Abstract
Porous coordination polymers have received intensive attention for pollution abatement, such as dye removal, because of their high porosity and specific surface areas. However, the commonly used water-stable porous coordination polymers are microporous and synthesized within organic solvents, which deters seriously their widespread application. In this report, we developed a facile strategy for the synthesis of mesoporous Zr-based coordination polymer (Zr-BDC-CP) within aqueous solutions. The morphology and structure of Zr-BDC-CP were characterized with scanning electron microscopy, powder X-ray diffraction, and Fourier transform infrared spectroscopy. Pore size distribution analysis confirms that the as-synthesized material is mesoporous, which allows the efficient adsorption of methylene blue, 2.6 times higher than that of the microporous coordination polymer, UiO-66. The decolorization ratio can reach higher than 93.5% in the range of 10 and 400 mg/L for methylene blue solutions. This Zr-based coordination polymer shows wonderful pH stability, where no significant loss of adsorption capacities was observed between pH values of 3 and 11. The simulation of adsorption isotherm indicates that the Freundlich model can fit the adsorption isotherm very well, which reflects that the surface of adsorbents is inhomogeneous. Fitting of kinetic curves shows that the dye adsorption by Zr-BDC-CP follows the pseudo-second-order model, which confirms that the rate-determining step may be a chemisorption process involving valence forces because of the defects within the frameworks of the mesoporous coordination polymer. Zr-BDC-CP also shows desirable recyclability without significant capacity loss. This work presents a facile and sustainable method for the preparation of mesoporous Zr-based coordination polymer for dye removal with excellent stability and recyclability, which could further push the porous coordination polymers for application in the areas of pollution abatement.
Introduction
Organic dyes, such as methyl orange, methylene blue, and rhodamine B, are widely employed in paper, food, and textile industry.1,2 At the same time, the discharge of dyes leads to serious environmental problems3 on account of their highly toxic and non-biodegradable nature.4 Their removal from waste water has been widely studied by various approaches.5 The conventional methods of dye wastewater treatment include the chemical method,6 the physical adsorption method,7 and the biological method.8,9 Among these methods, adsorption has received much attention owing to its high efficiency, low operation cost, and easy regeneration/recycling of adsorbents.10 Therefore, it is of significant importance to design an exceptional adsorbent with high selectivity and adsorption capacity, fast kinetics, and good recycling properties.11
Porous coordination polymers (PCPs), or the so-called metal organic frameworks, one of the exciting class of multifunctional crystalline porous materials,12,13 are constructed by the mutual connection between metal clusters and organic linkers.14 PCPs exhibit highly ordered porosity and high specific surface area,15 which make these materials suitable for selective removal of dye molecules, organic isomers, and even fullerenes. In particular, a Zr-based PCP, UIO-66, has been extensively investigated for pollute removal16 because of its high surface area (>1000 m2/g) and relatively higher thermal and hydrodynamic stabilities even under acidic/basic conditions.1 For example, Yao et al.1 reported that acetic acid-promoted UiO-66 could selectively adsorb methyl orange and methylene blue. Liu et al.17 fabricated a hybrid material through assembling a two-dimensional Tb-based coordination polymer on UiO-66-NH2, which achieved simultaneous adsorption18 and detection of organic dyes. However, UiO-66 is a typical microporous coordination polymer, with a moderate pore window size of 0.7 nm, which is almost similar to the size of typical organic dyes19 (the width of cross-section of methylene blue is around 0.7 nm). On the other hand, Zr-based coordination polymers are often synthesized within N,N-dimethylformamide (DMF),20 an expensive organic solvent. Therefore, it is important and also a challenge to synthesize mesoporous coordination polymers with high adsorption capacity under environmental benign conditions.
In this work, we develop a convenient strategy for the aqueous synthesis of mesoporous Zr-based coordination polymers (abbreviated as Zr-BDC-CP) at room temperature. Herein, water is used to replace the organic solvent, DMF, and NaOH is added to deprotonate the ligand, which can initiate the reaction to produce precipitates within several minutes at room temperature. The as-prepared coordination polymer is mesoporous with an average pore size of 6 nm, which shows an enhanced adsorption capacity of 9.78 mg/g toward methylene blue at a concentration of 100 mg/L, around 3 times higher than UiO-66 (3.71 mg/g). The mesoporous material shows stable adsorption ability at a wide pH range between 3 and 11 and can be recycled without a significant capacity loss.
Results and Discussion
Characterization of Zr-BDC-CP
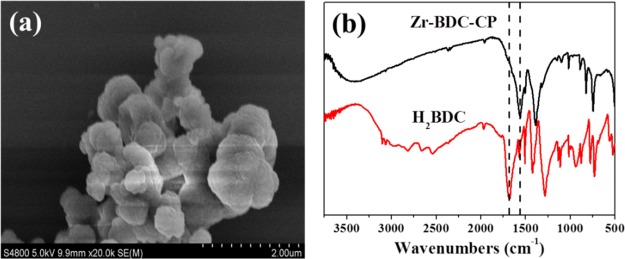
The morphology of Zr-BDC-CP was characterized with scanning electron microscopy (SEM) as shown in Figure 1a, which shows that the as-prepared sample is an aggregate of nanoparticles. The structure of the coordination polymer was characterized with powder X-ray diffraction (PXRD) (Figure S2), however, the PXRD pattern is indicative of the nature of amorphous material. The formation coordination polymer was confirmed with Fourier transform infrared (FTIR) spectroscopy (Figure 1b), where a peak at 1678 cm–1 attributed to H2BDC disappeared, and a peak at 1556 cm–1 becomes the main peak for Zr-BDC-CP.21 Zr-BDC-CP also exhibits excellent thermal stability, which is stable up to 450 °C under an Ar atmosphere (Figure S1).
Figure 1.
(a) SEM image of Zr-BDC-CP and (b) FTIR spectra of Zr-BDC-CP and H2BDC.
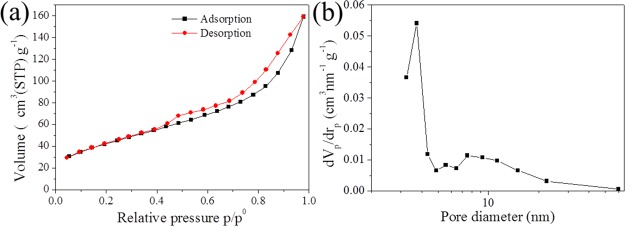
The porosity of Zr-BDC-CP was analyzed by N2 adsorption/desorption isotherms. As shown in Figure 2a, Zr-BDC-CP shows a typical type IV isotherm with an H3 hysteresis loop, which is the characteristic of mesoporous materials because of the gap resulting from nanoparticle aggregation. This indicates that the formation of mesopores within Zr-BDC-CP results from the aggregation of nanoparticles, which is consistent with the observation in SEM images. Further pore size distribution analysis confirms that the mesoporous coordination polymer is formed with an average pore diameter of 6.4 nm. The specific surface area and pore volume are 157 m2/g and 0.51 cm3/g, respectively.
Figure 2.
Nitrogen adsorption/desorption isotherm (a) and pore size distribution (b) of Zr-BDC-CP.
Dye Adsorption Performance of Zr-BDC-CP
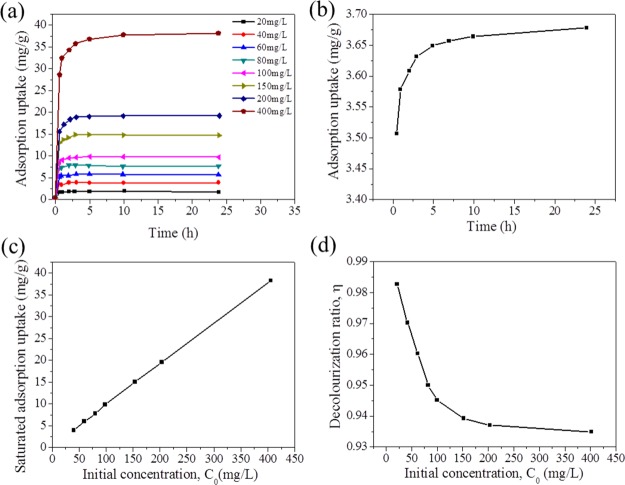
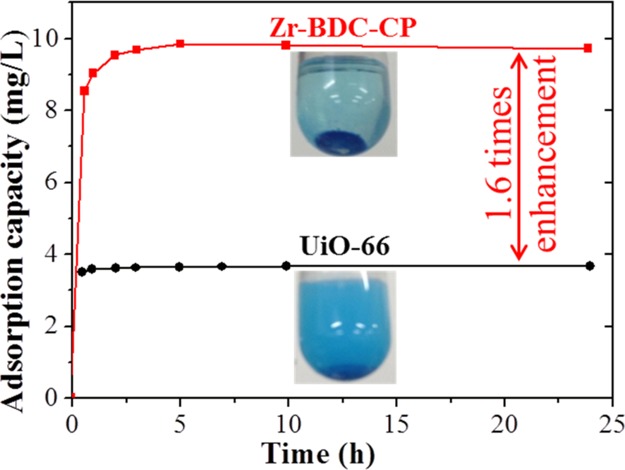
Methylene blue is a typical dye, which is widely applied in the area of chemical indicators, printing and dyeing, and pharmacy; therefore, this dye was selected as a model to assess the dye adsorption performance of as-prepared samples. Adsorption isotherms of Zr-BDC-CP toward methylene blue aqueous solutions with different concentrations (20, 40, 60, 80, 100, 150, 200, and 400 mg/L) were first measured to assess the adsorption capacity and kinetics, as shown in Figure 3a. It is found that Zr-BDC-CP can arrive at the saturated adsorption capacity within 5 h. We observe that the saturated adsorption capacity is linear with the initial concentration of the dye, and the maximum adsorption capacity is 38.17 mg/g in the tested range. We also compare the adsorption performance with that of typical microporous UiO-66 at the initial dye concentration of 100 mg/L. More interestingly, the saturated adsorption capacity of Zr-BDC-CP is 9.78 mg/g, which is 2.6 times higher than that of UiO-66 (3.71 mg/g). The significant improvement can be attributed to the presence of mesopores in Zr-BDC-CP, whereas surface adsorption occurs mainly for UiO-66 because of the nature of microporous materials. The enhanced adsorption efficiency of Zr-BDC-CP other than UiO-66 can be directly observed from the photos of the dye solutions after the adsorption saturation (Figure S4). The color of dye solution after adsorption with Zr-BDC-CP is very shallow, whereas that for UiO-66 is still very deep, which can obviously confirm that Zr-BDC-CP shows excellent dye removal ability. The decolorization ratio of Zr-BDC-CP was also calculated, as shown in Figure 3d. The decolorization efficiency is quite high, and the decolorization ratio can reach 98.4% at a low concentration (20 mg/L) and 93.5% at an even higher concentration (400 mg/L).
Figure 3.
(a) Adsorption isotherms of Zr-BDC-CP toward methylene blue at different initial dye concentrations; (b) adsorption isotherm of UiO-66 at an initial dye concentration of 100 mg/L; (c) effect of initial concentration on the adsorption capacity of methylene blue by Zr-BDC-CP; and (d) effect of initial concentration on the decolorization ratio of Zr-BDC-CP for removal of methylene blue.
The adsorption capacity of adsorbents with different pH values is also an important parameter, which determines their application range. Hence, we also measured the adsorption performance of Zr-BDC-CP in dye solutions with different pH values, including 1, 3, 5, 7, 9, 11, and 14. Figure 4 shows that Zr-BDC-CP possesses the highest adsorption capacity at a pH value of 7, and no significant capacity loss was observed between 3 and 11 for Zr-BDC-CP. However, the capacity decreases sharply when the pH was decreased to 1, which is possibly because of the structure collapse under highly acidic conditions. The photo of dye solutions after adsorption at different pH values clearly confirms the above-mentioned conclusion. These results indicate that the as-prepared mesoporous coordination polymer displays desirable adsorption capacity at the relatively wide pH range, which can enhance the practical application of this material.
Figure 4.
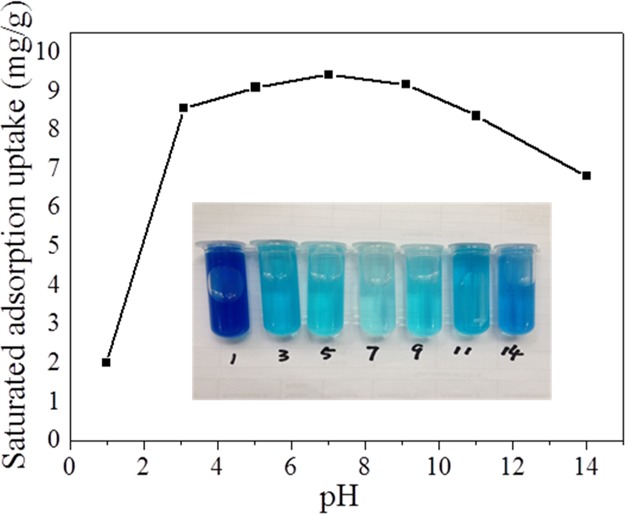
Influence of the pH value on the adsorption performance of Zr-BDC-CP (inset is the photo of dye solutions after adsorption at different pH values).
Adsorption Isotherm of Zr-BDC-CP for Dye Removal
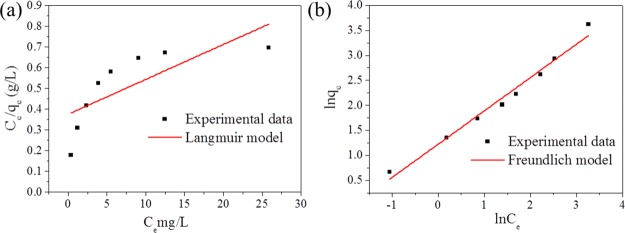
The adsorption mechanism of Zr-BDC-CP was disclosed through the analysis of the adsorption isotherm for methylene blue removal. Two commonly used models were employed to simulate the adsorption isotherms of Zr-BDC-CP: Langmuir and Freundlich models. The fitting curves according to Langmuir and Freundlich models are shown in Figure 5 and the fitting parameters are tabulated in Table 1. The fitting curves and correlation coefficients (R2) reveal that the Freundlich model is more appropriate for fitting the adsorption isotherm. This result is different from that of the microporous coordination polymer, such as UiO-66 and UiO-67.22,23 In the case of microporous coordination polymers, the pore is highly ordered, homogenous, and mostly in the range of 0.5–1 nm, merely allowing the monolayer adsorption of dyes within the pores, so that the adsorption isotherm can be fitted with the Langmuir model. However, in our case, the as-prepared material is mesoporous, and the pore surface is not homogenous like the microporous coordination polymers, where multilayer adsorption is possible so that the Langmuir model is not suitable for fitting the adsorption. The fitting parameters (KF and n) from the Freundlich model reflect the interaction between the adsorbates and adsorbents. KF is related to the adsorption capacity of the adsorbent and represents the strength of the adsorptive bond, and the value of n reflects the bond distribution adsorption and the type of isotherm to be favorable (n > 1) or unfavorable (0 < n < 1). Compared with UiO-67 reported previously, Zr-BDC-CP shows higher adsorption capacity and favorable adsorption.23
Figure 5.
Fitting of the adsorption isotherm of Zr-BDC-CP for methylene blue according to the Langmuir model (a) and Freundlich model (b).
Table 1. Langmuir and Freundlich Parameters of Zr-BDC-CP for Methylene Blue Adsorption.
| Langmuir
model |
Freundlich
model |
||||
|---|---|---|---|---|---|
| kL (L mg–1) | qmax (mg g–1) | R2 | KF (mg1–n Ln g–1) | n | R2 |
| 0.0446 | 59.773 | 0.5007 | 16.81 | 1.5 | 0.9773 |
Adsorption Kinetics of Zr-BDC-CP
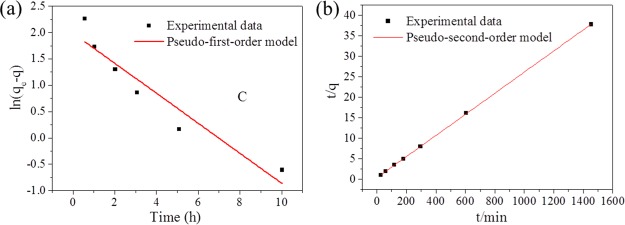
To understand the adsorption kinetics of Zr-BDC-CP for removal of methylene blue, we fitted the relationship between the adsorption capacity and adsorption time according to two typical models: pseudo-first-order model and pseudo-second-order model (Figure 6a). The kinetic parameters and correlation coefficients of Zr-BDC-CP for dye adsorption are listed in Table 2. The fitting curves, kinetic parameters, and correlation coefficients confirm that adsorption of methylene blue by Zr-BDC-CP follows the pseudo-second-order model. The equilibrium adsorption amount calculated from the pseudo-second-order kinetics matches very well with the experimental data (38.17 mg/g). The pseudo-second-order kinetics was developed assuming that the rate-determining step may be a chemisorption process involving valence forces via exchanging or sharing electrons between the adsorbates and adsorbents. Zr-BDC-CP was synthesized rapidly in an aqueous solution, possibly producing more defects in the frameworks, which could improve the interaction between methylene blue and Zr-BDC-CP.
Figure 6.
Adsorption kinetics of Zr-BDC-CP for methylene blue according to the pseudo-first-order model (a) and pseudo-second-order model (b). (The initial concentration of methylene blue is 400 mg/L.)
Table 2. Kinetic Parameters of Pseudo-First-Order and Pseudo-Second-Order Models of Zr-BDC-CP for Methylene Blue Adsorption.
| pseudo-first-order model |
pseudo-second-order model |
||||
|---|---|---|---|---|---|
| k1 (min–1) | qe (mg g–1) | R2 | k2 (min–1) | qe (mg g–1) | R2 |
| 0.2849 | 7.30 | 0.8941 | 0.0018 | 38.77 | 0.9999 |
Recyclability of Zr-BDC-CP for Removal of Methylene Blue
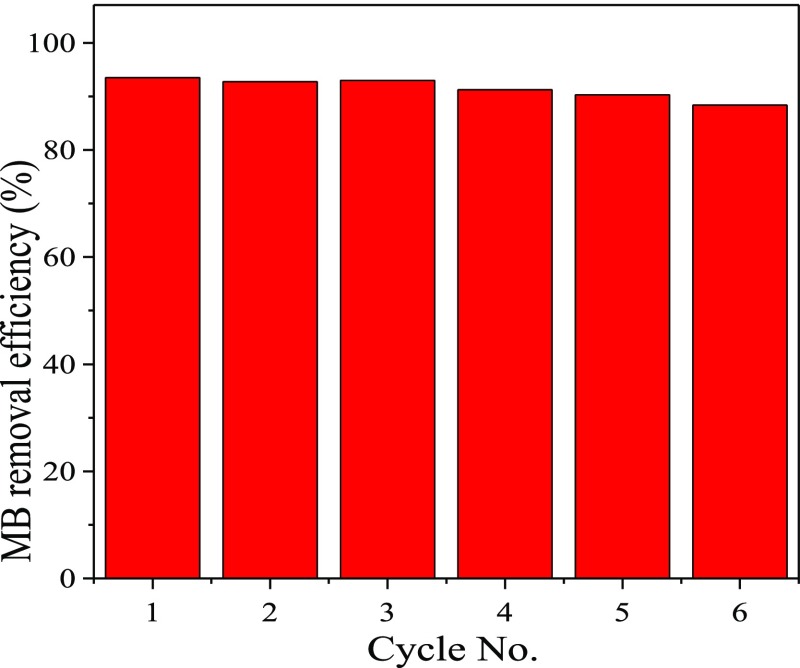
Recyclability is one of the important parameters for judging the adsorption performance of an adsorbent; therefore, we also tested the recyclability of Zr-BDC-CP. After adsorption of methylene blue, Zr-BDC-CP was regenerated through washing with sodium hydroxide aqueous solution and ethanol for several times, and then the regenerated sample was applied for adsorption of methylene blue again. The dye removal efficiency of Zr-BDC-CP versus recycling times is shown in Figure 7. It is found that the dye removal efficiency showed no obvious decrease for first three times, and only a slight decrease was observed after recycling for six times (from 93.5% at the first time to 90.0% at the sixth time). The slight decrease possibly resulted from the mass loss during the washing step. The results clearly demonstrate that Zr-BDC-CP can be easily recycled for the removal of methylene blue, which is definitely beneficial to its practical application.
Figure 7.
Adsorption isotherms of Zr-BDC-CP for the first and second removal of methylene blue (initial dye concentration is 400 mg/L).
Conclusions
In summary, we have successfully developed an aqueous synthesis of mesoporous Zr-based coordination polymers for removal of methylene blue. The synthetic strategy is straightforward and sustainable without use of organic solvents. The as-prepared coordination polymer exhibits an average diameter of 6.4 nm, which allows the efficient adsorption of dyes, 2.6 times higher than that of microporous UiO-66. The decolorization ratio can reach higher than 93.5% in the range of 10 and 400 mg/L for methylene blue solutions. The adsorption experiments in dye solutions with different pH values demonstrate that the mesoporous Zr-based coordination polymers can be utilized for dye removal between pH values of 3 and 11 without significant loss. The simulation of isotherms indicates that the Freundlich model can fit the adsorption isotherm very well, which reflects that the surface of adsorbents is not homogeneous. Fitting of kinetic curves shows that the dye adsorption by Zr-BDC-CP follows the pseudo-second-order model, which confirms that the rate-determining step may be a chemisorption process involving valence forces because of the defects within the frameworks of mesoporous coordination polymers. Zr-BDC-CP also shows desirable recyclability without a significant capacity loss after washing the materials saturated with methylene blue with the alkaline solution and ethanol. This work presents a facile and sustainable method for the preparation of mesoporous Zr-based coordination polymers for dye removal with excellent stability and recyclability, which could further push the PCPs for application in the areas of pollution abatement.
Experimental Section
Reagents and Chemicals
Zirconium tetrachloride (ZrCl4), terephthalic acid (H2BDC), methylene blue (C16H18ClN3S), sodium hydroxide (NaOH), trimethylamine (C6H15N), acetone (C3H6O), and DMF (C3H7NO) were of analytical reagent grade, commercial, and used without further purification.
Preparation of Mesoporous Zr-Based Coordination Polymer (Zr-BDC-CP)
Mesoporous Zr-based coordination polymer was synthesized as follows: 0.16 g of ZrCl4, 0.114 g of terephthalic acid (H2BDC), and 0.055 g of NaOH were dissolved in 10 mL of deionized water. Two solutions were mixed together under stirring for 24 h under room temperature. After the reaction, the products were washed repeatedly with trimethylamine aqueous solution and ethanol three times and then dried at 60 °C overnight in a vacuum drying oven. Typically, UiO-66 was synthesized according to the literature.16
Characterization
The phase identification and crystallinity of the samples were performed on a powder X-ray diffractometer (D8 ADVANCE) using Cu Kα radiation, where the scanning range of the diffraction angle (2θ) was 5–40°. The scanning rate was 4° min–1, and the step width was 0.02° with 40 mA operation current and 40 kV voltage. Thermogravimetric analysis (TGA) was performed using a HTG-1 (HENVEN) instrument, and the sample was heated from room temperature to 800 °C at a rate of 10 °C min–1 under an Ar atmosphere. SEM measurements were made on a Hitachi S-4800 thermal field emission scanning electron microscope at an accelerating voltage of 5, 10, or 15 kV. Samples for SEM measurements were attached to the stub using the carbon paste and then sputter-coated with a thin layer of conductive gold to improve electrical conductivity. The nitrogen adsorption–desorption isotherms were measured at 77 K with a JW-BK200C (Beijing JWGB Sci. & Tech. Co., Ltd.) gas adsorption analyzer after the sample was first degassed at 120 °C overnight. Specific surface areas were determined by the Brunauer–Emmett–Teller method, mesopore size distribution was obtained based on the Barrett–Joyner–Halenda analysis of the desorption branches of the isotherms, and the total pore volumes were determined using the desorption branch of the N2 isotherm at p/p0 = 0.99 (single point). FTIR spectra were measured by compressing the samples into KBr pellets and recording FTIR spectra in the 250–4000 cm–1 range using a Bruker TENSOR 27 FTIR spectrometer. Ultraviolet–visible (UV–vis) spectra were recorded on an Agilent Cary 300 spectrophotometer.
Adsorption Experiments
Methylene blue was used as a probe dye at different concentrations to evaluate the adsorption capacity of the as-prepared samples. Typically, 50 mg of Zr-BDC-CP was added into 5 mL of 100 mg/L dye solution at 25 °C in a constant temperature shaker. The adsorption capacities were calculated by monitoring the intensity of the supernatant liquid with an UV–vis spectrophotometer at given intervals. (The supernatant liquid was obtained by filtration with a 0.22 μm filter.) The adsorption capacity of UiO-66 was measured by the same method as that for Zr-BDC-CP.
Regeneration of Zr-BDC-CP after Dye Removal
After adsorption of methylene blue, Zr-BDC-CP was regenerated through washing with 0.4 mol/L of sodium hydroxide aqueous solution and ethanol for three times separately, until the color of mesoporous materials turned white, which were then dried at 60 °C overnight in a vacuum oven. The concentration of the dye solution used to measure the recyclability is 400 mg/L, and the adsorption time is 10 h.
Adsorption Isotherms of Zr-BDC-CP
The mass concentration, adsorption capacity, and decolorization rate of methylene blue solution were determined by the UV–vis spectrophotometer. The data obtained were calculated according to the following formula
wherein C0 is the initial mass concentration of methylene blue solution (mg/L); Ce is the mass concentration (mg/L) of methylene blue24 solution after equilibrium; qe is the equilibrium adsorption capacity (mg/g); m is the mass of the adsorbent used (g); V is the volume of waste water (L); and η is the decolorization rate of methylene blue solution (%).
Adsorption isotherm refers to the curve of the relationship between the mass concentration of the dye and the adsorption capacity of the adsorbent after adsorption reaches equilibrium,25,26 which can reflect the interaction between the adsorbents and adsorbates.27 The typical models used to simulate adsorption isotherms of solid–liquid adsorption include Langmuir and Freundlich adsorption models.28 It should be noted that the Langmuir model is a theoretical, monolayer adsorption one, whereas the Freundlich model is empirical. Langmuir model is based on the assumption that the adsorption is monolayer adsorption on the surface of adsorbents and adsorption locations are evenly distributed on the solid surface. The Freundlich adsorption model is often used to describe heterogeneous adsorption systems. The linear fitting equations based on these two models are shown as follows:
Linear Langmuir equation
Linear Freundlich equation
wherein qe is the equilibrium adsorption capacity (mg/g), qmax is the maximum adsorption capacity (mg/g), Ce is the equilibrium concentration (mg/L), KL is a constant related to adsorption energy, Kf is the Freundlich coefficient, and n is the Freundlich constant.
Adsorption Kinetics of Zr-BDC-CP
The main reason for studying adsorption kinetics is to investigate the quality of minimum adsorbents required for complete removal in a certain period, which is also an important factor to assess the adsorbent performance.29 The kinetic equation can directly reflect the relationship between the adsorption capacity and time during an adsorption process, and it can well explain the reaction mechanism and reaction path, as well as reliably predicting the adsorption result and process according to the adsorption kinetic model, which provides valuable insights into the treatment of organic dyes.30 There are two commonly used adsorption kinetic models, namely the pseudo-first-order and the pseudo-second- order kinetic models. The difference is whether the limiting factor of adsorption is mass-transfer resistance or not. The pseudo-first-order kinetic model considers that the limiting factor of adsorption is the mass-transfer resistance from the interior of the adsorbents. However, the pseudo-second-order kinetic model considers that the limiting factor of adsorption is actually the adsorption mechanism instead of the mass-transfer resistance.
Pseudo-first-order kinetic equation
Pseudo-second order kinetic equation
K1 is the rate constant of the first-order kinetic model (min–1), K2 is the rate constant of the second-order kinetic model (g/(mg·min)), q is the adsorption capacity of adsorbents at a certain time (mg/g), qe is the equilibrium adsorption capacity (mg/g), and t is the adsorption time (min).
Acknowledgments
The authors acknowledge support from the National Natural Science Foundation of China (grant nos. 21573063 and 51503062), the Provincial Natural Science Foundation of Hunan (grant no. 2017JJ3025), the Shenzhen Science and Technology Innovation Committee (grant no. JCYJ20170306141630229), and the Fundamental Research Funds for the Central Universities.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.9b03192.
TGA, PXRD patterns, and optical images (PDF)
Author Contributions
§ C.L. and J.R. authors contributed equally to this paper.
The authors declare no competing financial interest.
Supplementary Material
References
- Qiu J.; Feng Y.; Zhang X.; Jia M.; Yao J. Acid-promoted synthesis of UiO-66 for highly selective adsorption of anionic dyes: Adsorption performance and mechanisms. J. Colloid Interface Sci. 2017, 499, 151–158. 10.1016/j.jcis.2017.03.101. [DOI] [PubMed] [Google Scholar]
- Hasan Z.; Jhung S. H. Removal of hazardous organics from water using metal-organic frameworks (MOFs): Plausible mechanisms for selective adsorptions. J. Hazard. Mater. 2015, 283, 329–339. 10.1016/j.jhazmat.2014.09.046. [DOI] [PubMed] [Google Scholar]
- He J.; Li J.; Du W.; Han Q.; Wang Z.; Li M. A mesoporous metal-organic framework: Potential advances in selective dye adsorption. J. Alloys Compd. 2018, 750, 360–367. 10.1016/j.jallcom.2018.03.393. [DOI] [Google Scholar]
- Yao T.; Guo S.; Zeng C.; Wang C.; Zhang L. Investigation on efficient adsorption of cationic dyes on porous magnetic polyacrylamide microspheres. J. Hazard. Mater. 2015, 292, 90–97. 10.1016/j.jhazmat.2015.03.014. [DOI] [PubMed] [Google Scholar]
- Li C.-P.; Zhou H.; Wang S.; Yuan H.-H.; Zhang S.-Z.; Du M. A nanoporous Ag(i) coordination polymer for selective adsorption of carcinogenic dye Acid Red 26. Chem. Commun. 2017, 53, 4767–4770. 10.1039/c7cc02005h. [DOI] [PubMed] [Google Scholar]
- Azbar N.; Yonar T.; Kestioglu K. Comparison of various advanced oxidation processes and chemical treatment methods for COD and color removal from a polyester and acetate fiber dyeing effluent. Chemosphere 2004, 55, 35–43. 10.1016/j.chemosphere.2003.10.046. [DOI] [PubMed] [Google Scholar]
- Wang S.; Li H.; Xie S.; Liu S.; Xu L. Physical and chemical regeneration of zeolitic adsorbents for dye removal in wastewater treatment. Chemosphere 2006, 65, 82–87. 10.1016/j.chemosphere.2006.02.043. [DOI] [PubMed] [Google Scholar]
- Ghoreishi S. M.; Haghighi R. Chemical catalytic reaction and biological oxidation for treatment of non-biodegradable textile effluent. Chem. Eng. J. 2003, 95, 163–169. 10.1016/s1385-8947(03)00100-1. [DOI] [Google Scholar]
- Robinson T.; McMullan G.; Marchant R.; Nigam P. Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2001, 77, 247–255. 10.1016/s0960-8524(00)00080-8. [DOI] [PubMed] [Google Scholar]
- Yan T.; Li N.; Qiao Z.; Li W.; Wang H.; Jing Z.; Yu Y.; Jiang Z. Ultrathin sodium ferric silicate 2D nanosheets: A novel and robust adsorbent for selective removal of cationic dyes in wastewater. J. Alloys Compd. 2019, 784, 256–265. 10.1016/j.jallcom.2019.01.030. [DOI] [Google Scholar]
- Hui M.; Shengyan P.; Yaqi H.; Rongxin Z.; Anatoly Z.; Wei C. A highly efficient magnetic chitosan “fluid” adsorbent with a high capacity and fast adsorption kinetics for dyeing wastewater purification. Chem. Eng. J. 2018, 345, 556–565. 10.1016/j.cej.2018.03.115. [DOI] [Google Scholar]
- Chen L.; Ding X.; Huo J.; El Hankari S.; Bradshaw D. Facile synthesis of magnetic macroporous polymer/MOF composites as separable catalysts. J. Mater. Sci. 2019, 54, 370–382. 10.1007/s10853-018-2835-x. [DOI] [Google Scholar]
- Huo J.; Marcello M.; Garai A.; Bradshaw D. MOF-Polymer Composite Microcapsules Derived from Pickering Emulsions. Adv. Mater. 2013, 25, 2717–2722. 10.1002/adma.201204913. [DOI] [PubMed] [Google Scholar]
- Jin P.; Tan W.; Huo J.; Liu T.; Liang Y.; Wang S.; Bradshaw D. Hierarchically porous MOF/polymer composites via interfacial nanoassembly and emulsion polymerization. J. Mater. Chem. A 2018, 6, 20473–20479. 10.1039/c8ta06766j. [DOI] [Google Scholar]
- Kitagawa S.; Kitaura R.; Noro S.-i. Functional porous coordination polymers. Angew. Chem., Int. Ed. 2004, 43, 2334–2375. 10.1002/anie.200300610. [DOI] [PubMed] [Google Scholar]
- Jasuja H.; Zang J.; Sholl D. S.; Walton K. S. Rational Tuning of Water Vapor and CO2Adsorption in Highly Stable Zr-Based MOFs. J. Phys. Chem. C 2012, 116, 23526–23532. 10.1021/jp308657x. [DOI] [Google Scholar]
- Zhang Q.; Jiang X.; Kirillov A. M.; Zhang Y.; Hu M.; Liu W.; Yang L.; Fang R.; Liu W. Covalent Construction of Sustainable Hybrid UiO-66-NH2@Tb-CP Material for Selective Removal of Dyes and Detection of Metal Ions. ACS Sustainable Chem. Eng. 2019, 7, 3203–3212. 10.1021/acssuschemeng.8b05146. [DOI] [Google Scholar]
- Li G.; Liu Q.; Xia B.; Huang J.; Li S.; Guan Y.; Zhou H.; Liao B.; Zhou Z.; Liu B. Synthesis of stable metal-containing porous organic polymers for gas storage. Eur. Polym. J. 2017, 91, 242–247. 10.1016/j.eurpolymj.2017.03.014. [DOI] [Google Scholar]
- Katz M. J.; Brown Z. J.; Colón Y. J.; Siu P. W.; Scheidt K. A.; Snurr R. Q.; Hupp J. T.; Farha O. K. A facile synthesis of UiO-66, UiO-67 and their derivatives. Chem. Commun. 2013, 49, 9449–9451. 10.1039/c3cc46105j. [DOI] [PubMed] [Google Scholar]
- Kandiah M.; Nilsen M. H.; Usseglio S.; Jakobsen S.; Olsbye U.; Tilset M.; Larabi C.; Quadrelli E. A.; Bonino F.; Lillerud K. P. Synthesis and stability of tagged UiO-66 Zr-MOFs. Chem. Mater. 2010, 22, 6632–6640. 10.1021/cm102601v. [DOI] [Google Scholar]
- Huo J.; Wang L.; Irran E.; Yu H.; Gao J.; Fan D.; Li B.; Wang J.; Ding W.; Amin A. M.; Li C.; Ma L. Hollow Ferrocenyl coordination polymer microspheres with micropores in shells prepared by Ostwald ripening. Angew. Chem., Int. Ed. 2010, 49, 9237–9241. 10.1002/anie.201004745. [DOI] [PubMed] [Google Scholar]
- Li Y.; Liu Y.; Gao W.; Zhang L.; Liu W.; Lu J.; Wang Z.; Deng Y.-J. Microwave-assisted synthesis of UiO-66 and its adsorption performance towards dyes. CrystEngComm 2014, 16, 7037–7042. 10.1039/c4ce00526k. [DOI] [Google Scholar]
- Zhu X.; Li B.; Yang J.; Li Y.; Zhao W.; Shi J.; Gu J. Effective adsorption and enhanced removal of organophosphorus pesticides from aqueous solution by Zr-based MOFs of UiO-67. ACS Appl. Mater. Interfaces 2015, 7, 223–231. 10.1021/am5059074. [DOI] [PubMed] [Google Scholar]
- Hameed B. H.; Ahmad A. L.; Latiff K. N. A. Adsorption of basic dye (methylene blue) onto activated carbon prepared from rattan sawdust. Dyes Pigm. 2007, 75, 143–149. 10.1016/j.dyepig.2006.05.039. [DOI] [Google Scholar]
- Lee J.-W.; Choi S.-P.; Thiruvenkatachari R.; Shim W.-G.; Moon H. Evaluation of the performance of adsorption and coagulation processes for the maximum removal of reactive dyes. Dyes Pigm. 2006, 69, 196–203. 10.1016/j.dyepig.2005.03.008. [DOI] [Google Scholar]
- Uzun I. Kinetics of the adsorption of reactive dyes by chitosan. Dyes Pigm. 2006, 70, 76–83. 10.1016/j.dyepig.2005.04.016. [DOI] [Google Scholar]
- Kinniburgh D. G. General purpose adsorption isotherms. Environ. Sci. Technol. 1986, 20, 895–904. 10.1021/es00151a008. [DOI] [PubMed] [Google Scholar]
- Başar C. Applicability of the various adsorption models of three dyes adsorption onto activated carbon prepared waste apricot. J. Hazard. Mater. 2006, 135, 232–241. 10.1016/j.jhazmat.2005.11.055. [DOI] [PubMed] [Google Scholar]
- Bulut Y.; Aydın H. A kinetics and thermodynamics study of methylene blue adsorption on wheat shells. Desalination 2006, 194, 259–267. 10.1016/j.desal.2005.10.032. [DOI] [Google Scholar]
- Ho Y. S.; McKay G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. 10.1016/s0032-9592(98)00112-5. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.