Abstract
Axicabtagene ciloleucel is the first U.S. Food and Drug Administration–approved autologous anti‐CD19 chimeric antigen receptor (CAR) T‐cell therapy for the treatment of patients with relapsed or refractory large B‐cell lymphoma after ≥2 prior systemic therapies. Although axicabtagene ciloleucel is administered only at authorized treatment centers, community oncologists play a critical role in the CAR T‐cell treatment journey, recognizing potentially eligible patients for referral and then, after treatment, closely collaborating with treatment centers to monitor and manage patients long term. ZUMA‐1, the pivotal, multicenter, phase I/II study of 108 patients treated with axicabtagene ciloleucel, resulted in an objective response rate of 83%, including 58% complete responses. With a 27.1‐month median follow‐up, 39% of patients had ongoing responses. CAR T‐cell therapy is associated with the potentially life‐threatening adverse events (AEs) of cytokine release syndrome and neurologic events, which generally occur early after treatment. In ZUMA‐1, cytokine release syndrome and neurologic events were generally reversible and grade ≥3 cytokine release syndrome and neurologic events occurred in 11% and 32% of patients, respectively. Frequent prolonged AEs included hypogammaglobulinemia, B‐cell aplasia, and cytopenias requiring supportive care until recovery of hematopoietic function. Rate of treatment‐related mortality was low, at <2%. With appropriate management of common AEs, axicabtagene ciloleucel offers the potential for long‐term durable responses in patients who otherwise lack curative treatment options.
Implications for Practice
Community oncologists should be familiar with key aspects of chimeric antigen receptor (CAR) T‐cell indications and eligibility to help recognize and refer potential patients for this paradigm‐changing treatment option at the appropriate time during the disease course. To ensure optimal long‐term outcomes for patients who have been treated with CAR T‐cell therapy, oncologists must also be familiar with common prolonged AEs and their monitoring and management.
Keywords: Diffuse large B‐cell lymphoma, Transformed follicular lymphoma, Primary mediastinal B‐cell lymphoma, High‐grade B‐cell lymphoma, Immunotherapy
Short abstract
This article reviews information relevant to oncologists who are caring for patients who have been treated with CAR T cells or who are considering referring a patient with large B‐cell lymphoma for CAR T‐cell therapy.
Axicabtagene Ciloleucel for Relapsed or Refractory Large B‐Cell Lymphoma
Large B‐cell lymphomas are a group of common and aggressive subtypes of non‐Hodgkin lymphoma, with diffuse large B‐cell lymphoma (DLBCL) being the most common, with an incidence rate of 7.0 per 100,000 persons annually in the U.S. 1. Newly diagnosed patients with aggressive B‐cell lymphomas are treated with immunochemotherapy, commonly rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone 2. Approximately 20%–40% of patients may fail initial treatment or relapse 3, 4, 5. Patients who fail first‐line treatment but are eligible to receive intensive treatment are given salvage chemotherapy. If these patients have disease that is chemosensitive to salvage chemotherapy, they are offered high‐dose chemotherapy consolidation with autologous stem cell transplantation (ASCT) 2. Only 40%–60% of patients respond to salvage chemotherapy, and randomized studies have failed to show the superiority of any single salvage chemotherapy 3, 4. Patients who are refractory to immunochemotherapy or who relapse within 1 year of initial diagnosis are less likely to respond to standard salvage chemotherapy and, thus, less likely to proceed to ASCT 6. At least 50% of patients who do proceed to transplantation may relapse 7, 8. Patients who are refractory to or relapse following second‐line therapy have a very poor prognosis 9.
SCHOLAR‐1, a pooled analysis of 636 patients with refractory large B‐cell lymphoma, demonstrated an objective response rate (ORR) of 26%, with a complete response (CR) rate of 7% and a median overall survival (OS) of only 6.3 months 9. Overall, it is estimated that only 10%–25% of patients with relapsed or refractory large B‐cell lymphoma are cured 2, 6. Prior to chimeric antigen receptor (CAR) T‐cell therapy, treatment options for these patients were limited to trials of different salvage chemotherapies or other investigational treatment with very low likelihood of success 9. Allogeneic hematopoietic stem cell transplantation is offered to a small subset of patients who may have already failed ASCT but were able to achieve remission with another salvage regimen 10, 11. Thus, a clear unmet need exists for a more effective therapy for patients with refractory large B‐cell lymphoma to achieve remission and, hopefully, cure. To address this unmet need, ZUMA‐1 was the first multicenter study to investigate an autologous anti‐CD19 CAR T‐cell therapy, axicabtagene ciloleucel, for the treatment of patients with refractory large B‐cell lymphoma 12.
Axicabtagene ciloleucel was designed to overcome the limitations of the immune system in eradicating cancer, including potential suboptimal T‐cell activity and insufficient numbers of tumor‐specific T cells 13. It accomplishes this by using a second‐generation CAR construct that incorporates both a CD3ζ activation domain and a CD28 costimulatory domain to maximize CAR T‐cell activation in response to CD19 antigen (Fig. 1). In addition, a large number of CAR T cells can be manufactured and expanded in culture prior to infusion into the patient to ensure that sufficient tumor‐target T cells are present to generate a robust immune response. Conditioning regimens, administered before CAR T‐cell infusion, have been optimized to additionally enhance expansion of CAR T cells in the body. The construct used for axicabtagene ciloleucel was developed through pioneering work at the National Cancer Institute (NCI) 14, 15, 16. In long‐term follow‐up of an early phase I study at the NCI, four of five patients with CR maintained responses, which were still ongoing for 38–56 months 17.
Figure 1.
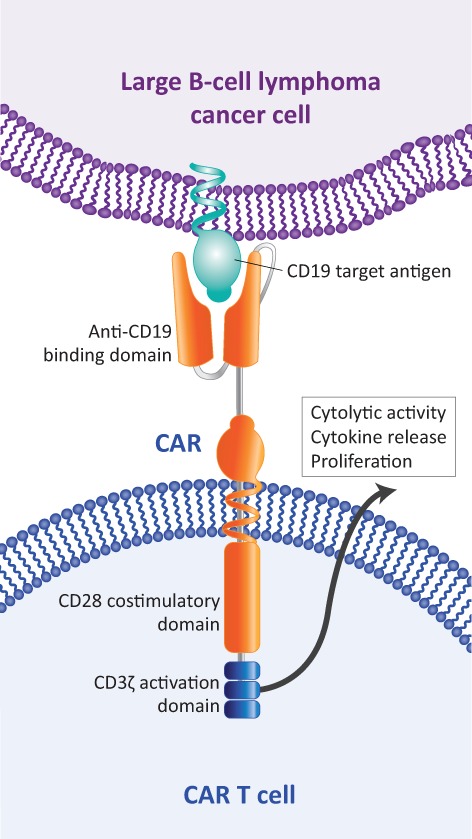
Axicabtagene ciloleucel structure.
Abbreviation: CAR, chimeric antigen receptor.
The pivotal, multicenter, phase I/II study of axicabtagene ciloleucel, ZUMA‐1, demonstrated a high rate of durable responses in patients with refractory large B‐cell lymphoma 12, 18. In 101 patients treated with axicabtagene ciloleucel in the phase II portion of ZUMA‐1, the best ORR was 83%, including a 58% rate of CR 18. At a median follow‐up of 27.1 months, ongoing responses were observed in 39% of patients, with a median duration of response of 11.1 months. Median OS had not yet been reached, and 24‐month survival was 50.5% (Fig. 2). In the phase I portion of ZUMA‐1, long‐term responses were observed in three of seven treated patients who maintained ongoing CR at 24 months 12. CAR T‐cell therapies are associated with potentially life‐threatening adverse events (AEs), notably cytokine release syndrome (CRS) and neurologic events 19. CRS has also been associated with some monoclonal antibodies and bispecific T‐cell engagers, and results when inflammatory cytokines are released from a large number of activated immune cells 19, 20, 21. Fever is considered a hallmark of CRS presentation. Other common symptoms include hypotension, tachycardia, hypoxia, and chills 19, 20, 22. At its most severe, CRS can manifest as atrial fibrillation, cardiac arrest, renal insufficiency, capillary leak syndrome, and hemophagocytic lymphohistiocytosis. Neurologic events can present with or without, or subsequent to, CRS, and the exact etiology of neurologic events is not well understood 19. CAR T cells have been observed in the cerebrospinal fluid, and elevation of certain cytokines has been demonstrated to be associated with neurologic events, suggesting they could possibly be due to a cytokine‐mediated mechanism 23, 24. Common neurologic events include encephalopathy, tremor, dizziness, aphasia, and delirium; serious neurologic events include seizures, leukoencephalopathy, and cerebral edema 19, 22. In ZUMA‐1, grade ≥3 CRS and neurologic events occurred in 11% and 32%, respectively, of the 108 patients and were, in general, fully reversible 12, 25. Common and serious symptoms of CRS and neurologic events, as well as the typical time during which these events commonly presented, are shown in Figure 3 12, 18, 20. These AEs usually present acutely, associated with the expansion and activity of CAR T cells in the patient. The median onset of CRS in ZUMA‐1 was day 2 after CAR T‐cell infusion, with resolution by day 8 12. Similarly, the median onset of neurologic events occurred early, on day 5, and resolved by day 17 after CAR T‐cell infusion 12. The rate of treatment‐related mortality was low, at <2%.
Figure 2.
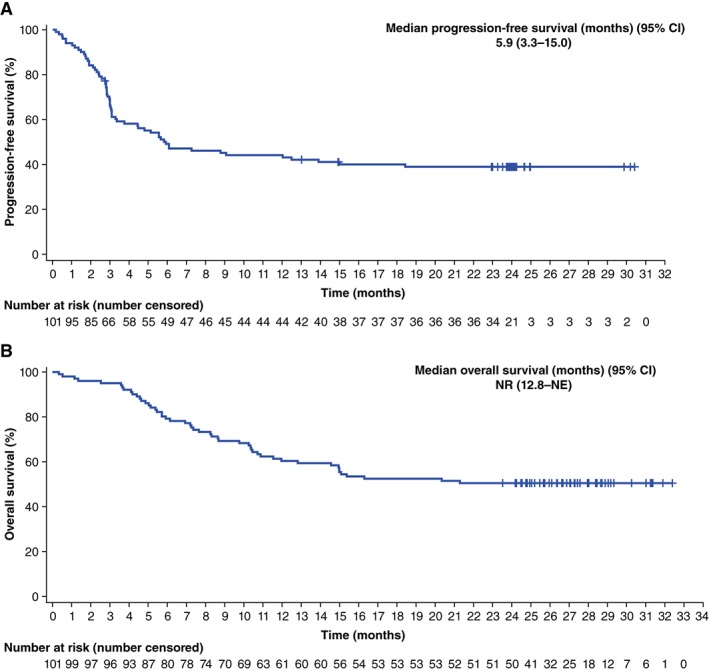
Survival outcomes with axicabtagene ciloleuecel in patients with large B‐cell lymphoma. Overall survival (A) and progression‐free survival (B) in ZUMA‐1.
Abbreviations: CI, confidence interval; NE, not evaluable; NR, not reached.
Reprinted from The Lancet Oncology, 20(1):31–42, FL Locke et al., “Long‐term safety and activity of axicabtagene ciloleucel in refractory large B‐cell lymphoma (ZUMA‐1): A single‐arm, multicentre, phase 1‐2 trial,” Copyright 2019, with permission from Elsevier 18.
Figure 3.
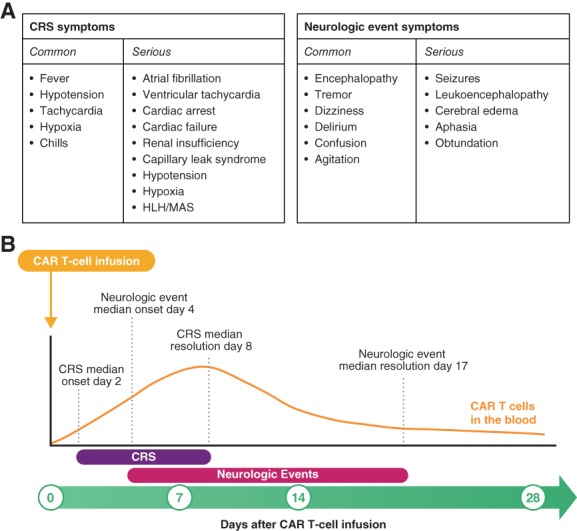
Overview of CRS and neurologic events. Common and serious symptoms (A) and median timing of onset and resolution (B) of CRS and neurologic events with axicabtagene ciloleucel.
Abbreviations: CAR, chimeric antigen receptor; CRS, cytokine release syndrome; HLH, hemophagocytic lymphohistiocytosis; MAS, macrophage activation syndrome.
Adapted from Journal of the Advanced Practitioner in Oncology, 10(suppl 3):21–28, S Adkins, “CAR T‐cell therapy: Adverse events and management,” Copyright 2019, with permission from Harborside 36.
Based on results from ZUMA‐1, axicabtagene ciloleucel became the first autologous anti‐CD19 CAR T‐cell therapy approved by the U.S. Food and Drug Administration for the treatment of patients with relapsed or refractory large B‐cell lymphoma after ≥2 prior systemic therapies (Table 1) 22. Large B‐cell lymphoma comprises a variety of subtypes, and axicabtagene ciloleucel is specifically indicated for treating DLBCL not otherwise specified, primary mediastinal B‐cell lymphoma, high‐grade B‐cell lymphoma, and DLBCL arising from follicular lymphoma. Patients do not need to test CD19‐positive to be treated with axicabtagene ciloleucel. In ZUMA‐1, 50% (5/10) of patients with CD19‐negative disease and 43% (33/77) of patients with CD19‐positive disease at baseline had ongoing responses to axicabtagene ciloleucel, with a minimum of 1 year of follow‐up 25. Patients with high‐risk genetics, including double‐hit, triple‐hit, or double‐expressor lymphoma, were not excluded from treatment with axicabtagene ciloleucel. Patients with T‐cell/histiocyte‐rich large B‐cell lymphoma were also not excluded from ZUMA‐1. Axicabtagene ciloleucel is contraindicated for patients with primary central nervous system lymphoma 22. Treatment centers have varied practices as to which specific central nervous system evaluation may be required prior to CAR T‐cell treatment.
Table 1.
Anti‐CD19 CAR T‐cell therapies approved or under investigation in relapsed/refractory large B‐cell lymphoma
| Therapy | Axicabtagene ciloleucel(Yescarta) [22] | Tisagenlecleucel(Kymriah) [26] | Lisocabtagene ciloleucel [29] |
|---|---|---|---|
| Patient population |
Approved for the treatment of adult patients with relapsed or refractory large B‐cell lymphoma after two or more lines of systemic therapy, including DLBCL not otherwise specified, primary mediastinal large B‐cell lymphoma, high‐grade B‐cell lymphoma, and DLBCL arising from follicular lymphoma |
Approved for the treatment of adult patients with relapsed or refractory large B‐cell lymphoma after two or more lines of systemic therapy, including DLBCL not otherwise specified, high‐grade B‐cell lymphoma, and DLBCL arising from follicular lymphoma | Under investigation in patients with DLBCL after two or more lines of therapy, including DLBCL not otherwise specified (de novo or transformed follicular lymphoma) and high‐grade B‐cell lymphoma (double‐/triple‐hit) |
| CAR | Anti‐CD19 scFV, CD28 costimulatory domain, CD3ζ activation domain | Anti‐CD19 scFV, 4‐1BB costimulatory domain, CD3ζ activation domain | Anti‐CD19 scFV, 4‐1BB costimulatory domain, CD3ζ activation domain; manufactured in a 1:1 ratio of CD4:CD8 T cells |
| Pivotal trial | ZUMA‐1 [12, 18] (NCT02348216) | JULIET 28 (NCT02445248) | TRANSCEND NHL 001 [29] (NCT02631044) |
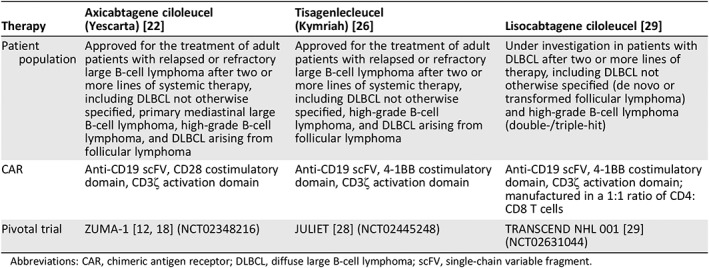
Abbreviations: CAR, chimeric antigen receptor; DLBCL, diffuse large B‐cell lymphoma; scFV, single‐chain variable fragment.
At the time of this publication, axicabtagene ciloleucel represents one of two approved anti‐CD19 CAR T‐cell treatment options for refractory large B‐cell lymphoma (Table 1). Tisagenlecleucel has indications similar to those of axicabtagene ciloleucel, except that it is not approved for primary mediastinal B‐cell lymphoma because patients with this lymphoma subtype were not included in the pivotal trial, JULIET 26, 27, 28. The phase II JULIET trial of tisagenlecleucel demonstrated an ORR of 54%, including a CR of 40% in 99 evaluable patients, with a median follow‐up of 19 months 27. Median OS was 11.1 months, and 12‐ and 18‐month survival rates were 48% and 43%, respectively. Grade ≥3 CRS occurred in 23% of patients, and grade ≥3 neurologic events occurred in 18% of patients 26. Additionally, lisocabtagene maraleucel is an anti‐CD19 CAR T‐cell therapy under investigation in patients with relapsed or refractory aggressive non‐Hodgkin lymphoma 29. TRANSCEND NHL 001, the pivotal study of lisocabtagene maraleucel, has demonstrated an ORR of 80% and a CR rate of 59% in 73 patients, with a median of 12 months of follow‐up. Median OS has not been reached in TRANSCEND, and the 12‐month survival rate was 63%. Grade ≥3 CRS has occurred in 1% of patients, and grade ≥3 neurologic events have occurred in 15% of patients. Overall, the clinical development and approvals of anti‐CD19 CAR T‐cell therapies, including axicabtagene ciloleucel, represent an important advance in the treatment of relapsed or refractory large B‐cell lymphoma. In addition to the commercial approval for patients with ≥2 prior therapies, an international, multicenter, randomized study, ZUMA‐7, is investigating axicabtagene ciloleucel versus standard of care for patients who have failed or relapsed after first‐line immunochemotherapy and intend to proceed to high‐dose therapy with ASCT (NCT03391466). Additionally, real‐world data on patients treated with commercially available CAR T‐cell therapy are emerging and appear to support findings from clinical trials demonstrating high rates of responses, with similar safety profiles 30, 31.
The CAR T‐Cell Therapy Treatment Journey
Evaluation
Commercial CAR T‐cell therapy is currently available for patients only after ≥2 prior therapies, and only through a Yescarta (axicabtagene ciloleucel; Kite, a Gilead Company, Santa Monica, CA) and Kymriah (tisagenlecleucel; Novartis Pharmaceuticals Corporation, Hanover, NJ) Risk Evaluation and Mitigation Strategy program–certified center, which ensures that health care providers who prescribe or administer these therapies are able to manage CRS and neurologic toxicities 22, 26. However, academic centers, such as those that are authorized treatment centers for CAR T‐cell therapy, may also have access to clinical trials evaluating the efficacy of CAR T‐cell therapy in earlier treatment settings, such as after failure of front‐line chemotherapy. The optimal timing to refer patients to academic centers can be as soon as suspicion of primary refractory disease or upon recognition of relapse after first‐line therapy, before second‐line treatment is initiated.
Following second‐line systemic treatment failure or relapse, patients can be evaluated for eligibility for commercial CAR T‐cell therapy (Fig. 4). Insurance coverage is available for patients, but approvals may take several weeks to obtain. Therefore, for patients who are reasonable candidates, the process of obtaining coverage can often be initiated during evaluation, which is important for limiting treatment delays.
Figure 4.
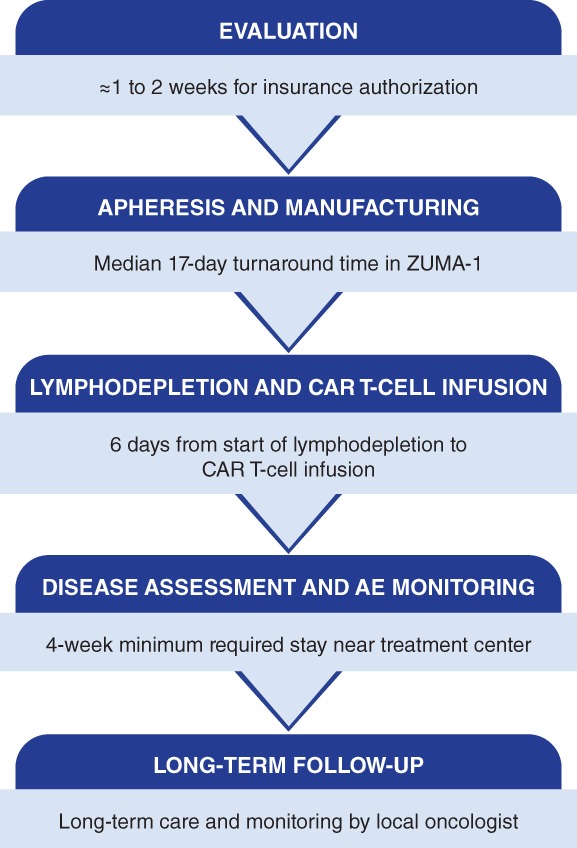
The CAR T‐cell treatment journey.
Abbreviations: AE, adverse event; CAR, chimeric antigen receptor.
Apheresis
Axicabtagene ciloleucel manufacturing begins with leukapheresis (Fig. 5). A patient's apheresis material is collected at the authorized treatment center. This process takes approximately 3–4 hours, during which time 10–20 L of blood is recirculated and approximately 100–500 mL of apheresis material is collected. The apheresis material collection bag is shipped cold to the central manufacturing facility 13.
Figure 5.
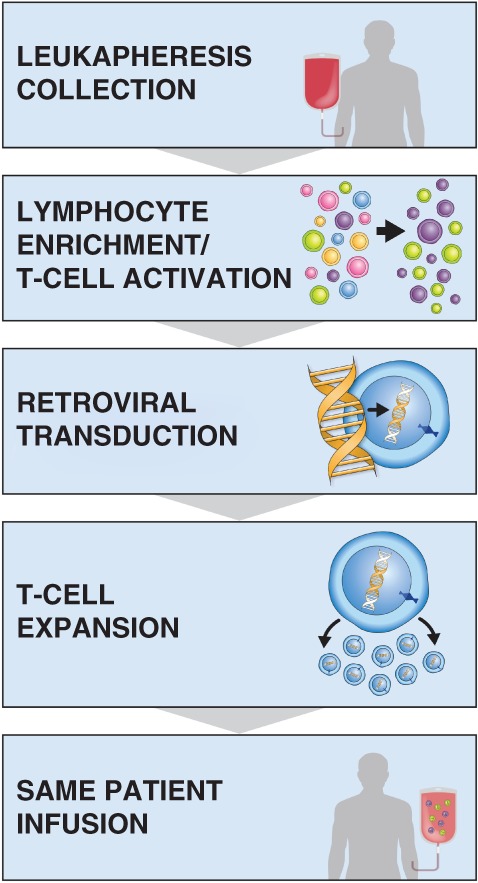
The manufacturing process for axicabtagene ciloleucel.
Manufacturing
Axicabtagene ciloleucel is manufactured in a robust, quality‐controlled process. T cells are enriched from the apheresis material, transduced with a retroviral vector containing the CAR gene, and then expanded in culture until they reach the target dose, at which point the cells are washed and cryopreserved for shipment back to the treatment center. Each lot undergoes a series of tests to ensure it meets certain standards prior to release 13. Manufacturing took a median of 17 days from apheresis to delivery back to the treatment site in ZUMA‐1 12. Although this describes the axicabtagene ciloleucel manufacturing process, specific details of manufacturing and turnaround times vary across the anti‐CD19 CAR T‐cell therapies. Although bridging therapy was not allowed on ZUMA‐1, in the commercial setting, some patients may benefit from bridging therapy during the manufacturing period 30, 31. Patients are monitored closely during this period to evaluate their disease status, determine their need for a bridging therapy, and confirm that they are still functionally able to receive CAR T‐cell therapy based on factors such as Eastern Cooperative Oncology Group performance status, organ function, and/or evidence of infection. We have experience using bridging therapies, such as corticosteroids, chemotherapy, and radiation. Bridging therapy was also allowed in the JULIET study but was not required 28. Further study is needed to determine optimal management of patients during the period between leukapheresis and CAR T‐cell infusion.
Lymphodepletion and CAR T‐Cell Infusion
The treatment center coordinates with the manufacturer to estimate the date of CAR T‐cell product arrival and to organize treatment logistics in advance. Once the treatment center receives the CAR T‐cell product from the manufacturing facility, the patient undergoes lymphodepleting chemotherapy. Lymphodepletion consists of a regimen of fludarabine (30 mg/m2) and cyclophosphamide (500 mg/m2) for 3 days (day −5, day −4, day −3), which can be administered in an outpatient setting at the authorized treatment center 22. Currently, centers usually admit patients prior to certain CAR T‐cell infusions. Axicabtagene ciloleucel is administered 3 days after completion of lymphodepletion (day 0) and infused at a target dose of 2 × 106 CAR T cells/kg 22. Before lymphodepletion and again before axicabtagene ciloleucel infusion, patients are carefully monitored and evaluated for any signs of inflammation to ensure they are not treated while they may have an underlying infection.
Disease Assessment and AE Monitoring
Following treatment, patients are closely monitored for signs and symptoms of AEs, particularly CRS and neurologic events, which can have rapid onset after CAR T‐cell administration. Multidisciplinary care teams are thoroughly trained on standard operating procedures for AE management and escalations of care, which are tailored at each treatment center, because early identification and intervention are critical for optimizing outcomes of patients who experience AEs. The majority of patients receiving CAR T‐cell therapy require inpatient care to manage these potentially serious acute AEs. During the first month after CAR T‐cell therapy, patients are closely monitored on a weekly basis at minimum.
In ZUMA‐1, most patients experienced hypogammaglobulinemia and B‐cell aplasia, and 31% received intravenous immunoglobulin (IVIG) therapy at some point after CAR T‐cell infusion 18. Patients may also experience delayed (i.e., normal counts at discharge and early in the postinfusion period) or prolonged cytopenias after CAR T‐cell therapy. Beginning at the treatment center, immunoglobulin levels may be monitored monthly, and blood counts of patients with cytopenia may be monitored up to twice a week, as needed. The benefit of IVIG in the absence of infections remains unclear but may be considered in patients with immunoglobulin G levels lower than approximately 400 mg/dL. Hypogammaglobulinemia is common during and after lymphoma therapy, but few patients require IVIG support, suggesting there may be some inherent predisposition for patients who do.
For patients with ongoing neutropenia after discharge, granulocyte colony‐stimulating factor support may be considered until absolute neutrophil counts have recovered to at least 1,000 cells/μL. Patients with cytopenias more than 1 month after CAR T‐cell infusion may undergo a repeat bone marrow biopsy to check bone marrow recovery. For patients with prolonged cytopenias lasting more than 2 months, other interventions, such as eltrombopag for transfusion‐dependent thrombocytopenia, may be considered on a case‐by‐case basis. Conditioning chemotherapy prior to CAR T‐cell therapy can result in T‐cell depletion in addition to severe neutropenia, leading to increased risk for infections. Infection prophylaxis including antibacterial prophylaxis with ciprofloxacin or levofloxacin and antifungal prophylaxis with fluconazole until absolute neutrophil counts recover to at least 1,000 cells/μL, Pneumocystis pneumonia prophylaxis until CD4+ cell counts reach at least 200 cells/μL or for 3 months after CAR T‐cell infusion (whichever is shorter), and herpes simplex virus prophylaxis with acyclovir or valacyclovir for 3–6 months after CAR T‐cell infusion (or longer depending on the degree of immunosuppression) should be considered.
Disease may be assessed as early as 1 month after CAR T‐cell infusion by a computed tomography (CT) or positron emission tomography‐computed tomography (PET‐CT) scan. Because responses may not always be evident at 1 month and may improve over time, PET‐CT may be performed at 3 months after infusion. Initial response assessment is generally performed by the authorized treatment center, and practices vary. In the ZUMA‐1 trial, 39% of patients with partial response or stable disease at 1 month subsequently achieved a CR on follow‐up imaging 18.
For commercially available CAR T‐cell therapies, patients are required to stay near the treatment center for at least 4 weeks after infusion so that any early complications that arise can be quickly responded to at the primary treatment center. Patients should not drive or operate heavy machinery for at least 8 weeks after infusion 22, 26, and many centers require the patient to stay locally for up to 8 weeks. This can be a major financial burden for some patients; however, housing support may be available through insurance or patient support organizations, and a social worker at the treatment center can help patients and their caregivers navigate access to these resources.
Long‐Term Follow‐Up in the Community Setting
After CAR T‐cell infusion, patients are provided a wallet card at discharge that they are directed to carry with them at all times. The wallet card includes symptoms that could be indicative of a serious AE for which to seek immediate medical treatment as well as contact information for the CAR T‐cell treating oncologist. Patients not experiencing any serious symptoms 4 weeks after CAR T‐cell infusion can return home. Treatment centers coordinate with a patient's local oncologist on key aspects of long‐term care. This is especially pertinent for care of common prolonged and late‐onset AEs such as hypogammaglobulinemia, cytopenias, and infections that may require ongoing supportive care and monitoring after the patient returns home.
Practical Guidance for Referring Patients for CAR T‐Cell Therapy
To maximize a patient's chance of receiving CAR T‐cell therapy, it is important for community oncologists to refer patients early and broadly. Patients with relapsed or refractory large B‐cell lymphoma who have failed two or more prior lines of therapy have very poor outcomes, and no standard of care exists for their management. In addition, as mentioned above, patients should be referred to academic centers after failure of or relapse following first‐line immunochemotherapy. Academic centers that are authorized treatment centers for CAR T‐cell therapy can provide the broadest range of care options for these patients, such as clinical trials and evaluation for transplant, based on their disease and individual preferences. Clinical trials may also be available for patients with DLBCL arising from indolent lymphoma other than follicular lymphoma or from chronic lymphocytic leukemia.
For a patient who may be a reasonable candidate for CAR T‐cell therapy, referring as early as possible is important for getting the evaluation process started. From the time a patient is referred to an authorized treatment center for CAR T‐cell therapy to the time of CAR T‐cell infusion, the process can take 4–6 weeks. Monitoring of patients may help facilitate prompt identification of treatment failure or disease relapse. For patients with stage III–IV DLBCL who have achieved complete remission as assessed by end‐of‐treatment PET‐CT, the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) recommend surveillance imaging (chest/abdomen/pelvic CT scan) no more than once every 6 months for up to 2 years after completion of therapy and then only as clinically indicated 32. For patients who have questionable residual uptake (by PET‐CT) at the end of treatment, it is important to follow up with either a biopsy or repeat imaging with PET or CT within 4–8 weeks depending on clinical suspicion.
Although patients may benefit from CAR T‐cell therapy regardless of the number of prior lines of therapy 33, postponing CAR T‐cell treatment until further in the disease course after additional treatment failures runs the risk of the patient becoming too frail as the result of advanced disease or effects of prior treatments. Patients who progress on first‐line therapy should be referred directly to academic centers whenever possible for management because high rates of relapse are observed with second‐line treatments. Academic centers are equipped to facilitate a smooth and rapid transition to the next line of therapy, especially CAR T‐cell therapy, if patients are already receiving treatment there, which may be particularly important for patients with rapidly progressing disease. It can be helpful to periodically confirm which academic centers in your state offer CAR T‐cell therapy, because the number of authorized treatment centers continues to expand.
Although patients may benefit from CAR T‐cell therapy regardless of the number of prior lines of therapy, postponing CAR T‐cell treatment until further in the disease course after additional treatment failures runs the risk of the patient becoming too frail as the result of advanced disease or effects of prior treatments. Patients who progress on first‐line therapy should be referred directly to academic centers whenever possible for management because high rates of relapse are observed with second‐line treatments.
Although overlap exists between patients who are candidates for transplant and patients who are candidates for CAR T‐cell therapy, some patients ineligible for transplant can receive CAR T‐cell therapy. The evaluation process for CAR T‐cell therapy eligibility differs from that for transplant. Importantly, patients who are refractory to chemotherapy are generally ineligible for transplant but may be able to receive CAR T‐cell therapy. Patients as old as 76 years were treated on ZUMA‐1 12, and clinical experience has shown that patients older than 65 years can receive similar clinical benefits of CAR T‐cell therapy in the commercial setting without significant risk for additional toxicities 34. Patients older than 80 years have been treated with commercial CAR T‐cell therapy 31. These are patients who traditionally would be considered too advanced in age to undergo transplantation. We also had success administering commercial CAR T‐cell therapy to patients with a broader range of organ function than reflected in the criteria for eligibility for ZUMA‐1. Only an individual evaluation at an authorized treatment center can determine whether a patient may be a candidate for CAR T‐cell therapy.
Key Considerations for Care of Patients After CAR T‐Cell Therapy
The community oncologist is a critical partner with an active role in the long‐term management of patients who have been treated with CAR T‐cell therapy after they transition home from the treatment center. Patients in response who are not experiencing serious AEs can return home after their 4‐ to 8‐week stay near the treatment center after CAR T‐cell infusion 22. Because CD19 is expressed by normal B cells, patients can experience prolonged hypogammaglobulinemia and B‐cell aplasia after CAR T‐cell treatment, and some patients may require supportive care with IVIG. Patients can also experience prolonged cytopenias, and blood counts should be periodically monitored. These patients may be receiving supportive care for low blood counts or infection prophylaxis. Because of treatment‐related immunosuppression, patients are at ongoing risk for serious infections after discharge following CAR T‐cell therapy. In ZUMA‐1, a total of 10 patients experienced serious AEs at least 6 months after CAR T‐cell infusion, 8 of whom had infections that occurred 7.1–18.6 months after treatment 12. Coordination and communication between the local oncologist and CAR T‐cell treatment oncologist are important during the months after patients return home from their minimum 4‐week stay near the treatment center. After this period, the authorized treatment center, in coordination with the local oncologist, may have patient follow‐ups every 2 weeks until month 3, then decreasing in frequency to 6 months and 12 months after CAR T‐cell infusion, then yearly until 5 years after CAR T‐cell infusion. During this period, routine laboratory monitoring, administration of supportive care, clinical surveillance of lymphoma, and imaging are largely conducted by the local oncologist.
Numerous strategies are under development for salvaging patients who relapse after CAR T‐cell therapy, and management of these patients remains an active area of clinical investigation. Immunotherapies have been shown to modulate CAR T‐cell activity, and for a patient who may have some level of persistent CAR T cells even after relapse, subsequent treatments must be carefully considered and their effects monitored.
Additionally, if a patient treated with CAR T‐cell therapy experiences disease relapse, it is important to send the patient back to the authorized treatment center where he or she initially received CAR T‐cell therapy, ideally before a biopsy is performed, so it can be done at the treating center. Numerous strategies are under development for salvaging patients who relapse after CAR T‐cell therapy, and management of these patients remains an active area of clinical investigation. Immunotherapies have been shown to modulate CAR T‐cell activity 35, and for a patient who may have some level of persistent CAR T cells even after relapse, subsequent treatments must be carefully considered and their effects monitored.
Conclusion
CAR T‐cell therapy represents a personalized, cellular immunotherapy for cancer treatment. The community oncologist plays a fundamental role in the CAR T‐cell treatment journey, first by referring patients to an academic center that is an authorized treatment center for CAR T‐cell therapy at an appropriate point in their disease course and then by providing long‐term support of patients through management of prolonged AEs and disease monitoring. Axicabtagene ciloleucel and other anti‐CD19 CAR T‐cell therapies offer the potential for durable remissions in a significant proportion of patients with relapsed or refractory large B‐cell lymphoma.
Author Contributions
Conception/design: Caron A. Jacobson, Umar Farooq, Armin Ghobadi
Collection and/or assembly of data: Caron A. Jacobson, Umar Farooq, Armin Ghobadi
Manuscript writing: Caron A. Jacobson, Umar Farooq, Armin Ghobadi
Final approval of manuscript: Caron A. Jacobson, Umar Farooq, Armin Ghobadi
Disclosures
Caron A. Jacobson: Kite, a Gilead Company, Bayer, Pfizer, Precision Biosciences, Novartis (H), Kite, a Gilead Company, Bayer, Pfizer, Novartis (other–travel support); Umar Farooq: Celgene (H), Kite, a Gilead Company (other–travel support); Armin Ghobadi: Kite, a Gilead Company (H), Kite, a Gilead Company, Amgen, Celgene, Atara Biotherapeutics (C/A).
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
Medical writing support was provided by Jennifer Leslie, Ph.D., of Nexus Global Group Science LLC, sponsored by Kite, a Gilead Company.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Noone AM, Howlader N, Krapcho M. et al., eds. SEER Cancer Statistics Review (CSR) 1975‐2015. Bethesda, MD: National Cancer Institute, 2018. Available at https://seer.cancer.gov/csr/1975_2015/. Accessed March 26, 2019. [Google Scholar]
- 2. Friedberg JW. Relapsed/refractory diffuse large B‐cell lymphoma. Hematology Am Soc Hematol Educ Program 2011;2011:498–505. [DOI] [PubMed] [Google Scholar]
- 3. Crump M, Kuruvilla J, Couban S et al. Randomized comparison of gemcitabine, dexamethasone, and cisplatin versus dexamethasone, cytarabine, and cisplatin chemotherapy before autologous stem‐cell transplantation for relapsed and refractory aggressive lymphomas: NCIC‐CTG LY.12. J Clin Oncol 2014;32:3490–3496. [DOI] [PubMed] [Google Scholar]
- 4. Gisselbrecht C, Glass B, Mounier N et al. Salvage regimens with autologous transplantation for relapsed large B‐cell lymphoma in the rituximab era. J Clin Oncol 2010;28:4184–4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vitolo U, Trněný M, Belada D et al. Obinutuzumab or rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in previously untreated diffuse large B‐cell lymphoma. J Clin Oncol 2017;35:3529–3537. [DOI] [PubMed] [Google Scholar]
- 6. Farooq U, Maurer MJ, Thompson CA et al. Clinical heterogeneity of diffuse large B cell lymphoma following failure of front‐line immunochemotherapy. Br J Haematol 2017;179:50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hamadani M, Hari PN, Zhang Y et al. Early failure of frontline rituximab‐containing chemo‐immunotherapy in diffuse large B cell lymphoma does not predict futility of autologous hematopoietic cell transplantation. Biol Blood Marrow Transplant 2014;20:1729–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gisselbrecht C, Schmitz N, Mounier N et al. Rituximab maintenance therapy after autologous stem‐cell transplantation in patients with relapsed CD20(+) diffuse large B‐cell lymphoma: Final analysis of the collaborative trial in relapsed aggressive lymphoma. J Clin Oncol 2012;30:4462–4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Crump M, Neelapu SS, Farooq U et al. Outcomes in refractory diffuse large B‐cell lymphoma: Results from the international SCHOLAR‐1 study. Blood 2017;130:1800–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fenske TS, Ahn KW, Graff TM et al. Allogeneic transplantation provides durable remission in a subset of DLCBL patients relapsing after autologous transplantation. Br J Haematol 2016;174:235–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rezvani AR, Norasetthada L, Gooley T et al. Non‐myeloablative allogeneic haematopoietic cell transplantation for relapsed diffuse large B‐cell lymphoma: A multicentre experience. Br J Haematol 2008;143:395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Neelapu SS, Locke FL, Bartlett NL et al. Axicabtagene ciloleucel CAR T‐cell therapy in refractory large B‐cell lymphoma. N Engl J Med 2017;377:2531–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roberts ZJ, Better M, Bot A et al. Axicabtagene ciloleucel, a first‐in‐class CAR T cell therapy for aggressive NHL. Leuk Lymphoma 2018;59:1785–1796. [DOI] [PubMed] [Google Scholar]
- 14. Kochenderfer JN, Dudley ME, Feldman SA et al. B‐cell depletion and remissions of malignancy along with cytokine‐associated toxicity in a clinical trial of anti‐CD19 chimeric‐antigen‐receptor‐transduced T cells. Blood 2012;119:2709–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kochenderfer JN, Dudley ME, Kassim SH et al. Chemotherapy‐refractory diffuse large B‐cell lymphoma and indolent B‐cell malignancies can be effectively treated with autologous T cells expressing an anti‐CD19 chimeric antigen receptor. J Clin Oncol 2015;33:540–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kochenderfer JN, Feldman SA, Zhao Y et al. Construction and preclinical evaluation of an anti‐CD19 chimeric antigen receptor. J Immunother 2009;32:689–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kochenderfer JN, Somerville RPT, Lu T et al. Long‐duration complete remissions of diffuse large B cell lymphoma after anti‐CD19 chimeric antigen receptor T cell therapy. Mol Ther 2017;25:2245–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Locke FL, Ghobadi A, Jacobson CA et al. Long‐term safety and activity of axicabtagene ciloleucel in refractory large B‐cell lymphoma (ZUMA‐1): A single‐arm, multicentre, phase 1‐2 trial. Lancet Oncol 2019;20:31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Neelapu SS, Tummala S, Kebriaei P et al. Chimeric antigen receptor T‐cell therapy — assessment and management of toxicities. Nat Rev Clin Oncol 2018;15:47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee DW, Gardner R, Porter DL et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014;124:188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Topp MS, Gökbuget N, Zugmaier G et al. Phase II trial of the anti‐CD19 bispecific T cell‐engager blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory B‐precursor acute lymphoblastic leukemia. J Clin Oncol 2014;32:4134–4140. [DOI] [PubMed] [Google Scholar]
- 22.Yescarta (axicabtagene ciloleucel) [package insert]. Santa Monica, CA: Kite Pharma, Inc; 2019.
- 23. Locke FL, Neelapu SS, Bartlett NL et al. Preliminary results of prophylactic tocilizumab after axicabtagene ciloleucel (axi‐cel; KTE‐C19) treatment for patients with refractory, aggressive non‐Hodgkin lymphoma (NHL). Blood 2017;130(suppl 1):1547a. [Google Scholar]
- 24. Gust J, Taraseviciute A, Turtle CJ. Neurotoxicity associated with CD19‐targeted CAR‐T cell therapies. CNS Drugs 2018;32:1091–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Neelapu SS, Locke FL, Bartlett NL et al. Long‐term follow‐up of ZUMA‐1: A pivotal trial of axicabtagene ciloleucel (axi‐cel; KTE‐C19) in patients with refractory aggressive non‐Hodgkin lymphoma. Blood 2017;130(suppl 1):578a. [Google Scholar]
- 26.Kymriah (tisagenlecleucel) [package insert]; Hanover, NJ: Novartis Pharmaceuticals Corporation; 2018.
- 27. Schuster SJ, Bishop MR, Tam C et al. Sustained disease control for adult patients with relapsed or refractory diffuse large B‐cell lymphoma: An updated analysis of JULIET, a global, pivotal, phase 2 trial of tisagenlecleucel. Blood 2018;132(suppl 1):1684a. [Google Scholar]
- 28. Schuster SJ, Bishop MR, Tam CS et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B‐cell lymphoma. N Engl J Med 2019;380:45–56. [DOI] [PubMed] [Google Scholar]
- 29. Abramson JS, Gordon LI, Palomba ML et al. Updated safety and long term clinical outcomes in TRANSCEND NHL 001, pivotal trial of lisocabtagene maraleucel (JCAR017) in R/R aggressive NHL. J Clin Oncol 2018;36(suppl):7505a. [Google Scholar]
- 30. Jacobson CA, Hunter B, Armand P et al. Axicabtagene ciloleucel in the real world: Outcomes and predictors of response, resistance and toxicity. Blood 2018;132(suppl 1):92a. [Google Scholar]
- 31. Nastoupil LJ, Jain MD, Spiegel JY et al. Axicabtagene ciloleucel (axi‐cel) CD19 chimeric antigen receptor (CAR) T‐cell therapy for relapsed/refractory large B‐cell lymphoma: Real world experience. Blood 2018;132(suppl 1):91a. [Google Scholar]
- 32.Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for B‐Cell Lymphomas V.2.2019. © National Comprehensive Cancer Network, Inc. 2019. All rights reserved. Accessed March 6, 2019. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way. [Google Scholar]
- 33. Locke FL, Ghobadi A, Lekakis LJ et al. Outcomes by prior lines of therapy (LOT) in ZUMA‐1, the pivotal phase 2 study of axicabtagene ciloleucel (axi‐cel) in patients (pts) with refractory large B‐cell lymphoma. J Clin Oncol 2018;36(suppl):3039a. [Google Scholar]
- 34. Sano D, Nastoupil LJ, Fowler NH et al. Safety of axicabtagene ciloleucel CD19 CAR T‐cell therapy in elderly patients with relapsed or refractory large B‐cell lymphoma. Blood 2018;132(suppl 1):96a. [Google Scholar]
- 35. Jacobson CA, Locke FL, Miklos DB et al. End of phase 1 results from ZUMA‐6: Axicabtagene ciloleucel (axi‐cel) in combination with atezolizumab for the treatment of patients with refractory diffuse large B cell lymphoma. Blood 2018;132(suppl 1):4192a. [Google Scholar]
- 36. Adkins S. CAR T‐cell therapy: Adverse events and management. J Adv Pract Oncol 2019;10(suppl 3):21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]


