Short abstract
Background
Gadolinium-perfusion magnetic resonance (MR) identifies gray matter abnormalities in early multiple sclerosis (MS), even in the absence of structural differences. These perfusion changes could be related to the cognitive disability of these patients, especially in the working memory. Arterial spin labeling (ASL) is a relatively recent perfusion technique that does not require intravenous contrast, making the technique especially attractive for clinical research.
Purpose
To verify the perfusion alterations in early MS, even in the absence of cerebral volume changes. To introduce the ASL sequence as a suitable non-invasive method in the monitoring of these patients.
Material and Methods
Nineteen healthy controls and 28 patients were included. The neuropsychological test EDSS and SDMT were evaluated. Cerebral blood flow and bolus arrival time were collected from the ASL study. Cerebral volume and cortical thickness were obtained from the volumetric T1 sequence. Spearman’s correlation analyzed the correlation between EDSS and SDMT tests and perfusion data. Differences were considered significant at a level of P < 0.05.
Results
Reduction of the cerebral blood flow and an increase in the bolus arrival time were found in patients compared to controls. A negative correlation between EDSS and thalamus transit time, and between EDSS and cerebral blood flow in the frontal cortex, was found.
Conclusion
ASL perfusion might detect changes in MS patients even in absent structural volumetric changes. More longitudinal studies are needed, but perfusion parameters could be biomarkers for monitoring these patients.
Keywords: Perfusion magnetic resonance imaging, arterial spin labeling, multiple sclerosis perfusion, gray matter-multiple sclerosis, cognitive disability, bolus arrival time
Introduction
There have been great advances in the diagnosis and treatment of multiple sclerosis (MS) in recent years. Magnetic resonance (MR) continues to be the cornerstone in the monitoring of the therapeutic response and clinical evolution of these patients. Nevertheless, the correlation between lesion volume in conventional MR sequences and clinical features, especially cognitive disability, is low (1–6). Cognitive decline affects 40%–65% of patients with MS and is one of the main causes of disability (7). Volumetric MR studies are the most commonly used in the assessment of brain atrophy. Many studies have shown an association between cognitive decline in patients with MS and brain atrophy, especially in the cortical gray matter, spinal gray matter, and thalamus (7–13). However, these volumetric changes occur late and only partially demonstrate the damage in gray and white cerebral matter, and their correlation with clinical evolution is not complete either (14,15). Other non-structural MR sequences such as spectroscopy, diffusion imaging, or functional MR have been used to detect metabolic, biochemical, or functional changes in these patients (16–19), but again they usually appear after the structural damage.
Recent studies have demonstrated the potential of perfusion MR imaging (MRI) in identifying cortical abnormalities, even in the absence of structural differences. Cerebral perfusion can assess the oxygenation and the metabolism of different parts of the brain and could, indirectly, analyze the neurodegeneration occurring in this disease (17–20). Dynamic susceptibility contrast MRI (DSC-MRI) evaluates the flow of a paramagnetic contrast agent along cerebral tissue and remains the most widely used method (21). But perfusion can also be assessed using the perfusion sequence called arterial spin labeling (ASL), without needing contrast agents (22–26), making this sequence suitable for research studies. Only a few reports have already shown a correlation between ASL perfusion changes in gray matter and cognitive and physical disability in MS (27–32).
In our study, an ASL sequence was used to quantify absolute perfusion changes, cerebral blood flow (CBF), and bolus arrival time (BAT) within areas implicated in the working memory and executive functions (frontal gray matter and deep basal ganglia) in patients with relapsing-remitting multiple sclerosis (RRMS) and secondary-progressive multiple sclerosis (SPMS). The data showed that patients with SPMS and RRMS had lower CBF and increased BAT compared with controls in superior frontal gray matter and thalamus, even in the absence of structural changes. We hypothesized that frontal lobe and thalamus perfusion changes could be a clinically relevant marker in cognitive dysfunction and disability.
Material and Methods
Patients
We designed a cross-sectional study where we included healthy controls and patients with RRMS—according to the McDonald 2010 criteria (33) and SPSM according to the Lublin 2013 criteria (34)—attending the demyelinating diseases unit at our center, who gave their consent to participate in the study. We excluded patients with primary-progressive multiple sclerosis, patients with a relapse, or corticosteroid treatment during the last month. The study included a total of 19 healthy controls and 28 patients (20 patients with RRMS and eight with SPMS), from whom we collected the following variables: gender; age; age at onset; age at diagnosis; and Expanded Disability Status Scale (EDSS). Cognitive function was evaluated with the Symbol Digit Modalities test (SDMT) (35). Written consent was obtained from all participants.
Image acquisition
MRI was performed on a 1.5-T MR450w MR scanner (General Electric Healthcare, Milwaukee, WI, USA) with an eight-channel phased-array head coil. The acquisitions included our routine structural protocol: isovolumetric sagittal T1 (3D-SPGR); TR = 8.5 ms; TE = 3.2 ms; TI = 700 ms; flip angle (FA) = 12; bandwidth = 31.25 kHz; acquisition matrix = 258 × 258; full brain coverage; reconstructed matrix = 1 × 1 × 1 mm; isovolumetric-FLAIR (CUBE); coronal T2-weighted and a post-gadolinium isovolumetric T1 sequence. Before the contrast gadolinium-enhanced images, we included a prototype sequence to measure perfusion called enhanced ASL. This three-dimensional (3D) stack of spirals sequence (32) has been designed to improve signal-to-noise ratio, reduce artefacts, and acquire multiple post-label delay times that allows us to obtain transit delay time maps, as well as transit time corrected perfusion maps. This sequence was used with the following parameters: TE = 11.5 ms; TR = 5981 ms; post-labeling delay = 1000 ms; perfusion labeling time = 3500 ms; number of delays = 7 (1000 ms, 1220 ms, 1480 ms, 1780 ms, 2150 ms, 2630 ms, and 3320 ms); bandwidth = 62.5 kHz; FA = 155, field of view = 22 cm; spiral acquisition = 640 points × 4 arms; reconstructed image = 128 × 128; and slice thickness = 4.5 mm.
Image processing
To evaluate disability and cognitive dysfunction in working memory and processing speed, our areas of interest were the superior, middle, and inferior gyrus, the caudate nucleus, and the thalamus, areas where a major degree of atrophy is seen in patients with great disability (36–39). From the T1-weighted (T1W) sequences, we computed cortical thickness (CTh) and cerebral volume (CV). Data obtained from the perfusion maps were the corrected CBF and BAT, also called arterial transit delay time, defined as the time from labeling of blood in feeding arteries to its first arrival in the capillary network of the voxel of interest. To evaluate perfusion and BAT in our areas of interest, the structural T1W volumes were previously segmented using FreeSurfer (https://surfer.nmr.mgh.harvard.edu/). The result is a label map of isotropic voxel size containing a plethora of brain regions, along with meaningful anatomical information—volume, area, and cortical thickness—for each of these regions. Among all the brain regions present in the output label map, 18 regions of interest (ROI) considered significant to our study were selected. In addition, white matter lesions were automatically segmented from each FLAIR volume using the Lesion Segmentation Toolbox (LST) from SPM12 (www.fil.ion.ucl. ac.uk/spm/software/spm12/). The result is a lesion probability map, in which the value of each voxel corresponds to its probability of being a lesion. Each voxel was finally labeled as lesion if its probability ≥0.65. The raw ASL volume and FLAIR volume of each patient were rigidly co-registered and resliced to the T1W volumes using SPM12, selecting normalized mutual information as the cost function and b-spline interpolation of order 4. The label map obtained from FreeSurfer was rigidly registered to the T1 volume to recover the original resolution. In this case, a nearest neighbor interpolation was chosen to preserve the label values in the voxels. The transformation resulting from the co-registration of the ASL volume to the T1 volume was applied to the perfusion and BAT maps. Similarly, the transformation obtained for the FLAIR volume was applied to the lesion segmentation map.
After this step, the BAT, CBF, and lesion segmentation maps are all co-registered to the anatomical segmentation map, which allows to evaluate these features locally per region. Quantification of perfusion (mL/100g/min) and bolus arrival time (ms) were assessed by calculating the mean and SD for each of the ROIs. Quantification of lesion load was assessed by estimating the lesion volume for each of the ROIs. All calculations required in the quantification step were conducted by a dedicated software, which was developed for this purpose.
Statistical analysis
Due to the exploratory nature of the study and the low sample size, statistical analyses were carried out using non-parametric techniques and without multivariate analysis. Quantitative variables were summarized as median and interquartile range (IQR) and categorical variables as number and percentage. Mann–Whitney U test was used for independent group comparison. Spearman’s correlation was used to analyze the correlation between the results of the EDSS and SDMT tests and CBF, BAT, and CV. Differences were considered significant at a level of P < 0.05. We used the IBM SPSS statistical software program, version 21.0 (IBM, Armonk, NY, USA).
Results
A total of 19 controls and 28 patients were included in the study. The median age of patients with MS was 45 years (age range = 39–52 years) and the median age of controls was 44 years (age range = 28–55 years) (P = 0.46). Of the 28 patients and 19 controls, 64.3% and 63.2%, respectively, were women (P = 0.94). The median time since diagnosis of MS in patients with MS was eight years (range = 5–14 years). With regard to the results of tests, the median EDSS was 1 (range = 0.3–3.6) and the median SMDT was 51 (range = 42–62).
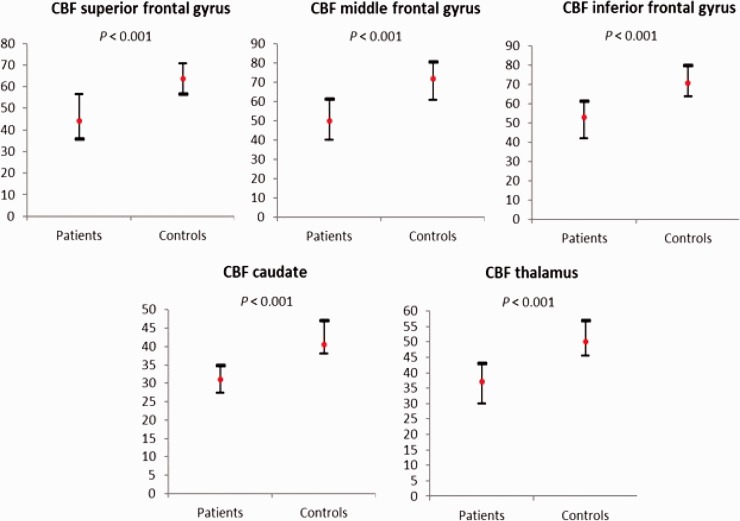
We found statistically significant differences in the CBF (Fig. 1) between patients with MS and controls in the superior frontal gyrus (median = 44.2 vs 63.36, P < 0.001), middle frontal gyrus (49.9 vs. 71.8, P < 0.001), inferior frontal gyrus (53 vs. 70.6, P < 0.001), caudate (31 vs. 40.5, P < 0.001), and thalamus (37.1 vs. 50, P < 0.001) (Fig. 1). There were no significant differences in brain volume values and BAT between patients with MS and controls in any of the areas studied (Table 1).
Fig. 1.
CBF in mL/100g/min: differences between patients with MS and controls. Median (red point) and IQR. CBF, cerebral blood flow, MS, multiple sclerosis.
Table 1.
BAT and CV: differences between patients with MS and controls.
| Patients with MS (n = 28) | Controls (n = 19) | P | |
|---|---|---|---|
| BAT superior frontal gyrus | 1290.8 (245.3–1403.2) | 1321.9 (1283–1424.7) | 0.43 |
| BAT middle frontal gyrus | 1401.6 (1301.6–1503.2) | 1387.3 (1327.2–1448) | 0.74 |
| BAT inferior frontal gyrus | 1210.6 (1166.8–1291.1) | 1206.5 (1176.5–1304.4) | 0.66 |
| BAT caudate | 1130.8 (1087.7–1201.3) | 1155.3 (1113.9–1249.6) | 0.15 |
| BAT thalamus | 1240.4 (1194.8–1311.9) | 1264.5 (1203.9–1355.9) | 0.26 |
| CV superior frontal gyrus | 22,993 (20,968.8–24,993) | 21,983 (19,426–26,163) | 0.91 |
| CV middle frontal gyrus | 22,378.5 (20,274.8–23,827) | 23,014 (19,786–25,419) | 0.39 |
| CV inferior frontal gyrus | 10,745 (10,072.2–11,732) | 10,704 (9758–12,394) | 0.7 |
| CV caudate | 3526.5 (3118.3–3922) | 3307.4 (3060.7–3779.9) | 0.32 |
| CV thalamus | 7601.5 (6813.5–8754.5) | 8344.2 (7725.9–8854.1) | 0.07 |
Values are given as median (IQR).
BAT, bolus arrival time (in ms); CV, cerebral volume (in mm3); EDSS: Expanded Disability Status Scale; IQR, interquartile range; MS, multiple sclerosis; SDMT: Symbol Digit Modalities Test.
The differences between patients with RRMS and patients with SPMS are shown in Table 2. As expected, patients with RRMS were younger (P = 0.002), with shorter diseases (P = 0.004), lower disability in the EDSS (P < 0.001), and better cognitive functioning (P < 0.001). We found statistically significant differences in the CBF between RRMS and SPMS in the superior frontal gyrus (median = 49.4 vs, 37.8, P = 0.033), middle frontal gyrus (59.5 vs. 39.7, P = 0.01), inferior frontal gyrus (56.1 vs. 41, P = 0.025), and thalamus (39.2 vs. 31.5, P = 0.028). There were no significant differences in the BAT data, although the values were higher in patients with SPMS in all areas studied. Brain volume values were lower in all areas in patients with SPMS, but we found no statistically significant differences (Table 2).
Table 2.
Comparative analysis between clinical forms.
| Clinical forms |
|||
|---|---|---|---|
| RRMS (n = 20) | SPMS (n = 8) | P | |
| Age (years) | 41 (37–47) | 51 (48–57) | 0.002 |
| Time since diagnosis (years) | 6 (5–10) | 13 (11–18) | 0.004 |
| EDSS | 1 (0–1) | 4.5 (4–6.3) | <0.001 |
| SDMT | 58 (50–64) | 36 (24–43) | <0.001 |
| CBF superior frontal gyrus | 49.4 (39.4–59.2) | 37.8 (29.6–42.8) | 0.033 |
| CBF middle frontal gyrus | 59.5 (42.3–67.1) | 39.7 (29.8–47.7) | 0.01 |
| CBF inferior frontal gyrus | 56.1 (47.3–64.4) | 41 (33.6–53) | 0.025 |
| CBF caudate | 31.8 (28–35.3) | 28.1 (26.7–30.5) | 0.182 |
| CBF thalamus | 39.2 (30.8–46.9) | 31.5 (26–37.1) | 0.028 |
| BAT superior frontal gyrus | 1288.2 (1246.7–1380.1) | 1348.8 (1251.2–1464.5) | 0.328 |
| BAT middle frontal gyrus | 1347.8 (1292.9–1469.3) | 1458.7 (1382.1–1538.9) | 0.182 |
| BAT inferior frontal gyrus | 1185.3 (1149.9–1258) | 1228.1 (1209.6–1327.9) | 0.165 |
| BAT caudate | 1115.1 (1080.5–1192) | 1145.9 (1126.5–1228.4) | 0.136 |
| BAT thalamus | 1210.4 (1164.7–1304.7) | 1298.4 (1251.60–1327.6) | 0.07 |
| CV superior frontal gyrus | 23,003 (2207–25,775) | 22,105 (16,649–24,761) | 0.231 |
| CV middle frontal gyrus | 22,531.5 (21,416.5–23,783.5) | 20,404.5 (17,965.5–23,401.5) | 0.15 |
| CV inferior frontal gyrus | 10,839 (10,277–11,894) | 9902.5 (9198.5–11,123) | 0.079 |
| CV caudate | 3597 (3225–3983.5) | 3315 (3018–3735) | 0.237 |
| CV thalamus | 7669.5 (7097.5–9087.5) | 6313 (6125.5–8391) | 0.079 |
Values are given as median (IQR).
Values in bold represent statistically significant differences.
BAT, bolus arrival time (in ms); CBF, cerebral blood flow (in mL/100g/min); CV, cerebral volume (in mm3); EDSS, Expanded Disability Status Scale; IQR, interquartile range; RRMS, relapsing-remitting multiple sclerosis; SDMT, Symbol Digit Modalities Test; SPMS, secondary progressive multiple sclerosis.
We found a negative correlation between EDSS and CBF in the superior frontal gyrus (Spearman’s rho = –0.52, P = 0.005), middle frontal gyrus (rho = –0.53, P = 0.003), inferior frontal gyrus (rho = –0.49, P = 0.007), thalamus (rho = –0.47, P = 0.01), and caudate (rho = –0.40, P = 0.04). There was no correlation between CBF and the SDMT. A negative correlation was found between BAT in the thalamus and EDDS (rho = –0.43, P = 0.02) but not with the SDMT, although there was a tendency towards a statistically significant correlation (rho = –0.35, P = 0.07). A moderate correlation between SDMT and CV in the superior frontal gyrus (rho = 0.4, P = 0.048) was observed (Table 3).
Table 3.
Correlation analysis between perfusion results and neurological test (SDMT and EDSS) in patients with MS.
| SDMTn = 27 | EDSSn = 28 | ||
|---|---|---|---|
| CBF superior frontal gyrus | rho | –0.07 | –0.52 |
| P | 0.75 | 0.005 | |
| CBF middle frontal gyrus | rho | –0.02 | –0.53 |
| P | 0.92 | 0.003 | |
| CBF inferior frontal gyrus | rho | –0.06 | –0.49 |
| P | 0.76 | 0.007 | |
| CBF caudate | rho | –0.15 | –0.40 |
| P | 0.45 | 0.04 | |
| CBF thalamus | rho | –0.08 | –0.47 |
| P | 0.68 | 0.01 | |
| BAT superior frontal gyrus | rho | –0.15 | 0.19 |
| P | 0.45 | 0.33 | |
| BAT middle frontal gyrus | rho | –0.17 | 0.22 |
| P | 0.40 | 0.26 | |
| BAT inferior frontal gyrus | rho | –0.16 | 0.31 |
| P | 0.44 | 0.11 | |
| BAT caudate | rho | –0.27 | 0.26 |
| P | 0.17 | 0.19 | |
| BAT thalamus | rho | –0.35 | 0.43 |
| P | 0.07 | 0.02 | |
| CV superior frontal gyrus | rho | 0.4 | –0.25 |
| P | 0.048 | 0.21 | |
| CV middle frontal gyrus | rho | 0.16 | –0.23 |
| P | 0.42 | 0.27 | |
| CV inferior frontal gyrus | rho | 0.37 | –0.39 |
| P | 0.06 | 0.04 | |
| CV caudate | rho | 0.26 | –0.25 |
| P | 0.18 | 0.21 | |
| CV thalamus | rho | 0.33 | –0.32 |
| P | 0.09 | 0.1 | |
Values in bold represent statistically significant correlations.
BAT, bolus arrival time; CBF, cerebral blood flow; CV, cerebral volume; EDSS, Expanded Disability Status Scale; SDMT, Symbol Digit Modalities Test.
We also analyzed the correlation between the data of the superior frontal gyrus cortical thickness (CTh) and neuropsychological tests. There was no significant correlation either with EDSS test (rho = –0.37, P = 0.054) or with the SDMT (rho = 0.24, P = 0.24). In the same way, we found no statistically significant correlation between cortical thickness and CBF values in the superior frontal gyrus (rho = 0.21, P = 0.28). These results support the hypothesis that changes in perfusion occur before loss of cortical volume.
Discussion
In recent years, several studies have demonstrated the potential for perfusion MRI in identifying cortical and deep gray matter abnormalities in patients with MS, even in the absence of structural differences (29–32). Almost all studies have used contrast media agents to perform the perfusion sequences. Patients with early RRMS with relatively preserved clinical and cognitive functions have shown multiple regions with reduced cerebral blood volume, but no loss of gray matter volume has been commonly found.
The goal of our study was to confirm these previously published results using a perfusion sequence that does not require any contrast media, the ASL sequence. It uses the water in arterial blood as an endogenous, freely diffusible contrast medium. The main physiological parameters measured with ASL are the CBF and the BAT (33). Its non-invasive and quantitative nature makes the technique especially attractive for longitudinal studies and clinical research. There is currently sufficient evidence to support its clinical application, with clear advantages in terms of improved and earlier diagnosis.
A statistically significant reduction in CBF has been found in the frontal cortices, the thalamus, and the caudate in patients with MS, with no evidence of loss of gray matter volume and no reduction in cortical thickness. The data included in this study reveal that these abnormalities are more evident in SPMS as compared with RRMS. Regarding disability, a negative correlation was discovered between EDSS and CBF in the frontal cortex and the basal ganglia, as well as between EDSS and the BAT in the thalamus.
The reasons for these changes in cerebral perfusion in MS are not fully understood. There are several hypotheses that may address these findings. First, hypoperfusion could be related to a neuroaxonal loss. However, most of the studies have not found a relationship between perfusion and brain atrophy (18,19,25,38), while other articles have only described a partial association with T2 lesion load (23,39). In this study, the reduction of perfusion was not associated with measures of brain atrophy either, so our results reinforce the idea that these changes are caused by other mechanisms. Other possible explanations include a reduction in energy demand, or metabolic consumption (39,40), primary ischemia (41), dysfunction of cerebrovascular reactivity (42–44), mitochondria dysfunction (45–47), or even a previous step in the neurodegeneration process before tissue loss is established. In this case, this parameter could allow for greater therapeutic opportunities than the detection of a more intense and widespread damage (48).
Moreover, we did not find a positive correlation between SDMT—an indirect measure of cognitive impairment—with the CBF or BAT, as previously reported (31). This fact could be due to methodological issues. On the one hand, we had a low percentage of cognitive dysfunction. Thus, only three patients with RRMS and seven with SPMS showed medium to low scores in SDMT. Even in this situation, we observed a tendency towards a statistically significant correlation between SDMT score, the CBF of the superior frontal gyrus, and the BAT in the thalamus, suggesting that they are interesting parameters for this component of the disease.
Nevertheless, there is growing evidence of the alterations in cerebral perfusion in patients with MS that would be independent from or before the structural volumetric changes. Our results, as well as the data from other studies, support the idea that this technique could be useful for monitoring the neurodegeneration occurring in MS, with a progressive reduction in the CBF and an increase in BAT, as the disease evolves. In this regard, the ASL sequence could be a suitable method, as it is a non-invasive, feasible, and reproducible technique (49,50).
It remains to be decided which measures or which brain areas would offer the most relevant information to be implemented as a routine evaluation. Methods and results are very variable in this regard (21,29,30). In our study, as pointed out previously, these regions could be the frontal cortex and the thalamus, where we obtained a negative correlation between EDSS and thalamus transit time, and between EDSS and CBF in different areas of the frontal cortex.
In order to better understand these perfusion alterations in patients with MS, and to clarify their possible uses in clinical practice, further studies with longitudinal designs and more participants are needed.
In conclusion, early perfusion changes occurring in patients with MS can be assessed with ASL sequence, even in those with absent or minimal structural changes. The perfusion data from the frontal cortex and the thalamus could offer relevant information to evaluate the cognitive disability in these patients.
Acknowledgements
The authors thank Peter Bonney for his support in proofreading this article.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
Mar Jiménez de la Peña https://orcid.org/0000-0001-5281-6448
Miguel López Gavilán https://orcid.org/0000-0002-2142-3948
References
- 1.Filippi M1, Grossman RI. MRI techniques to monitor MS evolution: the present and the future. Neurology 2002; 23:1147–1153. [DOI] [PubMed] [Google Scholar]
- 2.Li DK, Held U, Petkau J, et al. MRI T2 lesion burden in multiple sclerosis: a plateauing relationship with clinical disability. Neurology 2006; 9:1384–1389. [DOI] [PubMed] [Google Scholar]
- 3.Fisniku LK, Brex PA, Altmann DR, et al. Disability and T2 MRI lesions: a 20-year follow-up of patients with relapse onset of multiple sclerosis. Brain 2008; 131:808–817. [DOI] [PubMed] [Google Scholar]
- 4.Enzinger C, Fuchs S, Pichler A, et al. Predicting the severity of relapsing-remitting MS: the contribution of cross-sectional and short-term follow-up MRI data. Mult Scler 2011; 17:695–701. [DOI] [PubMed] [Google Scholar]
- 5.Rudick RA, Lee JC, Simon J, et al. Significance of T2 lesions in multiple sclerosis: A 13-year longitudinal study. Ann Neurol 2006; 60:236–242. [DOI] [PubMed] [Google Scholar]
- 6.Minneboo A, Uitdehaag BM, Jongen P, et al. Association between MRI parameters and the MS severity scale: a 12 year follow-up study. Mult Scler 2009; 15:632–637. [DOI] [PubMed] [Google Scholar]
- 7.Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol 2008; 7:1139–1151. [DOI] [PubMed] [Google Scholar]
- 8.Kearney H, Rocca MA, Valsasina P, et al. Magnetic resonance imaging correlates of physical disability in relapse onset multiple sclerosis of long disease duration. Mult Scler 2014; 20:72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Popescu V, Agosta F, Hulst HE, et al. Brain atrophy and lesion load predict long term disability in multiple sclerosis. J Neurol Neurosurg Psychiatry 2013; 84:1082–1091. [DOI] [PubMed] [Google Scholar]
- 10.Pérez-Miralles FC, Sastre-Garriga J, Vidal-Jordana A, et al. Predictive value of early brain atrophy on response in patients treated with interferon β. Neurol Neuroimmunol Neuroinflamm 2015; 2:e132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deloire MS, Ruet A, Hamel D, et al. MRI predictors of cognitive outcome in early multiple sclerosis. Neurology 2011; 29:1161–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benedict RH, Hulst HE, Bergsland N, et al. Clinical significance of atrophy and white matter mean diffusivity within the thalamus of multiple sclerosis patients. Mult Scler 2013; 19:1478–1484. [DOI] [PubMed] [Google Scholar]
- 13.Calabrese M1, Rinaldi F, Grossi P, et al. Cortical pathology and cognitive impairment in multiple sclerosis. Expert Rev Neurother 2011; 11:425–432. [DOI] [PubMed] [Google Scholar]
- 14.Azevedo CJ, Overton E, Khadka S, et al. Early CNS neurodegeneration in radiologically isolated syndrome. Neurol Neuroimmunol Neuroinflamm 2015; 9:e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rocca MA, Comi G, Filippi M. The role of T1-weighted derived measures of neurodegeneration for assessing disability progression in multiple sclerosis. Front Neurol 2017; 4:433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giorgio A, De Stefano N. Advanced structural and functional brain MRI in multiple sclerosis. Semin Neuro 2016; 36:163–176. [DOI] [PubMed] [Google Scholar]
- 17.Solana E, Martinez-Heras E, Martinez-Lapiscina EH, et al. Magnetic resonance markers of tissue damage related to connectivity disruption in multiple sclerosis. Neuroimage Clin 2018; 12:161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rovaris M, Gass A, Bammer R, et al. Diffusion MRI in multiple sclerosis. Neurology 2005; 22:1526–1532. [DOI] [PubMed] [Google Scholar]
- 19.Pantano P, Mainero C, Caramia F. Functional brain reorganization in multiple sclerosis: evidence from fMRI studies. J Neuroimaging 2006; 16:104–114. [DOI] [PubMed] [Google Scholar]
- 20.Vollmer T, Huynh L, Kelley C, et al. Relationship between brain volume loss and cognitive outcomes among patients with multiple sclerosis: a systematic literature review. Neurol Sci 2016; 37:165–179. [DOI] [PubMed] [Google Scholar]
- 21.Hojjat SP, Kincal M, Vitorino R, et al. Cortical perfusion alteration in normal-appearing gray matter is most sensitive to disease progression in relapsing-remitting multiple sclerosis. AJNR Am J Neuroradiol 2016; 37:1454–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rashid W, Parkes LM, Ingle GT, et al. Abnormalities of cerebral perfusion in multiple sclerosis. J Neurol Neurosurg Psychiatry 2004; 75:1288–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inglese M, Park SJ, Johnson G, et al. Deep gray matter perfusion in multiple sclerosis: dynamic susceptibility contrast perfusion magnetic resonance imaging at 3 T. Arch Neurol 2007; 64:196–202. [DOI] [PubMed] [Google Scholar]
- 24.Hojjat SP, Cantrell CG, Carroll TJ, et al. Perfusion reduction in the absence of structural differences in cognitively impaired versus unimpaired RRMS patients. Mult Scler 2016; 22:1685–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vitorino R, Hojjat SP, Cantrell CG, et al. Regional frontal perfusion deficits in relapsing-remitting multiple sclerosis with cognitive decline. AJNR Am J Neuroradiol 2016; 10:1800–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ingrisch M, Sourbron S, Herberich S, et al. Dynamic contrast-enhanced magnetic resonance imaging suggests normal perfusion in normal-appearing white matter in multiple sclerosis. Invest Radiol 2017; 52:135–141. [DOI] [PubMed] [Google Scholar]
- 27.Havsteen I, Damm Nybing J, Christensen H, et al. Arterial spin labeling: a technical overview. Acta Radiol 2018; 9:1232–1238. [DOI] [PubMed] [Google Scholar]
- 28.Telischak NA, Detre JA, Zaharchuk G. Arterial spin labeling MRI: clinical applications in the brain. Magn Reson Imaging 2015; 41:1165–1180. [DOI] [PubMed] [Google Scholar]
- 29.Ota M, Sato N, Nakata Y, et al. Abnormalities of cerebral blood flow in multiple sclerosis: A pseudocontinuous arterial spin labeling MRI study. Magn Reson Imaging 2013; 31:990–995. [DOI] [PubMed] [Google Scholar]
- 30.Paling D, Thade Petersen E, Tozer DJ, et al. Cerebral arterial bolus arrival time is prolonged in multiple sclerosis and associated with disability. J Cereb Blood Flow Metab 2014; 34:34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Debernard L, Melzer TR, Van Stockum S, et al. Reduced grey matter perfusion without volume loss in early relapsing-remitting multiple sclerosis. J Neurol Neurosurg Psychiatry 2014; 85:544–551. [DOI] [PubMed] [Google Scholar]
- 32.Koudriavtseva T, Plantone D, Renna R, et al. Brain perfusion by arterial spin labeling MRI in multiple sclerosis. J Neurol 2015; 262:1769–1771. [DOI] [PubMed] [Google Scholar]
- 33.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011; 69:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology 2014; 15:278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parmenter BA, Weinstock-Guttman B, Garg N, et al. Screening for cognitive impairment in multiple sclerosis using the Symbol digit Modalities Test. Mult Scler 2007; 13:52–57. [DOI] [PubMed] [Google Scholar]
- 36.Lansley J, Mataix-Cols D, Grau M, et al. Localized grey matter atrophy in multiple sclerosis: a meta-analysis of voxel-based morphometry studies and associations with functional disability. Neurosci Biobehav Rev 2013; 37:819–830. [DOI] [PubMed] [Google Scholar]
- 37.Tao G, Datta S, He R, et al. Deep gray matter atrophy in multiple sclerosis: a tensor based morphometry. J Neurol Sci 2009; 282:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rocca MA, Mesaros S, Pagani E, et al. Thalamic damage and long-term progression of disability in multiple sclerosis. Radiology 2010; 257:463–469. [DOI] [PubMed] [Google Scholar]
- 39.Audoin B, Zaaraoui W, Reuter F, et al. Atrophy mainly affects the limbic system and the deep grey matter at the first stage of multiple sclerosis. J Neurol Neurosurg Psychiatry 2010; 81:690–695. [DOI] [PubMed] [Google Scholar]
- 40.Dai W, Shankaranarayanan A, Alsop DC. Volumetric measurement of perfusion and arterial transit delay using Hadamard encoded continuous arterial spin labeling. Magn Reson Med 2013; 69:1014–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Francis PL, Jakubovic R, O’Connor P, et al. Robust perfusion deficits in cognitively impaired patients with secondary-progressive multiple sclerosis. AJNR Am J Neuroradiol 2013; 34:62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aviv RI, Francis PL, Tenenbein R, et al. Decreased frontal lobe gray matter perfusion in cognitively impaired patients with secondary progressive multiple sclerosis detected by the bookend technique. AJNR Am J Neuroradiol 2012; 33:1779–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adhya S, Johnson G, Herbert J, et al. Pattern of hemodynamic impairment in multiple sclerosis: dynamic susceptibility contrast perfusion MR imaging at 3.0 T. Neuroimage 2006; 33:1029–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koudriavtseva T, Sbardella E, Mainero C. Brain perfusion and vasoreactivity in multiple sclerosis. AJNR Am J Neuroradiol 2015; 36:E27–E28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dutta R, McDonough J, Yin X, et al. Mitochondrial dysfunction as a cause of axonal degeneration in multiple sclerosis patients. Ann Neurol 2006; 59:478–489. [DOI] [PubMed] [Google Scholar]
- 46.Campbell GR, Worrall JT, Mahad DJ. The central role of mitochondria in axonal degeneration in multiple sclerosis. Mult Scler 2014; 20:1806–1813. [DOI] [PubMed] [Google Scholar]
- 47.Mahad D, Lassmann H, Turnbull D. Review: Mitochondria and disease progression in multiple sclerosis. Neuropathol Appl Neurobiol 2008; 34:577–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nikić I, Merkler D, Sorbara C, et al. A reversible form of axon damage in experimental autoimmune encephalomyelitis and multiple sclerosis. Nat Med 2011; 17:495–499. [DOI] [PubMed] [Google Scholar]
- 49.Gevers S, van Osch MJ, Bokkers RP, et al. Intra- and multicenter reproducibility of pulsed, continuous and pseudo-continuous arterial spin labeling methods for measuring cerebral perfusion. J Cereb Blood Flow Metab 2011; 31:1706–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petersen ET, Mouridsen K, Golay X; all named co-authors of the QUASAR test-retest study. The QUASAR reproducibility study, Part II: Results from a multi-center Arterial Spin Labeling test-retest study. Neuroimage 2010; 49:104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]