Abstract
Aims
Over the last decades, the profile of chronic coronary syndrome has changed substantially. We aimed to determine characteristics and management of patients with chronic coronary syndrome in the contemporary era, as well as outcomes and their determinants.
Methods and results
Data from 32 703 patients (45 countries) with chronic coronary syndrome enrolled in the prospective observational CLARIFY registry (November 2009 to June 2010) with a 5-year follow-up, were analysed. The primary outcome [cardiovascular death or non-fatal myocardial infarction (MI)] 5-year rate was 8.0% [95% confidence interval (CI) 7.7–8.3] overall [male 8.1% (7.8–8.5); female 7.6% (7.0–8.3)]. A cox proportional hazards model showed that the main independent predictors of the primary outcome were prior hospitalization for heart failure, current smoking, atrial fibrillation, living in Central/South America, prior MI, prior stroke, diabetes, current angina, and peripheral artery disease. There was an interaction between angina and prior MI (P = 0.0016); among patients with prior MI, angina was associated with a higher primary event rate [11.8% (95% CI 10.9–12.9) vs. 8.2% (95% CI 7.8–8.7) in patients with no angina, P < 0.001], whereas among patients without prior MI, event rates were similar for patients with [6.3% (95% CI 5.4–7.3)] or without angina [6.4% (95% CI 5.9–7.0)], P > 0.99. Prescription rates of evidence-based secondary prevention therapies were high.
Conclusion
This description of the spectrum of chronic coronary syndrome patients shows that, despite high rates of prescription of evidence-based therapies, patients with both angina and prior MI are an easily identifiable high-risk group who may deserve intensive treatment.
Clinical registry
ISRCTN43070564
Keywords: Chronic coronary syndrome, Coronary artery disease, Angina, Myocardial infarction
Introduction
Over the last decades, the management and prognosis of chronic coronary syndrome has improved considerably and consequently cardiovascular mortality has declined steadily.1 Outcomes following acute myocardial infarction (MI) have improved following the advent of reperfusion therapy with thrombolysis or primary angioplasty2 and widespread implementation of evidence-based secondary prevention medications,3 including aspirin and other antiplatelet agents,4,5 statins,6 beta-blockers,7 and renin–angiotensin antagonists.8,9 In addition, the profile of these patients has changed with the use of coronary revascularization, particularly percutaneous coronary intervention (PCI) as well as the improved survival of patients experiencing an acute coronary syndrome (ACS). Whereas in the past, patients with chronic coronary syndrome were largely defined as patients with ‘stable angina’,10–12 today these patients are a heterogeneous group encompassing patients with or without symptoms of angina pectoris, with or without a history of coronary revascularization, and with or without a history of prior and often remote ACS.13,14 While there is a wealth of information regarding the acute and short-term outcomes of ACS patients, less is known regarding the contemporary characteristics, management, and long-term prognosis of the broad chronic coronary syndrome population.
The CLARIFY registry (ProspeCtive observational LongitudinAl RegIstry oF patients with stable coronary arterY disease; ISRCTN43070564) was established to provide information regarding the profile and prognosis of chronic coronary syndrome in clinical practice, across a broad range of geographic regions.
The aim of the present study was to describe the characteristics and management of chronic coronary syndrome patients and to establish the determinants of their long-term prognosis with a focus on assessing the relative importance of angina and a history of MI.
Methods
Study design and participants
The rationale, design, and preliminary baseline characteristics of the CLARIFY registry have been described in detail previously.13,15,16 Briefly, between 26 November 2009 and 30 June 2010, 32 703 chronic coronary syndrome patients were enrolled in 45 countries (Supplementary material online, Table S1), encompassing patients from high/middle/low-income countries organized in six geographical areas: Western/Central Europe, Eastern Europe, Middle East, Asia, Central/South America, and some Commonwealth countries (Australia/Canada/South Africa/UK).
Patients were eligible for enrolment if they fulfilled ≥1 of the following criteria (not mutually exclusive): documented MI for more than 3 months before enrolment, coronary artery bypass grafting (CABG) or PCI for more than 3 months before enrolment, chest pain with proven myocardial ischaemia, or previous coronary angiography showing at least one coronary stenosis of more than 50%. Exclusion criteria were hospitalization for cardiovascular disease within the previous 3 months, planned revascularization, and conditions interfering with 5-year follow-up, including severe heart failure (HF). Severe HF was left to the investigator opinion without providing an left ventricular ejection fraction (LVEF) threshold (which would not take into account HF with preserved ejection fraction), or New York Heart Association class (which can transiently improve).
Patients were enrolled over a brief period to minimize the risk of selection bias and were followed-up yearly for up to 5 years. Medical care was at the discretion of each physician. Outcomes were not adjudicated, but investigators were provided with definitions for each outcome in case report forms. Yearly, 1% of sites were randomly selected for onsite audit of 100% of the data.
CLARIFY was conducted in accordance with the Declaration of Helsinki and local ethical approval was obtained in each country. All patients gave informed consent.
Outcomes
The primary outcome was the composite of cardiovascular death or non-fatal MI. Secondary outcomes were cardiovascular death, non-cardiovascular death, all-cause death, the triple composite of cardiovascular death, non-fatal MI or non-fatal stroke, fatal MI, fatal stroke, non-fatal MI, non-fatal stroke, hospitalization for HF, coronary angiography, PCI, and CABG.
Statistical analyses
Baseline characteristics were analysed according to history of MI and/or angina at baseline, using mean (±standard deviation) for continuous variables, and counts (and percentages) for categorical variables. Continuous and categorical variables were compared across groups using Student’s and χ2 tests, respectively. Baseline characteristics were also analysed across geographical areas and compared using analyses of variance and χ2 tests, where appropriate.
Five-year rates with 95% confidence intervals (CIs) were estimated by Kaplan–Meier method in the total population, according to gender as well as in clinical subgroups (according to history of prior MI and/or angina status at baseline, independently or combined). Comparisons across subgroups were performed using the log-rank tests.
To determine the main predictors of cardiovascular death or non-fatal MI, an univariate and a multivariable analysis without selection (cause-specific Cox proportional hazards model) determined hazard ratios (HRs) and 95% CI, including as potential predictors: (i) cardiovascular risk factors [age, sex, diabetes, smoking status (current/former/never), and treated hypertension]; (ii) medical history [prior MI, PCI, CABG, or hospitalization for HF, asthma/chronic obstructive pulmonary disease, atrial fibrillation/flutter, prior stroke, cerebrovascular disease, peripheral artery disease (PAD)]; (iii) clinical parameters [current angina, blood pressure (BP) <140/90 mmHg]; (iv) treatments (aspirin, statins); and (v) geographical zones. Only factors showing a P-value of significance lower than 0.2 in univariate models were introduced in the multivariable model. The interaction between prior MI and angina status was assessed using unadjusted and adjusted Cox models. The effect of this interaction to the predictors of the primary outcome was assessed by adding the interaction between prior MI and angina in the previous multivariable model.
Analyses were performed using SAS software version 9.3 (SAS Institute Inc., Cary, NC, USA).
Role of the funding source
The CLARIFY registry is supported by Servier. The sponsor had no role in the study design, data analysis and interpretation, or decision to submit the manuscript for publication, but assisted with the set-up, data collection, and management of the study in each country. The corresponding author had full access to all data and had the final responsibility for the decision to submit for publication.
Results
A total of 33 299 patients were screened. Five hundred and ninety-six withdrew consent and/or did not meet the inclusion criteria and/or did not have baseline information available, leaving a study population of 32 703 participants with planned follow-up up to 5 years (median follow-up 5.0 years; interquartile range 4.8–5.1) (Supplementary material online, Figure S1).
Baseline characteristics of the entire CLARIFY population were previously described.13 Briefly, patients had a mean age of 64.2 ± 10.5 years, 22.4% were women, 71.0% had a history of hypertension. As expected from the inclusion criteria, prior MI (59.9%) and prior revascularization (PCI = 58.6%; CABG = 23.6%) were common.
A minority of the patients had angina (22.1%), HF symptoms (15.1%), or HF symptoms and LVEF ≥50% (10.2%). The prevalence of HF symptoms with LVEF ≥50% was higher in female (13.8%) than in male (9.0%), P < 0.001. Rates for use of secondary prevention therapies, such as antiplatelet agents, statins, beta-blockers, and renin–angiotensin system (RAS) inhibitors (angiotensin-converting enzyme inhibitors or angiotensin receptor blockers) were: 95.2%, 82.9%, 75.3%, and 76.3%, respectively (Supplementary material online, Table S2).
Compared with patients without prior MI, patients with a history of prior MI were younger, more often males and current or former smokers, had a lower prevalence of treated hypertension, a higher prevalence of family history of premature coronary disease, and history of PCI. They had more symptoms of angina or HF, and lower LVEF. They were more likely to receive guideline-recommended secondary prevention therapies (Table 1).
Table 1.
Baseline characteristics according to history of MI status or according to current angina status
| Prior MI (N = 19 595) | No prior MI (N = 13 100) | P-value | Angina at baseline (N = 7212) | No angina at baseline (N = 25 479) | P-value | |
|---|---|---|---|---|---|---|
| Risk factors and lifestyle | ||||||
| Age (years) | 63.1 ± 10.7 (19 587) | 65.8 ± 9.4 (13 093) | <0.001 | 63.04 ± 10.2 (7208) | 64.5 ± 10.5 (25 468) | <0.001 |
| Male | 80.8% (15 825/19 589) | 72.8% (9534/13 096) | <0.001 | 71.5% (5154/7210) | 79.3% (20 203/25 471) | <0.001 |
| Treated hypertension | 69.1% (13 538/19 591) | 73.8% (9669/13 099) | <0.001 | 78.5% (5658/7212) | 68.9% (17 549/25 477) | <0.001 |
| Diabetes | 28.6% (5601/19 589) | 29.7% (3895/13 099) | 0.026 | 28.6% (2062/7212) | 29.2% (7433/25 474) | 0.332 |
| Dyslipidaemia | 75.9% (14 877/19 591) | 73.5% (9622/13 099) | <0.001 | 78.8% (5683/7212) | 73.9% (18 815/25 476) | <0.001 |
| Smoking status | ||||||
| Current | 14.3% (2805/19 594) | 9.7% (1272/13 099) | <0.001 | 15.0% (1078/7212) | 11.8% (2999/25 479) | <0.001 |
| Former | 49.2% (9641/19 594) | 41.7% (5463/13 099) | 41.4% (2982/7212) | 47.6% (12 121/25 479) | ||
| Never | 36.5% (7148/19 594) | 48.6% (6364/13 099) | 43.7% (3152/7212) | 40.7% (10 359/25 479) | ||
| Family history of premature coronary disease | 29.1% (5704/19 590) | 27.7% (3622/13 098) | 0.004 | 35.4% (2552/7212) | 26.6% (6773/25 476) | <0.001 |
| No physical activity | 15.5% (3040/19 588) | 17.2% (2246/13 095) | <0.001 | 14.6% (1056/7211) | 16.6% (4230/25 472) | <0.001 |
| Medical history | ||||||
| Myocardial infarction | 100.0% (19 595/19 595) | 0.0% (0/13 100) | NA | 62.5% (4507/7211) | 59.2% (15 084/25 478) | <0.001 |
| PCI | 60.3% (11 812/19 594) | 56.1% (7348/13 099) | <0.001 | 41.9% (3018/7211) | 63.4% (16 140/25 477) | <0.001 |
| CABG | 21.5% (4217/19 592) | 26.6% (3484/13 099) | <0.001 | 19.3% (1392/7210) | 24.8% (6308/25 476) | <0.001 |
| Cerebrovascular disease | ||||||
| Stroke | 4.1% (801/19 593) | 3.9% (513/13 100) | 0.437 | 5.3% (383/7211) | 3.7% (931/25 477) | <0.001 |
| Transient ischemic attack | 2.8% (541/19 592) | 3.5% (460/13 100) | <0.001 | 4.8% (345/7211) | 2.6% (656/25 476) | <0.001 |
| Carotid disease | 6.7% (1312/19 594) | 8.9% (1162/13 100) | <0.001 | 9.6% (694/7211) | 7.0% (1779/25 478) | <0.001 |
| Peripheral arterial disease | ||||||
| Lower extremity artery disease | 9.7% (1900/19 590) | 10.2% (1338/13 099) | 0.126 | 13.0% (938/7212) | 9.0% (2301/25 475) | <0.001 |
| Aortic abdominal aneurysm | 1.6% (311/19 592) | 1.5% (193/13 099) | 0.412 | 1.2% (89/7211) | 1.6% (415/25 475) | 0.016 |
| Hospitalization for HF | 5.5% (1085/19 593) | 3.4% (445/13 100) | <0.001 | 7.5% (537/7211) | 3.9% (993/25 477) | <0.001 |
| Atrial fibrillation/flutter | 6.4% (1255/19 593) | 8.1% (1057/13 100) | <0.001 | 7.4% (531/7211) | 7.0% (1781/25 477) | 0.275 |
| Asthma/COPD | 7.3% (1437/19 593) | 7.5% (982/13 100) | 0.584 | 9.1% (654/7211) | 6.9% (1765/25 477) | <0.001 |
| Clinical examination | ||||||
| Angina symptoms | 23.0% (4507/19 591) | 20.6% (2704/13 098) | <0.001 | 100.0% (7212/7212) | 0.0% (0/25 479) | NA |
| HF symptoms | 18.5% (3628/19 590) | 10.0% (1296/13 098) | <0.001 | 40.4% (2912/7211) | 7.9% (2013/25 478) | <0.001 |
| Systolic BP (mmHg) | 130.1 ± 16.6 (19 576) | 132.5 ± 16.6 (13 094) | <0.001 | 133.5 ± 17.5 (7211) | 130.3 ± 16.4 (25 455) | <0.001 |
| Diastolic BP(mmHg) | 77.3 ± 10.1 (19 576) | 77.3 ± 9.8 (13 094) | 0.940 | 79.5 ± 10.7 (7211) | 76.6 ± 9.7 (25 455) | <0.001 |
| Pulse (b.p.m.) | 68.3 ± 10.5 (19 577) | 68.1 ± 10.7 (13 091) | 0.074 | 69.8 ± 10.9 (7212) | 67.8 ± 10.5 (25 452) | <0.001 |
| Body mass index (kg/m²) | 28.1 ± 4.7 (19 573) | 27.6 ± 4.5 (13 081) | <0.001 | 28.6 ± 4.8 (7202) | 27.7 ± 4.5 (25 448) | <0.001 |
| Paraclinical parameters | ||||||
| LVEF (%) | 53.7 ± 11.2 (13 963) | 60.0 ± 9.6 (8554) | <0.001 | 55.3 ± 10.4 (5422) | 56.3 ± 11.3 (17 091) | <0.001 |
| Coronary arteries involveda | ||||||
| Left main | 7.9% (1555/19 585) | 9.9% (1293/13 097) | <0.001 | 8.7% (624/7210) | 8.7% (2224/25 469) | 0.837 |
| LAD | 55.9% (10 947/19 585) | 62.0% (8113/13 097) | <0.001 | 44.5% (3211/7210) | 62.2% (15 848/25 469) | <0.001 |
| Cx | 34.9% (6834/19 585) | 37.9% (4959/13 097) | <0.001 | 29.5% (2128/7210) | 37.9% (9663/25 469) | <0.001 |
| RCA | 44.5% (8711/19 585) | 42.2% (5520/13 097) | <0.001 | 36.8% (2655/7210) | 45.4% (11 574/25 469) | <0.001 |
| Bypass graft | 7.7% (1510/19 585) | 8.6% (1120/13 097) | 0.006 | 7.5% (543/7210) | 8.2% (2087/25 469) | 0.068 |
| No significant stenosis | 3.1% (608/19 585) | 3.4% (449/13 097) | 0.105 | 3.9% (284/7210) | 3.0% (773/25 469) | <0.001 |
| Angiography not done <12 months | 16.5% (3223/19 585) | 11.8% (1540/13 097) | <0.001 | 31.7% (2282/7210) | 9.7% (2481/25 469) | <0.001 |
| Baseline medications | ||||||
| Aspirin | 89.1% (17 446/19 587) | 85.8% (11 236/13 097) | <0.001 | 89.1% (6423/7212) | 87.4% (22 258/25 471) | <0.001 |
| Any antiplatelet therapy | 95.8% (18 761/19 590) | 94.2% (12 342/13 097) | <0.001 | 95.0% (6854/7212) | 95.2% (24 248/25 474) | 0.598 |
| Dual antiplatelet therapy | 28.8% (5647/19 590) | 26.7% (3496/13 097) | <0.001 | 23.8% (1717/7212) | 29.2% (7425/25 474) | <0.001 |
| Oral anticoagulants | 8.1% (1590/19 573) | 8.2% (1078/13 089) | 0.716 | 7.9% (569/7208) | 8.3% (2101/25 453) | 0.324 |
| Lipid lowering agents | 93.6% (18 333/19 590) | 90.5% (11 853/13 097) | <0.001 | 91.3% (6584/7212) | 92.7% (23 601/25 474) | <0.001 |
| Statins | 84.7% (16 583/19 590) | 80.3% (10 512/13 097) | <0.001 | 82.9% (5978/7212) | 82.9% (21 115/25 474) | 0.998 |
| Beta-blockers | 79.0% (15 471/19 591) | 69.8% (9136/13 096) | <0.001 | 78.8% (5681/7212) | 74.3% (18 924/25 474) | <0.001 |
| Calcium antagonists | 23.0% (4504/19 588) | 33.6% (4405/13 095) | <0.001 | 31.8% (2296/7212) | 26.0% (6613/25 470) | <0.001 |
| Ivabradine | 10.3% (2015/19 588) | 9.2% (1203/13 097) | 0.001 | 21.3% (1533/7211) | 6.6% (1685/25 473) | <0.001 |
| Nitrates or other antianginal drugsb | 30.6% (5990/19 581) | 30.0% (3930/13 096) | 0.262 | 55.9% (4030/7209) | 23.1% (5887/25 467) | <0.001 |
| ACE-inhibitors or ARB | 79.6% (15 588/19 584) | 71.3% (9333/13 095) | <0.001 | 80.3% (5794/7212) | 75.1% (19 127/25 473) | <0.001 |
| Diuretics | 30.5% (5962/19 585) | 27.6% (3621/13 097) | <0.001 | 36.2% (2609/7212) | 27.4% (6975/25 469) | <0.001 |
| NSAID | 4.8% (929/19 581) | 5.2% (684/13 097) | 0.051 | 6.4% (459/7211) | 4.5% (1155/25 465) | <0.001 |
| Amiodarone/dronedarone | 3.2% (623/19 581) | 2.6% (338/13 096) | 0.002 | 3.7% (265/7211) | 2.7% (697/25 465) | <0.001 |
| Insulin in diabetics | 21.6% (1209/5599) | 21.5% (838/3894) | 0.932 | 23.3% (481/2062) | 21.1% (1566/7432) | 0.932 |
| Oral diabetic drugs in diabetics | 68.4% (3834/5599) | 70.4% (2742/3894) | 0.044 | 68.1% (1404/2062) | 69.6% (5174/7432) | <0.001 |
Groups according to history of prior MI or angina are non-mutually exclusive.
Data are expressed in mean ± standard deviation for continuous variables or % for categorical variables. For categorical data, the number of concerned patients/number of available data are indicated in brackets. For continuous data, the number of available data are indicated in brackets.
Categorical and continuous variables were compared across groups by χ2 and Student’s t-test, respectively. Continuous variables are presented by mean or median according to the distribution.
ACE, angiotensin converting enzyme; ARB, angiotensin receptor blockers; BP, blood pressure; CABG, coronary artery bypass graft; COPD, chronic obstructive coronary disease; HF, heart failure; LAD, left anterior descending artery; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NA, not applicable; NSAID, non-steroidal anti-inflammatory drugs; PCI, percutaneous coronary intervention; RCA, right coronary artery; SD, standard deviation.
With stenosis >50% at coronary angiography or having required revascularization in the past.
Other antiaginal drugs: molsidomine, nicorandil, ranolazine, or trimetazidine.
Compared with patients without angina, patients with angina were younger, more frequently females, had a higher prevalence of cardiovascular risk factors and history of hospitalization for HF, lower LVEF, higher rates of prior MI, lower extremity artery disease or cerebrovascular disease, and fewer prior coronary revascularization procedures. The use of medication was similar for single antiplatelet therapy and statins, but higher for antianginal drugs and RAS inhibitors in the angina subgroup. Rate of non-current smoking was lower in patients with angina (Table 1).
Baseline characteristics according to combined prior MI and angina status at study entry are reported in Supplementary material online, Table S3.
Across geographical regions, substantial variations in clinical profiles were observed (Supplementary material online, Table S4). Patients enrolled in Eastern Europe were the youngest and had the lowest prevalence of diabetes, but the highest prevalence of treated hypertension, current smoking, prior MI, prior hospitalization for HF, current angina, or HF symptoms, and the highest rate of RAS inhibitors use. In participating centres from the Middle East, patients had the lowest rate of current HF symptoms, the highest body mass index and the highest prevalence of diabetes. They had the lowest rate of lower extremity artery disease and family history of coronary disease. They had also the lowest LVEF. The overall use of secondary prevention therapy was the highest. Centres from Asia had the lowest prevalence of prior MI, the highest rate of prior PCI, but the lowest rate of CABG, the highest rate of stroke, and the smallest body mass index, and the lowest rate of use of statins and RAS inhibitors. Central/South America enrolled the highest proportion of females and the lowest rate of current smokers, prior stroke, or angina patients, but the highest rate of BP <140/90 mmHg. Commonwealth countries were characterized by the oldest population, with the highest rate of family history of premature coronary disease, prior transient ischaemic attack, or CABG, and the lowest rate of use of single antiplatelet therapy and beta-blockers. Western/Central Europe had the highest proportion of males and the highest prevalence of lower extremity artery disease.
In the CLARIFY cohort, the 5-year crude rate of cardiovascular death or non-fatal MI was 8.0% (95% CI 7.7–8.3), the rate of cardiovascular death, non-fatal MI, or non-fatal stroke was 9.5% (95% CI 9.2–9.9), and the cardiovascular death rate was 5.5% (95% CI 5.3–5.8). Approximately 20% of cardiovascular deaths were due to MI and nearly 10% to stroke. All-cause death was 8.5% (95% CI 8.2–8.9) and non-cardiovascular death was 3.2% (95% CI 3.0–3.4). Approximately 15% of patients required coronary angiography during follow-up, of which half resulted in PCI and close to 10% in CABG (Table 2).
Table 2.
Five-year event rates (95% CI) in the entire cohort and according to history of prior MI or the presence of anginal symptoms at baseline
| All CLARIFY (N = 32 703) | Prior MI (N = 19 595) | No prior MI (N = 13 100) | P-value | Angina (N = 7212) | No angina (N = 25 479) | P-value | |
|---|---|---|---|---|---|---|---|
| Primary outcome | |||||||
| CV death or Non-fatal MI | 8.0 (7.7–8.3) | 9.1 (8.7–9.5) | 6.4 (6.0–6.9) | <0.001 | 9.8 (9.1–10.5) | 7.5 (7.1–7.8) | <0.001 |
| Secondary outcomes | |||||||
| CV death | 5.5 (5.3–5.8) | 6.3 (5.9–6.6) | 4.4 (4.0–4.8) | <0.001 | 6.3 (5.7–6.9) | 5.3 (5.0–5.6) | 0.001 |
| Non-CV death | 3.2 (3.0–3.4) | 3.3 (3.1–3.6) | 3.0 (2.7–3.3) | 0.096 | 3.0 (2.6–3.5) | 3.3 (3.0–3.5) | 0.295 |
| All-cause death | 8.5 (8.2–8.9) | 9.4 (9.0–9.8) | 7.3 (6.8–7.8) | <0.001 | 9.1 (8.4–9.8) | 8.4 (8.0–8.8) | 0.050 |
| CV death non-fatal MI or non-fatal stroke | 9.5 (9.2–9.9) | 10.7 (10.3–11.2) | 7.7 (7.2–8.2) | <0.001 | 11.6 (10.8–12.4) | 8.9 (8.5–9.3) | <0.001 |
| Fatal MI | 1.1 (1.0–1.2) | 1.4 (1.3–1.6) | 0.7 (0.5–0.8) | <0.001 | 1.5 (1.2–1.8) | 1.0 (0.9–1.2) | 0.001 |
| Fatal stroke | 0.6 (0.5–0.7) | 0.7 (0.6–0.8) | 0.5 (0.4–0.6) | 0.034 | 0.9 (0.7–1.2) | 0.5 (0.4–0.6) | <0.001 |
| Non-fatal MI | 2.8 (2.6–3.0) | 3.2 (2.9–3.5) | 2.2 (1.9–2.5) | <0.001 | 3.9 (3.5–4.4) | 2.5 (2.3–2.7) | <0.001 |
| Non-fatal stroke | 1.9 (1.7–2.0) | 2.0 (1.8–2.3) | 1.6 (1.4–1.9) | 0.006 | 2.3 (1.9–2.7) | 1.7 (1.6–1.9) | 0.006 |
| Hospitalization for heart failure | 5.4 (5.2–5.7) | 6.4 (6.1–6.8) | 3.9 (3.6–4.3) | <0.001 | 10.7 (10.0–11.5) | 3.9 (3.6–4.1) | <0.001 |
| Coronary angiography | 15.0 (14.6–15.4) | 14.2 (13.7–14.8) | 16.1 (15.4–16.8) | <0.001 | 19.7 (18.7–20.7) | 13.6 (13.2–14.1) | <0.001 |
| PCI | 7.5 (7.2–7.8) | 7.1 (6.8–7.5) | 7.9 (7.5–8.5) | 0.006 | 9.6 (8.9–10.3) | 6.8 (6.5–7.2) | <0.001 |
| CABG | 1.5 (1.4–1.7) | 1.6 (1.4–1.8) | 1.4 (1.2–1.7) | 0.345 | 2.5 (2.2–2.9) | 1.2 (1.1–1.4) | <0.001 |
Groups according to history of prior MI or angina are non-mutually exclusive.
All risks are described as Kaplan–Meier estimates with their 95% CI. P-values for group comparisons were estimated by log-ranks tests.
CABG, coronary artery bypass graft; CI, confidence interval; CV, cardiovascular; MI, myocardial infarction; PCI, percutaneous coronary intervention.
The primary event rate was 7.6% (95% CI 7.0–8.3) for female and 8.1% (95% CI 7.8–8.5) for male. Overall, 5-year event rates were similar regardless of the gender, but female were significantly less revascularized than male: 6.6% (95% CI 6.1–7.3) vs. 7.7% (95% CI 7.4–8.1), respectively for PCI (Supplementary material online, Table S5).
Patients with prior MI experienced worse outcomes than patients without prior MI. Rates of cardiovascular death or non-fatal MI were 9.1% (95% CI 8.7–9.5) and 6.4% (95% CI 6.0–6.9), respectively (P < 0.001). They also had a significantly higher rate of cardiovascular death and of the triple composite endpoint. Compared with patients without angina, patients with angina had a higher rate of cardiovascular death or non-fatal MI with rates of 9.8% (95% CI 9.1–10.5) and 7.5% (95% CI 7.1–7.8), respectively (P < 0.001). They also had higher rates of cardiovascular death and triple composite endpoint (Table 2).
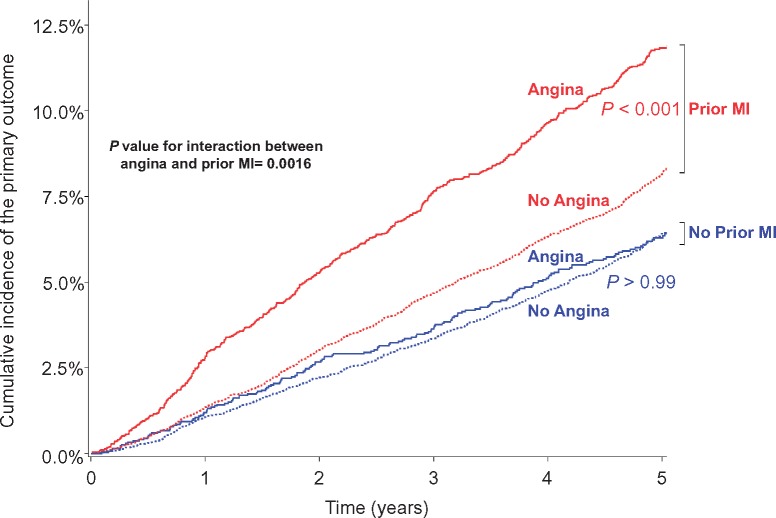
There was an interaction between angina and history of MI regarding the primary endpoint (P = 0.0016 with multivariable analysis), and the main secondary endpoints. This interaction was not time-dependent (Supplementary material online, Table S6). After stratification into four mutually exclusive subgroups according to the combined presence of angina at baseline and history of prior MI, angina was associated with a worse prognosis only in patients with a history of prior MI (Take home figure). In the subset of patients with prior MI, the 5-year rate of cardiovascular death or non-fatal MI was 11.8% (95% CI 10.9–12.9) for angina patients vs. 8.2% (95% CI 7.8–8.7) for non-angina patients (P < 0.001). In the subset of patients without prior MI, this rate was 6.3% (95% CI 5.4–7.3) for angina patients vs. 6.4% (95% CI 5.9–7.0) for patients without angina (P > 0.99). Similar results were found for most secondary outcomes (Table 3).
Take home figure.
Kaplan–Meier estimate curves for cardiovascular death or myocardial infarction according to angina status and history of prior myocardial infarction. P-value for interaction between angina and prior myocardial infarction in multivariable Cox model. CV, cardiovascular; MI, myocardial infarction.
Table 3.
Five-year outcomes according to combined history of prior MI and angina status
| Prior MIa (N = 19 595) |
P-value | No prior MIb (N = 13 100) |
P-value | |||
|---|---|---|---|---|---|---|
| Angina (N = 4507) | No angina (N = 15 084) | Angina (N = 2704) | No angina (N = 10 394) | |||
| 5-year event rate % (95% CI) | 5-year event rate % (95% CI) | 5-year event rate % (95% CI) | 5-year event rate % (95% CI) | |||
| Primary outcome | ||||||
| CV death or non-fatal MI | 11.8 (10.9–12.9) | 8.2 (7.8–8.7) | <0.001 | 6.3 (5.4–7.3) | 6.4 (5.9–7.0) | >0.99 |
| Secondary outcomes | ||||||
| Cardiovascular death | 7.7 (7.0–8.6) | 5.8 (5.4–6.2) | <0.001 | 3.7 (3.1–4.6) | 4.6 (4.2–5.1) | 0.111 |
| Non-CV death | 3.3 (2.8–3.9) | 3.3 (3.0–3.7) | >0.99 | 2.5 (1.9–3.2) | 3.1 (2.8–3.5) | 0.069 |
| All-cause death | 10.8 (9.9–11.8) | 8.9 (8.5–9.5) | <0.001 | 6.1 (5.2–7.1) | 7.6 (7.1–8.2) | 0.017 |
| CV death non-fatal MI or non-fatal stroke | 13.7 (12.7–14.8) | 9.8 (9.3–10.3) | <0.001 | 8.0 (7.0–9.1) | 7.6 (7.1–8.2) | 0.448 |
| Fatal MI | 2.0 (1.6–2.5) | 1.2 (1.1–1.5) | <0.001 | 0.6 (0.4–1.0) | 0.7 (0.5–0.9) | 0.939 |
| Fatal stroke | 1.2 (0.9–1.6) | 0.5 (0.4–0.7) | <0.001 | 0.5 (0.3–0.8) | 0.5 (0.4–0.7) | 0.860 |
| Non-fatal MI | 4.6 (4.0–5.3) | 2.7 (2.5–3.0) | <0.001 | 2.8 (2.2–3.5) | 2.0 (1.8–2.4) | 0.034 |
| Non-fatal stroke | 2.5 (2.0–3.0) | 1.9 (1.7–2.1) | 0.019 | 1.9 (1.4–2.5) | 1.5 (1.3–1.8) | 0.189 |
| Hospitalization for heart failure | 13.0 (12.0–14.1) | 4.4 (4.0–4.7) | <0.001 | 6.8 (5.9–7.9) | 3.1 (2.8–3.5) | <0.001 |
| Coronary angiography | 19.0 (17.8–20.3) | 12.8 (12.2–13.4) | <0.001 | 20.7 (19.2–22.4) | 14.9 (14.2–15.7) | <0.001 |
| PCI | 9.2 (8.3–10.1) | 6.5 (6.1–7.0) | <0.001 | 10.2 (9.1–11.5) | 7.3 (6.8–7.9) | <0.001 |
| CABG | 2.5 (2.1–3.1) | 1.3 (1.1–1.5) | <0.001 | 2.5 (1.9–3.2) | 1.2 (0.9–1.4) | <0.001 |
Groups according to history of prior MI or angina are mutually exclusive.
Among the subset with prior MI angina status was missing for four patients.
Among the subset without prior MI angina status was missing for two patients.
All risks are described as Kaplan–Meier estimates with their 95% CI. P-values for group comparisons were estimated by log-rank tests.
CABG, coronary artery bypass graft; CI, confidence interval; CV, cardiovascular; MI, myocardial infarction; PCI, percutaneous coronary intervention.
There were substantial variations in crude event rates across geographical zones. Asia had the lowest rate of primary outcome and the lowest mortality (all-cause, cardiovascular, and non-cardiovascular). Conversely, Central/South America had the highest rate of primary outcome and the highest mortality, driven by the highest cardiovascular death (Supplementary material online, Table S7).
Multivariable analysis identified the main independent predictors of the primary composite outcome of cardiovascular death or non-fatal MI (Figure 1) as history of hospitalization for HF (HR = 2.13; 95% CI 1.87–2.42), current smoking (HR = 1.74; 95% CI 1.51–1.99), atrial fibrillation/flutter (HR = 1.61; 95% CI 1.42–1.82), living in Central/South America (HR = 1.61; 95% CI 1.38–1.88), prior MI (HR = 1.50; 95% CI 1.37–1.65), prior stroke (HR = 1.45; 95% CI 1.20–1.76), diabetes (HR = 1.40; 95% CI 1.28–1.53), current angina (HR = 1.30; 95% CI 1.18–1.45), PAD (HR = 1.29; 95% CI 1.15–1.45), and former smoker (HR = 1.29; 95% CI 1.17–1.42). Age was also an independent predictor (HR = 1.04; 95% CI 1.03–1.04 for each 1-year increase). A lower risk of cardiovascular death or non-fatal MI was independently predicted by prior PCI (HR = 0.85; 95% CI 0.78–0.93) and BP <140/90 mmHg (HR = 0.90; 95% CI 0.83–0.99). Gender was not an independent predictor of cardiovascular death or non-fatal MI (HR = 0.95; 95% CI 0.85–1.06, for female) as illustrated in Figure 1. Univariate/multivariable analyses are reported in Supplementary material online, Table S8. After adjustment with interaction between prior MI and angina, the effect of these predictors was similar (Supplementary material online, Figure S2).
Figure 1.
Forest plot for multivariable analysis to determine the main predictors of cardiovascular death or myocardial infarction in the entire CLARIFY population. BP, blood pressure; CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; CV, cardiovascular; HF, heart failure; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Discussion
This large, international, observational, cohort of chronic coronary syndrome patients provides several important observations with direct clinical implications.
First, since prior registries, such as Euro Heart Survey on stable angina12 and REACH17,18 were started, the clinical characteristics of patients with chronic coronary syndrome have changed. Compared with the Euro Heart Survey on stable angina (enrolment 2002), and with the REACH registry (enrolment 2003–2004), more patients in CLARIFY were well treated in terms of exposure to secondary prevention therapies, regardless of the clinical profile or geographical area of enrolment. Moreover, patients in CLARIFY had more diverse clinical profiles than in the Euro Heart Survey, which was focused exclusively on patients with angina and without revascularization, and than in the REACH registry, which enrolled patients at risk for or with established atherothrombosis, including chronic coronary syndrome but which did not encompass the full spectrum of this syndrome. For example, REACH did not enroll patients with documented coronary disease but without prior MI or PCI. This observation highlights the changing picture of chronic coronary artery disease, a population which used to be defined largely via its angina symptoms, but which now covers a broad spectrum of clinical presentations, and prior medical history.
Second, in this cohort without severe HF, major cardiovascular event rates were overall relatively low compared with previous registries,12,18 but encompassed substantial variation across geographic zones. This may be related to secular trends in cardiovascular disease but also to different selection criteria resulting in enrolment of a lower risk population. In the CORONOR registry (enrolment 2010–2011), which recruited an older population (mean age 66.9 ± 11.6 years), half of deaths were non-cardiovascular and the rate of cardiovascular death was slightly higher (1.3 per 100 patient-years) than in the present study.19 Compared with contemporary randomized clinical trials (RCTs), CLARIFY patients were overall at lower risk than those from COMPASS20 (who required coronary artery disease enrichment criteria to be enrolled), from FOURIER21 (with a higher prevalence of prior MI) or from CANTOS22 (where all patients had a prior MI).
Third, major predictors of cardiovascular death or non-fatal MI were a history of hospitalization for HF and other comorbidities, such as atrial fibrillation or PAD, suggesting that these comorbidities must not be considered as incidental in chronic coronary syndrome patients and need to be more carefully targeted and treated. Conventional cardiovascular risk factors were also predictors of poor outcomes, highlighting that prevention and treatment of these factors remains a major goal.
Fourth, the profile of women and men with chronic coronary syndrome differ substantially15 with a higher rate of HF with preserved LVEF for women. However, gender was not an independent predictor of the primary outcome and 5-year event rates were overall similar regardless of the gender, other than a lower incidence of revascularization in women. These results highlight the need of further analyses to better understand gender determinants of outcome.
Fifth, history of prior MI and angina symptoms were both major determinants of adverse cardiovascular outcomes, with a significant statistical interaction. These results complement the previous findings where angina was shown to be prognostically important, while silent ischaemia without angina was not associated with a worse prognosis at 1 year.14
While a history of prior MI was a determinant of poor prognosis regardless of angina symptoms, angina had different implications depending on the patient profile. Although angina was associated with a poor prognosis, this was only true in patients with prior MI but not in patients without prior MI. Patients with angina usually have relatively infrequent episodes of ischaemia often occurring in response to exercise or emotional stress, and in the absence of a suitable substrate unlikely to cause harm. A previous MI is a circumstance where such a normally benign episode of ischaemia may lead to a devastating outcome with a much higher risk to develop serious ventricular arrhythmias or acute HF.
Moreover, the state of coronary arteries is an important determinant of outcome in patients with angina. Given the expected higher prevalence of vulnerable plaques in patients with previous MI, it is expected that the latter will fare worst irrespective of the severity or even extent of atheromatous disease. The implications of this finding are clear: patients with prior MI and angina symptoms represent 14% of the chronic coronary syndrome population and are at highest risk. They deserve more intensive monitoring and management and particular attention should be devoted to implementing secondary prevention strategies and ensuring targets are met.23–26 A clearer understanding of which patient profiles have the worst outcomes is necessary to focus follow-up efforts and appropriately target new therapies that are either expensive or can have carry risks and side effect (e.g. rivaroxaban,20 PCSK9 inhibitors21, or canakinumab22). In contrast, patients with angina but no prior MI may not deserve aggressive non-invasive testing.
When attempting to improve patient care, RCTs and registries are both useful. While RCTs are the gold-standard to evaluate new therapies, they are often restricted to highly selected populations and may not reflect daily practice.27 In contrast, registries are poorly suited to determine treatment efficacy or safety but provide important data on large number of patients from routine clinical practice in terms of describing patient characteristics, management and outcomes and identifying gaps between evidence and practice. Guidelines on secondary prevention of chronic patients are available, but largely rely on relatively old data pertaining to trials done in MI survivors.28,29 Large observational studies in patients with chronic coronary syndrome are scarce, or are often focused on patients with angina,30 or restricted to a single geographic area.31–33
Despite the size, scope, and quality of the CLARIFY registry, this analysis has several limitations, some of which are inherent to observational studies. Outcomes were not adjudicated and, given the difficulty in ascribing death to a specific cause in long-term outpatient studies, the incidence of cardiovascular mortality, in contrast to total mortality, should be taken with caution. Monitoring was limited each year to a random selection of 1% of the centres. These results refer only to enrolled patients and reflect the CLARIFY inclusion criteria. They do not necessarily apply to all chronic coronary syndrome patients encountered in daily practice. This study may not be extrapolated to patients managed in the USA, as there were no patients enrolled in this country due to lack of local sponsor. Despite a broad geographic scope encompassing 45 countries, CLARIFY only reflects countries and regions with high- or middle-income. The results cannot be extrapolated to important and large areas, such as most of Africa and regions in Asia where access to expensive medical care is limited. Causes of two-thirds of cardiovascular death (non-related to an MI or a stroke) were classified as other cardiovascular death and consequently were assumed rather than proven cardiovascular death, as is often the case in long-term outpatient studies where many deaths can occur out of hospital and where documentation is often limited or absent.
In conclusion, this international study provides helpful information to characterize the spectrum of patients with chronic coronary syndrome and inform patient management and future studies in this population, across various geographic regions. In this broad population with chronic coronary syndrome, rates of major cardiovascular events were lower than those observed in historical datasets, which may reflect improved global medical care particularly the high rates of use of evidence-based therapies. Although angina was associated with a poor prognosis, this was only true in patients with prior MI. Patients with both angina and prior MI are an easily identifiable high-risk group which may deserve more intensive treatment.
Conflict of interest: E.S. reports personal fees and non-financial support from Servier, during the conduct of the study; personal fees and non-financial support from Novartis, personal fees and non-financial support from Bayer, personal fees and non-financial support from Astra-Zeneca, personal fees and non-financial support from Merck Sharpe & Dohme, personal fees from BMS, outside the submitted work. K.M.F. reports personal fees and non-financial support from Servier, during the conduct of the study; personal fees from AstraZeneca, personal fees from Taurx, personal fees and non-financial support from Broadview Ventures, personal fees from CellAegis, personal fees from Celixir, outside the submitted work; and Director of Vesalius Trials Ltd. Y.E. has nothing to disclose. N.D. reports personal fees from Servier, during the conduct of the study; grants, personal fees and non-financial support from Amgen, grants, personal fees and non-financial support from AstraZeneca, grants, personal fees and non-financial support from Bayer, grants, personal fees and non-financial support from BMS, personal fees from Boehringer Ingelheim, personal fees from Intercept, grants and personal fees from MSD, grants and personal fees from Pfizer, grants and personal fees from Sanofi, personal fees from Servier, outside the submitted work. P.D. reports personal fees from Servier Inc., during the conduct of the study. R.F. reports grants and personal fees from Servier International, personal fees from Merck Serono, personal fees from Bayer, personal fees from Boehringer Ingelheim, grants and personal fees from Novartis, personal fees from CIPLA, personal fees from Lupin, outside the submitted work. I.F. reports grants and personal fees from Servier, during the conduct of the study. N.G. reports grants from Servier during the conduct of the study. P.R.K. reports personal fees from Servier, during the conduct of the study; personal fees from Abbott, personal fees from AstraZeneca, from Boston Scientific, personal fees from Napp, personal fees from Novartis, personal fees from Novo Nordisk, grants and personal fees from Vifor Pharma, grants from Pharmacosmos, outside the submitted work. Z.P. reports personal fees from Servier, during the conduct of the study. S.S. has nothing to disclose. JCT reports grants and personal fees from Servier, during the conduct of the study; grants and personal fees from Amarin, grants from Astra Zeneca, grants, personal fees and minor equity interest from DalCor, grants from Esperion, grants from Ionis, grants and personal fees from Pfizer, grants and personal fees from Sanofi, grants and personal fees from Servier, outside the submitted work; in addition, Dr. Tardif is an author of a patent on ‘Pharmacogenomics-guided CETP inhibition’ issued. M.T. reports personal fees from Servier, during the conduct of the study; personal fees from Bayer, personal fees from Cadila Pharmaceuticals, personal fees from Janssen-Cilag, personal fees from Kowa, personal fees from PERFUSE Group, personal fees from Servier, personal fees from UCB Pharmaceuticals, outside the submitted work. J.Z. reports grants from Abbott, grants from Phillips, personal fees from Bayer, grants from Amgen, outside the submitted work. E.V.P. reports non-financial support from Servier, outside the submitted work. P.G.S. reports grants and personal fees from Servier, during the conduct of the study; grants and personal fees from Bayer/Janssen, grants and personal fees from Merck, grants and personal fees from Sanofi, grants and personal fees from Amarin, personal fees from Amgen, personal fees from Bristol-Myers Squibb, personal fees from Boehringer-Ingelheim, personal fees from Pfizer, personal fees from Novartis, personal fees from Regeneron, personal fees from Lilly, personal fees from AstraZeneca, personal fees from Servier, personal fees from Novo Nordisk, personal fees from Idorsia, outside the submitted work.
Supplementary Material
See page 356 for the editorial comment on this article (doi: 10.1093/eurheartj/ehz739)
References
- 1. Timmis A, Townsend N, Gale C, Grobbee R, Maniadakis N, Flather M, Wilkins E, Wright L, Vos R, Bax J, Blum M, Pinto F, Vardas PESC Scientific Document Group. European Society of Cardiology: cardiovascular Disease Statistics 2017. Eur Heart J 2018;39:508–579. [DOI] [PubMed] [Google Scholar]
- 2. Puymirat E, Simon T, Cayla G, Cottin Y, Elbaz M, Coste P, Lemesle G, Motreff P, Popovic B, Khalife K, Labeque JN, Perret T, Le Ray C, Orion L, Jouve B, Blanchard D, Peycher P, Silvain J, Steg PG, Goldstein P, Gueret P, Belle L, Aissaoui N, Ferrieres J, Schiele F, Danchin NUSIK, USIC 2000, and FAST-MI investigators. Acute myocardial infarction: changes in patient characteristics, management, and 6-month outcomes over a period of 20 years in the FAST-MI Program (French Registry of Acute ST-Elevation or Non-ST-Elevation Myocardial Infarction) 1995 to 2015. Circulation 2017;136:1908–1919. [DOI] [PubMed] [Google Scholar]
- 3. Jernberg T, Johanson P, Held C, Svennblad B, Lindback J, Wallentin LSWEDEHEART/RIKS-HIA. Association between adoption of evidence-based treatment and survival for patients with ST-elevation myocardial infarction. JAMA 2011;305:1677–1684. [DOI] [PubMed] [Google Scholar]
- 4. Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, Buring J, Hennekens C, Kearney P, Meade T, Patrono C, Roncaglioni MC, Zanchetti A.. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009;373:1849–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA, Investigators P, Freij A, Thorsen M.. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009;361:1045–1057. [DOI] [PubMed] [Google Scholar]
- 6. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994;344:1383–1389. [PubMed] [Google Scholar]
- 7. Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial. Lancet 2001;357:1385–1390. [DOI] [PubMed] [Google Scholar]
- 8. Fox KM. Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double-blind, placebo-controlled, multicentre trial (the EUROPA study). Lancet 2003;362:782–788. [DOI] [PubMed] [Google Scholar]
- 9. van Vark LC, Bertrand M, Akkerhuis KM, Brugts JJ, Fox K, Mourad JJ, Boersma E.. Angiotensin-converting enzyme inhibitors reduce mortality in hypertension: a meta-analysis of randomized clinical trials of renin-angiotensin-aldosterone system inhibitors involving 158,998 patients. Eur Heart J 2012;33:2088–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kannel WB, Feinleib M.. Natural history of angina pectoris in the Framingham study. Prognosis and survival. Am J Cardiol 1972;29:154–163. [DOI] [PubMed] [Google Scholar]
- 11. Emond M, Mock MB, Davis KB, Fisher LD, Holmes DR Jr, Chaitman BR, Kaiser GC, Alderman E, Killip T 3rd. Long-term survival of medically treated patients in the Coronary Artery Surgery Study (CASS) registry. Circulation 1994;90:2645–2657. [DOI] [PubMed] [Google Scholar]
- 12. Daly CA, De Stavola B, Sendon JL, Tavazzi L, Boersma E, Clemens F, Danchin N, Delahaye F, Gitt A, Julian D, Mulcahy D, Ruzyllo W, Thygesen K, Verheugt F, Fox KM; Euro Heart Survey Investigators. Predicting prognosis in stable angina—results from the Euro heart survey of stable angina: prospective observational study. BMJ 2006;332:262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sorbets E, Greenlaw N, Ferrari R, Ford I, Fox KM, Tardif JC, Tendera M, Steg PG.. Rationale, design, and baseline characteristics of the CLARIFY registry of outpatients with stable coronary artery disease. Clin Cardiol 2017;40:797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Steg PG, Greenlaw N, Tendera M, Tardif JC, Ferrari R, Al-Zaibag M, Dorian P, Hu D, Shalnova S, Sokn FJ, Ford I, Fox KM.. Prevalence of anginal symptoms and myocardial ischemia and their effect on clinical outcomes in outpatients with stable coronary artery disease: data from the International Observational CLARIFY Registry. JAMA Intern Med 2014;174:1651–1659. [DOI] [PubMed] [Google Scholar]
- 15. Steg PG, Greenlaw N, Tardif JC, Tendera M, Ford I, Kaab S, Abergel H, Fox KM, Ferrari R.. Women and men with stable coronary artery disease have similar clinical outcomes: insights from the international prospective CLARIFY registry. Eur Heart J 2012;33:2831–2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vidal-Petiot E, Ford I, Greenlaw N, Ferrari R, Fox KM, Tardif JC, Tendera M, Tavazzi L, Bhatt DL, Steg PG.. Cardiovascular event rates and mortality according to achieved systolic and diastolic blood pressure in patients with stable coronary artery disease: an international cohort study. Lancet 2016;388:2142–2152. [DOI] [PubMed] [Google Scholar]
- 17. Bhatt DL, Steg PG, Ohman EM, Hirsch AT, Ikeda Y, Mas JL, Goto S, Liau CS, Richard AJ, Rother J, Wilson PWREACH Registry Investigators. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA 2006;295:180–189. [DOI] [PubMed] [Google Scholar]
- 18. Bhatt DL, Eagle KA, Ohman EM, Hirsch AT, Goto S, Mahoney EM, Wilson PW, Alberts MJ, D'Agostino R, Liau CS, Mas JL, Rother J, Smith SC Jr, Salette G, Contant CF, Massaro JM, Steg PG.. Comparative determinants of 4-year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA 2010;304:1350–1357. [DOI] [PubMed] [Google Scholar]
- 19. Bauters C, Tricot O, Meurice T, Lamblin N.. Long-term risk and predictors of cardiovascular death in stable coronary artery disease: the CORONOR study. Coron Artery Dis 2017;28:636–641. [DOI] [PubMed] [Google Scholar]
- 20. Eikelboom JW, Connolly SJ, Bosch J, Dagenais GR, Hart RG, Shestakovska O, Diaz R, Alings M, Lonn EM, Anand SS, Widimsky P, Hori M, Avezum A, Piegas LS, Branch KRH, Probstfield J, Bhatt DL, Zhu J, Liang Y, Maggioni AP, Lopez-Jaramillo P, O'Donnell M, Kakkar AK, Fox KAA, Parkhomenko AN, Ertl G, Stork S, Keltai M, Ryden L, Pogosova N, Dans AL, Lanas F, Commerford PJ, Torp-Pedersen C, Guzik TJ, Verhamme PB, Vinereanu D, Kim JH, Tonkin AM, Lewis BS, Felix C, Yusoff K, Steg PG, Metsarinne KP, Cook Bruns N, Misselwitz F, Chen E, Leong D, Yusuf SCOMPASS Investigators. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med 2017;377:1319–1330. [DOI] [PubMed] [Google Scholar]
- 21. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TRFOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017;376:1713–1722. [DOI] [PubMed] [Google Scholar]
- 22. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJCANTOS Trial Group. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 23. Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, Hoes AW, Jennings CS, Landmesser U, Pedersen TR, Reiner Z, Riccardi G, Taskinen MR, Tokgozoglu L, Verschuren WMM, Vlachopoulos C, Wood DA, Zamorano JLCooney MTV. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J 2016;37:2999–3058. [DOI] [PubMed] [Google Scholar]
- 24. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, Goldberg R, Heidenreich PA, Hlatky MA, Jones DW, Lloyd-Jones D, Lopez-Pajares N, Ndumele CE, Orringer CE, Peralta CA, Saseen JJ, Smith SC Jr, Sperling L, Virani SS, Yeboah J.. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol. Circulation 2019;139:e1082–e1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais IESC Scientific Document Group. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J 2018;39:3021–3104. [DOI] [PubMed] [Google Scholar]
- 26. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr.. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018;71:e127–e248. [DOI] [PubMed] [Google Scholar]
- 27. Rothwell PM. External validity of randomised controlled trials: “to whom do the results of this trial apply?”. Lancet 2005;365:82–93. [DOI] [PubMed] [Google Scholar]
- 28. Task Force MembersMontalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Bugiardini R, Crea F, Cuisset T, Di Mario C, Ferreira JR, Gersh BJ, Gitt AK, Hulot JS, Marx N, Opie LH, Pfisterer M, Prescott E, Ruschitzka F, Sabaté M, Senior R, Taggart DP, van der Wall EE, Vrints CJ;ESC Committee for Practice GuidelinesZamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker SDocument ReviewersKnuuti J, Valgimigli M, Bueno H, Claeys MJ, Donner-Banzhoff N, Erol C, Frank H, Funck-Brentano C, Gaemperli O, Gonzalez-Juanatey JR, Hamilos M, Hasdai D, Husted S, James SK, Kervinen K, Kolh P, Kristensen SD, Lancellotti P, Maggioni AP, Piepoli MF, Pries AR, Romeo F, Rydén L, Simoons ML, Sirnes PA, Steg PG, Timmis A, Wijns W, Windecker S, Yildirir A, Zamorano JL.. 2013 ESC Guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 2013;34:2949–3003. [DOI] [PubMed] [Google Scholar]
- 29. Fihn SD, Blankenship JC, Alexander KP, Bittl JA, Byrne JG, Fletcher BJ, Fonarow GC, Lange RA, Levine GN, Maddox TM, Naidu SS, Ohman EM, Smith PK.. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2014;130:1749–1767. [DOI] [PubMed] [Google Scholar]
- 30. Daly CA, Clemens F, Sendon JL, Tavazzi L, Boersma E, Danchin N, Delahaye F, Gitt A, Julian D, Mulcahy D, Ruzyllo W, Thygesen K, Verheugt F, Fox KM.. The clinical characteristics and investigations planned in patients with stable angina presenting to cardiologists in Europe: from the Euro Heart Survey of Stable Angina. Eur Heart J 2005;26:996–1010. [DOI] [PubMed] [Google Scholar]
- 31. Puymirat E, Simon T, Steg PG, Schiele F, Gueret P, Blanchard D, Khalife K, Goldstein P, Cattan S, Vaur L, Cambou JP, Ferrieres J, Danchin NUSIK USIC 2000 Investigators; FAST MI Investigators. Association of changes in clinical characteristics and management with improvement in survival among patients with ST-elevation myocardial infarction. JAMA 2012;308:998–1006. [DOI] [PubMed] [Google Scholar]
- 32. Carrero JJ, Evans M, Szummer K, Spaak J, Lindhagen L, Edfors R, Stenvinkel P, Jacobson SH, Jernberg T.. Warfarin, kidney dysfunction, and outcomes following acute myocardial infarction in patients with atrial fibrillation. JAMA 2014;311:919–928. [DOI] [PubMed] [Google Scholar]
- 33. Lemesle G, Tricot O, Meurice T, Lallemant R, Delomez M, Equine O, Lamblin N, Bauters C.. Incident myocardial infarction and very late stent thrombosis in outpatients with stable coronary artery disease. J Am Coll Cardiol 2017;69:2149–2156. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.