Abstract
Fibroblast growth factor 21 (FGF21) is an endocrine hormone derived from the liver that exerts pleiotropic effects on the body to maintain overall metabolic homeostasis. During the past decade, there has been an enormous effort made to understand the physiological roles of FGF21 in regulating metabolism and to identify the mechanism for its potent pharmacological effects to reverse diabetes and obesity. Through both human and rodent studies, it is now evident that FGF21 levels are dynamically regulated by nutrient sensing, and consequently FGF21 functions as a critical regulator of nutrient homeostasis. In addition, recent studies using new genetic and molecular tools have provided critical insight into the actions of this endocrine factor. This review examines the numerous functions of FGF21 and highlights the therapeutic potential of FGF21-targeted pathways for treating metabolic disease.
Keywords: FGF21, macronutrient, β-klotho, adipose, thermogenesis, alcohol
1. INTRODUCTION TO FGF21
Fibroblast growth factors (FGFs) have diverse roles in signaling and development across a wide array of tissues. In addition to their vital roles in regulating cell growth, altered FGF function may also contribute to diseases ranging from cancer to bone disorders (8). Mammalian genomes encode 22 FGFs, which are classically considered paracrine, autocrine, or intracrine. The majority of FGFs are secreted and interact with heparan sulfate glycosaminoglycans (HSGAGs), which sequester the proteins to the extracellular matrix and prevent their dispersion from their cell of origin (8). FGFs interact with one of four FGF receptors (FGFR1–4), which are classical tyrosine kinase receptors that dimerize upon interaction with FGFs and initiate intracellular signaling. Unlike traditional FGFs, however, the FGF19 subfamily of FGFs, consisting of FGF19, FGF21, and FGF23, has reduced affinity for HSGAGs and diffuses into circulation to act on distal tissues (9, 106). Thus, these FGFs function as endocrine hormones. Importantly, since FGF receptors are ubiquitously expressed, endocrine FGFs require a coreceptor to provide signaling specificity, which for FGF21 and FGF19 is the coreceptor β-klotho (71, 99, 140). Due to the potent metabolic actions and therapeutic potential of FGF21, this review focuses on its diverse functions.
FGF21 was first identified as a novel FGF with high expression in the liver and thymus (98). Subsequently, the metabolic role of FGF21 was identified through a screen for factors that induced glucose uptake in 3T3L1 adipocytes (64). A myriad of studies ensued demonstrating that administering FGF21 to obese or diabetic rodents and primates markedly reduced body weight and significantly improved carbohydrate and lipid homeostasis (21, 64, 65, 141). Clinical trials with FGF21-based analogs revealed that many of the metabolic effects of FGF21 observed in rodents also translate to humans (41, 66, 126). In addition to the pharmacological studies, numerous studies have uncovered physiological functions of FGF21, and with these have come the unraveling of unique endocrine pathways regulating nutrient homeostasis.
The expression levels and metabolic functions of FGF21 are dynamically impacted by nutritional status (37, 102). The expression of Fgf21 messenger RNA (mRNA) is detectable in multiple tissues, including in the liver, thymus, white adipose tissue (WAT), brown adipose tissue (BAT), pancreas, and heart (39, 98). Notably, despite the expression of Fgf21 mRNA in these tissues, under most physiological conditions, circulating levels of FGF21 are derived primarily from the liver (46, 86). As is discussed in subsequent sections, the induction of FGF21 occurs in specific tissues during diverse physiological states, ranging from fasting to overfeeding (88). FGF21 functions in target tissues that express the traditional FGF receptor FGFR1c and the obligate coreceptor β-klotho (2, 27, 71, 99, 124, 140). While FGFR1c is ubiquitously expressed, β-klotho expression is limited to metabolic tissues and confers specificity for FGF21 signaling. β-klotho is expressed in the liver, WAT, BAT, pancreas (39), and distinct regions of the brain, including the area postrema, nucleus tractus solitarii, suprachiasmatic nucleus (12), and the paraventricular nucleus (PVN) (81). FGF21 initiates signaling to target tissues by binding to the extracellular domain of β-klotho via the C terminus of FGF21, which then facilitates the interaction of FGF21 with FGFR1c (77, 89, 144, 145). This trimeric receptor complex leads to the dimerization of FGFR1c, transphosphorylation of the receptor, and initiation of a signaling cascade (64, 145). Currently, the best readouts for FGF21 signaling in target tissues are general signals of growth factors, including increased phosphorylation of FRS2α and ERK1/2 (64). FGF21-specific signaling cascades have yet to be identified. The relative contribution of FGF21-mediated signaling in specific tissues to its metabolic effects is examined in later sections.
2. THE PHYSIOLOGICAL EFFECTS OF FGF21
While the metabolic effects of pharmacological FGF21 administration have been clear, the physiological effects of FGF21 have been less straightforward. This is likely due to the complexity of the conditions that regulate FGF21 production, the hormonal milieux that impact FGF21 function, and differences in the experimental design and animal models used in studies that have examined physiological FGF21 function. In the following sections, we examine what we consider to be the major physiological functions of FGF21 and explore the biological networks that contribute to its role in regulating nutrient and energy homeostasis.
2.1. Physiological Role of FGF21 During Fasting and Refeeding
The liver is a critical regulator of energy and nutrient homeostasis. During states of nutrient deprivation, such as fasting, the liver maintains energy availability for peripheral tissues through increased production of hepatic glucose and ketones. As fasting progresses, fuel utilization shifts from carbohydrates to ketone bodies. To accomplish this, WAT undergoes lipolysis to release glycerol and free fatty acids to be used by the liver for gluconeogenesis and ketogenesis, respectively. In the liver, the nuclear hormone receptor peroxisome proliferator–activated receptor-α (PPARα) becomes activated by fatty acids, leading to a transcriptional orchestration of the hepatic fasting response for glucose and lipid metabolism (62). Multiple groups discovered that PPARα-mediated induction of FGF21 is important for the adaptive fasting response (7, 54). The Fgf21 promoter contains PPARα response elements that lead to the induction of hepatic and circulating levels of FGF21 during prolonged fasting (54). These circulating levels of FGF21 are derived from the liver, as the genetic loss of Fgf21 specifically from the liver ablates plasma FGF21 levels during fasting (86). Consistent with data from rodents, circulating levels of FGF21 are also induced in humans during prolonged fasting (33, 42). The significance of FGF21 induction during fasting was demonstrated by gain- and loss-of-functions studies. The overexpression of FGF21 was sufficient to increase hepatic gluconeogenesis, β-oxidation, and ketogenesis, whereas the loss of FGF21 prevented fasting-induced hepatic gluconeogenesis, β-oxidation, and ketogenesis in mice (105). While fasting plasma glucose levels were slightly decreased in two different Fgf21 knockout mouse lines (81, 105), this was not observed in another Fgf21 knockout line (6). However, liver-specific Fgf21 knockout mice also exhibited reduced plasma glucose levels during fasting (86).
An important observation made during these studies was that FGF21 does not act directly on the liver to mediate these effects on hepatic metabolism. The administration of FGF21 to primary hepatocytes or infusion into an isolated perfused liver failed to recapitulate the induction of hepatic gluconeogenic gene expression observed in wild-type mice administered FGF21 (105). A subsequent study validated these results and also revealed that FGF21 elicits its effects on hepatic gluconeogenesis through actions in the hypothalamus that activate the hypothalamic–pituitary–adrenal axis to release corticosterone (81). Loss of FGF21 impaired hepatic gluconeogenesis due to blunted corticosterone production (81). Importantly, the FGF21 coreceptor β-klotho is expressed in the hypothalamus (12, 81), and FGF21 crosses the blood–brain barrier (50). Administration of FGF21 to the PVN of the hypothalamus restored corticosterone production and reversed the impaired hepatic gluconeogenesis observed in Fgf21 knockout mice (81). Therefore, FGF21 coordinates the hepatic fasting response through a liver-to-brain signaling axis (Figure 1).
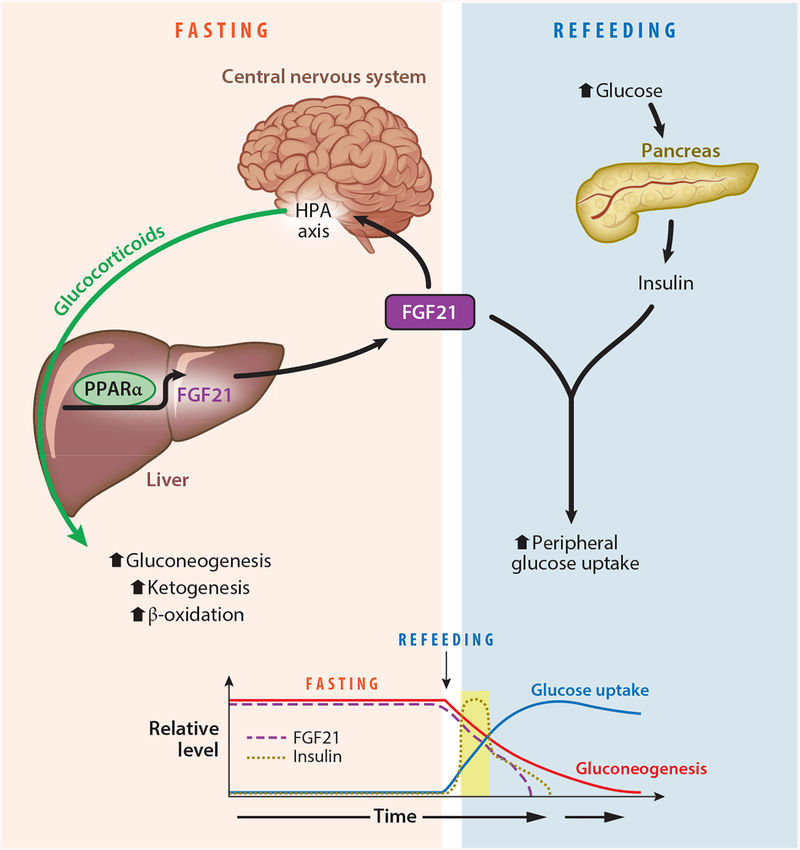
Figure 1.
The role of fibroblast growth factor 21 (FGF21) during fasting and refeeding. During fasting, FGF21 expression is induced by peroxisome proliferator–activated receptor-α (PPARα) in the liver. Circulating FGF21 then activates the hypothalamic–pituitary–adrenal (HPA) axis to increase glucocorticoid release and increase gluconeogenesis. When nutrients become available during refeeding, insulin is released from the pancreas, and while both FGF21 and insulin are in circulation, FGF21 functions to enhance insulin-stimulated glucose uptake in peripheral tissues.
FGF21 also functions to coordinate the transition from the fasting to the refed state (Figure 1). FGF21 is well known to be a potent and rapid insulin sensitizer (27, 142), which is not congruent with its actions as simply a fasting factor. During fasting, elevated fatty acid levels in circulation induce physiological peripheral insulin resistance to spare glucose utilization for the brain. However, when nutrients become available following a prolonged fast, FGF21 functions as a metabolic switch to facilitate the transition to the refed state by enhancing insulin-stimulated glucose uptake while acutely maintaining the actions of the fasting response (86). Plasma FGF21 levels are still present acutely during refeeding, and liver-specific Fgf21 knockout mice have elevated plasma glucose levels and impaired insulin sensitivity during refeeding (86). In addition, in humans, plasma FGF21 levels have been shown to be induced by increases in both glucose and insulin levels (31, 84, 110, 133), supporting a role during refeeding. Thus, FGF21 functions as a critical mediator of nutrient homeostasis during both the fasted and refed states (Figure 1).
2.2. FGF21-Mediated Lipid Metabolism on a Ketogenic Diet
High-fat, low-carbohydrate ketogenic diets lead to a unique metabolic state that promotes lipid oxidation and weight loss (23). Similar to fasting, low-carbohydrate ketogenic diets decrease overall carbohydrate levels, thereby promoting lipid oxidation and increased ketogenesis. FGF21 is markedly induced by ketogenic diets in rodents and has been proposed to be a key mediator of hepatic lipid metabolism during ketotic states (6, 7). Knockdown of hepatic Fgf21 using adenovirus-mediated expression of short hairpin RNAs directed against Fgf21 mRNA resulted in significantly elevated hepatic triglyceride and plasma lipid levels, and impaired ketosis (7). Consistent with these data, Fgf21 total knockout mice fed a ketogenic diet developed hepatosteatosis and exhibited impaired ketogenesis and glucose control (6). However, these conclusions have been controversial because other groups have reported that FGF21 is not required for regulating glucose homeostasis and ketosis induced by ketogenic diets (97, 123). Importantly, it is unclear whether there is any translational relevance of FGF21 to the beneficial effects of ketogenic diets in humans because circulating FGF21 levels fail to increase in humans in response to a ketogenic diet (19, 42, 73).
2.3. FGF21 Signals Protein Restriction
Low-protein diets (73, 85), amino acid starvation (25, 121), and the amino acid–depleting drug asparaginase (138) also induce plasma FGF21 levels. Interestingly, protein restriction promotes systemic energy expenditure through this elevation in plasma FGF21 levels (73, 85). In order to maintain macronutrient balance, animals modify their caloric intake to reach a sufficient amount of protein in the body, a process termed protein leverage (118). That is, to meet daily protein requirements, animals consume more of a diet low in protein, which also results in an increase in calorie consumption. However, to maintain energy balance, a corresponding increase in energy expenditure is required to offset this increase in caloric intake needed to meet protein demands (85, 118). Elevation of circulating FGF21 levels in response to low protein levels increases both the browning of adipose tissues and energy expenditure (73), likely to offset this increased caloric intake (Figure 2). FGF21 is absolutely necessary in mice to mediate the effects of low-protein diets (72, 73, 85). Both Fgf21 total knockout (73) and Fgf21 liver–specific knockout mice (85) fail to increase energy expenditure and food intake in response to protein dilution.
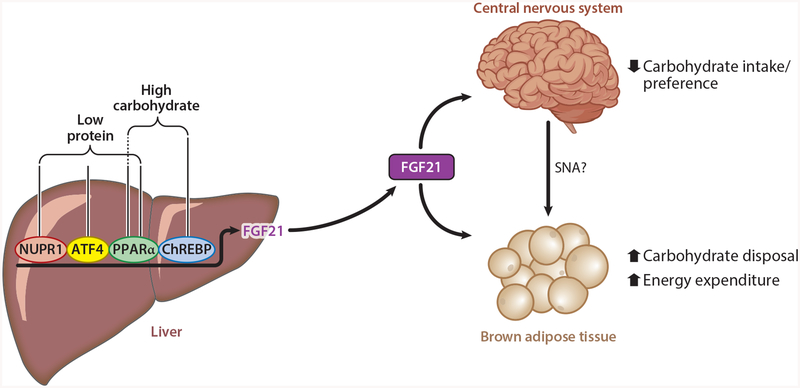
Figure 2.
Nutritional regulation of FGF21 production. The production of circulating levels of FGF21 is regulated by PPARα and ChREBP in response to high carbohydrate levels and by ATF4 and PPARαin response to amino acid starvation in the liver. NUPR1 is also critical for protein dilution–mediated increases in hepatic FGF21. In response to these nutritional cues, circulating FGF21 decreases carbohydrate intake through actions on the central nervous system. In addition, FGF21 enhances carbohydrate disposal by direct action on brown adipose tissue, and increases energy expenditure through central nervous system–mediated regulation of adipose tissue function. Abbreviations: ATF4, activating transcription factor 4; ChREBP, carbohydrate-response element-binding protein; FGF21, fibroblast growth factor 21; NUPR1, nuclear protein 1; PPARα, peroxisome proliferator–activated receptor-α; SNA, sympathetic nerve activity.
FGF21 is markedly induced in response to protein restriction (25, 73, 85) but not caloric restriction (73, 148). Interestingly, the increase in circulating FGF21 levels induced by a ketogenic diet in rodents also appears to be largely due to reduced protein intake rather than to high lipid intake (73). The addition of protein to a ketogenic diet reduces plasma FGF21 levels, although it does not completely return plasma FGF21 levels to baseline (73). Notably in humans, unlike a ketogenic diet, the administration of a low-protein diet for 28 days significantly increases plasma FGF21 levels (73), demonstrating that in humans amino acid starvation is an important regulator of FGF21 production. Low-protein diets reduce essential amino acid levels, leading to the activation of the amino acid sensor general control nonderepressible 2 (GCN2) (136). GCN2 phosphorylates eukaryotic translation initiation factor 2α (eIF2α), which subsequently leads to the activation of activating transcription factor 4 (ATF4) (90). The Fgf21 promoter has multiple ATF4 binding sites, and GCN2-mediated activation of ATF4 leads to the induction of hepatic and circulating levels of FGF21 (24, 68, 72). Interestingly, the induction of FGF21 during protein restriction requires both the GCN2–ATF4 and PPARα signaling pathways (73), consistent with the observation that adding protein to a ketogenic diet does not completely restore plasma FGF21 levels to baseline (73). In addition, recent studies have identified the liver-integrated stress response–driven nuclear protein 1 (NUPR1) as being required for normal FGF21 production and associated metabolic remodeling in response to low protein levels (85). Thus, FGF21 is a key mediator of energy homeostasis under conditions of macronutrient challenge, including protein dilution (Figure 2).
2.4. Regulation of Macronutrient Intake and Sweet Taste Preference by FGF21
The regulation of food intake is complex and integrates both peripheral and central cues (92). While the mechanisms controlling total caloric intake have been explored extensively, little is known regarding the regulation of macronutrient intake. Since there is evidence that the consumption of major macronutrients has a genetic component (107), common genetic variants associated with macronutrient intake have been explored through genome-wide association studies of carbohydrate, fat, and protein intake. Two independent studies revealed that single nucleotide polymorphisms (SNPs) at the FGF21 locus are associated with changes in macronutrient intake (20, 127). Specifically, these studies reported that variants rs838133 and rs838145 were associated with higher carbohydrate intake and that FGF21 was a candidate gene in the region (20, 127). A third report also identified SNPs at the FGF21 locus associating with a preference for sweets (120). Although variants around the FGF21 locus are associated with changes in macronutrient intake, they are not associated with obesity or type 2 diabetes (120). However, while changes in caloric intake can affect body weight and metabolic homeostasis, changes in macronutrient intake alone can affect insulin signaling, cardiometabolic health, and longevity (122). Importantly, an additional study using data from 451,000 individuals in the UK Biobank found that not only is the rs838133 allele associated with higher carbohydrate intake but it is also associated with stronger effects on body fat distribution and higher blood pressure than with effects on body mass (40). Thus, FGF21 may regulate nutrient homeostasis and metabolic health beyond changes in body weight.
Consistent with these data in humans, Fgf21 total knockout mice exhibit an increased preference for simple sugars, but not lipids or protein (133). In addition, this alteration in macronutrient preference did not result in a change in overall caloric intake (133). Ad libitum feeding of sucrose for 6–24 hours gradually increases hepatic and plasma FGF21 levels in wild-type mice, and this effect is lost in liver-specific Fgf21 knockout mice (133). Consistent with total Fgf21 knockout mice, liver-specific Fgf21 knockout mice exhibited a preference for a high-carbohydrate diet (133). Simple sugars also increase plasma FGF21 levels in humans. The ingestion of a single bolus of fructose or glucose induces plasma FGF21 levels in both lean healthy participants and patients with metabolic syndrome. While fructose markedly and acutely increased plasma FGF21 levels in humans, glucose caused a delayed and gradual increase in plasma FGF21 levels (31). The difference in the time course for FGF21 induction by these two sugars is likely due to differences in their uptake. The uptake of absorbed glucose and fructose differs between tissues, with glucose largely escaping first-pass removal by the liver, whereas fructose uptake occurs primarily in the liver (114). Thus, a high fructose load results in higher carbohydrate levels in the liver compared with a high glucose load, which may result in a more potent induction of FGF21 by fructose. A separate study examined plasma FGF21 levels in individuals fed a carbohydrate-rich diet without reductions in protein intake (84). Notably, without affecting protein levels, excess carbohydrate led to an approximately eightfold increase in circulating FGF21 levels, an effect not observed with increased fat intake (84). Finally, an infusion of dextrose used to induce hyperglycemia in healthy individuals also significantly increased circulating plasma FGF21 levels (133). Together, these data reveal that FGF21 is induced in response to excess levels of carbohydrate and that the loss of FGF21 signaling increases carbohydrate preference.
The stimulation of FGF21 production by carbohydrates is mediated by the transcription factor carbohydrate-response element-binding protein (ChREBP). In response to increased carbohydrate intake, particularly fructose, increased hepatocyte carbohydrate concentrations drive substrate into the pentose phosphate pathway. An intermediate in this pathway, xylulose-5-phosphate, activates ChREBP (1) and promotes its binding to the carbohydrate response element sites in the promoters of target genes, including FGF21 (53, 130). Mice that have been fasted and refed a high-carbohydrate diet have markedly elevated hepatic and plasma FGF21 levels (112), which can be recapitulated by treating hepatocytes with simple sugars (53, 130, 133). Mice lacking ChREBP fail to show an increase in plasma FGF21 levels in response to simple sugar intake (35, 133). Recent work also shows that the loss of PPARα disrupts ChREBP-mediated induction of FGF21 and the regulation of sucrose preference (55). This dependence on PPARα for the full induction of FGF21 in response to carbohydrate is reminiscent of the mechanism for the protein restriction–mediated induction of FGF21 (Figure 2).
Based on the FGF21 loss-of-function data, which showed an increase in carbohydrate preference (133), the administration of FGF21 would be predicted to suppress carbohydrate preference. Indeed, the administration of recombinant FGF21 to mice markedly reduces carbohydrate preference without affecting the preferences for lipid and protein (133). Moreover, the overexpression of FGF21 decreases sucrose preference (125, 133) but not the preference for lipid and protein (133). Interestingly, FGF21 also suppresses the intake of noncaloric sweeteners (125, 133). In fact, FGF21 has a more pronounced effect on reducing the intake of noncaloric sweeteners, such as sucralose, than it does on sucrose intake (133). Due to its energetic value and pre- and postabsorptive mechanisms that drive sucrose intake, these data suggest that FGF21 suppresses some, but not all, of the rewarding effects of sucrose. FGF21 mediates these effects on simple sugar and sweet taste preference not by affecting sweet taste sensing, but rather by affecting taste processing. Chorda tympani nerve recordings in wild-type mice administered FGF21 were not different from those of vehicle-treated mice in response to various tastants, including simple sugars and noncaloric sweeteners (133). Instead, intracerebroventricular administration of FGF21 was sufficient to suppress sucrose preference (133), and the loss of the obligate FGF21 coreceptor β-klotho in the brain abolished the ability of FGF21 to suppress sweet taste preference (125). Importantly, loss of β-klotho in the PVN of the hypothalamus, but not the suprachiasmatic nucleus, impaired FGF21-mediated suppression of sucrose intake (133). Together, these data identified a novel liver-to-brain negative feedback loop mediated by FGF21 to regulate carbohydrate homeostasis and sweet taste preference, possibly as a mechanism to protect the liver from carbohydrate overload (Figure 2).
FGF21 also regulates sweet taste preference in primates and humans. When an FGF21 analog, PF-05231023, was administered to monkeys, they rapidly and significantly reduced their saccharin intake (125). To explore the relevance of these observations to humans, variants in the FGF21 locus were examined in participants from the Danish Inter99 cohort to study the relationship of these genotypes to a detailed range of ingestive behaviors. As was observed with increased carbohydrate intake (20, 127), statistically significant associations were identified between the FGF21 rs838133 allele and increased candy consumption (120).
Multiple conditions, diets, and nutritional states affect circulating levels of FGF21. To examine the nutritional context for the regulation of FGF21 production, the Geometric Framework for Nutrition was utilized to assess the influence of macronutrient and energy intakes on hepatic and plasma FGF21 levels (121). The framework was composed of 25 diets varying in macronutrient (carbohydrate, protein, and lipid) and energy density. This study found that FGF21 was elevated robustly by low protein and that this induction was maximized when low-protein intake was coupled with high-carbohydrate intake (121). While FGF21 may be induced by excess carbohydrate to suppress simple sugar intake and prevent liver injury, it may be maximally induced when carbohydrate consumption progresses to a point of low protein availability. Thus, this FGF21 signaling pathway may be utilized to prevent liver damage as well as to promote the protein leverage effect.
2.5. Alcohol, FGF21, and Liver Function
Similar to excess fructose consumption, binge ethanol intake can be a source of liver damage that may progress to fatty liver disease. Acute binge consumption of ethanol significantly increases plasma FGF21 levels in both rodents and humans (26), and FGF21 protects against alcohol-induced liver injury. Mice that lack FGF21 develop marked liver steatosis and injury in response to chronic alcohol consumption despite being able to clear ethanol as effectively as wild-type controls (26). Fgf21 knockout mice exhibit increased hepatic lipogenesis and decreased β-oxidation following alcohol exposure (83). In addition, FGF21 regulates alcohol-induced adipose tissue lipolysis (149). Importantly, pharmacological administration of FGF21 for 5 days to wild-type mice that were chronically fed alcohol significantly attenuated hepatic steatosis and liver injury (83).
FGF21 also regulates alcohol intake. The FGF21 rs838133 variant associates with increased alcohol intake (40), and the rs11940694 SNP in the β-Klotho (KLB) locus associates with alcohol consumption (116). Interestingly, administering recombinant FGF21 (116) or the overexpression of FGF21 (125) significantly reduced alcohol preference but not plasma ethanol concentrations. This effect of FGF21 is lost is mice lacking β-klotho in the central nervous system (CNS; Klbfl/fl;CamK2a-Cre) (116). FGF21 may mediate these effects by altering dopamine signaling (125), but additional studies are necessary to determine the potential mechanism for these effects. Finally, as would be predicted from the human SNP data, Klbfl/fl;CamK2a-Cre mice consume more alcohol and prefer alcohol more than wild-type control mice (116).
While FGF21 decreases alcohol intake, it should be noted that the effect on alcohol preference is impacted by the fact that FGF21 administration also increases total fluid intake through increased water intake in the presence of alcohol (125). This effect of FGF21 to increase total fluid intake, and thus preference, was not observed in sucrose preference studies in lean, wild-type mice administered FGF21 for 1–3 days (133; M.J. Potthoff, unpublished data). An increase in water intake in response to FGF21, however, was observed in lean and diet-induced obese wild-type mice administered FGF21 by minipump for 1 week (14). This increase in fluid intake in response to FGF21 may be due to increased energy expenditure. In rodents, pharmacological levels of FGF21 increase energy expenditure (64, 141), which results in increased food intake (54, 64). Rodents and humans tightly control drinking behavior during feeding to ensure an adequate water supply for food ingestion and digestion (151). This regulation of drinking by eating is termed prandial thirst (151). Increases in total fluid intake in response to FGF21 may be secondary to increased energy expenditure as opposed to a direct regulation of thirst. Alternatively, FGF21-induced thermogenesis may affect visceral sensory information and that could influence thirst (91). A possible explanation for the FGF21-stimulated induction of total fluid intake during alcohol consumption, but not sucrose consumption, may be that sucrose is rewarding and itself increases total fluid intake compared with water alone. In contrast, alcohol consumption can lead to dehydration. Thus, FGF21’s effect on fluid intake needs to be examined in a model in which FGF21-mediated increases in energy expenditure are abrogated.
2.6. FGF21 and Adaptive Thermogenesis
One of the earliest physiological functions attributed to FGF21 was its ability to regulate adipose tissue thermogenesis. Since FGF21 administration increases energy expenditure and the browning of adipose tissues in vivo (21, 64, 141), it was hypothesized that FGF21 regulates adaptive thermogenesis in response to cold. Fgf21 mRNA expression is upregulated in both WAT (36, 52) and BAT (15, 36, 49, 52) in response to cold. However, while an increase in circulating levels of FGF21 has been reported in response to cold in mice (49), this has not been observed in other studies (36, 52). In brown adipocytes, norepinephrine activates β-adrenergic receptors and cyclic adenosine monophosphate (cAMP)-dependent, protein kinase A (PKA)- and p38 mitogen-activated protein kinase (MAPK)-mediated mechanisms to regulate ATF2 binding to the Fgf21 promoter to induce FGF21 production (Figure 3a) (49). Similar mechanisms may upregulate FGF21 expression in white adipocytes during cold, but FGF21 expression is also regulated by both PPARγ (32, 49, 94) and PPARα (49) in white adipocytes. Consistent with its role in regulating thermogenesis, FGF21 treatment is sufficient to increase thermogenic gene expression in mouse primary white adipocytes (36). In addition, treating human primary neck adipocytes with FGF21 increases both thermogenic gene expression and cellular respiration (76). This has led to the hypothesis that adipose FGF21 may act in an autocrine manner to regulate adipose thermogenesis (Figure 3a).
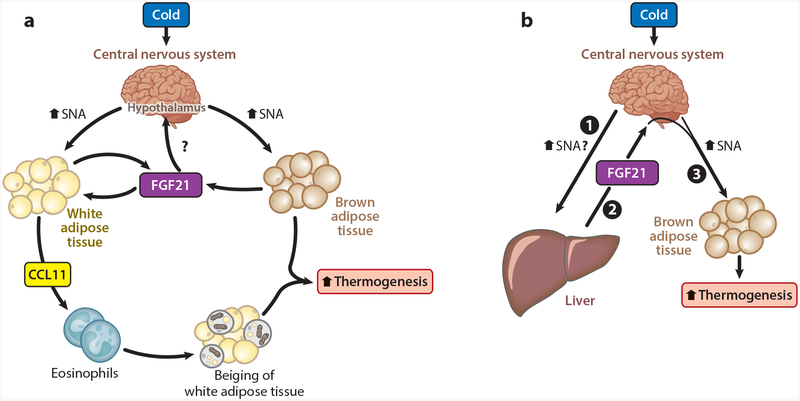
Figure 3.
Fibroblast growth factor 21 (FGF21) coordinates adipose tissue thermogenesis during acute cold. Two models are shown for the physiological regulation of energy homeostasis by FGF21 during acute cold exposure. (a) Exposure to cold stimulates sympathetic nerve activity (SNA) to adipose tissues. Increased SNA to brown adipose tissue increases thermogenesis and the production of FGF21, which enhances thermogenesis. Increased SNA to white adipose tissue stimulates FGF21 to act in an autocrine fashion on adipose tissue to induce the production of the cytokine CCL11. CCL11 recruits eosinophils and macrophages to promote the beiging of subcutaneous white adipose tissue and increase adipose tissue thermogenesis. (b) During acute cold exposure, FGF21 production from the liver is increased through an unknown mechanism, but it may involve central nervous system (CNS)–mediated sympathetic output to the liver (❶). Elevated circulating levels of FGF21 (❷) then act centrally to increase sympathetic nerve output to drive brown adipose tissue thermogenesis (❸).
Consistent with a functional role for FGF21 during cold, both Fgf21 total knockout mice (36) and adipose-specific Fgf21 knockout mice (52) exhibit reduced body temperature in response to acute cold exposure. During chronic cold exposure, however, FGF21 is dispensable for adaptive thermogenesis (60). During acute cold, FGF21 signals directly to adipose tissue to regulate adipose thermogenesis by inducing the expression and secretion of the chemokine CCL11 (52). CCL11 then functions to recruit eosinophils to subcutaneous adipose tissues, which promotes the beiging of WAT depots (52). These studies provide a novel pathway linking sympathetic nervous system activation to the activation of type 2 immunity and subcutaneous WAT thermogenesis (Figure 3a). In addition, these studies illustrate another role for FGF21 in maintaining energy homeostasis.
In contrast to the studies supporting a role for adipose-derived FGF21 in regulating thermogenesis, recent work from our lab has found that liver-derived, but not adipose-derived, FGF21 regulates core body temperature during cold exposure. Liver-specific, but not adipose-specific, Fgf21 knockout mice are susceptible to acute cold exposure (lasting 1–3 days) (M. Ameka, K.M. Markan & M.J. Potthoff, unpublished data). Plasma FGF21 levels are induced acutely from the liver in response to cold exposure and liver-derived FGF21 signals to the CNS to increase sympathetic nerve activity to BAT and regulate core body temperature (Figure 3b) (M. Ameka, K.M. Markan & M.J. Potthoff, unpublished data). Therefore, our data suggest that liver-derived FGF21 regulates energy homeostasis during acute cold exposure by signaling to the CNS to, at least in part, regulate sympathetic nerve activity to BAT. Additional studies are needed to evaluate the relative importance of adipose versus liver-derived FGF21 in regulating energy homeostasis.
2.7. Induction of FGF21 by Cellular and Mitochondrial Stress
Cells are naturally exposed to a wide range of environmental stressors and thus have developed responses to specific stimuli to adapt to these situations. These hazardous stimuli can lead to conditions of mitochondrial stress, autophagy, endoplasmic reticulum stress, or activation of the unfolded protein response. Although FGF21 is induced under conditions of metabolic stress (e.g., fasting, cold), it is also induced under many conditions of cellular stress, leading FGF21 to be referred to in some contexts as a stress hormone (69). For example, FGF21 production from skeletal muscle (56, 61, 103, 128), BAT (59), and cardiac tissue (143) is increased in genetic mouse models of cellular or mitochondrial stress. However, while FGF21 can be induced in extrahepatic tissues in these genetic mouse models, the physiological relevance of this induction is unclear. For example, while chronic feeding of a high-fat diet clearly represents a state of cellular and mitochondrial stress for skeletal muscle, skeletal muscle does not contribute to circulating levels of FGF21 during feeding of a high-fat diet; instead, circulating levels of FGF21 are derived exclusively from the liver under these conditions (86). In addition, in humans in response to exercise, FGF21 is produced from the liver not muscle (46). Thus, it is unclear whether there is a physiological context for the extrahepatic production of FGF21 or whether this is simply a by-product arising from artificial genetic stress. Nevertheless, these studies demonstrate that extrahepatic tissues have the capacity to contribute to circulating levels of FGF21 and that the activation of pathways to induce FGF21 production from muscle may be a beneficial therapeutic strategy.
3. THE PHARMACOLOGICAL EFFECTS OF FGF21
When administered to diabetic and obese animal models, FGF21 provides multiple metabolic benefits and has been shown to decrease blood glucose levels and body weight, improve insulin and leptin sensitivity, improve plasma lipid profiles, and decrease hepatic steatosis (21, 64, 141, 142). Importantly, in animal models FGF21 treatment not only prevents (64) but also reverses diabetes and obesity (21, 141). Pharmacological administration of FGF21 has both acute and chronic effects on metabolism. A single injection of FGF21 to diabetic and obese animal models is sufficient to decrease plasma glucose levels by more than 50% (142). This effect of FGF21 can be recapitulated in wild-type lean mice through coinjection with insulin (11, 27), suggesting that FGF21 functions to directly enhance insulin sensitivity. In contrast to the acute metabolic effects, repeated daily administration of FGF21 increases metabolic rate, promotes weight loss, and thus secondarily improves insulin sensitivity and glucose homeostasis (141). It is clear that FGF21 treatment elicits significant metabolic changes in many tissues, including the liver and adipose tissues (21, 141). However, whether these changes result from the direct actions of FGF21 on these particular tissues has been greatly debated (63). Thus, significant efforts have been made to identify the primary cellular targets and downstream pathways responsible for the metabolic actions of FGF21. While this topic has been contentious in the past, studies within the past couple of years from multiple groups have revealed the direct target tissues that regulate these specific actions of FGF21. In the following sections, we review the cellular targets of FGF21 action and the mechanisms regulating FGF21 activity.
3.1. FGF21 Signals to Adipose Tissues to Acutely Increase Insulin Sensitivity
The acute pharmacological administration of FGF21 increases insulin sensitivity and lowers plasma glucose levels, primarily by stimulating peripheral glucose disposal, while also slightly reducing hepatic glucose production (27, 142). Multiple lines of evidence implicate adipose tissues as important regulators of these acute effects of FGF21 (reviewed in 88). Initial studies exploring the significance of direct FGF21 signaling to adipose tissues used ap2-Cre transgenic mice crossed with mice with a conditional Klb (27) or Fgfr1 allele (4). Both Fgfr1fl/fl;ap2-Cre and Klbfl/fl;ap2-Cre mice were refractory to the acute insulin-sensitizing effects of FGF21 (4, 27). However, these studies were confounded by the fact that ap2-Cre transgenic mice also express Cre recombinase in nonadipose tissues, including in the CNS (47). Thus, to explore the significance of FGF21 signaling directly to adipose tissues, investigators crossed mice with a conditional Klb allele with Adiponectin-Cre transgenic mice (11), which drive Cre expression specifically in adipose tissues (75). Consistent with the earlier studies, while FGF21 significantly increased insulin sensitivity in both lean and diet-induced obese wild-type mice, this effect was completely lost in Klbfl/fl;Adiponectin-Cre mice (11). These findings were subsequently confirmed in a separate study using hyperinsulinemic–euglycemic clamps (74). Thus, FGF21 signals directly to adipose tissue to acutely increase insulin sensitivity (Figure 4).
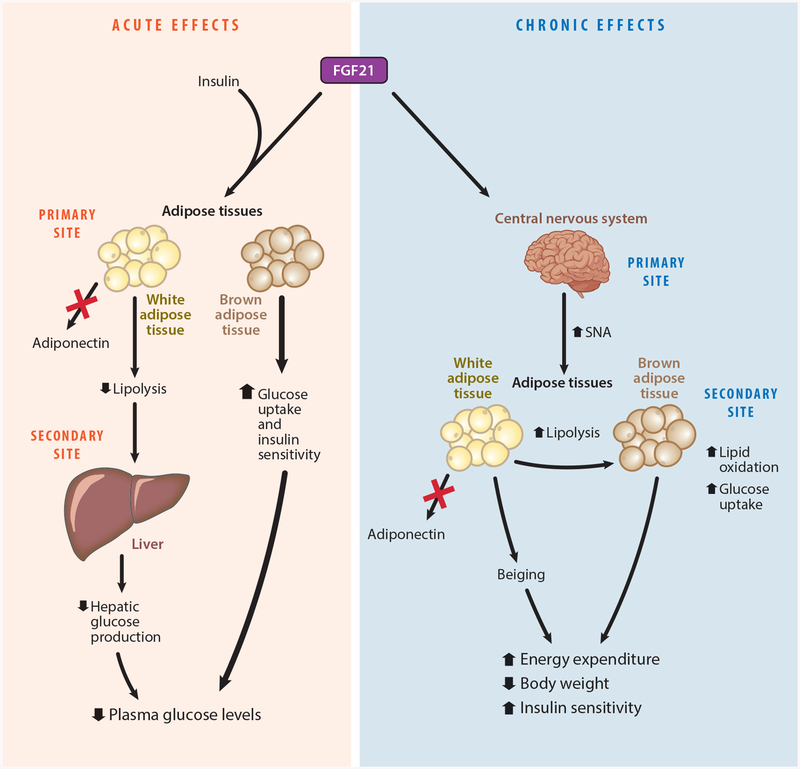
Figure 4.
Dissociation of the pharmacological actions of fibroblast growth factor 21 (FGF21). FGF21 has acute and chronic effects on metabolism. Primary, or direct targets, and secondary sites of FGF21 action are indicated. The acute insulin-sensitizing effects are mediated through direct signaling to adipose tissue in the presence of insulin signaling, but do not require adiponectin (red X). FGF21 signaling to brown adipose tissue is primarily responsible for the acute glucose-lowering effect of FGF21. The chronic effects of FGF21 are mediated through direct signaling in the central nervous system (CNS). The activation of specific FGFR1 and β-klotho neurons by FGF21 leads to increased energy expenditure through increased sympathetic nerve activity (SNA) to brown adipose tissue and the beiging of white adipose tissue. These effects result in decreased body weight and a secondary improvement in insulin sensitivity. figure adapted from Reference 11.
There are multiple types of adipocytes in the body that differ in function and arise from distinct lineages. Whereas white adipocytes function to store energy, brown and beige adipocytes expend energy through heat dissipation via activation of the mitochondrial uncoupling protein 1 (UCP1) (108). Multiple studies have demonstrated that FGF21 stimulates glucose uptake in BAT in vivo (11, 27, 86, 115). In addition, FGF21 enhances insulin sensitivity in primary brown adipocytes (86) but not in primary white adipocytes (64, 86). Thus, to determine the relative contribution of direct FGF21 signaling to white versus brown adipocytes, investigators crossed mice with a conditional Klb allele with UCP1-Cre transgenic mice to ablate FGF21 signaling to brown and beige adipocytes. Notably, as observed with Klbfl/fl;Adiponectin-Cre mice, mice lacking β-klotho in UCP1+ adipocytes (Klbfl/fl;UCP1-Cre) failed to show enhanced insulin sensitivity in response to acute FGF21 administration (11). Positron emission tomography and computed tomography imaging revealed that acute administration of FGF21 with insulin markedly induced glucose uptake in interscapular BAT compared to insulin alone, and this effect is impaired in mice lacking β-klotho in BAT (11). Together, these data demonstrate that FGF21 signals directly to brown adipocytes to acutely enhance insulin sensitivity (Figure 4).
The exact mechanism for the insulin-sensitizing effects of FGF21 has not been elucidated. Two studies reported that the adipokine adiponectin is induced in response to acute FGF21 administration and that adiponectin is essential for the insulin-sensitizing effects of FGF21 (48, 82). However, more recent studies have challenged these findings by failing to observe increases in plasma adiponectin levels in response to acute FGF21 administration (11, 95). Moreover, in contrast to previous studies, both FGF21 (11) and a bispecific activating antibody for the FGFR1–β-klotho receptor complex (70) were found to efficiently retain their acute insulin-sensitizing effects in adiponectin knockout mice (Figure 4). Instead, insulin sensitization specifically in adipose tissues is required for the acute insulin-sensitizing effects of FGF21 (11). Mice lacking the insulin receptor specifically in adipose tissues (IRfl/fl;Adiponectin-Cre) do not respond to the acute insulin-sensitizing effects of FGF21 (11). Increased insulin sensitization in adipose tissues results in increased glucose uptake in BAT (11) and attenuation of lipolysis in WAT (5). Thus, FGF21 increases insulin sensitivity in adipose tissues to lower plasma glucose levels.
3.2. FGF21 and the Central Nervous System
One of the main areas of controversy regarding FGF21 biology is the site of action responsible for the beneficial metabolic effects of chronic FGF21 administration on body weight and glucose homeostasis. Multiple studies have implicated adipose tissues, liver, and the CNS as direct targets of FGF21 action (reviewed in 88). The following studies have addressed this controversy and have identified the target tissue mediating the beneficial metabolic effects of FGF21. Last year, it was demonstrated that the acute insulin-sensitizing effects of FGF21 can be dissociated from the chronic metabolic effects of FGF21 (Figure 4). Although FGF21 signals directly to adipose tissues to acutely enhance insulin sensitivity, the chronic effects of FGF21 on body weight and improved insulin sensitivity are retained in adipose-specific Klb knockout mice (Klbfl/fl;Adiponectin-Cre) (11, 74), demonstrating that direct signaling to adipose tissues is not required for the chronic effects of FGF21 (Figure 4). In addition, the potent effects of FGF21 on lipid metabolism (115) are also present in adipose-specific Klb knockout mice (11). Consistent with these results, adipose-specific Fgfr1 knockout mice (Fgfr1fl/fl;Adiponectin-Cre) were also recently shown to respond to chronic FGF21 administration similarly to control mice (17) in contrast to the results obtained with Fgfr1fl/fl;ap2-Cre mice (4). In addition to the loss-of-function studies, independent groups also found that the activation of FGFR1-dependent signaling specifically in adipose tissues is not sufficient to induce the beneficial metabolic effects of FGF21 in vivo (11, 17). Neither the inducible expression of a constitutively active FGFR1 protein specifically in adipose tissue (11) nor antibody-mediated activation of a chimeric human FGFR1 receptor in brown adipocytes (17) is sufficient to increase energy expenditure or affect body weight in vivo.
FGF21 crosses the blood–brain barrier and acts centrally to regulate multiple processes (50). It is now clear that the chronic metabolic effects of FGF21 and FGFR1 agonists are mediated through actions in the CNS. The chronic metabolic effects of FGF21 on body weight and glucose homeostasis are lost when β-klotho is deleted from the CNS (Klbfl/fl;Camk2a-Cre) (101, 126). In contrast, the loss of β-klotho in hepatocytes (74) or adipocytes (11, 74) does not impair FGF21-mediated effects on body weight. Consistent with recombinant FGF21 administration, the administration of a bispecific FGFR1–β-klotho agonist to diet-induced obese wild-type mice promotes weight loss and improves glucose homeostasis, effects that are lost when β-klotho is removed from the CNS (74). FGF21 administration increases sympathetic nerve activity to BAT (101) and WAT (28), and browning of WAT depots (28, 101). Interestingly, FGF21-mediated increases in sympathetic nerve activity to BAT appear to require hypothalamic corticotropin-releasing hormone. The administration of a corticotropin-releasing hormone antagonist blocks FGF21-mediated increases in sympathetic nerve activity to BAT (101). Together, these data demonstrate that the chronic metabolic effects of FGF21 are mediated through effects in the CNS (Figure 4).
Although direct FGF21 signaling to adipose tissues is not required for its chronic effects on metabolism, adipose tissue is still important for mediating the metabolic effects of FGF21 (Figure 4). Lipodystrophic mice, which lack mature adipocytes, are refractory to the metabolic effects of FGF21 (131) and FGFR1-based agonists (38). It is unclear whether this is due to the loss of adipose tissue metabolism or to the loss of an adipose-derived factor. Extended FGF21 administration increases energy expenditure, promotes lipid and glucose uptake in BAT, and increases adipose tissue metabolism (88). However, while adipose tissues are critical for these metabolic effects, UCP1 is dispensable for FGF21-mediated increases in energy expenditure (17, 85, 111, 132). Studies within the past few years have demonstrated that adipose tissue depots possess alternative futile cycles for increasing energy expenditure independent of UCP1 (10, 58). Thus, compensatory adipose tissue thermogenic pathways may be utilized in the absence of UCP1 to maintain energy homeostasis. In addition to centrally activated adipose-tissue thermogenesis, adipose tissues may contribute to FGF21-mediated increases in energy expenditure through the production of adipokines such as leptin. While lipodystrophic mice do not respond to FGF21 administration, the transplantation of WAT from wild-type mice increases leptin levels and restores FGF21 responsiveness (131). In addition, disrupting leptin signaling using ob/ob or db/db mice abrogates the beneficial effects of FGF21 in lowering body weight in response to extended FGF21 administration (3, 45, 51, 67, 139). Finally, FGF21 administration enhances leptin-mediated decreases in body weight (96, 131). Thus, future studies are needed to elucidate the mechanism of the CNS–adipose circuit (or circuits) targeted by FGF21 that regulates energy homeostasis.
3.3. Mechanisms Regulating FGF21 Activity
Numerous studies have explored the mechanisms regulating FGF21 induction, but much less is known regarding the mechanisms regulating FGF21 activity. Circulating levels of FGF21 are significantly elevated in humans during pathophysiological states, including type 2 diabetes (16, 18), obesity (22, 30, 93), hepatosteatosis (30, 79, 146), pancreatitis (117, 137), and mitochondrial dysfunction (78). These elevations in circulating FGF21 levels are paradoxical, given the potent pharmacological actions of FGF21. However, although levels of FGF21 are increased during these different states, the form of FGF21 in circulation may actually be nonfunctional due to proteolytic processing. Alternatively, FGF21 signaling may be impaired by some pathological stimulus that results in FGF21 resistance, similar to insulin or leptin resistance. Indeed, obesity has been proposed to be a FGF21-resistant state (34). However, the concept of FGF21 resistance is controversial, with another group failing to observe FGF21 resistance in mouse models of obesity and insulin resistance (45). A proposed mechanism for FGF21 resistance is the marked downregulation of β-klotho protein expression observed in WAT during obesity. Although one study found that overexpression of β-klotho in adipose tissues provided protection against diet-induced weight gain via increased energy expenditure (109), another study found that maintaining physiological β-klotho expression levels in adipose tissues during diet-induced obesity failed to prevent weight gain, glucose intolerance, or insulin resistance (87). Since adipose tissues are not the target tissue responsible for chronic FGF21-mediated effects on body weight and glucose homeostasis (11, 17, 74), it seems likely that changes in adipose tissue β-klotho levels would not affect systemic FGF21 sensitivity. Additional studies are needed to examine the existence and etiology of FGF21 resistance.
In addition to regulating FGF21 sensitivity, FGF21 activity could also be regulated by altering its stability or half-life. The half-life of FGF21 is approximately 0.4–2 hours, depending on the species and method of delivery (51, 65, 142). Circulating human FGF21 is composed of 181 amino acids and is proteolytically cleaved, and inactivated, between Pro-171 and Ser-172 by the endopeptidase fibroblast activation protein (FAP) (29, 150). The deletion of these 10 residues on the C terminus of human FGF21 disrupts its ability to interact with β-klotho and signal to target tissues (89, 144). Interestingly, mouse FGF21 is neither cleaved nor inactivated by FAP because mouse FGF21 has a glutamate residue at the corresponding glycine residue at position 170, which disrupts the stringent glycine–proline sequence required for FAP-mediated cleavage (29, 150). In humans, however, it is unclear what percentage of circulating FGF21 is active during disease states (e.g., obesity, diabetes). Since FGF21 is cleaved and inactivated by FAP, the administration of FAP inhibitors may represent a therapeutic strategy to potentiate FGF21 function in vivo by prolonging the endogenous half-life (43, 113).
FGF21 levels can also be regulated by adipose-derived exosomes. Although circulating levels of FGF21 are derived from the liver, adipose-derived circulating microRNAs are packaged into exosomes and regulate plasma FGF21 levels (129). Since FGF21 acts directly and indirectly on adipocytes, this may be a potential feedback mechanism for regulating FGF21 signaling in vivo.
4. TRANSLATING THE METABOLIC EFFECTS OF FGF21
Multiple FGF21 analogs have been developed or are in development for treating metabolic disease (44). Clinical trials using FGF21 analogs have demonstrated the therapeutic potential of this pathway in treating obesity and diabetes. A study from Eli Lilly and Company was the first to report the effects of an FGF21 analog, LY2405319, in obese subjects with type 2 diabetes. Administering this analog for 28 days resulted in decreased body weight, improved lipid profiles, lowered fasting insulin levels, and a trend toward reduced plasma glucose levels (41). A subsequent clinical trial, performed by Pfizer Inc., used the FGF21 analog PF-05231023 in subjects with type 2 diabetes (126). PF-05231023 significantly decreased body weight and markedly reduced plasma cholesterol and lipid levels. However, unlike the previous study, PF-05231023 had no significant effect on plasma insulin or glucose levels (126). Notably, this FGF21 analog also significantly reduced body weight and metabolic profiles in nonhuman primates, but did so by reducing their food intake rather than by increasing the browning of subcutaneous WAT (126). Thus, while the effects of FGF21 may translate to humans, the mechanism for the effects may be species specific (104). Since humans possess relatively less BAT than rodents, adipose tissue thermogenesis may contribute less to overall energy homeostasis in humans. Instead, FGF21 may activate a central circuit that regulates energy homeostasis in both species, manifesting functionally as a change in food intake in humans and nonhuman primates, but as an increase in energy expenditure in rodents.
While FGF21-based therapies have beneficial effects, they may also cause adverse effects. One controversial concern is whether FGF21 affects bone loss. In mice, FGF21 has been reported to promote bone loss (134, 135); however, this finding could not be replicated (80). When PF-05231023 was administered to obese cynomolgus monkeys, it decreased body weight and mean abdominal fat content but did not affect bone mineral content (126). In humans, PF-05231023 treatment resulted in changes in markers of bone turnover (126). However, it is unclear whether this is a direct effect of FGF21 or an indirect effect on bone turnover that is known to occur with weight loss. In the same study, PF-05231023 did not affect systolic or diastolic blood pressure, pulse rate, or electrocardiogram readings. Instead, the most frequent adverse effect was diarrhea (126). A subsequent clinical study was conducted with PF-05231023 in obese participants with hypertriglyceridemia who were taking atorvastatin, with or without type 2 diabetes (66). Similar to the previous study (126), once-weekly administration of PF-05231023 markedly improved plasma lipid profiles without significantly affecting fasting plasma glucose levels (66). However, in contrast to the previous study, no significant changes in body weight were observed (66). In addition, the latter study found that PF-05231023 caused multiple adverse effects. The majority of adverse effects were gastrointestinal, but unlike the previous study, systolic and diastolic blood pressure, and pulse rate, increased in a dose-dependent manner (66). Interestingly, similar effects on blood pressure were observed in rats but not in monkeys (66).
FGF21 has also been shown to affect female reproduction (100, 119). Female mice that over-express FGF21 are infertile, but whether this is a direct effect of FGF21 on fertility or rather a secondary consequence of FGF21-induced energy expenditure and relative caloric insufficiency has been disputed. One study found that FGF21 signals to the brain to suppress vasopressin–kisspeptin signaling, which impairs the proestrus surge in luteinizing hormone (100). However, a separate study suggests that this phenotype is due to caloric insufficiency from chronic energy expenditure (119). While Fgf21 transgenic mice are infertile on a chow diet, this infertility is reversed by high-fat diet feeding (119). In addition, the direct infusion of FGF21 into the brain at concentrations sufficient to acutely increase energy expenditure failed to alter the estrus cycle (119), suggesting that FGF21 may not function acutely to regulate female fertility. Future studies are necessary to determine whether FGF21-based therapies affect female fertility.
5. CONCLUSIONS
After more than a decade of research, tremendous progress has been made in identifying the physiological actions of FGF21 and assessing its therapeutic potential to treat metabolic disease. Going forward, additional work is necessary to determine the mechanisms for both the beneficial and adverse effects of FGF21. Since FGF21 increases sympathetic nerve activity (28, 101) and affects blood pressure (66), it is unclear whether FGF21 will suffer the same fate as previous sympathomimetics that promoted weight loss at the cost of cardiovascular complications (57). As for the gastrointestinal issues, extended exposure to elevated levels of FGF21 has been shown to increase bile acid biosynthesis by opposing the actions of another endocrine FGF, FGF15/19 (147). Additional studies are needed to determine whether bile acid–induced diarrhea (13) is the cause of the gastrointestinal issues in humans associated with the administration of FGF21 analogs. Finally, it is now evident that the metabolic effects of FGF21 that lower body weight and improve carbohydrate and lipid homeostasis are mediated through actions on the CNS. In addition, FGF21 regulates macronutrient preference, sweet taste preference, and alcohol intake through central mechanisms. Therefore, the future of FGF21 lies in unraveling the complex neural networks mediating the effects of this therapeutically promising endocrine factor.
ACKNOWLEDGMENTS
We would like to thank members of the Potthoff lab for valuable comments and Teresa Ruggle for assistance with graphic design. This work was supported by the National Institutes of Health (grants R01DK106104 to M.J.P. and T32 GM067795 to L.D.B.) and the University of Iowa Carver College of Medicine (funds to M.J.P.). The authors apologize to colleagues whose relevant work was not cited due to limits on the number of references.
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Abdul-Wahed A, Guilmeau S, Postic C. 2017. Sweet sixteenth for ChREBP: established roles and future goals. Cell Metab. 26:324–41 [DOI] [PubMed] [Google Scholar]
- 2.Adams AC, Cheng CC, Coskun T, Kharitonenkov A. 2012. FGF21 requires βklotho to act in vivo. PLOS ONE 7:e49977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams AC, Coskun T, Rovira AR, Schneider MA, Raches DW, et al. 2012. Fundamentals of FGF19 & FGF21 action in vitro and in vivo. PLOS ONE 7:e38438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams AC, Yang C, Coskun T, Cheng CC, Gimeno RE, et al. 2012. The breadth of FGF21’s metabolic actions are governed by FGFR1 in adipose tissue. Mol. Metab 2:31–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arner P, Pettersson A, Mitchell PJ, Dunbar JD, Kharitonenkov A, Ryden M. 2008. FGF21 attenuates lipolysis in human adipocytes—a possible link to improved insulin sensitivity. FEBS Lett. 582:1725–30 [DOI] [PubMed] [Google Scholar]
- 6.Badman MK, Koester A, Flier JS, Kharitonenkov A, Maratos-Flier E. 2009. Fibroblast growth factor 21–deficient mice demonstrate impaired adaptation to ketosis. Endocrinology 150:4931–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. 2007. Hepatic fibroblast growth factor 21 is regulated by PPARα and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 5:426–37 [DOI] [PubMed] [Google Scholar]
- 8.Beenken A, Mohammadi M. 2009. The FGF family: biology, pathophysiology and therapy. Nat. Rev. Drug Discov 8:235–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beenken A, Mohammadi M. 2012. The structural biology of the FGF19 subfamily. Adv. Exp. Med. Biol 728:1–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bertholet AM, Kazak L, Chouchani ET, Bogaczynska MG, Paranjpe I, et al. 2017. Mitochondrial patch clamp of beige adipocytes reveals UCP1-positive and UCP1-negative cells both exhibiting futile creatine cycling. Cell Metab. 25:811–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.BonDurant LD, Ameka M, Naber MC, Markan KR, Idiga SO, et al. 2017. FGF21 regulates metabolism through adipose-dependent and -independent mechanisms. Cell Metab. 25:935–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bookout AL, de Groot MH, Owen BM, Lee S, Gautron L, et al. 2013. FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nat. Med 19:1147–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Camilleri M 2015. Bile acid diarrhea: prevalence, pathogenesis, and therapy. Gut Liver 9:332–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Camporez JP, Jornayvaz FR, Petersen MC, Pesta D, Guigni BA, et al. 2013. Cellular mechanisms by which FGF21 improves insulin sensitivity in male mice. Endocrinology 154:3099–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chartoumpekis DV, Habeos IG, Ziros PG, Psyrogiannis AI, Kyriazopoulou VE, Papavassiliou AG. 2011. Brown adipose tissue responds to cold and adrenergic stimulation by induction of FGF21. Mol. Med 17:736–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chavez AO, Molina-Carrion M, Abdul-Ghani MA, Folli F, Defronzo RA, Tripathy D. 2009. Circulating fibroblast growth factor-21 is elevated in impaired glucose tolerance and type 2 diabetes and correlates with muscle and hepatic insulin resistance. Diabetes Care 32:1542–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen MZ, Chang JC, Zavala-Solorio J, Kates L, Thai M, et al. 2017. FGF21 mimetic antibody stimulates UCP1-independent brown fat thermogenesis via FGFR1/βKlotho complex in non-adipocytes. Mol. Metab 6:1454–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen WW, Li L, Yang GY, Li K, Qi XY, et al. 2008. Circulating FGF-21 levels in normal subjects and in newly diagnosed patients with type 2 diabetes mellitus. Exp. Clin. Endocrinol. Diabetes 116:65–68 [DOI] [PubMed] [Google Scholar]
- 19.Christodoulides C, Dyson P, Sprecher D, Tsintzas K, Karpe F. 2009. Circulating fibroblast growth factor 21 is induced by peroxisome proliferator–activated receptor agonists but not ketosis in man. J. Clin. Endocrinol. Metab 94:3594–601 [DOI] [PubMed] [Google Scholar]
- 20.Chu AY, Workalemahu T, Paynter NP, Rose LM, Giulianini F, et al. 2013. Novel locus including FGF21 is associated with dietary macronutrient intake. Hum. Mol. Genet 22:1895–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, et al. 2008. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology 149:6018–27 [DOI] [PubMed] [Google Scholar]
- 22.Cuevas-Ramos D, Almeda-Valdes P, Gomez-Perez FJ, Meza-Arana CE, Cruz-Bautista I, et al. 2010. Daily physical activity, fasting glucose, uric acid, and body mass index are independent factors associated with serum fibroblast growth factor 21 levels. Eur. J. Endocrinol 163:469–77 [DOI] [PubMed] [Google Scholar]
- 23.Dashti HM, Mathew TC, Khadada M, Al-Mousawi M, Talib H, et al. 2007. Beneficial effects of ketogenic diet in obese diabetic subjects. Mol. Cell. Biochem 302:249–56 [DOI] [PubMed] [Google Scholar]
- 24.De Sousa-Coelho AL, Marrero PF, Haro D. 2012. Activating transcription factor 4–dependent induction of FGF21 during amino acid deprivation. Biochem. J 443:165–71 [DOI] [PubMed] [Google Scholar]
- 25.De Sousa-Coelho AL, Relat J, Hondares E, Perez-Marti A, Ribas F, et al. 2013. FGF21 mediates the lipid metabolism response to amino acid starvation. J. Lipid Res 54:1786–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Desai BN, Singhal G, Watanabe M, Stevanovic D, Lundasen T, et al. 2017. Fibroblast growth factor 21 (FGF21) is robustly induced by ethanol and has a protective role in ethanol associated liver injury. Mol. Metab 6:1395–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding X, Boney-Montoya J, Owen BM, Bookout AL, Coate KC, et al. 2012. βKlotho is required for fibroblast growth factor 21 effects on growth and metabolism. Cell Metab. 16:387–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Douris N, Stevanovic DM, Fisher FM, Cisu TI, Chee MJ, et al. 2015. Central fibroblast growth factor 21 browns white fat via sympathetic action in male mice. Endocrinology 156:2470–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dunshee DR, Bainbridge TW, Kljavin NM, Zavala-Solorio J, Schroeder AC, et al. 2016. Fibroblast activation protein cleaves and inactivates fibroblast growth factor 21. J. Biol. Chem 291:5986–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dushay JR, Chui PC, Gopalakrishnan GS, Varela-Rey M, Crawley M, et al. 2010. Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology 139:456–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dushay JR, Toschi E, Mitten EK, Fisher FM, Herman MA, Maratos-Flier E. 2015. Fructose ingestion acutely stimulates circulating FGF21 levels in humans. Mol. Metab 4:51–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dutchak PA, Katafuchi T, Bookout AL, Choi JH, Yu RT, et al. 2012. Fibroblast growth factor-21 regulates PPARγ activity and the antidiabetic actions of thiazolidinediones. Cell 148:556–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fazeli PK, Lun M, Kim SM, Bredella MA, Wright S, et al. 2015. FGF21 and the late adaptive response to starvation in humans. J. Clin. Investig 125:4601–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fisher FM, Chui PC, Antonellis PJ, Bina HA, Kharitonenkov A, et al. 2010. Obesity is a fibroblast growth factor 21 (FGF21)-resistant state. Diabetes 59:2781–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fisher FM, Kim M, Doridot L, Cunniff JC, Parker TS, et al. 2017. A critical role for ChREBP-mediated FGF21 secretion in hepatic fructose metabolism. Mol. Metab 6:14–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, et al. 2012. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 26:271–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fisher FM, Maratos-Flier E. 2016. Understanding the physiology of FGF21. Annu. Rev. Physiol 78:223–41 [DOI] [PubMed] [Google Scholar]
- 38.Foltz IN, Hu S, King C, Wu X, Yang C, et al. 2012. Treating diabetes and obesity with an FGF21-mimetic antibody activating the βKlotho/FGFR1c receptor complex. Sci. Transl. Med 4:162ra53. [DOI] [PubMed] [Google Scholar]
- 39.Fon Tacer K, Bookout AL, Ding X, Kurosu H, John GB, et al. 2010. Research resource: comprehensive expression atlas of the fibroblast growth factor system in adult mouse. Mol. Endocrinol 24:2050–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frayling TM, Beaumont R, Jones SE, Yaghootkar H, Tuke MA, et al. 2017. A common allele in FGF21 associated with preference for sugar consumption lowers body fat in the lower body and increases blood pressure. bioRxiv 214700 10.1101/214700 [DOI] [Google Scholar]
- 41.Gaich G, Chien JY, Fu H, Glass LC, Deeg MA, et al. 2013. The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metab. 18:333–40 [DOI] [PubMed] [Google Scholar]
- 42.Galman C, Lundasen T, Kharitonenkov A, Bina HA, Eriksson M, et al. 2008. The circulating metabolic regulator FGF21 is induced by prolonged fasting and PPARα activation in man. Cell Metab. 8:169–74 [DOI] [PubMed] [Google Scholar]
- 43.Gillum MP, Potthoff MJ. 2016. FAP finds FGF21 easy to digest. Biochem. J 473:1125–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gimeno RE, Moller DE. 2014. FGF21-based pharmacotherapy—potential utility for metabolic disorders. Trends Endocrinol. Metab 25:303–11 [DOI] [PubMed] [Google Scholar]
- 45.Hale C, Chen MM, Stanislaus S, Chinookoswong N, Hager T, et al. 2012. Lack of overt FGF21 resistance in two mouse models of obesity and insulin resistance. Endocrinology 153:69–80 [DOI] [PubMed] [Google Scholar]
- 46.Hansen JS, Clemmesen JO, Secher NH, Hoene M, Drescher A, et al. 2015. Glucagon-to-insulin ratio is pivotal for splanchnic regulation of FGF-21 in humans. Mol. Metab 4:551–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harno E, Cottrell EC, White A. 2013. Metabolic pitfalls of CNS Cre-based technology. Cell Metab. 18:21–28 [DOI] [PubMed] [Google Scholar]
- 48.Holland WL, Adams AC, Brozinick JT, Bui HH, Miyauchi Y, et al. 2013. An FGF21–adiponectin–ceramide axis controls energy expenditure and insulin action in mice. Cell Metab. 17:790–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hondares E, Iglesias R, Giralt A, Gonzalez FJ, Giralt M, et al. 2011. Thermogenic activation induces FGF21 expression and release in brown adipose tissue. J. Biol. Chem 286:12983–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hsuchou H, Pan W, Kastin AJ. 2007. The fasting polypeptide FGF21 can enter brain from blood. Peptides 28:2382–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang J, Ishino T, Chen G, Rolzin P, Osothprarop TF, et al. 2013. Development of a novel long-acting antidiabetic FGF21 mimetic by targeted conjugation to a scaffold antibody. J. Pharmacol. Exp. Ther 346:270–80 [DOI] [PubMed] [Google Scholar]
- 52.Huang Z, Zhong L, Lee JTH, Zhang J, Wu D, et al. 2017. The FGF21–CCL11 axis mediates beiging of white adipose tissues by coupling sympathetic nervous system to type 2 immunity. Cell Metab. 26:493–508.e4 [DOI] [PubMed] [Google Scholar]
- 53.Iizuka K, Takeda J, Horikawa Y. 2009. Glucose induces FGF21 mRNA expression through ChREBP activation in rat hepatocytes. FEBS Lett. 583:2882–86 [DOI] [PubMed] [Google Scholar]
- 54.Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, et al. 2007. Endocrine regulation of the fasting response by PPARα-mediated induction of fibroblast growth factor 21. Cell Metab. 5:415–25 [DOI] [PubMed] [Google Scholar]
- 55.Iroz A, Montagner A, Benhamed F, Levavasseur F, Polizzi A, et al. 2017. A specific ChREBP and PPARα cross-talk is required for the glucose-mediated FGF21 response. Cell Rep. 21:403–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Izumiya Y, Bina HA, Ouchi N, Akasaki Y, Kharitonenkov A, Walsh K. 2008. FGF21 is an Akt-regulated myokine. FEBS Lett. 582:3805–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kang JG, Park CY. 2012. Anti-obesity drugs: a review about their effects and safety. Diabetes Metab. J 36:13–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kazak L, Chouchani ET, Jedrychowski MP, Erickson BK, Shinoda K, et al. 2015. A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell 163:643–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Keipert S, Kutschke M, Lamp D, Brachthauser L, Neff F, et al. 2015. Genetic disruption of uncoupling protein 1 in mice renders brown adipose tissue a significant source of FGF21 secretion. Mol. Metab 4:537–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Keipert S, Kutschke M, Ost M, Schwarzmayr T, van Schothorst EM, et al. 2017. Long-term cold adaptation does not require FGF21 or UCP1. Cell Metab. 26:437–46.e5 [DOI] [PubMed] [Google Scholar]
- 61.Keipert S, Ost M, Johann K, Imber F, Jastroch M, et al. 2014. Skeletal muscle mitochondrial uncoupling drives endocrine cross-talk through the induction of FGF21 as a myokine. Am. J. Physiol. Endocrinol. Metab 306:E469–82 [DOI] [PubMed] [Google Scholar]
- 62.Kersten S, Seydoux J, Peters JM, Gonzalez FJ, Desvergne B, Wahli W. 1999. Peroxisome proliferator-activated receptor α mediates the adaptive response to fasting. J. Clin. Investig 103:1489–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kharitonenkov A, DiMarchi R. 2017. Fibroblast growth factor 21 night watch: advances and uncertainties in the field. J. Intern. Med 281:233–46 [DOI] [PubMed] [Google Scholar]
- 64.Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, et al. 2005. FGF-21 as a novel metabolic regulator. J. Clin. Investig 115:1627–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kharitonenkov A, Wroblewski VJ, Koester A, Chen YF, Clutinger CK, et al. 2007. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology 148:774–81 [DOI] [PubMed] [Google Scholar]
- 66.Kim AM, Somayaji VR, Dong JQ, Rolph TP, Weng Y, et al. 2017. Once-weekly administration of a long-acting fibroblast growth factor 21 analogue modulates lipids, bone turnover markers, blood pressure and body weight differently in obese people with hypertriglyceridaemia and in non-human primates. Diabetes Obes. Metab 19:1762–72 [DOI] [PubMed] [Google Scholar]
- 67.Kim HW, Lee JE, Cha JJ, Hyun YY, Kim JE, et al. 2013. Fibroblast growth factor 21 improves insulin resistance and ameliorates renal injury in db/db mice. Endocrinology 154:3366–76 [DOI] [PubMed] [Google Scholar]
- 68.Kim KH, Jeong YT, Oh H, Kim SH, Cho JM, et al. 2013. Autophagy deficiency leads to protection from obesity and insulin resistance by inducing Fgf21 as a mitokine. Nat. Med 19:83–92 [DOI] [PubMed] [Google Scholar]
- 69.Kim KH, Lee MS. 2014. FGF21 as a stress hormone: the roles of FGF21 in stress adaptation and the treatment of metabolic diseases. Diabetes Metab. J 38:245–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kolumam G, Chen MZ, Tong R, Zavala-Solorio J, Kates L, et al. 2015. Sustained brown fat stimulation and insulin sensitization by a humanized bispecific antibody agonist for fibroblast growth factor receptor 1/βKlotho complex. EBioMedicine 2:730–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kurosu H, Choi M, Ogawa Y, Dickson AS, Goetz R, et al. 2007. Tissue-specific expression of βKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J. Biol. Chem 282:26687–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Laeger T, Albarado DC, Burke SJ, Trosclair L, Hedgepeth JW, et al. 2016. Metabolic responses to dietary protein restriction require an increase in FGF21 that is delayed by the absence of GCN2. Cell Rep. 16:707–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Laeger T, Henagan TM, Albarado DC, Redman LM, Bray GA, et al. 2014. FGF21 is an endocrine signal of protein restriction. J. Clin. Investig 124:3913–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lan T, Morgan DA, Rahmouni K, Sonoda J, Fu X, et al. 2017. FGF19, FGF21, and an FGFR1/β-Klotho-activating antibody act on the nervous system to regulate body weight and glycemia. Cell Metab. 26:709–18.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee KY, Russell SJ, Ussar S, Boucher J, Vernochet C, et al. 2013. Lessons on conditional gene targeting in mouse adipose tissue. Diabetes 62:864–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee P, Linderman JD, Smith S, Brychta RJ, Wang J, et al. 2014. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 19:302–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee S, Choi J, Mohanty J, Sousa LP, Tome F, et al. 2018. Structures of β-klotho reveal a ‘zip code’-like mechanism for endocrine FGF signalling. Nature 553:501–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lehtonen JM, Forsstrom S, Bottani E, Viscomi C, Baris OR, et al. 2016. FGF21 is a biomarker for mitochondrial translation and mtDNA maintenance disorders. Neurology 87:2290–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li H, Fang Q, Gao F, Fan J, Zhou J, et al. 2010. Fibroblast growth factor 21 levels are increased in nonalcoholic fatty liver disease patients and are correlated with hepatic triglyceride. J. Hepatol 53:934–40 [DOI] [PubMed] [Google Scholar]
- 80.Li X, Stanislaus S, Asuncion F, Niu QT, Chinookoswong N, et al. 2017. FGF21 is not a major mediator for bone homeostasis or metabolic actions of PPARαand PPARγagonists. J. Bone Miner. Res 32:834–45 [DOI] [PubMed] [Google Scholar]
- 81.Liang Q, Zhong L, Zhang J, Wang Y, Bornstein SR, et al. 2014. FGF21 maintains glucose homeostasis by mediating the cross talk between liver and brain during prolonged fasting. Diabetes 63:4064–75 [DOI] [PubMed] [Google Scholar]
- 82.Lin Z, Tian H, Lam KS, Lin S, Hoo RC, et al. 2013. Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell Metab. 17:779–89 [DOI] [PubMed] [Google Scholar]
- 83.Liu Y, Zhao C, Xiao J, Liu L, Zhang M, et al. 2016. Fibroblast growth factor 21 deficiency exacerbates chronic alcohol-induced hepatic steatosis and injury. Sci. Rep 6:31026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lundsgaard AM, Fritzen AM, Sjoberg KA, Myrmel LS, Madsen L, et al. 2017. Circulating FGF21 in humans is potently induced by short term overfeeding of carbohydrates. Mol. Metab 6:22–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Maida A, Zota A, Sjoberg KA, Schumacher J, Sijmonsma TP, et al. 2016. A liver stress–endocrine nexus promotes metabolic integrity during dietary protein dilution. J. Clin. Investig 126:3263–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Markan KR, Naber MC, Ameka MK, Anderegg MD, Mangelsdorf DJ, et al. 2014. Circulating FGF21 is liver derived and enhances glucose uptake during refeeding and overfeeding. Diabetes 63:4057–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Markan KR, Naber MC, Small SM, Peltekian L, Kessler RL, Potthoff MJ. 2017. FGF21 resistance is not mediated by downregulation of β-klotho expression in white adipose tissue. Mol. Metab 6:602–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Markan KR, Potthoff MJ. 2016. Metabolic fibroblast growth factors (FGFs): mediators of energy homeostasis. Semin. Cell Dev. Biol 53:85–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Micanovic R, Raches DW, Dunbar JD, Driver DA, Bina HA, et al. 2009. Different roles of N- and C-termini in the functional activity of FGF21. J. Cell. Physiol 219:227–34 [DOI] [PubMed] [Google Scholar]
- 90.Morrison CD, Laeger T. 2015. Protein-dependent regulation of feeding and metabolism. Trends Endocrinol. Metab 26:256–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Morrison SF, Nakamura K. 2011. Central neural pathways for thermoregulation. Front. Biosci 16:74–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. 2006. Central nervous system control of food intake and body weight. Nature 443:289–95 [DOI] [PubMed] [Google Scholar]
- 93.Mraz M, Bartlova M, Lacinova Z, Michalsky D, Kasalicky M, et al. 2009. Serum concentrations and tissue expression of a novel endocrine regulator fibroblast growth factor-21 in patients with type 2 diabetes and obesity. Clin. Endocrinol 71:369–75 [DOI] [PubMed] [Google Scholar]
- 94.Muise ES, Azzolina B, Kuo DW, El-Sherbeini M, Tan Y, et al. 2008. Adipose fibroblast growth factor 21 is up-regulated by peroxisome proliferator-activated receptor γ and altered metabolic states. Mol. Pharmacol 74:403–12 [DOI] [PubMed] [Google Scholar]
- 95.Muise ES, Souza S, Chi A, Tan Y, Zhao X, et al. 2013. Downstream signaling pathways in mouse adipose tissues following acute in vivo administration of fibroblast growth factor 21. PLOS ONE 8:e73011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Muller TD, Sullivan LM, Habegger K, Yi CX, Kabra D, et al. 2012. Restoration of leptin responsiveness in diet-induced obese mice using an optimized leptin analog in combination with exendin-4 or FGF21. J. Pept. Sci 18:383–93 [DOI] [PubMed] [Google Scholar]
- 97.Murata Y, Nishio K, Mochiyama T, Konishi M, Shimada M, et al. 2013. Fgf21 impairs adipocyte insulin sensitivity in mice fed a low-carbohydrate, high-fat ketogenic diet. PLOS ONE 8:e69330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nishimura T, Nakatake Y, Konishi M, Itoh N. 2000. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim. Biophys. Acta 1492:203–6 [DOI] [PubMed] [Google Scholar]
- 99.Ogawa Y, Kurosu H, Yamamoto M, Nandi A, Rosenblatt KP, et al. 2007. βKlotho is required for metabolic activity of fibroblast growth factor 21. PNAS 104:7432–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Owen BM, Bookout AL, Ding X, Lin VY, Atkin SD, et al. 2013. FGF21 contributes to neuroendocrine control of female reproduction. Nat. Med 19:1153–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Owen BM, Ding X, Morgan DA, Coate KC, Bookout AL, et al. 2014. FGF21 acts centrally to induce sympathetic nerve activity, energy expenditure, and weight loss. Cell Metab. 20:670–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Owen BM, Mangelsdorf DJ, Kliewer SA. 2015. Tissue-specific actions of the metabolic hormones FGF15/19 and FGF21. Trends Endocrinol. Metab 26:22–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pereira RO, Tadinada SM, Zasadny FM, Oliveira KJ, Pires KMP, et al. 2017. OPA1 deficiency promotes secretion of FGF21 from muscle that prevents obesity and insulin resistance. EMBO J. 36:2126–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Potthoff MJ. 2017. FGF21 and metabolic disease in 2016: a new frontier in FGF21 biology. Nat. Rev. Endocrinol 13:74–76 [DOI] [PubMed] [Google Scholar]
- 105.Potthoff MJ, Inagaki T, Satapati S, Ding X, He T, et al. 2009. FGF21 induces PGC-1α and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. PNAS 106:10853–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Potthoff MJ, Kliewer SA, Mangelsdorf DJ. 2012. Endocrine fibroblast growth factors 15/19 and 21: from feast to famine. Genes Dev. 26:312–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rankinen T, Bouchard C. 2006. Genetics of food intake and eating behavior phenotypes in humans. Annu. Rev. Nutr 26:413–34 [DOI] [PubMed] [Google Scholar]
- 108.Rosen ED, Spiegelman BM. 2014. What we talk about when we talk about fat. Cell 156:20–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Samms RJ, Cheng CC, Kharitonenkov A, Gimeno RE, Adams AC. 2016. Overexpression of β-klotho in adipose tissue sensitizes male mice to endogenous FGF21 and provides protection from diet-induced obesity. Endocrinology 157:1467–80 [DOI] [PubMed] [Google Scholar]
- 110.Samms RJ, Lewis JE, Norton L, Stephens FB, Gaffney CJ, et al. 2017. FGF21 is an insulin-dependent postprandial hormone in adult humans. J. Clin. Endocrinol. Metab 102:3806–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Samms RJ, Smith DP, Cheng CC, Antonellis PP, Perfield JW 2nd, et al. 2015. Discrete aspects of FGF21 in vivo pharmacology do not require UCP1. Cell Rep. 11:991–99 [DOI] [PubMed] [Google Scholar]
- 112.Sanchez J, Palou A, Pico C. 2009. Response to carbohydrate and fat refeeding in the expression of genes involved in nutrient partitioning and metabolism: striking effects on fibroblast growth factor-21 induction. Endocrinology 150:5341–50 [DOI] [PubMed] [Google Scholar]
- 113.Sanchez-Garrido MA, Habegger KM, Clemmensen C, Holleman C, Muller TD, et al. 2016. Fibroblast activation protein (FAP) as a novel metabolic target. Mol. Metab 5:1015–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schaefer EJ, Gleason JA, Dansinger ML. 2009. Dietary fructose and glucose differentially affect lipid and glucose homeostasis. J. Nutr 139:1257S–62S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schlein C, Talukdar S, Heine M, Fischer AW, Krott LM, et al. 2016. FGF21 lowers plasma triglycerides by accelerating lipoprotein catabolism in white and brown adipose tissues. Cell Metab. 23:441–53 [DOI] [PubMed] [Google Scholar]
- 116.Schumann G, Liu C, O’Reilly P, Gao H, Song P, et al. 2016. KLB is associated with alcohol drinking, and its gene product β-klotho is necessary for FGF21 regulation of alcohol preference. PNAS 113:14372–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shenoy VK, Beaver KM, Fisher FM, Singhal G, Dushay JR, et al. 2016. Elevated serum fibroblast growth factor 21 in humans with acute pancreatitis. PLOS ONE 11:e0164351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Simpson SJ, Raubenheimer D. 2005. Obesity: the protein leverage hypothesis. Obes. Rev 6:133–42 [DOI] [PubMed] [Google Scholar]
- 119.Singhal G, Douris N, Fish AJ, Zhang X, Adams AC, et al. 2016. Fibroblast growth factor 21 has no direct role in regulating fertility in female mice. Mol. Metab 5:690–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Soberg S, Sandholt CH, Jespersen NZ, Toft U, Madsen AL, et al. 2017. FGF21 is a sugar-induced hormone associated with sweet intake and preference in humans. Cell Metab. 25:1045–53.e6 [DOI] [PubMed] [Google Scholar]
- 121.Solon-Biet SM, Cogger VC, Pulpitel T, Heblinski M, Wahl D, et al. 2016. Defining the nutritional and metabolic context of FGF21 using the Geometric Framework. Cell Metab. 24:555–65 [DOI] [PubMed] [Google Scholar]
- 122.Solon-Biet SM, McMahon AC, Ballard JW, Ruohonen K, Wu LE, et al. 2014. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum–fed mice. Cell Metab. 19:418–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Stemmer K, Zani F, Habegger KM, Neff C, Kotzbeck P, et al. 2015. FGF21 is not required for glucose homeostasis, ketosis or tumour suppression associated with ketogenic diets in mice. Diabetologia 58:2414–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Suzuki M, Uehara Y, Motomura-Matsuzaka K, Oki J, Koyama Y, et al. 2008. βKlotho is required for fibroblast growth factor (FGF) 21 signaling through FGF receptor (FGFR) 1c and FGFR3c. Mol. Endocrinol 22:1006–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Talukdar S, Owen BM, Song P, Hernandez G, Zhang Y, et al. 2016. FGF21 regulates sweet and alcohol preference. Cell Metab. 23:344–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Talukdar S, Zhou Y, Li D, Rossulek M, Dong J, et al. 2016. A long-acting FGF21 molecule, PF-05231023, decreases body weight and improves lipid profile in non-human primates and type 2 diabetic subjects. Cell Metab. 23:427–40 [DOI] [PubMed] [Google Scholar]
- 127.Tanaka T, Ngwa JS, van Rooij FJ, Zillikens MC, Wojczynski MK, et al. 2013. Genome-wide meta-analysis of observational studies shows common genetic variants associated with macronutrient intake. Am. J. Clin. Nutr 97:1395–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tezze C, Romanello V, Desbats MA, Fadini GP, Albiero M, et al. 2017. Age-associated loss of OPA1 in muscle impacts muscle mass, metabolic homeostasis, systemic inflammation, and epithelial senescence. Cell Metab. 25:1374–89.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Thomou T, Mori MA, Dreyfuss JM, Konishi M, Sakaguchi M, et al. 2017. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature 542:450–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Uebanso T, Taketani Y, Yamamoto H, Amo K, Ominami H, et al. 2011. Paradoxical regulation of human FGF21 by both fasting and feeding signals: Is FGF21 a nutritional adaptation factor? PLOS ONE 6:e22976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Veniant MM, Hale C, Helmering J, Chen MM, Stanislaus S, et al. 2012. FGF21 promotes metabolic homeostasis via white adipose and leptin in mice. PLOS ONE 7:e40164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Veniant MM, Sivits G, Helmering J, Komorowski R, Lee J, et al. 2015. Pharmacologic effects of FGF21 are independent of the “browning” of white adipose tissue. Cell Metab. 21:731–38 [DOI] [PubMed] [Google Scholar]
- 133.von Holstein-Rathlou S, BonDurant LD, Peltekian L, Naber MC, Yin TC, et al. 2016. FGF21 mediates endocrine control of simple sugar intake and sweet taste preference by the liver. Cell Metab. 23:335–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang X, Wei W, Krzeszinski JY, Wang Y, Wan Y. 2015. A liver–bone endocrine relay by IGFBP1 promotes osteoclastogenesis and mediates FGF21-induced bone resorption. Cell Metab. 22:811–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wei W, Dutchak PA, Wang X, Ding X, Wang X, et al. 2012. Fibroblast growth factor 21 promotes bone loss by potentiating the effects of peroxisome proliferator-activated receptor γ. PNAS 109:3143–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wek SA, Zhu S, Wek RC. 1995. The histidyl-tRNA synthetase-related sequence in the eIF-2α protein kinase GCN2 interacts with tRNA and is required for activation in response to starvation for different amino acids. Mol. Cell Biol 15:4497–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wente W, Efanov AM, Brenner M, Kharitonenkov A, Koster A, et al. 2006. Fibroblast growth factor-21 improves pancreatic β-cell function and survival by activation of extracellular signal-regulated kinase 1/2 and Akt signaling pathways. Diabetes 55:2470–78 [DOI] [PubMed] [Google Scholar]
- 138.Wilson GJ, Lennox BA, She P, Mirek ET, Al Baghdadi RJ, et al. 2015. GCN2 is required to increase fibroblast growth factor 21 and maintain hepatic triglyceride homeostasis during asparaginase treatment. Am. J. Physiol. Endocrinol. Metab 308:E283–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wu AL, Coulter S, Liddle C, Wong A, Eastham-Anderson J, et al. 2011. FGF19 regulates cell proliferation, glucose and bile acid metabolism via FGFR4-dependent and independent pathways. PLOS ONE 6:e17868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wu X, Ge H, Gupte J, Weiszmann J, Shimamoto G, et al. 2007. Co-receptor requirements for fibroblast growth factor-19 signaling. J. Biol. Chem 282:29069–72 [DOI] [PubMed] [Google Scholar]
- 141.Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, et al. 2009. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes 58:250–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Xu J, Stanislaus S, Chinookoswong N, Lau YY, Hager T, et al. 2009. Acute glucose-lowering and insulin-sensitizing action of FGF21 in insulin-resistant mouse models—association with liver and adipose tissue effects. Am. J. Physiol. Endocrinol. Metab 297:E1105–14 [DOI] [PubMed] [Google Scholar]
- 143.Yan Z, Kronemberger A, Blomme J, Call JA, Caster HM, et al. 2017. Exercise leads to unfavourable cardiac remodelling and enhanced metabolic homeostasis in obese mice with cardiac and skeletal muscle autophagy deficiency. Sci. Rep 7:7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yie J, Hecht R, Patel J, Stevens J, Wang W, et al. 2009. FGF21 N- and C-termini play different roles in receptor interaction and activation. FEBS Lett. 583:19–24 [DOI] [PubMed] [Google Scholar]
- 145.Yie J, Wang W, Deng L, Tam LT, Stevens J, et al. 2012. Understanding the physical interactions in the FGF21/FGFR/β-Klotho complex: structural requirements and implications in FGF21 signaling. Chem. Biol. Drug Des 79:398–410 [DOI] [PubMed] [Google Scholar]
- 146.Yilmaz Y, Eren F, Yonal O, Kurt R, Aktas B, et al. 2010. Increased serum FGF21 levels in patients with nonalcoholic fatty liver disease. Eur. J. Clin. Investig 40:887–92 [DOI] [PubMed] [Google Scholar]
- 147.Zhang J, Gupte J, Gong Y, Weiszmann J, Zhang Y, et al. 2017. Chronic over-expression of fibroblast growth factor 21 increases bile acid biosynthesis by opposing FGF15/19 action. EBioMedicine 15:173–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhang Y, Xie Y, Berglund ED, Coate KC, He TT, et al. 2012. The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. eLife 1:e00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhao C, Liu Y, Xiao J, Liu L, Chen S, et al. 2015. FGF21 mediates alcohol-induced adipose tissue lipolysis by activation of systemic release of catecholamine in mice. J. Lipid Res 56:1481–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhen EY, Jin Z, Ackermann BL, Thomas MK, Gutierrez JA. 2016. Circulating FGF21 proteolytic processing mediated by fibroblast activation protein. Biochem. J 473:605–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zimmerman CA, Leib DE, Knight ZA. 2017. Neural circuits underlying thirst and fluid homeostasis. Nat. Rev. Neurosci 18:459–69 [DOI] [PMC free article] [PubMed] [Google Scholar]