Abstract
Background
In the high-prevalence setting of Pakistan, screening, diagnosis and treatment services for chronic hepatitis C (CHC) patients are commonly offered in specialized facilities. We aimed to describe the cascade of care in a Médecins Sans Frontières primary health care clinic offering CHC care in an informal settlement in Karachi, Pakistan.
Methods
This was a retrospective cohort analysis using routinely collected data. Three different screening algorithms were assessed among patients with one or more CHC risk factors.
Results
Among the 87 348 patients attending the outpatient clinic, 5003 (6%) presented with one or more risk factors. Rapid diagnostic test (RDT) positivity was 38% overall. Approximately 60% of the CHC patients across all risk categories were in the early stage of the disease, with an aspartate aminotransferase:platelet ratio index score <1. The sequential delays in the cascade differed between the three groups, with the interval between screening and treatment initiation being the shortest in the cohort tested with GeneXpert onsite.
Conclusions
Delays between screening and treatment can be reduced by putting in place more patient-centric testing algorithms. New strategies, to better identify and treat the hidden at-risk populations, should be developed and implemented.
Keywords: hepatitis C, operational research, risk factors, SORT IT, testing and treatment algorithm, time delays
Introduction
Hepatitis C is a major public health problem worldwide, with a global seroprevalence of 1.6%.1 According to the 2017 World Health Organization (WHO) global hepatitis report, an estimated 71 million people are living with hepatitis C virus (HCV) infection, which corresponds to a global prevalence of 1.0%.2 The WHO Eastern Mediterranean region has the highest reported prevalence of HCV of 2.3%, followed by the European region at 1.5%.2 The burden remains particularly high in low- and middle-income countries (LMICs).3 Chronic hepatitis C (CHC) patients are at increased risk of developing hepatic fibrosis, cirrhosis and hepatocellular carcinoma, and it has been estimated that approximately 1.4 million untreated people die each year.3,4 While the new era of direct-acting antivirals has dramatically improved the treatment outcomes of CHC patients, and may in the long term even contribute to bringing the elimination of hepatitis C within reach, scaling up and streamlining testing services and linkage to care remain a major hurdle, mainly in LMICs.5,6 Testing services are particularly challenged by the asymptomatic or non-specific clinical presentation during acute and early stages of chronic HCV infections and by the fact that hepatitis C is typically most prevalent in marginalized at-risk populations.4,7
Pakistan is among the countries with the highest HCV seroprevalence, with approximately 10 million people carrying the infection.1 A survey conducted in 2007–2008 in Pakistan showed a prevalence of 4.8%, with the highest rates reported in Punjab and Sindh provinces.8,9 A systematic review done in 2010 reported the seroprevalence to be even higher, up to 6.8% in the general population.10 Unscreened blood transfusions and unsafe medical and dental procedures have been associated with hepatitis C transmission in settings where overuse and inappropriate use of injections in medical practice is common, as is the case in Pakistan.11 A plethora of other potential risk factors have been identified, such as injectable drug use (needle sharing), tattooing with reused needles, mother-to-child transmission and unprotected sex, all of which may play an important role in different strata of the population in Pakistan.11–13
Early diagnosis of CHC infection and providing necessary treatment are crucial to prevent or delay the onset of liver disease and prevent transmission.14 However, lack of provider and patient awareness, unavailability of testing sites, limited technical human resources, concerns about stigma and discrimination, and high medicine costs all contribute to poor diagnosis and linkage to care in Pakistan.4,15 HCV screening and treatment services are mainly provided at the tertiary care level by both private and public health care providers, but at high cost, which makes them inaccessible for the marginalized and at-risk populations who bear the burden of the disease.15,16 Several studies and systematic reviews have been conducted to describe the epidemiology of HCV and its associated risk factors in Pakistan, but limited evidence is available on screening and linkage to care in a primary health care setting.9,10,17,18 Additionally, while the contribution of different risk behaviours to HCV transmission dynamics in general has been described previously, risk behaviours among particular populations remain relatively underdocumented, and interventions towards addressing such risk behaviours are rarely implemented.17,19
The aim of our study was therefore to assess the performance of the hepatitis C screening programme in a Médecins Sans Frontières (MSF) primary health care clinic offering CHC diagnosis and treatment in an informal settlement in Karachi. The specific objectives were to describe the cascade of care from screening to treatment for the at-risk population, determine sociodemographic characteristics associated with seropositivity, describe disease staging per risk category and calculate the time interval between each step of the screening cascade.
Materials and methods
Study design
This was a retrospective cohort analysis of routinely collected project data.
General setting
Pakistan is a country with a population of 188.1 million and an estimated population increase of 41% over the last 25 y.20 Almost 62% of the country’s population lives in rural areas and the majority of the population belongs to low socio-economic strata, with low literacy rates.20 Huge health disparities and gaps exist between the rural and urban populations.15 The burden of communicable and non-communicable diseases is relatively high, at 38.3% and 50.5%, respectively.20 In Pakistan, health care delivery is implemented by the provincial government, with the federal government limited to providing technical assistance, training and policymaking. The state provides health care via a three-tiered delivery system: basic health units and rural health centres forming the core of the primary health care model; secondary care including first- and second-level referral facilities, such as the tehsil and district headquarters hospitals; and tertiary care teaching hospitals.15
Specific setting—Karachi and Machar Colony
Karachi is the capital of the Sindh province and represents a major financial and industrial hub of the country, with an estimated population of 14.9 million.21 As the largest city in Pakistan, it has a number of informal settlements with populations who live below the poverty line and have limited access to health care services.22 MSF started working in collaboration with the local non-governmental organization (NGO) SINA in the Machar Colony settlement in 2013, providing primary health care services to the population. SINA is a local NGO that runs similar clinics (25 in total) and services in other parts of Karachi. An assessment done by MSF in 2013 investigating the burden of hepatitis C in the community identified a high prevalence of key risk factors for HCV infection (reuse of needles and reports of intravenous drug use) in this slum area and recommended starting an HCV screening and treatment programme. The MSF hepatitis C clinic was started in February 2015, with the objective to provide free-of-cost access to HCV care for the residents of Machar Colony through a primary health care clinic using simplified diagnostic and treatment algorithms. Most of the population residing in this slum were reported to be undocumented migrants with very limited access to health care.
MSF hepatitis C clinic
All patients presenting to the primary health care clinic (6 d/week, 9 h/d) passed by the triage desk, except for pregnant patients, who went directly to the maternity unit. At the triage desk, patients were directed to the proper unit (general outpatient department [OPD], HCV clinic or the vaccination unit).
All the patients who were sent to the OPD were then routinely assessed for hepatitis C risk factors by the OPD doctors (Box 1). Patients who exhibited any signs or symptoms or presented with a history of any of the risk factors for HCV were further referred by the OPD doctor to the lab (situated within the clinic) for HCV antibody screening using an OraQuick (OraSure Technologies, Bethlehem, PA, USA) rapid diagnostic test (RDT).
Box 1.
Risk factors for screening
|
Patients who tested positive on the RDT were then tested by polymerase chain reaction (PCR) for confirmation of CHC. If the patient was positive on PCR, she/he was then referred to the HCV unit (within the same premises) to be assessed or evaluated by the hepatitis C doctor (general practitioner) for treatment initiation based on set eligibility criteria (including aspartate aminotransferase:platelet ratio index [APRI] score cut-offs, non-pregnancy, absence of mental health issues). The APRI score was used as a simple, non-invasive proxy serum marker to determine the stage of liver fibrosis.13,14 All patients who qualified for treatment were subsequently started on direct-acting antivirals such as sofosbuvir, daclatasvir and ribavirin according to the MSF clinical protocol based on the WHO hepatitis C guidelines.7
Patients who were eligible for treatment were also simultaneously started on patient support counselling, which included different sessions (eligibility session, treatment initiation, end of treatment session, sustained virological response [SVR] session). In Pakistan during the study period, MSF was the first and only international medical organization to treat patients with the new direct-acting antivirals (sofosbuvir and daclatasvir), which are known to have lesser side effects and reduced treatment duration. Sofosbuvir and daclatasvir were very expensive at that time, with no generics available in Pakistan. MSF procured this treatment regimen internationally and provided it for the eligible patients free of cost with importation approval from the drug regulatory authority of Pakistan (DRAP); at the time, it was not even registered in the country.
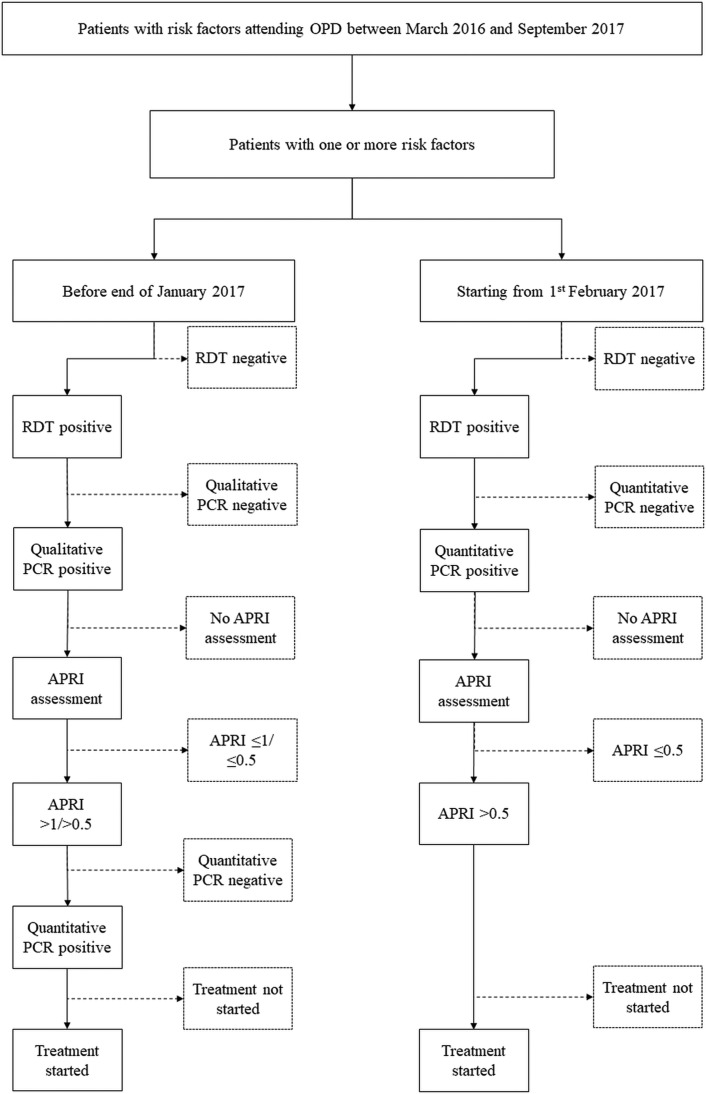
MSF screening and diagnostic algorithm
The screening and diagnostic algorithm adopted in different time periods is shown in Figure 1. Before February 2017, all RDT-positive patients were first tested by qualitative PCR at an external laboratory (Dow University of Health Sciences): if found to be positive, the patient underwent APRI scoring and was then tested for viral load through quantitative PCR, again at the external laboratory. As a cut-off to establish treatment eligibility, an APRI >1 was used initially, until October 2016; subsequently the eligibility threshold was decreased to an APRI >0.5, when it was observed that an APRI >1 resulted in the exclusion of many patients from the target population in Machar Colony, as they tended to have a lower APRI than patients from outside Machar Colony.
Figure 1.
Hepatitis C screening and diagnostic algorithm at the MSF clinic, Karachi, Pakistan, March 2016–September 2017.
After February 2017, RDT-positive patients were offered HCV PCR testing internally in the clinic using the GeneXpert HCV Viral Load assay (GeneXpert 1V system, Cepheid, Sunnyvale, CA, USA) on the same day as the RDT, followed by APRI staging. Using the APRI score threshold and the change in testing platform, we divided the study population into three groups: group 1 (RDT date before 1 October 2016; APRI cut-off >1), group 2 (RDT date between 1 October 2016 and 31 January 2017; APRI cut-off >0.5) and group 3 (RDT date between 1 February 2017 and 30 September 2017; testing by GeneXpert, APRI cut-off >0.5).
Study population
All patients presenting to the MSF OPD clinic in Machar Colony, Karachi between March 2016 and September 2017 having one or more risk factors for HCV were included. Patients who came with an externally provided hepatitis C diagnosis were excluded from the study. The study population was categorized as RDT APRI >1, RDT APRI >0.5 and GeneXpert cohorts, respectively, based on which diagnostic algorithm they underwent.
Data sources and variables
All screening data were extracted from the screening database, which was routinely entered by data staff in the project. The data for all CHC-positive patients were extracted from the Hepa-MUD electronic database, which is used as a routine monitoring and evaluation tool for hepatitis C patients in the project.
Statistical analysis
The clinic identification (ID) was used as a unique identifier to cross-link data from the screening database and Hepa-MUD in Excel (Microsoft, Redmond, WA, USA); the combined dataset was then imported into STATA version 14.2 (StataCorp, College Station, TX, USA) for analysis. Consistency checks were performed for each duplicate ID encountered; if any variable differed between duplicate IDs, they were coded as separate patients.
Positivity rates were calculated per risk group category and were expressed as proportions. For the two RDT cohorts, the median time intervals were calculated between the RDT date and qualitative PCR date, between the qualitative PCR date and quantitative PCR date and between the quantitative PCR date and treatment initiation date. For the GeneXpert group, we calculated the time interval between the RDT date and quantitative PCR/GeneXpert dates and between the quantitative PCR/GeneXpert dates and treatment initiation dates.
If there were missing dates for quantitative PCR/GeneXpert, we used the APRI score date instead, as quantitative PCR/GeneXpert testing and blood investigation for APRI scoring were conducted at the same time. The treatment initiation status was censored by 28 November 2017.
Associations between seropositivity and patient characteristics (age and sex) were assessed, using logistic regression and expressed as odds ratios (ORs) with 95% confidence intervals (CIs). All the study results were reported according to the Strengthening and Reporting of Observational Studies in Epidemiology (STROBE) guidelines.23
Results
Diagnostic cascade
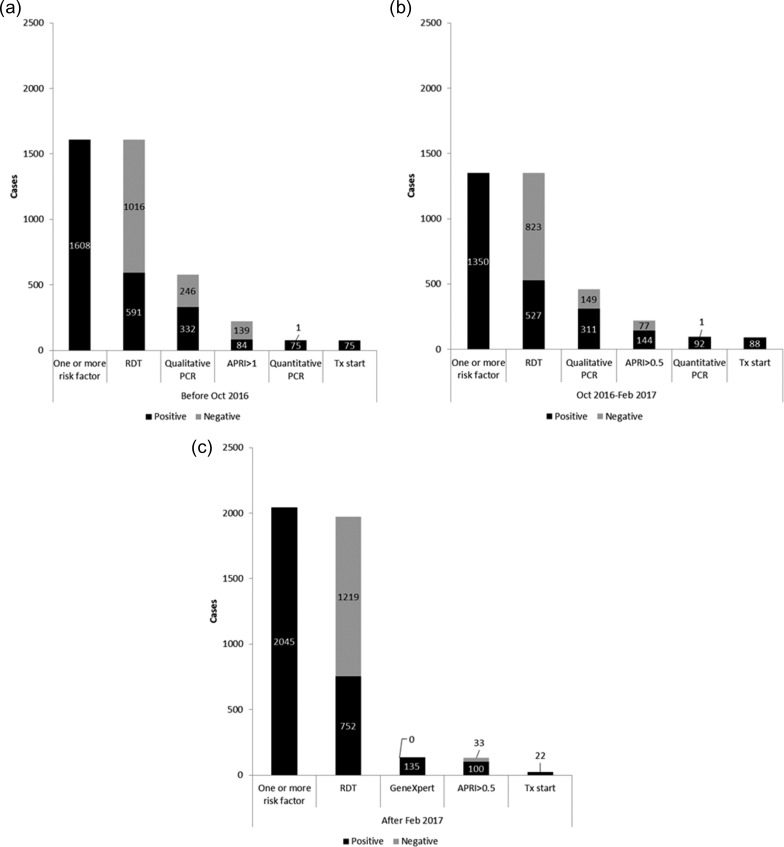
Of a total of 87 348 patients seen in the of the MSF-operated primary health clinic, 5003 (6%) were identified as having one or more HCV risk factors and 1901 (38%) of these had a positive RDT test for HCV. The diagnostic cascade, with positive, negative and untested proportions at each step, for each of the cohorts is shown in Figure 2A–C. The treatment initiation rates by the censor date of those eligible for treatment in the three cohorts were 75/75 (100%) for patients diagnosed using RDT APRI >1, 82/92 (96%) for patients diagnosed using RDT APRI >0.5 and 22/100 (22%) for patients diagnosed using GeneXpert. Among the patients testing positive by qualitative PCR in the RDT cohorts, the APRI score was not recorded in 33% and 29%, respectively. Among the 752 patients who were RDT-positive in the GeneXpert cohort, the quantitative PCR results were not recorded in the electronic database for 617 (82%) patients.
Figure 2.
Diagnostic and screening cascade for hepatitis C, with positivity rate at each diagnostic step, at the MSF Clinic, Karachi, Pakistan, March 2016–September 2017. (a) Diagnostic and screening cascade for hepatitis C, with positivity rate at each diagnostic step, for patients diagnosed using RDT APRI >1. (b) Diagnostic and screening cascade for hepatitis C, with positivity rate at each diagnostic step, for patients diagnosed using RDT APRI >0.5. (c) Diagnostic and screening cascade for hepatitis C, with positivity rate at each diagnostic step, for patients diagnosed using GeneXpert.
Risk for HCV positivity and disease staging
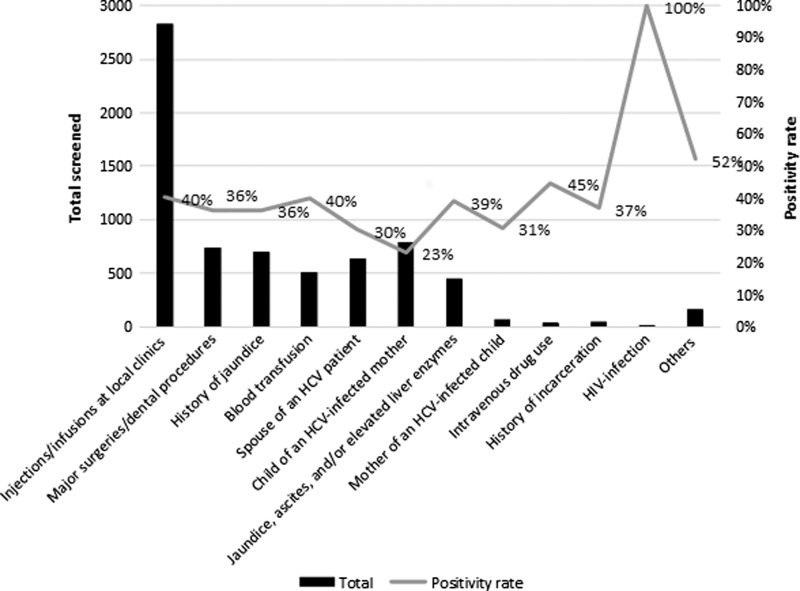
The overall number of at-risk individuals and the proportions of seropositive individuals per risk category are shown in Figure 3. Positivity rates were highest among human immunodeficiency virus (HIV)-positive patients (100%), intravenous drug users (45%), patients with a history of blood transfusion (40%) and patients having injections/infusions at local clinics (40%), while the lowest positivity rate was found in the risk group of children of hepatitis C–positive mothers (23%).
Figure 3.
Number and proportion of patients testing seropositive for hepatitis C, by risk group category, at the MSF clinic, Karachi, Pakistan, March 2016–September 2017.
Among the at-risk population, only sex and age were available as possible additional exposure variables. Men were more likely to be seropositive than women, at an adjusted odds ratio (aOR) of 1.20 (95% CI 1.14–1.26). The age groups 39–58 y and ≥59 y were at higher risk of seropositivity than the age group of 18–38 y, with an aOR of 1.37 (95% CI 1.27–1.48) and 1.61 (95% CI 1.44–1.80), respectively. Within this group of patients with a risk factor, individuals related to someone with confirmed HCV (parent, child or spouse) and individuals with a history of major surgeries or dental procedures were least at risk of HCV (Table 1).
Table 1.
Factors associated with hepatitis C seropositivity among all patients with an identified risk factor of hepatitis C at the MSF clinic, Karachi, Pakistan, March 2016–September 2017
| Characteristics | Total, N | Seropositive, n (%) | aOR (95% CI) | p-Value |
|---|---|---|---|---|
| Sex | ||||
| Female | 3464 | 1248 (36) | 1 | – |
| Male | 1528 | 599 (39) | 1.2 (1.1–1.3) | <0.001 |
| Age (y) | ||||
| <20 | 665 | 64 (10) | 0.1 (0.1–0.2) | <0.001 |
| 20–29 | 1074 | 398 (37) | 0.6 (0.5–0.7) | <0.001 |
| 30–39 | 1250 | 437 (35) | 1 | – |
| 40–49 | 1223 | 462 (38) | 1.4 (1.2–1.6) | <0.001 |
| 50–59 | 600 | 231 (39) | 1.4 (1.2–1.7) | 0.001 |
| ≥60 | 327 | 121 (37) | 2.0 (1.5–2.5) | <0.001 |
| Risk group | ||||
| Symptomatic patient | 450 | 176 (39) | 1.0 (0.8–1.2) | 0.99 |
| Intravenous drug use | 38 | 17 (45) | 1.2 (0.6–2.3) | 0.7 |
| HIV positive | 10 | 10 (100) | – | – |
| Spouse of HCV-positive patient | 639 | 193 (30) | 0.5 (0.4–0.7) | <0.001 |
| Child of HCV-positive mother | 790 | 184 (23) | 0.8 (0.6–1.0) | 0.03 |
| Mother of HCV-positive child | 68 | 21 (31) | 0.5 (0.3–0.9) | 0.02 |
| History of incarceration | 46 | 17 (37) | 1.0 (0.5–1.8) | 0.9 |
| History of jaundice | 703 | 255 (36) | 0.9 (0.7–1.0) | 0.09 |
| History of blood transfusion | 508 | 203 (40) | 1.0 (0.8–1.2) | 0.99 |
| Major surgeries/dental procedures | 741 | 269 (36) | 0.7 (0.6–0.8) | <0.001 |
| Injections/infusions at local clinics | 2833 | 1141 (40) | 1.1 (0.9–1.2) | 0.4 |
| Others | 165 | 86 (52) | 2.3 (1.6–3.3) | <0.001 |
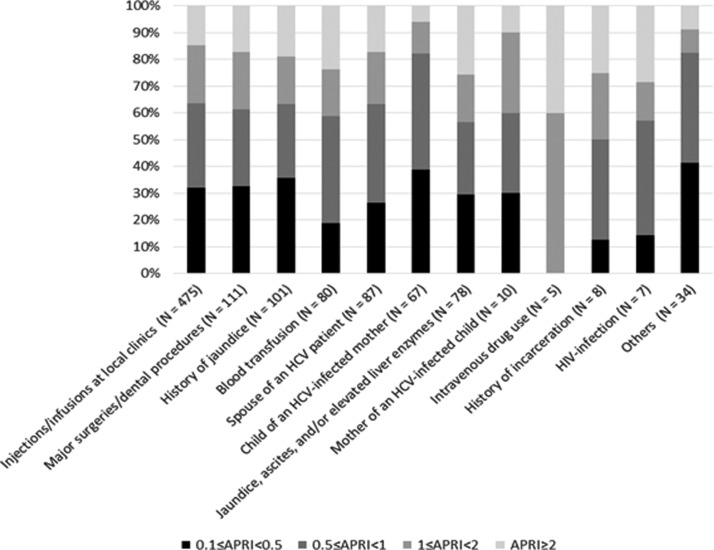
Disease staging according to risk category is shown in Figure 4: approximately 60% of the patients across all risk categories were in early-stage disease, with an APRI score <1.
Figure 4.
Disease staging for CHC infection, by risk category, at the MSF clinic, Karachi, Pakistan, March 2016–September 2017.
Diagnostic delays
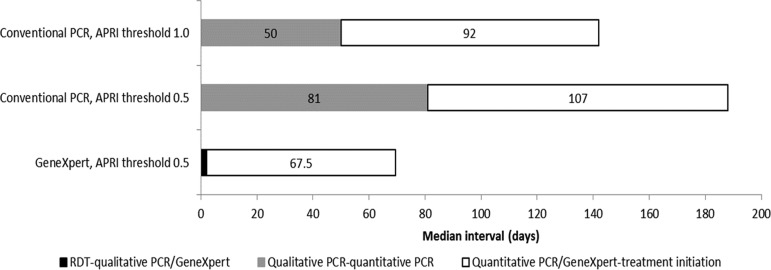
In the RDT cohorts, almost 90% of the patients were tested on the same day by RDT and qualitative PCR; the median interval between qualitative PCR/APRI staging and quantitative PCR was 50 d (interquartile range [IQR] 29–113) and 81 d (IQR 53–122), and between quantitative PCR and treatment initiation it was 92 d (IQR 27–133) and 107 d (IQR 64–139), respectively. In the GeneXpert cohort, the median interval between RDT and GeneXpert and between GeneXpert and treatment initiation was 2 d (IQR 0–74) and 68 d (IQR 56–120), respectively (Figure 5).
Figure 5.
Time interval in days between each step of the hepatitis C diagnostic and screening cascade at the MSF clinic, Karachi, Pakistan, March 2016–September 2017.
Discussion
The present study reports the feasibility of a simplified, primary health care-level, single entry point diagnostic and screening testing cascade for hepatitis C. Our study demonstrates how the cascade resulted in a high proportion of treatment-eligible patients starting treatment. For the cohort assessed through GeneXpert, only a quarter of the patients who were treatment eligible were initiated on treatment at the time of censoring; it is likely that most patients were still on the waiting list by the censor date, although some may have been lost. While this study did not assess the patient outcomes after treatment or the SVR, previous work at this clinic has shown a high treatment success rate of 83%.29
RDT positivity rates were high overall, at 38%—considerably higher than the reported seroprevalence of 6.8% in Pakistan,10 which reflects the at-risk profile of the study population. Positivity rates were higher in certain risk groups, including HIV-positive patients, intravenous drug users, patients with a history of blood transfusion and patients having injections/infusions at local clinics. These findings are in line with previous studies where seropositivity rates were found to be high among people who had a history of previous injections, a history of syringe reuse and a history of blood transfusion.9,17 Other studies in a variety of other settings have also shown high seroprevalence rates in intravenous drug users.24–28
In this study, men had a higher risk of being seropositive and this risk increased with age. This finding is comparable to a survey conducted in Pakistan in 2010.9 Being related to people with HCV carried the least risk, as well as having undergone major surgical or dental procedures, although even for these risk groups the positivity rates were considerably higher than the estimated HCV prevalence in Pakistan.
The time interval between point of entry (RDT testing) and care (treatment initiation) in all three algorithms was different and was shortest in the GeneXpert cohort, as onsite GeneXpert was implemented. Removing one step in the algorithm could reduce the time interval between the RDT test and treatment initiation from >180 d to 70 d. However, the increase of the delay in the first step of the cascade (from 0 d to 2 d) may reflect low lab capacity to process all samples in a timely manner, and other sites considering implementation of GeneXpert may need to consider the anticipated sample load when setting up their services.
The study did not come without certain limitations. First, certain exposure variables relevant to the project were not available, such as patient origin, marital status and employment status. In many patients, the results were not recorded in an electronic database and it was not possible to differentiate between patients who did not come back to the clinic (pretreatment attrition) and patients for whom data were not entered. Second, the study was undertaken in one MSF-supported site, where care was offered free of charge, and therefore the data may not be representative of public clinics in similar settings. Third, the risk factors recorded in the project were self-reported, and stigmatizing risk factors (HIV infection, intravenous drug use, history of incarceration) may thus have remained under-reported. Fourth, no prevalence study was conducted in the overall population attending the MSF-supported clinic, and the performance of the risk groups as a means of identifying individuals at exceptional risk for HCV could thus not be evaluated.
Our study findings suggest that the screening algorithm recommended by the WHO and implemented by the MSF project in a primary health care setting is a robust one and can work well in most settings.2 Streamlining and simplifying the diagnostic and testing algorithms can benefit patients and provide direct and easy access to the point of care. Additionally, decreasing the delays in the pathway to care will decrease pretreatment attrition, which has been shown earlier to be sizeable in this type of population.29 However, more work is needed to reduce the diagnostic process further to a single-stop diagnostic step, thus reducing the possibility of patient losses during the waiting periods between steps. Collaboration with programmes such as HIV/acquired immune deficiency syndrome (AIDS) and tuberculosis (TB) control programmes can significantly reduce the burden of buying and maintaining the GeneXpert machines. Additionally, such collaborations could lead to many patients within the HIV/AIDS and TB programmes being tested for HCV under one roof.
Several additional recommendations can be drawn from our study. Internally, from the programme perspective, the data recording system needs to be strengthened and routine data quality should be assured to minimize missing data. Additionally, specific issues on the risk criteria for hepatitis C were identified. Some of the groups with the highest positivity rates are also the least likely to self-declare their risk profile, such as individuals with a history of incarceration or people who inject drugs, and the overall numbers identified by applying these risk categories remain low. Other risk groups have a higher ‘yield’ of total positive cases but lower positivity rates. As the positivity rates per risk group stay well above the estimated prevalence of hepatitis C in Pakistan, they can be considered a tool of some worth in screening for CHC, but they remain too non-specific to contribute sizably to reducing the burden in a setting such as the informal settlements in Karachi. The high positivity rate in the ‘other’ risk group category suggests that this group merits further exploration. More specific definitions of populations at risk are urgently needed, as well as strategies to actively reach high-risk populations such as people who inject drugs and people living with HIV, but also the older age groups.
Improvement strategies could include integrating hepatitis C screening and testing services in risk group–targeted activities, such as needle and syringe exchange programmes for intravenous drug users, and HIV care and treatment centres. To be able to provide strong evidence on the effectiveness of the testing algorithms used, cost-effectiveness studies comparing the different screening strategies should also be considered.
Conclusions
This study documents for the first time the screening and diagnostic cascade of care for CHC integrated in a primary health clinic. It illustrates the time gains that can be achieved by bringing molecular diagnosis to the point of testing and the success of this strategy in linking patients to care. The use of risk categories to identify individuals who may benefit from screening is a key component in this process, and new strategies to better access existing but hidden at-risk populations are urgently needed. Further efforts are needed to reduce the delay between CHC confirmation and HCV treatment initiation, which was still long even when the delay between screening and linkage to care was dramatically reduced. This may be ensured by a greater availability of affordable new pan-genotypic direct-acting antiviral combinations and further simplification of treatment follow-up.
Authors’ contributions: GGK, KWYK and RVdB conceived and designed the experiments. GGK, SK and KA performed the experiments. GGK, KWYK, RVdB and AT analysed the data. KWYK, RVdB and AT contributed reagents, materials and analysis tools. GGK, RVdB and KWYK wrote the paper. All the authors approved the manuscript.
Acknowledgments: This research was conducted through the Structured Operational Research and Training Initiative (SORT IT), a global partnership led by the Special Program for Research and Training in Tropical Diseases at the World Health Organization (WHO/TDR). The model is based on a course developed jointly by the International Union Against Tuberculosis and Lung Disease (The Union) and Médecins sans Frontières (MSF [Doctors Without Borders]). The specific SORT IT program that resulted in this publication was jointly developed and implemented by The Union South-East Asia Office, New Delhi, India; the Center for Operational Research, The Union, Paris, France; The Union, Mandalay, Myanmar; MSF Luxembourg Operational Research (LuxOR); MSF Operational Center Brussels (MSF OCB); Institute of Medicine, University of Chester, UK; and the Department of Medical Research, Ministry of Health and Sports, Naypyidaw, Myanmar. The MSF OCB, Karachi hepatitis C project staff contributions are hereby also acknowledged.
Funding: The training program was funded by the Department for International Development (DFID), UK. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Competing interests: None declared.
Ethics approval: Ethics approval was obtained from the Ethics Advisory Group of The Union, Paris, France (EAG No: 36/17) and the National Bioethics Committee, Pakistan. The study fulfilled the exemption criteria set by the Ethics Review Board (ERB) of MSF (Geneva, Switzerland) for a posteriori analyses of routinely collected data and thus did not require formal MSF ERB review. It was conducted with permission from the Medical Director of the MSF Operational Centre, Brussels. As the study involved a review of existing records, a waiver for informed consent was sought and approved by the ethics committees.
References
- 1. Gower E, Estes C, Blach S, et al. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014;61(1 Suppl):S45–57. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization Global hepatitis report, 2017. Geneva: World Health Organization, 2017.
- 3. World Health Organization Guidelines on hepatitis B and C testing. Geneva: World Health Organization, 2017.
- 4. World Health Organization Global report on access to hepatitis C treatment. Geneva: World Health Organization, 2016. [Google Scholar]
- 5. Reipold EI, Trianni A, Krakower D, et al. Values, preferences and current hepatitis B and C testing practices in low- and middle-income countries: results of a survey of end users and implementers. BMC Infect Dis. 2017;17(Suppl 1):702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lim SG. HCV management in resource-constrained countries. Hepatol Int. 2017;11(3):245–54. [DOI] [PubMed] [Google Scholar]
- 7. World Health Organization Guidelines for the screening, care and treatment of persons with hepatitis C infection. Geneva: World Health Organization, 2014. [PubMed]
- 8. Qureshi H, Mohamud BK, Alam SE, et al. Treatment of hepatitis B and C through national programme—an audit. J Pak Med Assoc. 2013;63(2):220–4. [PubMed] [Google Scholar]
- 9. Qureshi H, Bile KM, Jooma R, et al. Prevalence of hepatitis B and C viral infections in Pakistan: findings of a national survey appealing for effective prevention and control measures. East Mediterr Health J. 2010;16(Suppl):S15–23. [PubMed] [Google Scholar]
- 10. Umer M, Iqbal M. Hepatitis C virus prevalence and genotype distribution in Pakistan: comprehensive review of recent data. World J Gastroenterol. 2016;22(4):1684–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Raglow GJ, Luby SP, Nabi N. Therapeutic injections in Pakistan: from the patients’ perspective. Trop Med Int Health. 2001;6(1):69–75. [DOI] [PubMed] [Google Scholar]
- 12. Warpakowski A. [Daclatasvir/sofosbuvir is effective also in advanced liver disease]. MMW Fortschr Med. 2016;158(6):78. [DOI] [PubMed] [Google Scholar]
- 13. Shafiq M, Nadeem M. Identification of risk factors for hepatitis B and C in Peshawar, Pakistan. HIV/AIDS (Auckl). 2015;7:223–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lim AG, Qureshi H, Mahmood H, et al. Curbing the hepatitis C virus epidemic in Pakistan: the impact of scaling up treatment and prevention for achieving elimination. Int J Epidemiol. 2018;47(2):550–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nishtar S, Jillani A, Ali Khan S, et al. The gateway paper: health systems in Pakistan, a way forward. Islamabad: Pakistan Health Policy Forum, 2006.
- 16.Ministry of Health Pakistan. Pakistan HCV treatment guidelines 2016. 2016:1–42.
- 17. Hamid S, Saeed Q, May MT, et al. Importance and contribution of community, social, and healthcare risk factors for hepatitis C infection in Pakistan. Am J Trop Med Hyg. 2017;97(6):1920–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Umar M, Bushra HT, Ahmad M, et al. Hepatitis C in Pakistan: a review of available data. Hepat Mon. 2010;10(3):205–14. [PMC free article] [PubMed] [Google Scholar]
- 19. Ahmed B, Ali T, Qureshi H, et al. Population-attributable estimates for risk factors associated with hepatitis B and C: policy implications for Pakistan and other South Asian countries. Hepatol Int. 2013;7(2):500–7. [DOI] [PubMed] [Google Scholar]
- 20. World Health Organization Pakistan health profile 2015. Geneva: World Health Organization, 2015.
- 21.Pakistan Bureau of Statistics. Population census 2017. http://www.pbscensus.gov.pk/ (last accessed January 2018).
- 22. Hasan A, Mohib M Urban slums reports: the case of Karachi, Pakistan. http://www.ucl.ac.uk/dpu-projects/Global_Report/pdfs/Karachi.pdf(last accessed March 2017).
- 23. Vandenbroucke JP, von Elm E, Altman DG, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):W-163–94. [DOI] [PubMed] [Google Scholar]
- 24. Eckhardt B, Winkelstein ER, Shu MA, et al. Risk factors for hepatitis C seropositivity among young people who inject drugs in New York City: implications for prevention. PLoS One. 2017;12(5):e0177341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Grebely J, Dore GJ, Morin S, et al. Elimination of HCV as a public health concern among people who inject drugs by 2030 – What will it take to get there? J Int AIDS Soc. 2017;20(1):22146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Belaunzarán-Zamudio PF, Mosqueda-Gomez JL, Macias-Hernandez A, et al. Risk factors for prevalent hepatitis C virus infection among inmates in a state prison system in Mexico. PLoS One. 2017;12(6):e0179931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reed JR, Jordan AE, Perlman DC, et al. The HCV care continuum among people who use drugs: protocol for a systematic review and meta-analysis. Syst Rev. 2016;5:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ali I, Siddique L, Rehman LU, et al. Prevalence of HCV among the high risk groups in Khyber Pakhtunkhwa. Virol J. 2011;8:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Capileno YA, Van den Bergh R, Donchunk D, et al. Management of chronic hepatitis C at a primary health clinic in the high-burden context of Karachi, Pakistan. PLoS One. 2017;12:e0175562. [DOI] [PMC free article] [PubMed] [Google Scholar]