Abstract
Purpose
Older patients with colon cancer (CC) are vulnerable to chemotherapy toxicity and death. Establishing simple scores specific for patients with CC to predict severe chemotoxicity or early death is needed to select the best treatment strategy.
Subjects, Materials, and Methods
This prospective multicenter study included patients aged ≥70 years with CC receiving adjuvant or first‐line metastatic chemotherapy. Frailty markers (nutrition, physical activity, energy, mobility, strength), comprehensive geriatric assessment (functional status, comorbidities, falls, nutrition, cognition, and depression), and usual laboratory parameters were collected. Logistic or Cox regression was used to examine at 500 days the association between frailty markers, comprehensive geriatric assessment, laboratory parameters, and grade 3–4 toxicity or death.
Results
A total of 97 patients (median age, 79.0 years) received adjuvant (37.1%) or metastatic (62.9%) chemotherapy. During the first 500 days, grade 3–4 toxicity occurred in 49.5%, and 30% died. The predictive model for grade 3–4 toxicity combined (polychemotherapy × 3) + (hypoalbuminemia <32 g/L × 2) + (abnormal grip strength × 1.5) + C‐reactive protein >11 mg/L + Eastern Cooperative Oncology Group performance status (ECOG‐PS), cutoff score >3. The predictive model for death combined (metastasis × 5) + (age × 2) + alkaline phosphatase >100 IU/mL + sex (female) + abnormal grip strength + ECOG‐PS, cutoff score >6. For chemotoxicity prediction, sensitivity was 81.6% and specificity 71.4%. For death prediction, sensitivity was 89.7% and specificity was 83.6%.
Conclusion
These simple and efficient “ColonPrediscores” will help to better identify older patients with CC with increased risk of chemotherapy‐related toxicity and/or death.
Implications for Practice
The two scores assessed in this study, called “ColonPrediscores”, offer a major advantage in that they do not need a previous complete geriatric assessment, which makes them an easy‐to‐use tool in oncologic settings.
Keywords: Colon cancer, Frailty markers, Grip strength, Geriatric assessment, Death, Chemotoxicity
Short abstract
Colon cancer mainly affects older patients who are at risk for chemotherapy‐related toxicities. This article describes the ColonPrediscores system for identifying patients with in increased risk for toxicity and/or death in this patient group.
Introduction
Colon cancer (CC) is mainly a disease of older individuals, as median age at diagnosis is 70 years, and it represents the second cause for all cancer deaths 1. The older cancer population is heterogeneous and requires specific workup in order to decide the best treatment strategy. Older patients seem to benefit from chemotherapy as much as younger ones 2. However, older age is a risk factor for chemotherapy toxicities 3.
The International Society of Geriatric Oncology task force on comprehensive geriatric assessment (CGA) recommends implementation of a geriatric assessment for older patients with cancer to identify patients who may benefit from treatment as well as to detect conditions that may interfere with cancer therapy 4. Some domains of CGA have been associated with cancer treatment toxicity (impairments in instrumental activities of daily living [IADL], comorbidity, depression, poor social support, and cognitive functioning) and all‐cause mortality (impairments in basic and IADL nutritional status, comorbidities, depression) 5, 6. However, a minority of patients with CC were included in these studies. In almost 1,000 older patients with cancer, one‐fifth of whom had CC (21.4%), severe comorbidities and malnutrition were geriatrics parameters significantly associated with death 7. Only two studies exclusively concern chemotherapy for colorectal cancer. Aparicio et al. 8 found in 123 patients that abnormal IADL score and Mini‐Mental Status Examination were independently associated with chemotoxicity. Ramsdale et al. 9 showed in a very small sample of older patients that the vulnerable elders survey (VES‐13) was the only significant predictive factor for death. To date, only two predictive scores for chemotherapy toxicity in older cancer patients (with 27% and 12% of whom had gastrointestinal cancers) have been published 10, 11. Altogether, the scarcity of studies and the potential ceiling effects of CGA in CC highlight the need for the search of additional markers and specific scores 12, 13, 14.
The frailty phenotype in older adults described by Fried et al. 15 identified five markers: nutrition, mobility, strength, energy, and physical activity. Individuals exhibiting three or more of these characteristics were classified as frail, those with 1 or 2 as prefrail, and those with none as nonfrail. Regardless of the number of frailty markers, the presence of at least one of these markers conferred an increased risk (death, institutionalization, disability, mobility impairment, etc.) compared with patients with none 16, 17, 18. In an oncology setting, Retornaz et al. 14. found that more than 80% of older patients with cancer had at least one frailty marker including IADL or ADL disability, whereas 42% presented with at least one frailty marker without any IADL or ADL disability. Some frailty markers predicted treatment toxicity (low grip strength) 19 and risk of early death (nutrition and mobility) 20. Thus, frailty phenotype could be a useful approach to detect potential vulnerability to cancer treatment and death in older patients with cancer.
The primary objective of this prospective longitudinal study was to develop two simple scores able predict grade 3–4 chemotoxicity and death during the 500 days follow‐up period in a cohort of older patients with CC cancer by using CGA, frailty markers, laboratory data, and oncologic parameters.
Subjects, Materials, and Methods
Study Design
This multicenter prospective longitudinal study in eight oncologic centers included from October 2010 to January 2013, 97 patients with CC, aged 70 years and older, referred for adjuvant or first‐line metastatic chemotherapy. The selection of the eligible patients was done in each center during the multidisciplinary team meeting. Eight oncologic centers participated in this study. Then, the research coordinator of each center approached the eligible patients. Unfortunately, we did not record the reasons for nonacceptance or the number of patients with exclusion criteria. We are not able to produce a flow diagram. Patients were excluded if they were terminally ill, had a life expectancy of less than 3 months, or had previously received any chemotherapy or hormone therapy regimen. All patients completed the informed consent form. The protocol was approved by the regional ethics committee and was conducted in accordance with the declaration of Helsinski, Good Clinical Practices, and local ethical and legal requirements (trial registration in http://clinicaltrials.gov: MOST no. NCT02148731).
Data Collection
The measurement tools were selected by a multidisciplinary team composed of geriatricians, oncologists, epidemiologists, and statisticians, based on a review of both the geriatric and oncology literature. The assessment was completed by a research coordinator using both self‐report and performance‐based measures, in addition to a medical chart review. Demographic data and oncologic parameters (stage of disease, metastasis location, chemotherapy regimen, K‐ras mutation, and Eastern Cooperative Oncology Group [ECOG]) performance status) were collected. CGA consisted of basic activities of daily living (ADL) 21, IADL 22, comorbidities (the Cumulative Illness Rating Scale for Geriatrics [CIRS‐G] 23, falls in the last 6 months, Mini Nutritional Assessment Short Form (MNA‐SF) 24, Mini‐Cog (cutoff score < 4) 25, and 4‐item Geriatric Depression Scale (mini GDS) 26. Lack of social support was defined as a negative answer to the question “Do you have a person who is able to take care of you if necessary?”
A frailty‐related phenotype was used 14 to assess the five frailty domains defined by Fried 15: (a) mobility: balance was considered abnormal if the patient was unable to balance on one leg for more than 5 seconds 27 or if the Timed Up and Go test 28 cutoff score was above 10 seconds; (b) grip strength: adjusted for sex and body mass index as described by Fried et al. 15; (c) energy (visual scale assessed less than 3) 29; (d) impaired physical activity (Canadian Study of Health and Aging Risk Factor Questionnaire assessed physical activities: no exercise or a low level of exercise was considered a positive marker of frailty for physical activity) 30; and (e) impaired nutrition (losing more than 4 kg unintentionally during the last year 15 and/or decrease in food intake during the last 3 months whatever the cause) 31.
CGA and frailty markers were collected by a research coordinator.
Laboratory data (serum hemoglobin, lymphocyte count, serum creatinine clearance, serum albumin, C‐reactive protein, and alkaline phosphatase levels) were recorded at inclusion.
Chemotherapy‐induced side‐effects were assessed at 3, 6, 9, and 12 months using the Common Terminology Criteria for Adverse Events (CTCAE) version 2.0 and collected by a research coordinator. Toxicities were graded on a 0–4 scale. Grade 3 or 4 toxicities, as well as any cause of death, were recorded.
Statistical Analysis
Statistical analysis was performed using SPSS software, version 17.0 (SPSS, Chicago, IL). The association between death or toxicity and the dichotomous variables or the continuous variables was examined using the χ2 test or the Mann‐Whitney U test, respectively. The relevant variables that reached a p value <.2 or that were of major interest were examined using multivariate logistic regression or Cox regression for toxicity or death, respectively; the optimal cut‐point for the continuous variables was determined using the Youden index. The combined set of risk factors with the highest sensitivity and specificity (estimated using the receiver operating characteristic [ROC] curve), the best goodness of fit (estimated using the Hosmer‐Lemeshow test or the −2 log likelihood for toxicity or death, respectively) was selected and internally validated using the bootstrap methodology. The risk score for each factor was the rounded adjusted odds ratio and hazard ratio, for toxicity or death risk, respectively. Some risk scores were adjusted to insure an optimal sensitivity and/or specificity. Interactions among the selected factors were evaluated by introducing interaction terms to the model one at a time in the multivariate logistic regression or Cox regression for toxicity or death, respectively. No significant interaction was found between the different risk factors of each model, implying that they were independent. The sum of the score values was calculated for each patient and a cutoff point was estimated using the Youden index. Differences between groups were estimated with logistic regression or Kaplan‐Meier analysis for toxicity or death, respectively.
Results
Patients
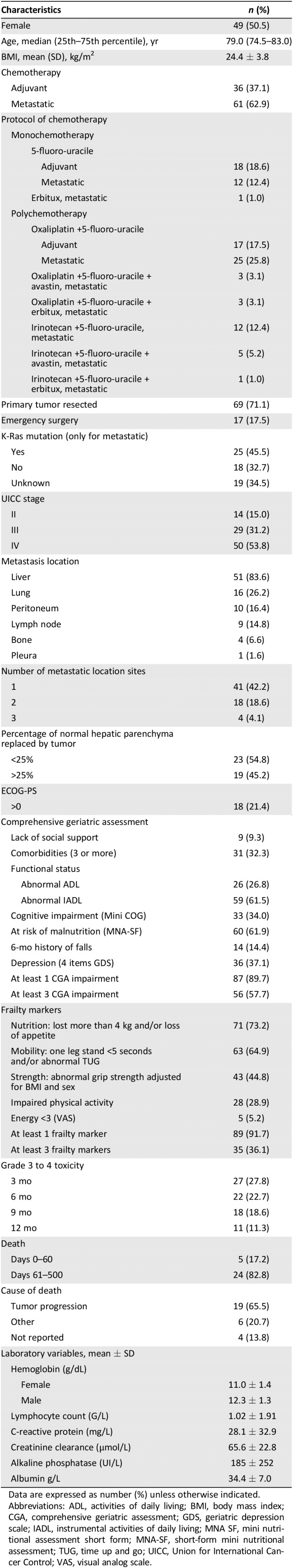
Ninety seven patients (median age 79.0 years; range, 70–90) received either adjuvant (n = 36, 37.1%) or first‐line metastatic (61, 62.9%) chemotherapy (Table 1). Almost one‐third received monotherapy.
Table 1.
Patients characteristics (n = 97)
| Characteristics | n (%) |
|---|---|
| Female | 49 (50.5) |
| Age, median (25th–75th percentile), yr | 79.0 (74.5–83.0) |
| BMI, mean (SD), kg/m2 | 24.4 ± 3.8 |
| Chemotherapy | |
| Adjuvant | 36 (37.1) |
| Metastatic | 61 (62.9) |
| Protocol of chemotherapy | |
| Monochemotherapy | |
| 5‐fluoro‐uracile | |
| Adjuvant | 18 (18.6) |
| Metastatic | 12 (12.4) |
| Erbitux, metastatic | 1 (1.0) |
| Polychemotherapy | |
| Oxaliplatin +5‐fluoro‐uracile | |
| Adjuvant | 17 (17.5) |
| Metastatic | 25 (25.8) |
| Oxaliplatin +5‐fluoro‐uracile + avastin, metastatic | 3 (3.1) |
| Oxaliplatin +5‐fluoro‐uracile + erbitux, metastatic | 3 (3.1) |
| Irinotecan +5‐fluoro‐uracile, metastatic | 12 (12.4) |
| Irinotecan +5‐fluoro‐uracile + avastin, metastatic | 5 (5.2) |
| Irinotecan +5‐fluoro‐uracile + erbitux, metastatic | 1 (1.0) |
| Primary tumor resected | 69 (71.1) |
| Emergency surgery | 17 (17.5) |
| K‐Ras mutation (only for metastatic) | |
| Yes | 25 (45.5) |
| No | 18 (32.7) |
| Unknown | 19 (34.5) |
| UICC stage | |
| II | 14 (15.0) |
| III | 29 (31.2) |
| IV | 50 (53.8) |
| Metastasis location | |
| Liver | 51 (83.6) |
| Lung | 16 (26.2) |
| Peritoneum | 10 (16.4) |
| Lymph node | 9 (14.8) |
| Bone | 4 (6.6) |
| Pleura | 1 (1.6) |
| Number of metastatic location sites | |
| 1 | 41 (42.2) |
| 2 | 18 (18.6) |
| 3 | 4 (4.1) |
| Percentage of normal hepatic parenchyma replaced by tumor | |
| <25% | 23 (54.8) |
| >25% | 19 (45.2) |
| ECOG‐PS | |
| >0 | 18 (21.4) |
| Comprehensive geriatric assessment | |
| Lack of social support | 9 (9.3) |
| Comorbidities (3 or more) | 31 (32.3) |
| Functional status | |
| Abnormal ADL | 26 (26.8) |
| Abnormal IADL | 59 (61.5) |
| Cognitive impairment (Mini COG) | 33 (34.0) |
| At risk of malnutrition (MNA‐SF) | 60 (61.9) |
| 6‐mo history of falls | 14 (14.4) |
| Depression (4 items GDS) | 36 (37.1) |
| At least 1 CGA impairment | 87 (89.7) |
| At least 3 CGA impairment | 56 (57.7) |
| Frailty markers | |
| Nutrition: lost more than 4 kg and/or loss of appetite | 71 (73.2) |
| Mobility: one leg stand <5 seconds and/or abnormal TUG | 63 (64.9) |
| Strength: abnormal grip strength adjusted for BMI and sex | 43 (44.8) |
| Impaired physical activity | 28 (28.9) |
| Energy <3 (VAS) | 5 (5.2) |
| At least 1 frailty marker | 89 (91.7) |
| At least 3 frailty markers | 35 (36.1) |
| Grade 3 to 4 toxicity | |
| 3 mo | 27 (27.8) |
| 6 mo | 22 (22.7) |
| 9 mo | 18 (18.6) |
| 12 mo | 11 (11.3) |
| Death | |
| Days 0–60 | 5 (17.2) |
| Days 61–500 | 24 (82.8) |
| Cause of death | |
| Tumor progression | 19 (65.5) |
| Other | 6 (20.7) |
| Not reported | 4 (13.8) |
| Laboratory variables, mean ± SD | |
| Hemoglobin (g/dL) | |
| Female | 11.0 ± 1.4 |
| Male | 12.3 ± 1.3 |
| Lymphocyte count (G/L) | 1.02 ± 1.91 |
| C‐reactive protein (mg/L) | 28.1 ± 32.9 |
| Creatinine clearance (μmol/L) | 65.6 ± 22.8 |
| Alkaline phosphatase (UI/L) | 185 ± 252 |
| Albumin g/L | 34.4 ± 7.0 |
Data are expressed as number (%) unless otherwise indicated.
Abbreviations: ADL, activities of daily living; BMI, body mass index; CGA, comprehensive geriatric assessment; GDS, geriatric depression scale; IADL, instrumental activities of daily living; MNA SF, mini nutritional assessment short form; MNA‐SF, short‐form mini nutritional assessment; TUG, time up and go; UICC, Union for International Cancer Control; VAS, visual analog scale.
Geriatric Assessment and Frailty Markers Results
See Table 1. One‐third of patients had more than three comorbidities. A total of 61.5% and 26.8% of patients, respectively, had IADL and ADL disabilities. Cognitive disorders and depression were observed in 34.0% and 37.1% of the patients, respectively. The most prevalent frailty markers were malnutrition (73.2%), mobility (64.9%), and strength (44.8%). Almost 90% of the patients presented at least one frailty marker, whereas 36.1% were frail (three or more frailty markers).
Chemotherapy Toxicities and Death
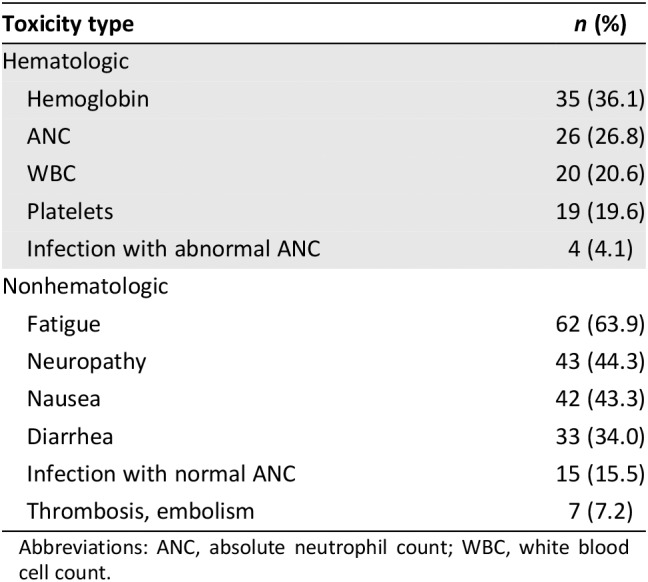
During the first 500 days, grade 3–4 toxicity occurred in 49 patients (50.5%) and death occurred in 29 (30.0%). The 60‐day mortality occurred in five patients (17.2%; Table 1). Grade 3–4 hematologic and nonhematologic toxicity occurred in 34.0 and 44.3% of the patients, respectively (Table 2). The most common hematologic toxicities were anemia (36.1%) and neutropenia (26.8%). The most common nonhematologic toxicities were fatigue (63.9%), neuropathy (44.3%), and nausea (43.3%).
Table 2.
Treatment‐related adverse events (grade 3–4)
| Toxicity type | n (%) |
|---|---|
| Hematologic | |
| Hemoglobin | 35 (36.1) |
| ANC | 26 (26.8) |
| WBC | 20 (20.6) |
| Platelets | 19 (19.6) |
| Infection with abnormal ANC | 4 (4.1) |
| Nonhematologic | |
| Fatigue | 62 (63.9) |
| Neuropathy | 43 (44.3) |
| Nausea | 42 (43.3) |
| Diarrhea | 33 (34.0) |
| Infection with normal ANC | 15 (15.5) |
| Thrombosis, embolism | 7 (7.2) |
Abbreviations: ANC, absolute neutrophil count; WBC, white blood cell count.
Factors Associated with Chemotherapy Toxicity and Death
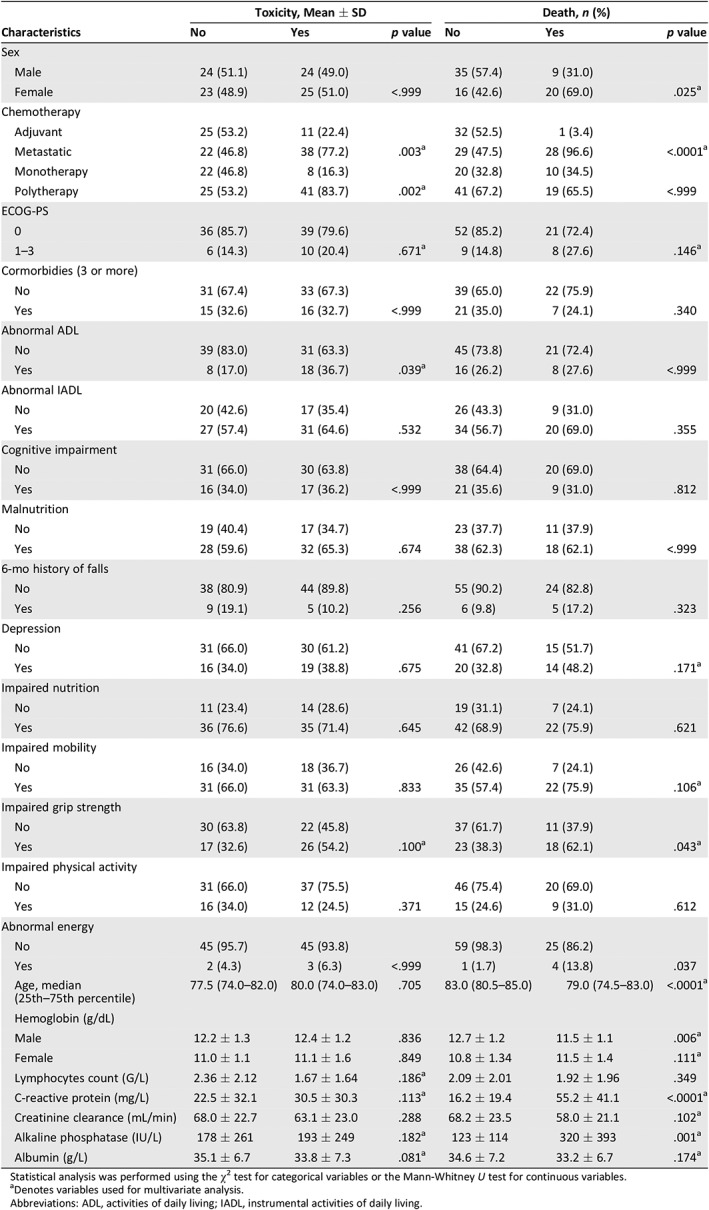
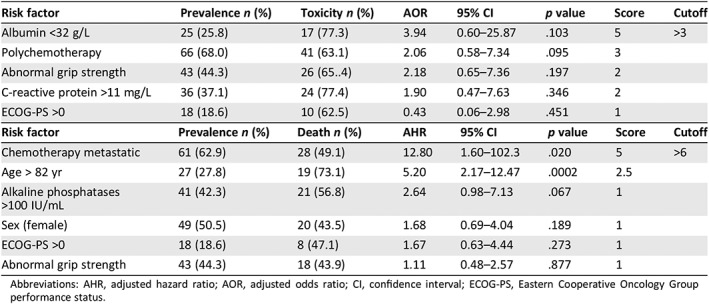
The risk factors associated with grade 3–4 toxicity in univariate analysis were metastatic chemotherapy, polychemotherapy, impaired grip strength, increased C‐reactive protein and alkaline phosphatase levels, and decreased lymphocytes count and hypoalbuminemia (Table 3). The risk factors associated with death were age, sex (female), metastatic chemotherapy, ECOG‐PS, depression, impaired mobility and grip strength, increased C‐reactive protein and alkaline phosphatase levels, and decreased hemoglobin, creatinine clearance, and hypoalbuminemia. In multivariate logistic regression, variables independently associated with toxicity were albuminemia <32 g/L, polychemotherapy, abnormal grip strength, C reactive protein >11 mg/L, and ECOG‐PS >0 (odds ratio: 3.94, 2.06, 2.18, 1.90, and 0.43, respectively). In Cox regression, variables independently associated with death were chemotherapy for metastatic disease, age > 82 years, alkaline phosphatase >100 IU/mL, sex (female), ECOG‐PS, and abnormal grip strength (hazard ratio: 12.80, 5.20, 2.64, 1.68, 1.67, and 1.11, respectively; Table 4).
Table 3.
Patient characteristics for toxicity or death
| Characteristics | Toxicity, Mean ± SD | Death, n (%) | ||||
|---|---|---|---|---|---|---|
| No | Yes | p value | No | Yes | p value | |
| Sex | ||||||
| Male | 24 (51.1) | 24 (49.0) | 35 (57.4) | 9 (31.0) | ||
| Female | 23 (48.9) | 25 (51.0) | <.999 | 16 (42.6) | 20 (69.0) | .025a |
| Chemotherapy | ||||||
| Adjuvant | 25 (53.2) | 11 (22.4) | 32 (52.5) | 1 (3.4) | ||
| Metastatic | 22 (46.8) | 38 (77.2) | .003a | 29 (47.5) | 28 (96.6) | <.0001a |
| Monotherapy | 22 (46.8) | 8 (16.3) | 20 (32.8) | 10 (34.5) | ||
| Polytherapy | 25 (53.2) | 41 (83.7) | .002a | 41 (67.2) | 19 (65.5) | <.999 |
| ECOG‐PS | ||||||
| 0 | 36 (85.7) | 39 (79.6) | 52 (85.2) | 21 (72.4) | ||
| 1–3 | 6 (14.3) | 10 (20.4) | .671a | 9 (14.8) | 8 (27.6) | .146a |
| Cormorbidies (3 or more) | ||||||
| No | 31 (67.4) | 33 (67.3) | 39 (65.0) | 22 (75.9) | ||
| Yes | 15 (32.6) | 16 (32.7) | <.999 | 21 (35.0) | 7 (24.1) | .340 |
| Abnormal ADL | ||||||
| No | 39 (83.0) | 31 (63.3) | 45 (73.8) | 21 (72.4) | ||
| Yes | 8 (17.0) | 18 (36.7) | .039a | 16 (26.2) | 8 (27.6) | <.999 |
| Abnormal IADL | ||||||
| No | 20 (42.6) | 17 (35.4) | 26 (43.3) | 9 (31.0) | ||
| Yes | 27 (57.4) | 31 (64.6) | .532 | 34 (56.7) | 20 (69.0) | .355 |
| Cognitive impairment | ||||||
| No | 31 (66.0) | 30 (63.8) | 38 (64.4) | 20 (69.0) | ||
| Yes | 16 (34.0) | 17 (36.2) | <.999 | 21 (35.6) | 9 (31.0) | .812 |
| Malnutrition | ||||||
| No | 19 (40.4) | 17 (34.7) | 23 (37.7) | 11 (37.9) | ||
| Yes | 28 (59.6) | 32 (65.3) | .674 | 38 (62.3) | 18 (62.1) | <.999 |
| 6‐mo history of falls | ||||||
| No | 38 (80.9) | 44 (89.8) | 55 (90.2) | 24 (82.8) | ||
| Yes | 9 (19.1) | 5 (10.2) | .256 | 6 (9.8) | 5 (17.2) | .323 |
| Depression | ||||||
| No | 31 (66.0) | 30 (61.2) | 41 (67.2) | 15 (51.7) | ||
| Yes | 16 (34.0) | 19 (38.8) | .675 | 20 (32.8) | 14 (48.2) | .171a |
| Impaired nutrition | ||||||
| No | 11 (23.4) | 14 (28.6) | 19 (31.1) | 7 (24.1) | ||
| Yes | 36 (76.6) | 35 (71.4) | .645 | 42 (68.9) | 22 (75.9) | .621 |
| Impaired mobility | ||||||
| No | 16 (34.0) | 18 (36.7) | 26 (42.6) | 7 (24.1) | ||
| Yes | 31 (66.0) | 31 (63.3) | .833 | 35 (57.4) | 22 (75.9) | .106a |
| Impaired grip strength | ||||||
| No | 30 (63.8) | 22 (45.8) | 37 (61.7) | 11 (37.9) | ||
| Yes | 17 (32.6) | 26 (54.2) | .100a | 23 (38.3) | 18 (62.1) | .043a |
| Impaired physical activity | ||||||
| No | 31 (66.0) | 37 (75.5) | 46 (75.4) | 20 (69.0) | ||
| Yes | 16 (34.0) | 12 (24.5) | .371 | 15 (24.6) | 9 (31.0) | .612 |
| Abnormal energy | ||||||
| No | 45 (95.7) | 45 (93.8) | 59 (98.3) | 25 (86.2) | ||
| Yes | 2 (4.3) | 3 (6.3) | <.999 | 1 (1.7) | 4 (13.8) | .037 |
| Age, median (25th–75th percentile) | 77.5 (74.0–82.0) | 80.0 (74.0–83.0) | .705 | 83.0 (80.5–85.0) | 79.0 (74.5–83.0) | <.0001a |
| Hemoglobin (g/dL) | ||||||
| Male | 12.2 ± 1.3 | 12.4 ± 1.2 | .836 | 12.7 ± 1.2 | 11.5 ± 1.1 | .006a |
| Female | 11.0 ± 1.1 | 11.1 ± 1.6 | .849 | 10.8 ± 1.34 | 11.5 ± 1.4 | .111a |
| Lymphocytes count (G/L) | 2.36 ± 2.12 | 1.67 ± 1.64 | .186a | 2.09 ± 2.01 | 1.92 ± 1.96 | .349 |
| C‐reactive protein (mg/L) | 22.5 ± 32.1 | 30.5 ± 30.3 | .113a | 16.2 ± 19.4 | 55.2 ± 41.1 | <.0001a |
| Creatinine clearance (mL/min) | 68.0 ± 22.7 | 63.1 ± 23.0 | .288 | 68.2 ± 23.5 | 58.0 ± 21.1 | .102a |
| Alkaline phosphatase (IU/L) | 178 ± 261 | 193 ± 249 | .182a | 123 ± 114 | 320 ± 393 | .001a |
| Albumin (g/L) | 35.1 ± 6.7 | 33.8 ± 7.3 | .081a | 34.6 ± 7.2 | 33.2 ± 6.7 | .174a |
Statistical analysis was performed using the χ2 test for categorical variables or the Mann‐Whitney U test for continuous variables.
Denotes variables used for multivariate analysis.
Abbreviations: ADL, activities of daily living; IADL, instrumental activities of daily living.
Table 4.
Multivariate logistic regression and Cox regression analysis for toxicity or death, respectively
| Risk factor | Prevalence n (%) | Toxicity n (%) | AOR | 95% CI | p value | Score | Cutoff |
|---|---|---|---|---|---|---|---|
| Albumin <32 g/L | 25 (25.8) | 17 (77.3) | 3.94 | 0.60–25.87 | .103 | 5 | >3 |
| Polychemotherapy | 66 (68.0) | 41 (63.1) | 2.06 | 0.58–7.34 | .095 | 3 | |
| Abnormal grip strength | 43 (44.3) | 26 (65..4) | 2.18 | 0.65–7.36 | .197 | 2 | |
| C‐reactive protein >11 mg/L | 36 (37.1) | 24 (77.4) | 1.90 | 0.47–7.63 | .346 | 2 | |
| ECOG‐PS >0 | 18 (18.6) | 10 (62.5) | 0.43 | 0.06–2.98 | .451 | 1 |
| Risk factor | Prevalence n (%) | Death n (%) | AHR | 95% CI | p value | Score | Cutoff |
|---|---|---|---|---|---|---|---|
| Chemotherapy metastatic | 61 (62.9) | 28 (49.1) | 12.80 | 1.60–102.3 | .020 | 5 | >6 |
| Age > 82 yr | 27 (27.8) | 19 (73.1) | 5.20 | 2.17–12.47 | .0002 | 2.5 | |
| Alkaline phosphatases >100 IU/mL | 41 (42.3) | 21 (56.8) | 2.64 | 0.98–7.13 | .067 | 1 | |
| Sex (female) | 49 (50.5) | 20 (43.5) | 1.68 | 0.69–4.04 | .189 | 1 | |
| ECOG‐PS >0 | 18 (18.6) | 8 (47.1) | 1.67 | 0.63–4.44 | .273 | 1 | |
| Abnormal grip strength | 43 (44.3) | 18 (43.9) | 1.11 | 0.48–2.57 | .877 | 1 |
Abbreviations: AHR, adjusted hazard ratio; AOR, adjusted odds ratio; CI, confidence interval; ECOG‐PS, Eastern Cooperative Oncology Group performance status.
Predictive Model for Chemotherapy Toxicity and Death
The predictive model for grade 3–4 toxicity combined (albumin <32 g/L × 5) + (polychemotherapy × 3) + (abnormal grip strength × 2) + (C‐reactive protein >11 mg/L × 2) + ECOG‐PS >0, cutoff score > 3. The predictive model for death combined (chemotherapy metastatic × 5) + (age > 82 years × 2.5) + alkaline phosphatase >100 IU/mL + sex (female) + ECOG‐PS >0 + abnormal grip strength, cutoff score > 6. Characteristics of both models are described Table 4 and Figures 1 and 2. No significant interaction between the variables was found. Both models showed a high goodness of fit (Hosmer‐Lemeshow test: χ2 = 5.751 p = .569, −2 log likelihood = 203, χ2 = 47.7, p < .0001 for toxicity or death model, respectively) and a good discrimination ability (area under the ROC curve = 0.774 ± 0.051; 95% CI, 0.674–0.855; p < .0001) and 0.925 ± 0.028 (95% CI, 0.849–0.970; p < .0001) for toxicity or death model, respectively (Table 5). Supplemental Data Table 1 describes the ability of the stratified risk score to predict chemotherapy toxicity.
Figure 1.
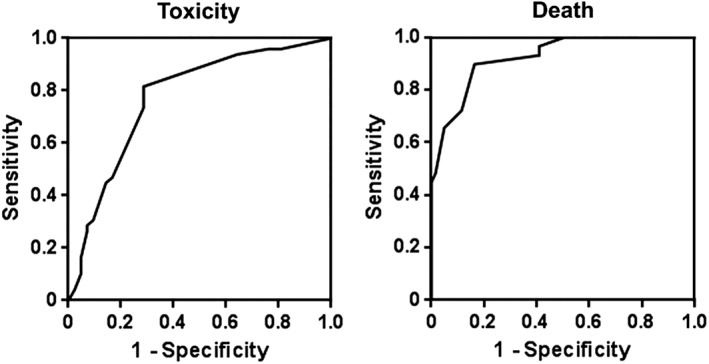
Receiver operating characteristic curve analysis of the predictive models of toxicity or death.
Figure 2.
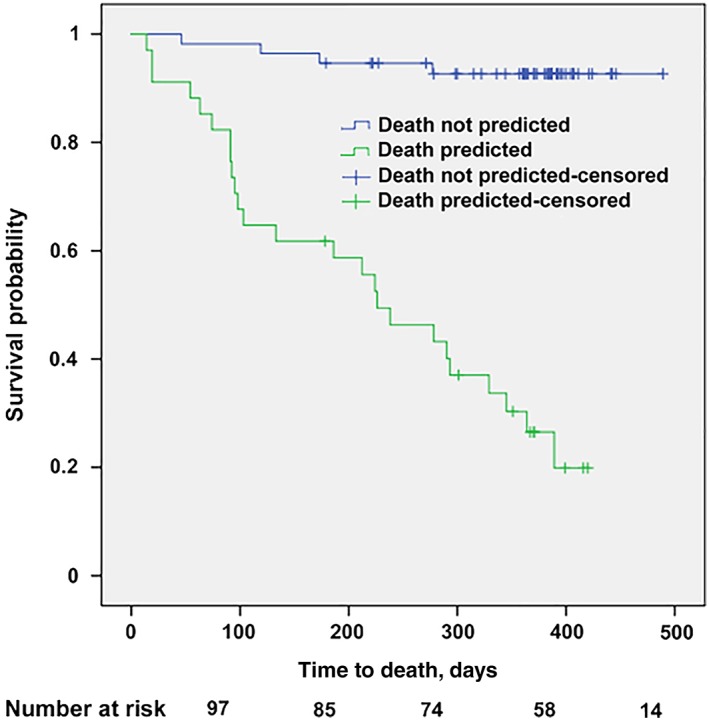
Kaplan‐Meier analysis of the predictive model of death.
Table 5.
Characteristics of the predictive models for toxicity or death.
| Risk | AUC ROC curve ± SD | 95% CI | p value | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|
| Toxicity | 0.774 ± 0.051 | 0.674–0.855 | <.0001 | 81.6 | 71.4 |
| Death | 0.925 ± 0.028 | 0.849–0.970 | <.0001 | 89.7 | 83.6 |

Abbreviations: AUC, area under the cure; CI, confidence interval; ROC, receiver operating characteristic.
Discussion
Predicting chemotoxicity and death is one of the main issues for oncologists when they prescribe chemotherapy in older patients, particularly in the adjuvant setting. Our study suggests that for patients greater than 70 years of age with CC, among the numerous geriatric, oncologic, and laboratory parameters, easy‐to‐obtain variables such as albumin, polychemotherapy, grip strength, C‐reactive protein, and ECOG‐PS predicted chemotoxicity, whereas chemotherapy for metastatic disease, age, alkaline phosphatase, sex, ECOG‐PS, and grip strength predicted death. A cutoff value >3 for toxicity and > 6 for death provided a good sensitivity (81.6 and 89.7%, respectively) and specificity (71.4 and 83.6%, respectively). Both models showed a robust goodness of fit (Hosmer‐Lemeshow test: χ2 = 5.751, p = .569 and –2 log likelihood = .203, χ2 = 47.7, p < .0001, for toxicity and death, respectively).
Only two studies have developed a predictive model for chemotoxicity in older populations with cancer. With data from more than 500 aged patients with various types of cancer, the Chemotherapy Risk Assessment Scale for High‐Age Patients (CRASH) score 11 was constructed along 2 subscores: hematological (H) toxicity and nonhematological (NH) toxicity. In their model, predictors of H toxicity were IADL score, lactate dehydrogenase levels, and diastolic blood pressure, and predictors of NH toxicity were Eastern Cooperative Oncology Group performance, Mini‐Mental Status score, and Mini‐Nutritional Assessment score, associated with a chemotherapy risk for both toxicities (H and NH). The CRASH score identified 4 risk categories of severe toxicity categories: low, medium‐low, medium‐high, and high. In 500 older patients with cancer, 53% of which had grade 3–5 chemotoxicity, Hurria et al. 10 developed a predictive model for grade 3–5 toxicity. Geriatric assessment variables (hearing impairment, fall, assistance in taking medications, limited walk, decreased social activities), laboratory test values (hemoglobin, creatinine clearance), and patient (age), tumor (gastrointestinal or genitourinary tumor), and treatment characteristics (standard dosing of chemotherapy, polychemotherapy) identified older adults at low, intermediate, or high risk of chemotherapy toxicity. These two studies included various types of cancer and chemotherapy whereas the proportion of CC was less than 25%. In addition, half the patients in one study 10 or more than two‐thirds in the other 11 were classified as intermediate risk, which lead to uncertainty for the oncologist when making treatment decisions. The scores of the present study identify two categories of older patients with CC: low‐ and high‐risk group, thus simplifying the decision‐making process.
Several prospective studies in oncology have demonstrated the predictive value of frailty markers for treatment toxicities. In two studies of older patients with CC, patients with at least three markers had higher risks of developing postoperative major complications 32 and early death 33. Whatever the number of frailty markers, it appears that some markers have their own predictive value. In the older cancer population, abnormal nutrition and poor mobility were significantly predictive for early deaths 7, 34, 35. Grip strength was also identified as an independent variable that predicted chemotoxicity 36 and various adverse outcomes such as functional decline and postoperative morbimortality 37, 38, 39. The International Database Inquiry on Frailty data from five studies of aging including almost 15,000 participants examined the importance and the interrelation of each frailty markers in explaining differences among participants. Researchers concluded that grip strength had the highest contribution overall in explaining differences among participants across the samples 40. Our study confirms the usefulness of grip strength to predict outcomes in older patients with CC.
Today, CGA is recommended for older patients with cancer to identify the patients who may benefit from treatment and may be hurt by treatment as well as conditions that may interfere with cancer treatment 4. However, implementation of CGA in oncologic setting presents some limitations. CGA is time consuming, costly in terms of resources, and is not standardized 1. Furthermore, some studies suggest than CGA would have a ceiling effect and is unable to detect vulnerability in a relatively highly functional population seen in oncology. In our model, simple laboratory data, frailty, and oncologic parameters are sufficient to complete the risk scores of both toxicity and death independently of a previous CGA 13, 41.
In fit patients with stage III colon cancer, adjuvant chemotherapy regimens with fluorouracil, leucovorin, and oxaliplatin (FOLFOX) or capecitabin and oxaliplatin (CAPOX) are the standard of care, with a 5‐year disease‐free survival rate of 73.3% 42, 43. For patients with metastatic colon cancer, several chemotherapy regimens can be administered at first line. The most frequently prescribed regimens are FOLFOX and FOLFIRI, combined with either antiangiogenic drugs or anti‐epidermal growth factor receptor monoclonal antibodies (for KRAS‐wild‐type patients). The median overall survival of these various regimens exceeds 20 months 44, 45, 46. Hence, assessing the risk of chemotherapy toxicity in the adjuvant and first‐line metastatic settings are a major concern for oncologists because of the relatively good prognosis of these categories of patients with colon cancer.
An important strength of this study is that it is the first one to propose two simple, efficient models able to predict toxicity and death specifically for patients with CC. Also, the variables used do no depend upon clinicians or nurses, can be objectively quantified, and do not rely on patient's interview in a context of possible cognitive impairment. There are limitations to this study. First, the number of patients was relatively small and treatment regimens were large. However, our study population was homogeneous, grouping patients with CC from several centers, thus reflecting the whole population. This study focused on grade 3–4 toxicity. However, some grade 2 toxicities (diarrhea, neuropathy) may also be relevant to the geriatric population. Finally, although the models were internally validated using the bootstrap methodology, an external validation cohort would be helpful to assess the potential of our scores as predictors for both chemotoxicity and death in older patients with CC.
Conclusion
In geriatric oncology, optimal management of older patients with cancer is challenging, as the assessment of the underlying vulnerability guides decision making. We demonstrate, using an homogeneous colon cancer cohort, that two simple scores combining patient characteristics and tumoral and biological indexes are powerful to predict severe chemotoxicity and death. These “ColonPrediscores” offer a major advantage in that they do not need a previous complete geriatric assessment, which makes them an easy‐to‐use tool in oncologic settings. An external validation of these scores is currently ongoing in an independent cohort.
Author Contributions
Study concepts: Frédérique Retornaz, Olivier Guillem, Frédérique Rousseau, Dany Gholam
Study design: Frédérique Retornaz, Olivier Guillem, Frédérique Rousseau, Dany Gholam
Data acquisition: Olivier Guillem, Frédérique Rousseau, Francois Morvan, Yves Rinaldi, Sophie Nahon, Chantal Castagna, Rabia Boulahssass, Dany Gholam
Quality control of data and algorithms: Frédérique Retornaz, Olivier Guillem, Michel Grino
Data analysis and interpretation: Frédérique Retornaz, Olivier Guillem, Frédérique Rousseau, Michel Grino, Dany Gholam
Statistical analysis: Frédérique Retornaz, Michel Grino
Manuscript preparation: Frédérique Retornaz, Michel Grino
Manuscript editing: Frédérique Retornaz, Olivier Guillem, Michel Grino, Dany Gholam
Manuscript review: Frédérique Retornaz, Olivier Guillem, Michel Grino, Dany Gholam
Disclosures
Francois Morvan: Novartis, Pfizer (SAB, ET, H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Table S1 Ability of the stratified risk score to predict chemotherapy toxicity
Acknowledgments
The authors thank Drs. J.F. Codoul, C. Bratisevic, N. Barrière, and O. Guerin for their help in collecting data. This work was supported by grants from Applied Molecular Genetics and Association Sud pour la Recherche en Oncogeriatrie. This study was presented in part at the annual meeting of the American Society of Clinical Oncology; June 1–5, 2018; Chicago, IL:10041a.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1.Cancer du côlon: Quelques chiffres. Institut National du Cancer. Available at http://www.e-cancer.fr/Patients-et-proches/Les-cancers/Cancer-du-colon/Quelques-chiffres. Accessed July 28, 2019.
- 2. Sargent DJ, Goldberg RM, Jacobson SD et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med 2001;345:1091–1097. [DOI] [PubMed] [Google Scholar]
- 3. Moth EB, Vardy J, Blinman P. Decision‐making in geriatric oncology: Systemic treatment considerations for older adults with colon cancer. Expert Rev Gastroenterol Hepatol 2016;10:1321–1340. [DOI] [PubMed] [Google Scholar]
- 4. Wildiers H, Heeren P, Puts M et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol 2014;32:2595–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hamaker ME, Vos AG, Smorenburg CH et al. The value of geriatric assessments in predicting treatment tolerance and all‐cause mortality in older patients with cancer. The Oncologist 2012;17:1439–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Puts MT, Santos B, Hardt J et al. Update on a systematic review of the use of geriatric assessment for older adults in oncology. Ann Oncol 2014;25:307–315. [DOI] [PubMed] [Google Scholar]
- 7. Ferrat E, Paillaud E, Laurent M et al.; ELPACA Study Group . Predictors of 1‐year mortality in a prospective cohort of elderly patients with cancer. J Gerontol A Biol Sci Med Sci 2015;70:1148–1155. [DOI] [PubMed] [Google Scholar]
- 8. Aparicio T, Gargot D, Teillet L et al. Geriatric factors analyses from FFCD 2001‐02 phase III study of first‐line chemotherapy for elderly metastatic colorectal cancer patients. Eur J Cancer 2017;74:98–108. [DOI] [PubMed] [Google Scholar]
- 9. Ramsdale E, Polite B, Hemmerich J et al. The Vulnerable Elders Survey‐13 predicts mortality in older adults with later‐stage colorectal cancer receiving chemotherapy: A prospective pilot study. J Am Geriatr Soc 2013;61:2043–2044. [DOI] [PubMed] [Google Scholar]
- 10. Hurria A, Togawa K, Mohile SG et al. Predicting chemotherapy toxicity in older adults with cancer: A prospective multicenter study. J Clin Oncol 2011;29:3457–3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Extermann M, Boler I, Reich RR et al. Predicting the risk of chemotherapy toxicity in older patients: The Chemotherapy Risk Assessment Scale for High‐Age Patients (CRASH) score. Cancer 2012;118:3377–3386. [DOI] [PubMed] [Google Scholar]
- 12. Carreca I, Balducci L, Extermann M. Cancer in the older person. Cancer Treat Rev 2005;31:380–402. [DOI] [PubMed] [Google Scholar]
- 13. Hurria A, Zuckerman E, Panageas KS et al. A Prospective longitudinal study of the functional status and quality of life of older patients with breast cancer receiving adjuvant chemotherapy. J Am Geriatr Soc 2006;54:1119–1124. [DOI] [PubMed] [Google Scholar]
- 14. Retornaz F, Monette J, Batist G et al. Usefulness of frailty markers in the assessment of health and functional status of older cancer patients referred for chemotherapy: A pilot study. J Gerontol A Biol Sci Med Sci 2008;63:518–522. [DOI] [PubMed] [Google Scholar]
- 15. Fried LP, Tangen CM, Walston J et al. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 16. Schuurmans H, Steverink N, Lindenberg S et al. Old or frail: What tells us more? J Gerontol A Biol Sci Med Sci 2004;59:M962–M965. [DOI] [PubMed] [Google Scholar]
- 17. Puts MT, Lips P, Deeg DJ. Static and dynamic measures of frailty predicted decline in performance‐based and self‐reported physical functioning. J Clin Epidemiol 2005;58:1188–1198. [DOI] [PubMed] [Google Scholar]
- 18. Mitnitski A, Song X, Skoog I et al. Relative fitness and frailty of elderly men and women in developed countries and their relationship with mortality. J Am Geriatr Soc 2005;53:2184–2189. [DOI] [PubMed] [Google Scholar]
- 19. Puts MT, Monette J, Girre V et al. Are frailty markers useful for predicting treatment toxicity and mortality in older newly diagnosed cancer patients? Results from a prospective pilot study. Crit Rev Oncol Hematol 2011;78:138–149. [DOI] [PubMed] [Google Scholar]
- 20. Soubeyran P, Fonck M, Blanc‐Bisson C et al. Predictors of early death risk in older patients treated with first‐line chemotherapy for cancer. J Clin Oncol 2012;30:1829–1834. [DOI] [PubMed] [Google Scholar]
- 21. Katz S. Assessing self‐maintenance: Activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc 1983;31:721–727. [DOI] [PubMed] [Google Scholar]
- 22. Fillenbaum GG, Smyer MA. The development, validity, and reliability of the OARS multidimensional functional assessment questionnaire. J Gerontol 1981;36:428–434. [DOI] [PubMed] [Google Scholar]
- 23. Miller MD, Towers A. A Manual of Guidelines for Scoring the Cumulative Illness Rating Scale For Geriatrics (CIRS‐G). Pittsburg, PA: University of Pittsburgh, 1991. [Google Scholar]
- 24. Rubenstein LZ, Harker JO, Salvà A et al. Screening for undernutrition in geriatric practice: Developing the short‐form mini‐nutritional assessment (MNA‐SF). J Gerontol Med Sci 2001;56:M366–M372. [DOI] [PubMed] [Google Scholar]
- 25. Borson S, Scanlan JM, Watanabe J et al. Simplifying detection of cognitive impairment: Comparison of the Mini‐Cog and Mini‐Mental State Examination in a multiethnic sample. J Am Geriatr Soc 2005;53:871–874. [DOI] [PubMed] [Google Scholar]
- 26. Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version In: Brink TL, ed. Clinical Gerontology: A Guide to Assessment and Intervention. New York, NY: The Haworth Press, 1986:165–173. [Google Scholar]
- 27. Vellas BJ, Wayne SJ, Romero L et al. One‐leg balance is an important predictor of injurious falls in older persons. J Am Geriatr Soc 1997;145:735–738. [DOI] [PubMed] [Google Scholar]
- 28. Podsiadlo D, Richardson S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991;39:142–148. [DOI] [PubMed] [Google Scholar]
- 29. Bandeen‐Roche K, Xue QL, Ferrucci L et al. Phenotype of frailty: Characterization in the women's health and aging studies. J Gerontol A Biol Sci Med Sci 2006;61:262–266. [DOI] [PubMed] [Google Scholar]
- 30. Davis HS, MacPherson K, Merry HR et al. Reliability and validity of questions about exercise in the Canadian Study of Health and Aging. Int Psychogeriatr. 2001;(supp 1):177–182. [DOI] [PubMed] [Google Scholar]
- 31. Cornali C, Franzoni S, Frisoni GB et al. Anorexia as an independent predictor of mortality. J Am Geriatr Soc 2005;53:354–355. [DOI] [PubMed] [Google Scholar]
- 32. Tan KY, Kawamura YJ, Tokomitsu A et al. Assessment for frailty is useful for predicting morbidity in elderly patients undergoing colorectal cancer resection whose comorbidities are already optimized. Am J Surg 2012;204:139–143. [DOI] [PubMed] [Google Scholar]
- 33. Kristjansson SR, Rønning B, Hurria A et al. A comparison of two pre‐operative frailty measures in older surgical cancer patients. J Geriat Oncol 2012;3:1–7. [Google Scholar]
- 34. Boulahssass R, Gonfrier S, Ferrero JM, et al. Predicting early death in older adults with cancer. Eur J Cancer 2018;100:65–74. [DOI] [PubMed] [Google Scholar]
- 35. Pamoukdjian F, Lévy V, Sebbane G et al. Slow gait speed is an independent predictor of early death in older cancer outpatients: Results from a prospective cohort study. J Nutr Health Aging 2017;21:202–206. [DOI] [PubMed] [Google Scholar]
- 36. Puts M, Monette J, Girre V et al. Are frailty markers useful for predicting treatment toxicity and mortality in older newly diagnosed patients? Results from a prospective pilot study. Crit Rev Oncol Hematol 2011;78:138–149. [DOI] [PubMed] [Google Scholar]
- 37. Owusu C, Margevicius S, Schluchter M et al. Short Physical Performance Battery, usual gait speed, grip strength and Vulnerable Elders Survey each predict functional decline among older women with breast cancer. J Geriatr Oncol 2017;8:356‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kilgour RD, Vigano A, Trutschnigg B et al. Handgrip strength predicts survival and is associated with markers of clinical and functional outcomes in advanced cancer patients. Support Care Cancer 2013;21:3261–3270. [DOI] [PubMed] [Google Scholar]
- 39. Versteeg KS, Blauwhoff‐Buskermolen S, Buffart LM et al. Higher muscle strength is associated with prolonged survival in older patients with advanced cancer. The Oncologist 2018;23;580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sourial N, Bergman H, Karunananthan S et al. Contribution of frailty markers in explaining differences among individuals in five samples of older persons. J Gerontol A Biol Sci Med Sci. 2012;67:1197–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Puts MT, Hardt J, Monette J et al. Use of geriatric assessment for older adults in the oncology setting: A systematic review. J Natl Cancer Inst 2012;104:1133–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. André T, Boni C, Navarro M et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 2009;27:3109–3116. [DOI] [PubMed] [Google Scholar]
- 43. Loree JM, Mulder KE, Ghosh S et al. CAPOX associated with toxicities of higher grade but improved disease‐free survival when compared with FOLFOX in the adjuvant treatment of stage III colon cancer. Clin Colorectal Cancer 2014;13:172–177. [DOI] [PubMed] [Google Scholar]
- 44. Hurwitz H, Fehrenbacher L, Novotny W et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335–2342. [DOI] [PubMed] [Google Scholar]
- 45. Cremolini C, Loupakis F, Antoniotti C et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first‐line treatment of patients with metastatic colorectal cancer: Updated overall survival and molecular subgroup analyses of the open‐label, phase 3 TRIBE study. Lancet Oncol 2015;16:1306–1315. [DOI] [PubMed] [Google Scholar]
- 46. Venook AP , Niedzwiecki D , Lenz HJ et al. Effect of first‐line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild‐type advanced or metastatic colorectal cancer: A randomized clinical trial. JAMA 2017. ; 317:2392–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Table S1 Ability of the stratified risk score to predict chemotherapy toxicity


