB, Basch E, Keating NL. For uninsured cancer patients, outpatient charges can be costly, putting treatments out of reach. Health Aff (Millwood) 2015;34:584–591. 10.1377/hlthaff.2014.0801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goldstein DA, Chen Q, Ayer T et al. First‐ and second‐line bevacizumab in addition to chemotherapy for metastatic colorectal cancer: A United States‐based cost‐effectiveness analysis. J Clin Oncol 2015;33:1112–1118. 10.1200/JCO.2014.58.4904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Leung HWC, Chan ALF, Muo CH et al. Cost‐effectiveness of pertuzumab combined with trastuzumab and docetaxel as a first‐line treatment for HER‐2 positive metastatic breast cancer. Expert Rev Pharmacoecon Outcomes Res 2018;18:207–213. 10.1080/14737167.2018.1386559 [DOI] [PubMed] [Google Scholar]
- 7. Shlomai A, Leshno M, Goldstein DA. Regorafenib treatment for patients with hepatocellular carcinoma who progressed on sorafenib—A cost‐effectiveness analysis. PLoS One 2018;13:e0207132 10.1371/journal.pone.0207132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carpenter WR, Reeder‐Hayes K, Bainbridge J et al. The role of organizational affiliations and research networks in the diffusion of breast cancer treatment innovation. Med Care 2011;49:172–179. 10.1097/MLR.0b013e3182028ff2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lipitz‐Snyderman A, Atoria CL, Schleicher SM et al. Practice patterns for older adult patients with advanced cancer: Physician office versus hospital outpatient setting. J Oncol Pract 2019;15:e30–e38. 10.1200/JOP.18.00315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lipitz‐Snyderman A, Sima CS, Atoria CL et al. Physician‐driven variation in nonrecommended services among older adults diagnosed with cancer. JAMA Intern Med 2016;176:1541–1548. 10.1001/jamainternmed.2016.4426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lipitz‐Snyderman A, Elkin EB, Atoria CL et al. Provider differences in use of implanted ports in older adults with cancer. Med Care 2015;53:646–652. 10.1097/MLR.0000000000000368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shahinian VB, Kuo Y, Freeman JL et al. Characteristics of urologists predict the use of androgen deprivation therapy for prostate cancer. J Clin Oncol 2007;25:5359–5365. 10.1200/JCO.2006.09.9580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Green JB, Shapiro MF, Ettner SL et al. Physician variation in lung cancer treatment at the end of life. Am J Manag Care 2017;23:216–223. [PMC free article] [PubMed] [Google Scholar]
- 14. Polite B, Conti RM, Ward JC. Reform of the buy‐and‐bill system for outpatient chemotherapy care is inevitable: Perspectives from an economist, a realpolitik, and an oncologist. Am Soc Clin Oncol Educ Book 2015:e75–e80. 10.14694/EdBook_AM.2015.35.e75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jung J, Xu WY, Kalidindi Y. Impact of the 340B drug pricing program on cancer care site and spending in Medicare. Health Serv Res 2018;53:3528–3548. 10.1111/1475-6773.12823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ellis SD, Chen RC, Dusetzina SB et al. Are small reimbursement changes enough to change cancer care? Reimbursement variation in prostate cancer treatment. J Oncol Pract 2016;12:e423–e436. 10.1200/JOP.2015.007344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mehrotra A, Reid RO, Adams JL et al. Physicians with the least experience have higher cost profiles than do physicians with the most experience. Health Aff (Millwood) 2012;31:2453–2463. 10.1377/hlthaff.2011.0252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Faurot KR, Jonsson Funk M, Pate V et al. Using claims data to predict dependency in activities of daily living as a proxy for frailty. Pharmacoepidemiol Drug Saf 2015;24:59–66. 10.1002/pds.3719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cuthbertson CC, Kucharska‐Newton A, Faurot KR et al. Controlling for frailty in pharmacoepidemiologic studies of older adults: Validation of an existing Medicare claims‐based algorithm. Epidemiology 2018;29:556–561. 10.1097/EDE.0000000000000833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jain RH, Bach PB. Hospital Outpatient versus Physician Office Cost for Physician Administered Cancer Drugs. New York, NY: Memorial Sloan Kettering Drug Pricing Lab; January 4, 2017. Available at https://drugpricinglab.org/wp-content/uploads/2017/01/Hospital-outpatient-versus-doctor-office-cost-for-physician-administered-cancer-drugs.pdf. Accessed April 23, 2018. [Google Scholar]
- 21. Fitch K, Pyenson B. Site of Service Cost Differences for Medicare Patients Receiving Chemotherapy. Seattle, WA: Milliman; October 19, 2011. Available at http://us.milliman.com/uploadedFiles/insight/health-published/site-of-service-cost-differences.pdf. Accessed April 23, 2018. [Google Scholar]
- 22. Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology 2000;11:550–560. [DOI] [PubMed] [Google Scholar]
- 23. Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol 2008;168:656–664. 10.1093/aje/kwn164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yang D, Dalton JE. A unified approach to measuring the effect size between two groups using SAS. Paper presented at SAS Global Forum 2012; April 22–25, 2012; Orlando, FL; 335‐2012. Available at http://support.sas.com/resources/papers/proceedings12/335-2012.pdf. Accessed July 25, 2018.
- 25. Stürmer T, Rothman KJ, Avorn J et al. Treatment effects in the presence of unmeasured confounding: Dealing with observations in the tails of the propensity score distribution–a simulation study. Am J Epidemiol 2010;172:843–854. 10.1093/aje/kwq198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Richardson DB, Kinlaw AC, MacLehose RF et al. Standardized binomial models for risk or prevalence ratios and differences. Int J Epidemiol 2015;44:1660–1672. 10.1093/ije/dyv137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Medicaid covered outpatient prescription drug reimbursement information by state, quarter ending September 2016. Medicaid Web site. Available at https://www.medicaid.gov/medicaid-chip-program-information/by-topics/prescription-drugs/downloads/xxxreimbursement-chart-current-qtr.pdf. Updated September 2016. Accessed January 8, 2017.
- 28. Jain RH, Schleicher SM, Coral AL et al. Part B Payment for Drugs in Medicare: Phase 1 of CMS's Proposed Pilot and Its Impact on Oncology Care. New York, NY: Memorial Sloan Kettering Drug Pricing Lab; April 11, 2016. Available at https://drugpricinglab.org/wp-content/uploads/2016/04/DPL-Part-B-phase-1-report.pdf. Accessed July 25, 2018. [Google Scholar]
- 29. Lawrence D, Maschio M, Leahy KJ et al. Economic analysis of bevacizumab, cetuximab, and panitumumab with fluoropyrimidine‐based chemotherapy in the first‐line treatment of KRAS wild‐type metastatic colorectal cancer (mCRC). J Med Econ 2013;16:1387–1398. 10.3111/13696998.2013.852097 [DOI] [PubMed] [Google Scholar]
- 30. Zafar SY. Financial toxicity of cancer care: It's time to intervene. J Natl Cancer Inst 2016;108:djv370 10.1093/jnci/djv370 [DOI] [PubMed] [Google Scholar]
- 31. Bach PB. Limits on Medicare's ability to control rising spending on cancer drugs. N Engl J Med 2009;360:626–633. 10.1056/NEJMhpr0807774 [DOI] [PubMed] [Google Scholar]
- 32. Desch CE, Blayney DW. Making the choice between academic oncology and community practice: The big picture and details about each career. J Oncol Pract 2006;2:132–136. 10.1200/jop.2006.2.3.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Malin JL, Weeks JC, Potosky AL et al. Medical oncologists' perceptions of financial incentives in cancer care. J Clin Oncol 2013;31:530–535. 10.1200/JCO.2012.43.6063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mitchell AP, Rotter JS, Patel E et al. Association between reimbursement incentives and physician practice in oncology: A systematic review. JAMA Oncol 2019;5:893–899. 10.1001/jamaoncol.2018.6196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Epstein AJ, Johnson SJ. Physician response to financial incentives when choosing drugs to treat breast cancer. Int J Health Care Finance Econ 2012;12:285–302. 10.1007/s10754-012-9117-y [DOI] [PubMed] [Google Scholar]
- 36. Jacobson M, O'Malley AJ, Earle CC et al. Does reimbursement influence chemotherapy treatment for cancer patients? Health Aff (Millwood) 2006;25:437–443. 10.1377/hlthaff.25.2.437 [DOI] [PubMed] [Google Scholar]
- 37. Conti RM, Rosenthal MB, Polite BN et al. Infused chemotherapy use in the elderly after patent expiration. J Oncol Pract 2012;8(suppl 3):e18s–e23s. 10.1200/JOP.2012.000541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Colla CH, Morden NE, Skinner JS et al. Impact of payment reform on chemotherapy at the end of life. J Oncol Pract 2012;8(suppl 3):e6s–e13s. 10.1200/JOP.2012.000539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bonner JA, Harari PM, Giralt J et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5‐year survival data from a phase 3 randomised trial, and relation between cetuximab‐induced rash and survival. Lancet Oncol 2010;11:21–28. 10.1016/S1470-2045(09)70311-0 [DOI] [PubMed] [Google Scholar]
- 40. Vermorken JB, Mesia R, Rivera F et al. Platinum‐based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 2008;359:1116–1127. 10.1056/NEJMoa0802656 [DOI] [PubMed] [Google Scholar]
- 41. Hurwitz H, Fehrenbacher L, Novotny W et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335–2342. 10.1056/NEJMoa032691 [DOI] [PubMed] [Google Scholar]
- 42. Saltz LB, Clarke S, Díaz‐Rubio E et al. Bevacizumab in combination with oxaliplatin‐based chemotherapy as first‐line therapy in metastatic colorectal cancer: A randomized phase III study. J Clin Oncol 2008;26:2013–2019. 10.1200/JCO.2007.14.9930 [DOI] [PubMed] [Google Scholar]
- 43. Van Cutsem E, Köhne CH, Láng I et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first‐line treatment for metastatic colorectal cancer: Updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol 2011;29:2011–2019. 10.1200/JCO.2010.33.5091 [DOI] [PubMed] [Google Scholar]
- 44. Douillard JY, Siena S, Cassidy J et al. Final results from PRIME: Randomized phase III study of panitumumab with FOLFOX4 for first‐line treatment of metastatic colorectal cancer. Ann Oncol 2014;25:1346–1355. 10.1093/annonc/mdu141 [DOI] [PubMed] [Google Scholar]
- 45. Sandler A, Gray R, Perry MC et al. Paclitaxel‐carboplatin alone or with bevacizumab for non‐small‐cell lung cancer. N Engl J Med 2006;355:2542–2550. 10.1056/NEJMoa061884 [DOI] [PubMed] [Google Scholar]
- 46. Winquist E, Agbassi C, Meyers BM et al.; Head and Neck Disease Site Group. Systemic therapy in the curative treatment of head and neck squamous cell cancer: A systematic review. J Otolaryngol Head Neck Surg 2017;46:29 10.1186/s40463-017-0199-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Petrelli F, Coinu A, Riboldi V et al. Concomitant platinum‐based chemotherapy or cetuximab with radiotherapy for locally advanced head and neck cancer: A systematic review and meta‐analysis of published studies. Oral Oncol 2014;50:1041–1048. 10.1016/j.oraloncology.2014.08.005 [DOI] [PubMed] [Google Scholar]
- 48. Jackman DM, Zhang Y, Dalby C et al. Cost and survival analysis before and after implementation of Dana‐Farber clinical pathways for patients with stage IV non‐small‐cell lung cancer. J Oncol Pract 2017;13:e346–e352. 10.1200/JOP.2017.021741 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1: Supporting Information


