Abstract
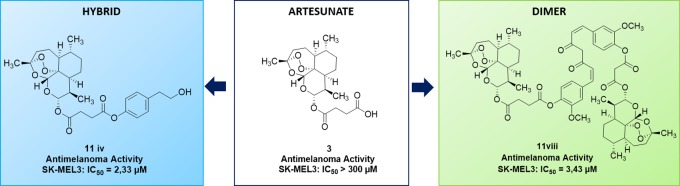
A library of hybrid and dimer compounds based on the natural scaffold of artemisinin was synthesized. These derivatives were obtained by coupling of artemisinin derivatives, artesunate, and dihydroartemisinin with a panel of phytochemical compounds. The novel artemisinin-based hybrids and dimers were evaluated for their anticancer activity on a cervical cancer cell line (HeLa) and on three complementary metastatic melanoma cancer cell lines (SK-MEL3, SK-MEL24, and RPMI-7951). Two hybrid compounds obtained by coupling of artesunate with eugenol and tyrosol, and one of the dimer compounds containing curcumin, emerged as the most active and cancer-selective derivatives.
Introduction
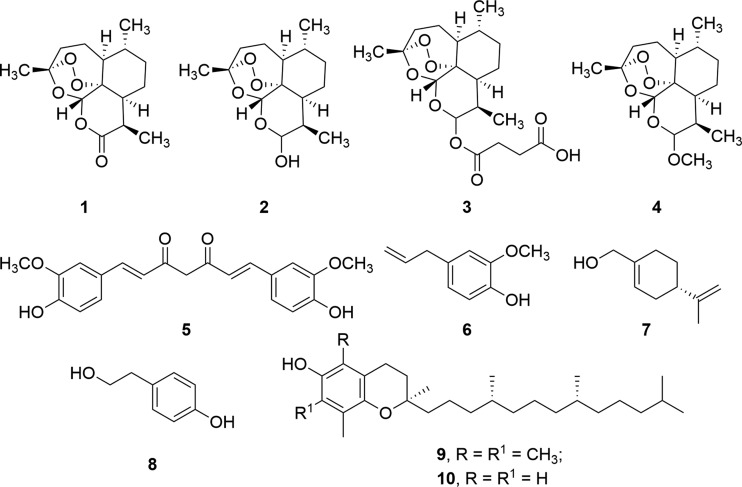
Artemisinin (1) is a sesquiterpene from Artemisia annua L. (sweet wormwood)1 characterized by a reactive 1,2,4-trioxane ring (endoperoxide bridge) and a lactone moiety as pharmacophores (Figure 1).2 This compound is applied in the treatment of different types of malaria.3 Semisynthetic derivatives of artemisinin, dihydroartemisinin 2, artesunate 3, and artemether 4, have been developed with the aim of increasing the pharmacological activity and the pharmacokinetic profile of the parent drug (Figure 1).4 In addition, artemisinin and artemisinin derivatives showed remarkable activity against cancer cell lines,5 including leukemia, melanoma, breast, ovarian, prostate, colon, and renal cancers, without inducing cytotoxicity in normal cells.6 This selectivity is due to the iron-mediated cleavage of the endoperoxide bridge in tumor cells containing a higher concentration of this metal with respect to their normal cell counterpart.7 Moreover, tumor cells usually show a diminished expression of antioxidant enzymes able to scavenge radical species.8 Iron catalyzes the ring opening of the endoperoxide bridge of artemisinin, with subsequent generation of free-radical reactive oxygen and carbon-centered species,9 followed by oxidative DNA damage. The mechanism is also responsible for the antimalarial effect of artemisinin, in which case the radical cascade is triggered by iron atoms delivered during the metabolic breakdown of hemoglobin in the vacuole of the parasite.10
Figure 1.
Artemisinin and semisynthetic derivatives of artemisinin and phytochemicals.
Recently, the synthesis of hybrid and dimer derivatives of bioactive natural substances turned out to be a useful strategy to increase both biological activity and pharmacokinetic profiles, avoiding drug-resistance phenomena with respect to the individual starting compounds.11 Dimers and hybrids of artemisinin and artemisinin derivatives in association with bioactive phytochemicals, such as thymoquinone, showed increased antileukemia and antimalarial activity.12 Different dimers and hybrids of artemisinin derivatives containing natural phenol and catechol residues, such as 2-(3-hydroxyphenyl)ethanol and 3-hydroxytyrosol, have also been patented.13
We report here the synthesis of a novel library of artemisinin-based hybrid and dimer derivatives obtained by chemical coupling of dihydroartemisinin 2 and artesunate 3 with chemopreventive phytochemicals, including curcumin 5, eugenol 6, perillyl alcohol 7, tyrosol 8, α-tocopherol 9, and δ-tocopherol 10, respectively (Figure 1).14 The products were evaluated on melanoma, the main cause of skin-cancer-related death and one of the most aggressive and lethal pathology in human.15 In this context, phytochemicals 5–10 showed antimelanoma activity as isolated compounds or components of natural extracts.16−19
Results and Discussion
Synthesis of Artemisinin-Based Hybrid and Dimer Derivatives
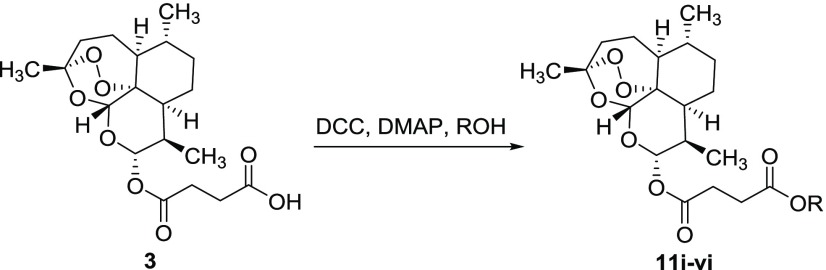
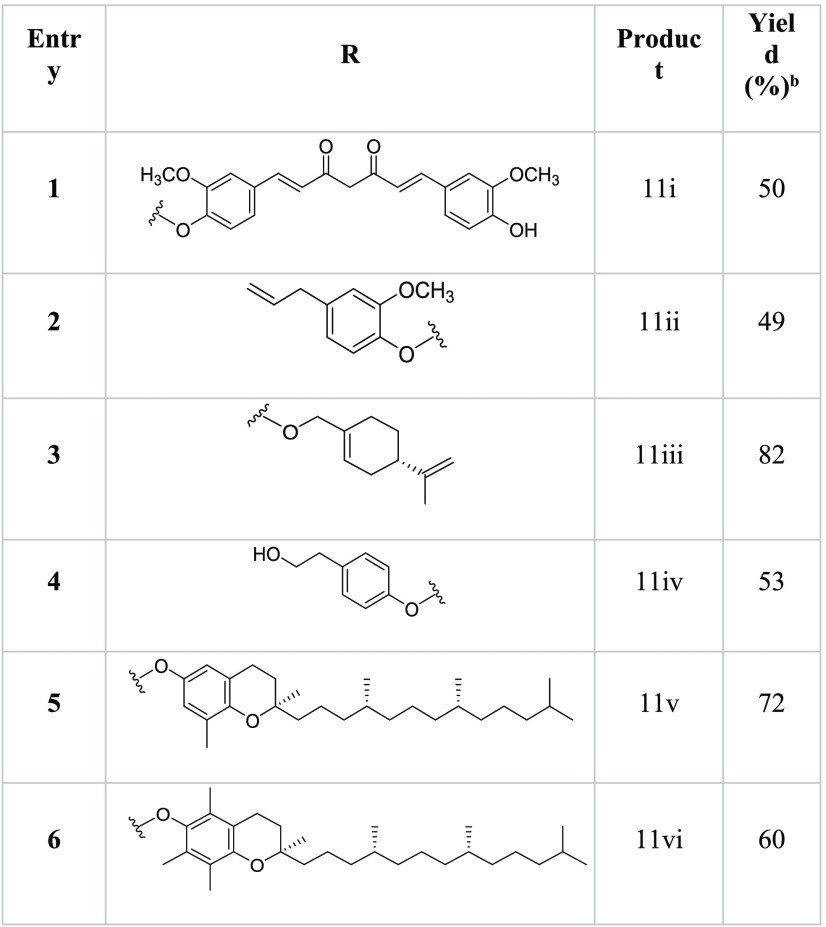
The hybrid derivatives of artemisinin, 11i–vi, were synthesized by a procedure involving the reaction between artesunate 3 (1.0 mmol) and the appropriate phytochemical, 5–10 (1.1 mmol), in the presence of N,N′-dicyclohexylcarbodiimide (DCC) (1.2 mmol) and 4-dimethylaminopyridine (DMAP) (catalytic amount) as coupling agents (Scheme 1). Compounds 11i–vi were obtained, as the only recovered products, besides the unreacted substrate, from acceptable to high yield (Table 1).
Scheme 1. Synthesis of Hybrid Derivatives 11i–iv.
Table 1. Yield of Hybrid Derivatives 11i–iv Obtained by the DCC/DMAP Coupling Reaction between Artesunate 3 and Phytochemicals 5–10a,b.
The reactions were performed by treating artesunate 3 (1.0 mmol) with DCC (1.2 mmol), DMAP (catalytic amount), and the appropriate phytochemical, 5–10 (1.1 mmol), at 25 °C for 18h.
Experiments were conducted in triplicate.
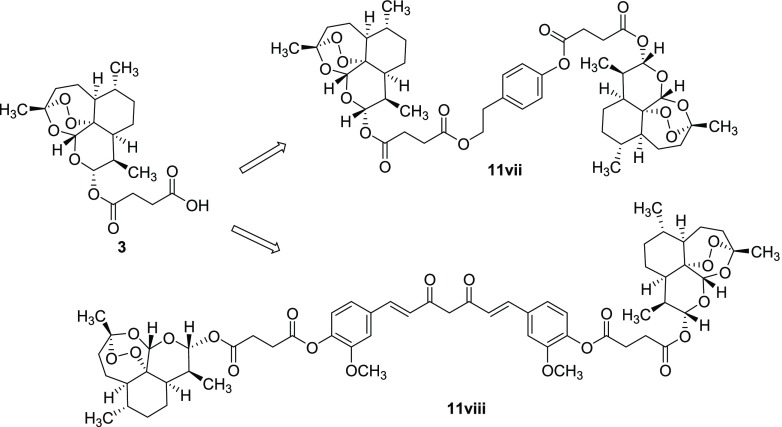
In addition, dimeric derivatives of artemisinin 11vii and 11viii were synthesized using phytochemicals bearing two reactive functional groups, such as curcumin 5 (two OH phenol moieties) and tyrosol 8 (OH phenol and primary alcohol moieties). In these latter cases, an excess of artesunate 3 (2.2 mmol) was required to optimize the yield of the desired product (Chart 1). Note that compound 11viii was characterized by a C-2 internal symmetry property.
Chart 1. Structures of Dimeric Derivatives 11vii and 11viii.
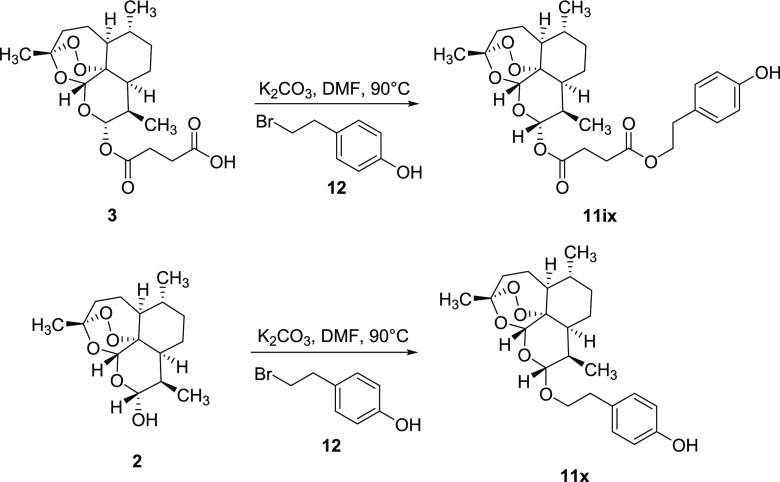
Finally, 4-hydroxyphenethyl bromide 12 was used as an electrophilic alkylating agent to obtain hybrid derivatives 11ix and 11x from artesunate 3 and dihydroartemisinin 2, respectively (Scheme 2). The reactions were performed under general basic catalysis conditions (K2CO3) in dimethylformamide (DMF, 10 mL) at 90 °C.
Scheme 2. Synthesis of Hybrid Derivatives 11ix (46%) and 11x (53%) from Artesunate 3 and Dihydroartemisinin 2, respectively.
In terms of structure relationships, compounds 11iv and 11ix were characterized by a different regioselectivity in the linkage of the tyrosol moiety to the artesunate scaffold, that is phenolic (11iv) versus primary alcohol (11ix) coupling site, respectively. The tyrosol moiety was linked to artesunate and dihydroartemisinin only by the primary alcoholic function in compounds 11ix and 11x.
Biological Activity
Artemisinin derivatives can inhibit metastasis20 and angiogenesis processes,21 influencing some cancer-related signaling pathways.22 We evaluated the anticancer activity of artemisinin 1, dihydroartemisinin 2, artesunate 3, and artemisinin derivatives 11i–x by cell survival [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide] (MTT) assay on a drug-resistant cervical cancer cell line (HeLa) and three complementary metastatic melanoma cancer cell lines, SK-MEL3, SK-MEL24, and RPMI-7951, respectively. The genetic characteristics of the SK-MEL3, SK-MEL24, and RPMI-7951 cell lines are summarized in Table 2. A normal human primary fibroblast cell line (C3PV) was used as a reference.
Table 2. Genetic Properties of Melanoma Cancer Cell Lines Used in the Biological Evaluations.
| name | mutant genea | zygosityb | gene Seq.c | protein seqd |
|---|---|---|---|---|
| SK-MEL3 | BRAF | Heterozygous | c.1799T>A | p.V600E |
| TP53 | Homozygous | c.799 C>T | p.R267W | |
| RPMI-7951 | BRAF | Heterozygous | c.1799T>A | p.V600E |
| CDKN2A | Homozygous | c.47T>G | p.L16R | |
| PTEN | Homozygous | c.1_79del79 | ||
| TP53 | Homozygous | c.497C>A | p.S166* | |
| SK-MEL24 | BRAF | Heterozygous | c.1799T>A | p.V600E |
| CDKN2A | Homozygous | c.1_471del471 | ||
| PTEN | Homozygous | c.80_164del85 |
These cell lines are characterized by mutations in different genes and gene regions that are representative of the metastatic melanoma.
Mutation state.
Mutation gene sequencing.
Mutation protein sequencing.
All three melanoma cell lines show the mutation of the B-RAF gene, which is involved in the regulation of the RAS/MAPK pathway, controlling cell proliferation, differentiation, migration, and apoptosis. The B-RAF gene mutation is highly frequent in both primary and metastatic melanoma cells, accounting for 50.9 and 66.3% of the total observed mutations, respectively.23 Two cell lines, SK-MEL-3 and RPMI-7951, are mutated on the TP53 gene, which controls the synthesis of the p53 protein, involved in cell division and prevention of cancer formation.24 Finally, the RPMI-7951 and SK-MEL24 cell lines were characterized by the cyclin-dependent kinase inhibitor 2A (CDKN2A)25 and phosphatase and tensin homolog (PTEN)26 gene mutations, controlling the production of tumor suppressor proteins.
Phytochemicals 5–10, and the well-known anticancer drug, paclitaxel (Taxol), were used as references. Table 3 reports the IC50 values (half-maximal inhibitory concentration) of the tested compounds. The stability of compound 11iv in the cell culture medium has been evaluated by thin-layer chromatographic (TLC) and high-performance liquid chromatography (HPLC) analyses as a selected example. No traces of tyrosol were observed during 48 h, confirming the stability of the ester bond under the reported experimental conditions (see the Supporting Information SI #1–2 and Figures S1–S3).
Table 3. Anticancer Activity of Artemisinin 1, Dihydroartemisinin 2, Artesunate 3, Hybrid Derivatives 11i-iv and 11ix-x, and Dimeric Derivatives 11vii and 11viiia.
| IC50 ± SDb |
||||||
|---|---|---|---|---|---|---|
| entry | CPD | C3PV | HeLa | SK-MEL3 | SK-MEL24 | RPMI-7951 |
| 1 | 1 | >300 ± 14.85 | 1.37 ± 0.59 | 3.43 ± 0.86 | 8.66 ± 4.56 | 3.62 ± 0.99 |
| 2 | 2 | 0.68 ± 0.19 | 1.32 ± 0.26 | 10.36 ± 0.61 | 3.23 ± 1.60 | 0.91 ± 0.45 |
| 3 | 3 | 1.68 ± 0.44 | 1.76 ± 0.38 | >300 ± 5.07 | 0.82 ± 0.37 | 1.08 ± 0.56 |
| 4 | 11i | 2.36 ± 0.24 | 1.15 ± 1.09 | 3.23 ± 0.37 | 5.88 ± 2.25 | 10.43 ± 1.60 |
| 5 | 11ii | 6.79 ± 0.15 | 1.44 ± 1.09 | 4.65 ± 1.80 | 5.44 ± 2.4 | 1.21 ± 0.63 |
| 6 | 11iii | >300 ± 35.35 | 1.76 ± 0.47 | 5.24 ± 2.29 | 0.06 ± 0.1 | 2.35 ± 1.52 |
| 7 | 11iv | >300 ± 10.71 | >300 ± 7.071 | 2.33 ± 1.18 | 2.32 ± 1.19 | 8.34 ± 3.06 |
| 8 | 11v | 0.85 ± 0.32 | 4.39 ± 0.77 | 2.61 ± 0.49 | >300 ± 21.21 | 3.06 ± 0.17 |
| 9 | 11vi | 10.18 ± 1.57 | 3.34 ± 0.66 | 1.23 ± 0.83 | 3.69 ± 0.91 | 1.90 ± 0.35 |
| 10 | 11vii | 6.10 ± 3.74 | 2.16 ± 0.54 | 32.78 ± 5.81 | 0.24 ± 0.12 | 0.49 ± 0.05 |
| 11 | 11viii | >300 ± 14.84 | 2.41 ± 0.56 | 3.43 ± 0.16 | 2.54 ± 0.47 | 1.37 ± 0.13 |
| 12 | 11ix | 132.20 ± 12.58 | 2.66 ± 0.82 | 1.28 ± 0.15 | 1.5 ± 0.85 | 0.09 ± 0.03 |
| 13 | 11x | 1.76 ± 0.31 | 2.73 ± 0.37 | 10.58 ± 1.14 | 3.01 ± 0.57 | 0.33 ± 0.08 |
| 14 | 5 | >300 ± 31.82 | 2.12 ± 0.94 | 1.69 ± 1.24 | 3.00 ± 1.35 | 3.95 ± 0.09 |
| 15 | 6 | 6.88 ± 0.085 | 4.68 ± 1.64 | 1.16 ± 0.32 | 2.61 ± 0.34 | 0.84 ± 0.34 |
| 16 | 7 | 3.80 ± 0.28 | 1.63 ± 0.40 | 1.37 ± 0.59 | 2.69 ± 1.3 | 1.71 ± 0.52 |
| 17 | 8 | 22.71 ± 1.83 | 1.41 ± 0.79 | 2.53 ± 1.41 | 1.69 ± 0.15 | 10.83 ± 1.40 |
| 18 | 9 | 74.09 ± 5.09 | 3.03 ± 1.5 | 1.22 ± 0.64 | 2.35 ± 1.05 | 4.11 ± 2.1 |
| 19 | 10 | 15.82 ± 0.97 | 4.01 ± 0.91 | 2.93 ± 0.15 | 5.57 ± 2.01 | 5.69 ± 1.91 |
| 20 | paclitaxel | 78.88 ± 0.79 | 0.006 ± 0.0014 | 4.73 ± 0.83 | 1.04 ± 0.31 | 0.013 ± 0.19 |
All experiments were conducted in triplicate.
IC50 ± SD (half-maximal inhibitory concentration ± standard deviation) values for all compounds are expressed in micromolar units.
In the case of the HeLa cell line, artemisinin derivatives were less active than paclitaxel, with an antiproliferative effect comparable to that of artemisinin. The dimer, 11vii, containing the tyrosol scaffold, showed a remarkable anticancer activity against both SK-MEL24 and RPMI-7951 melanoma cell lines, with IC50 values (0.24 and 0.49 μM, respectively) lower than, or comparable to, those of artemisinin and paclitaxel, respectively. Unfortunately, 11vii was also characterized by a significant activity against the C3PV cell line. The dimer, 11viii, containing the curcumin scaffold, was active against all melanoma cell lines studied, without any appreciable effect on the C3PV cell.
Note that perillyl alcohol 7 and tyrosol 8 showed higher activity and lower toxicity when incorporated into the artemisinin scaffold, as in the case of hybrids 11iii (Table 3, entry 6 versus entry 16) and 11ix (Table 3, entry 12 versus entry 17) against the SK-MEL24 and RPMI-7951 cell lines, respectively.
Compounds 11iv and 11ix showed antiproliferative activity against all studied melanoma cell lines, with acceptable tolerability toward the C3PV cell line. They were characterized by a toxicity higher than that of tyrosol 8, highlighting a different effect of the artemisinin scaffold on the toxicity of the hybrid, depending on the nature of the associated phytochemical.
Artemisinin 1 and the novel artemisinin derivatives, 11iii–iv, 11viii, and 11ix, were found to be cancer-selective, being not cytotoxic on normal cell line C3PV. Moreover, compound 11iv possessed a melanoma-specific cancer selectivity (inactive against HeLa).
As a general trend, curcumin 5 was the most effective phytochemical in the dimer-type derivatives, whereas perillyl alcohol 7 and tyrosol 8 were the most effective in the hybrid-type compounds. Moreover, artesunate appeared to be the most active artemisinin derivative. To obtain information about the mechanism of action of compounds 11iii–iv, 11viii, and 11ix, we repeated the cellular assays on the cancer lines in the presence of the iron chelating agent, deferoxamine (DFO),27,28 able to inhibit the redox activation of the endoperoxide ring.
As reported in Table 4, the presence of DFO significantly lowered the efficacy of artemisinin 1, confirming the beneficial role of iron in triggering the radical cascade mechanism responsible for the anticancer activity. A similar pathway was observed for compounds 11iv and 11ix containing the tyrosol scaffold. Instead, the anticancer activity of perillyl alcohol and curcumin in 11iii and 11viii was found to be independent from the presence of iron, suggesting the possibility of an alternative mechanism. Regarding the SK-MEL24 line, the activity of the four synthetic compounds seems to be not influenced by the iron chelation mediated by DFO.
Table 4. IC50 Values of Compounds 11iii, 11iv, 11viii, and 11ix on the RPMI-7951, SK-MEL3, and SK-MEL24 Cancer Cell Lines in the Presence or Absence of DFOa,b.
| IC50 ± SDc |
||||||
|---|---|---|---|---|---|---|
| RPMI-7951 |
SK-MEL3 |
SK-MEL24 |
||||
| CPD | without DFO | with DFO | without DFO | with DFO | without DFO | with DFO |
| 1 | 3.62 ± 1.6 | 28.97 ± 2.8 | 3.43 ± 0.8 | >300 ± 3.8 | 8.66 ± 0.9 | 27.8 ± 3.4 |
| 3 | 1.08 ± 0.6 | 1.09 ± 0.9 | >300 ± 5.6 | >300 ± 4.5 | 0.82 ± 0.05 | 0.90 ± 0.02 |
| 11iii | 2.35 ± 0.9 | 2.95 ± 0.7 | 5.24 ± 0.2 | 5.6 ± 0.6 | 0.06 ± 0.01 | 0.16 ± 0.04 |
| 11iv | 8.34 ± 2.7 | 81.7 ± 5.7 | 2.33 ± 0.09 | 24.5 ± 1.3 | 2.32 ± 0.9 | 2.5 ± 0.6 |
| 11viii | 1.37 ± 0.9 | 1.18 ± 0.7 | 2.41 ± 0.8 | 3.8 ± 0.8 | 2.54 ± 0.5 | 3.3 ± 0.8 |
| 11ix | 0.09 ± 0.05 | 24.5 ± 2.1 | 2.66 ± 0.9 | 10.5 ± 2.3 | 1.5 ± 0.04 | 6.0 ± 1.5 |
All experiments were conducted in triplicate.
The treatment time was 24 hours for all experiments.
IC50 ± SD (half-maximal inhibitory concentration ± standard deviation) values for all compounds are expressed in micromolar units.
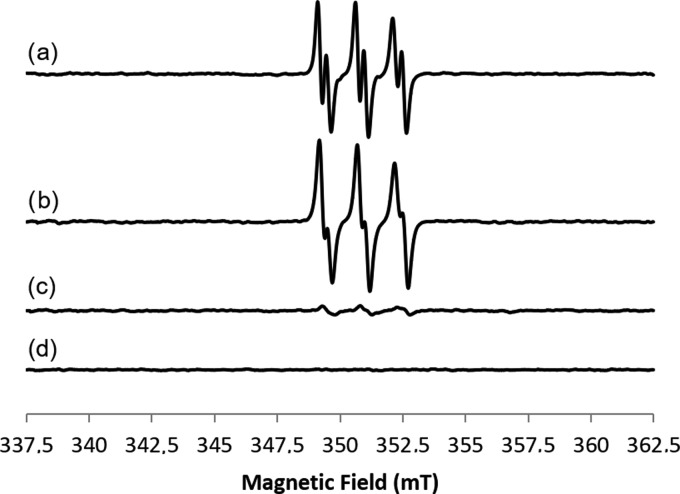
Electron paramagnetic resonance (EPR) analysis was further performed to characterize the radical species formed during the endoperoxide ring opening in the presence of Fe(II)SO4 as a catalyst (for the general procedure see SI #3). Figure 2 shows the EPR spectra of 2-methyl-nitrosopropane (MNP) adducts of artesunate derivatives 11iii, 11iv, 11ix, and 11viii, recorded as representative active samples (25 °C, t = 0), in comparison with the appropriate reference [that is, the compound and MNP without the Fe(II) salt].
Figure 2.
Room-temperature CW-EPR spectra of (a) 11iv, (b) 11iii, (c) 11ix, and (d) blank, all recorded at t = 0 in the presence of the spin trap MNP. Experimental conditions: ν = 9.86GHz, 0.1 mT modulation amplitude, and 0.63 mW microwave power.
The EPR spectra of hybrid derivatives 11iv and 11iii (Figure 2, lines a and b, respectively) show the same line shape with giso = 2.006 ± 0.0001, AN = 1.49 ± 0.1mT, and AH = 0.3±0.01mT. In accordance with the EPR results previously reported for artemisinin,29 the magnetic parameters of artesunate derivatives 11iv and 11iii clearly identified the formation of a secondary C-centered radical.30 The EPR signal decreased over time (Figures S4 and S5, respectively). For hybrid derivative 11ix (line c), the C-centered radical is present with a low intensity at t = 0, being stable during all recorded times (see Figure S6). Unexpectedly, in the case of dimer derivative 11viii (Figure S8), no radical formation was detected (only at t = 90 min a very weak signal appeared, but it was no more present at t = 150 min). Note that artesunate alone showed a different behavior with respect to hybrids 11iv and 11iii (Figure S7).
Even though a direct correlation between the in vitro EPR analysis of artemisinin derivatives and the in-cell anticancer activity cannot be made, in the case of compound 11iv, the formation of a secondary C-centered radical was in accordance with the role suggested for this type of intermediate in the biological activity of artemisinin.29 Moreover, the biological activity of 11iv dropped after treatment with DFO, whereas that of compound 11viii, deprived of an EPR signal, was unaffected by the DFO treatment.
Conclusions
A library of novel derivatives with hybrid and dimer structures were synthesized by coupling between artesunate and dihydroartemisinin, with six phytochemicals, curcumin, eugenol, perillyl alcohol, tyrosol, and α- and δ-tocopherol. Among the novel derivatives, 11iii–iv, 11viii, and 11ix showed a significant and selective anticancer activity. Moreover, the hybrid derivative, 11iv, was characterized by a specific melanoma selectivity, being inactive against HeLa cells. This compound showed the formation of a secondary C-centered radical in the EPR analysis. DFO studies on the role of endoperoxide ring opening in the antimelanoma activity of 11iv confirmed the relevant role of the formation of radical intermediates in the biological effect. Surprisingly, the anticancer activity of dimer 11viii was not dependent on the formation of radical species, leaving open the possibility for other mechanisms to be operative. Further studies aimed at the determination of topoisomerase I as a possible target for the activity of these novel derivatives, as well as transcriptome comparison with other efficient antimelanoma drugs are still ongoing.
Experimental Section
Chemistry: General Part
All reactions were performed in flame-dried glassware under a nitrogen atmosphere. Reagents were obtained from commercial suppliers (Sigma-Aldrich Srl, Milan, Italy) and used without further purification. TLC chromatography was performed on precoated aluminum silica gel SIL G/UV254 plates (Macherey-Nagel & Co.). The detection occurred via fluorescence quenching or development in a molybdato phosphate solution (10% in EtOH). Merck silica gel 60 was used for flash chromatography (23–400 mesh). All products were dried in high vacuum (10-3 mbar). 1H NMR and 13C NMR spectra were measured on a Bruker Avance DRX400 (400 MHz/100 MHz) and on a Bruker Avance DPX200 (200 MHz/50 MHz) spectrometers. Chemical shifts for protons are reported in parts per million (δ scale) and internally referenced to the CD3OD or CDCl3 signal at δ 3.33 and 7.28 ppm, respectively. Mass spectral (MS) data were obtained using an Agilent 1100 LC/MSD VL system (G1946C) with a 0.4 mL/min flow rate using a binary solvent system of 95:5 methyl alcohol/water. UV detection was monitored at 254 nm. Mass spectra were acquired in positive and negative mode scanning over the mass range. Elemental analyses (C, H, N) were performed in house. Artemisinin, dihydroartemisinin, and artesunate were obtained from Lachifarma s.r.l. (Zollino (LE), Italy).
General Procedure for the Synthesis of Derivatives 11i–vii
To a solution of artesunate (0.52 mmol, 1.0 equiv) in dry CH2Cl2 (3.0 mL), DCC (0.52 mmol, 1.0 equiv) and DMAP (0.16 mmol, 30 mol %) were added at room temperature. After the addition of the opportune phytochemical (0.52 mmol, 1.0 equiv), the reaction mixture was slowly stirred overnight (18 h) under a N2 atmosphere. The precipitated dicyclohexylurea was removed by filtration, and the solvent was removed under reduced pressure. The residue was purified by gradient column chromatography (hexane/EtOAc 1:1, 1:2).
Compound 11i
 Yield = 50%. Rf = 0.36 (hexane/EtOAc 1:2, molybdato phosphate). 1H NMR
(CDCl3, 400 MHz): δ = 7.62 (d, 1H, J = 7.2 Hz), 7.58 (d, 1H, J = 7.2 Hz), 7.16–7.06
(m, 5H), 6.94 (d, 1H, J = 8.0 Hz), 6.57–6.46
(m, 2H), 5.85–5.81 (m, 2H), 5.46 (s, 1H), 3.95 (s, 3H), 3.88
(s, 3H), 2.98–2.92 (m, 2H), 2.88–2.84 (m, 2H), 2.62–2.57
(m, 1H), 2.43–2.34 (m, 1H), 2.04–1.47 (m, 6H), 1.45
(s, 3H), 1.41–1.30 (m, 2H), 1.08–1.00 (m, 1H), 0.98
(d, 3H, J = 5.7 Hz), 0.86 (d, 3H, J = 7.2 Hz) ppm. 13C NMR (CDCl3, 100 MHz): δ
= 184.5, 183.3, 183.1, 181.9, 171.2, 170.9, 170.1, 151.4, 148.1, 147.9,
146.9, 141.1, 140.6, 134.1, 124.3, 123.0, 122.1, 114.9, 109.7, 104.5,
92.4, 92.3, 80.1, 60.4, 55.9, 51.6, 45.2, 37.3, 36.2, 34.1, 31.8,
29.1, 28.8, 25.9, 24.6, 22.1, 22.0, 21.0 20.2 12.0 ppm. MS (ESI): m/z for [C40H45O13]−: 733. Anal. calcd for C40H46O13: C, 65.38; H, 6.31; O, 28.31; found:
C, 65.34; H, 6.30; O, 28.28.
Yield = 50%. Rf = 0.36 (hexane/EtOAc 1:2, molybdato phosphate). 1H NMR
(CDCl3, 400 MHz): δ = 7.62 (d, 1H, J = 7.2 Hz), 7.58 (d, 1H, J = 7.2 Hz), 7.16–7.06
(m, 5H), 6.94 (d, 1H, J = 8.0 Hz), 6.57–6.46
(m, 2H), 5.85–5.81 (m, 2H), 5.46 (s, 1H), 3.95 (s, 3H), 3.88
(s, 3H), 2.98–2.92 (m, 2H), 2.88–2.84 (m, 2H), 2.62–2.57
(m, 1H), 2.43–2.34 (m, 1H), 2.04–1.47 (m, 6H), 1.45
(s, 3H), 1.41–1.30 (m, 2H), 1.08–1.00 (m, 1H), 0.98
(d, 3H, J = 5.7 Hz), 0.86 (d, 3H, J = 7.2 Hz) ppm. 13C NMR (CDCl3, 100 MHz): δ
= 184.5, 183.3, 183.1, 181.9, 171.2, 170.9, 170.1, 151.4, 148.1, 147.9,
146.9, 141.1, 140.6, 134.1, 124.3, 123.0, 122.1, 114.9, 109.7, 104.5,
92.4, 92.3, 80.1, 60.4, 55.9, 51.6, 45.2, 37.3, 36.2, 34.1, 31.8,
29.1, 28.8, 25.9, 24.6, 22.1, 22.0, 21.0 20.2 12.0 ppm. MS (ESI): m/z for [C40H45O13]−: 733. Anal. calcd for C40H46O13: C, 65.38; H, 6.31; O, 28.31; found:
C, 65.34; H, 6.30; O, 28.28.
Compound 11ii
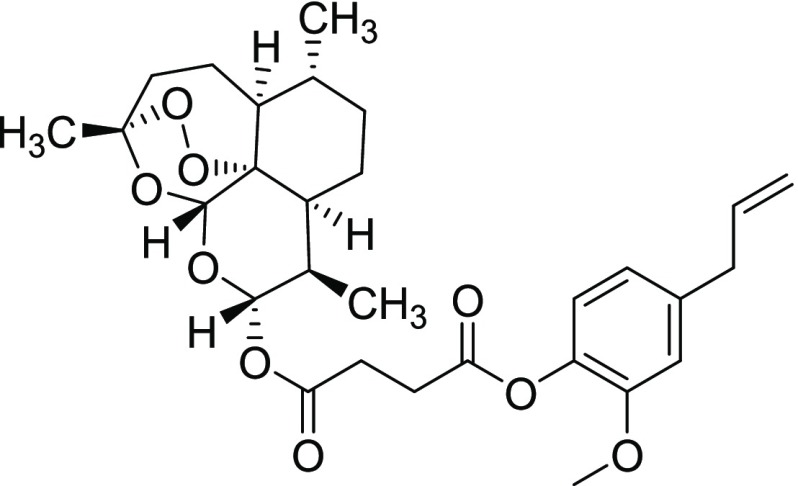 Yield = 49%. Rf = 0.70 (hexane/EtOAc 4:1, molybdato phosphate). 1H NMR
(CDCl3, 200 MHz): δ = 6.95 (d, 1H, J = 8.0 Hz), 6.74–6.70 (m, 2H), 5.99–5.85 (m, 1H), 5.77
(d, 1H, J = 9.8 Hz), 5.42 (s, 1H), 5.08 (d, 1H, J = 6.8 Hz), 5.02 (s, 1H), 3.77 (s, 3H), 3.34 (d, 2H, J = 6.6 Hz), 2.96–2.76 (m, 4H), 2.60–2.41
(m, 1H), 2.04–1.47 (m, 6H), 1.41 (s, 3H), 1.30–1.05
(m, 4H), 0.93 (d, 3H, J = 5.2 Hz), 0.81 (d, 3H, J = 7.2 Hz) ppm. 13C NMR (CDCl3, 50
MHz): δ = 170.9, 170.4, 138.9, 137.9, 137.0, 122.5, 120.6, 116.1,112.7,
104.4, 92.2, 91.5, 80.1, 55.8, 51.6, 45.2, 40.0, 37.3, 36.2, 34.1,
31.8, 29.3, 28.7, 25.9, 24.6, 21.9, 20.2, 12.0 ppm. MS (ESI): m/z for [C29H39O9]+: 532. Anal. calcd for C29H39O9: C, 65.64; H, 7.22; O, 27.14; found: C, 65.67; H, 7.24;
O, 27.16.
Yield = 49%. Rf = 0.70 (hexane/EtOAc 4:1, molybdato phosphate). 1H NMR
(CDCl3, 200 MHz): δ = 6.95 (d, 1H, J = 8.0 Hz), 6.74–6.70 (m, 2H), 5.99–5.85 (m, 1H), 5.77
(d, 1H, J = 9.8 Hz), 5.42 (s, 1H), 5.08 (d, 1H, J = 6.8 Hz), 5.02 (s, 1H), 3.77 (s, 3H), 3.34 (d, 2H, J = 6.6 Hz), 2.96–2.76 (m, 4H), 2.60–2.41
(m, 1H), 2.04–1.47 (m, 6H), 1.41 (s, 3H), 1.30–1.05
(m, 4H), 0.93 (d, 3H, J = 5.2 Hz), 0.81 (d, 3H, J = 7.2 Hz) ppm. 13C NMR (CDCl3, 50
MHz): δ = 170.9, 170.4, 138.9, 137.9, 137.0, 122.5, 120.6, 116.1,112.7,
104.4, 92.2, 91.5, 80.1, 55.8, 51.6, 45.2, 40.0, 37.3, 36.2, 34.1,
31.8, 29.3, 28.7, 25.9, 24.6, 21.9, 20.2, 12.0 ppm. MS (ESI): m/z for [C29H39O9]+: 532. Anal. calcd for C29H39O9: C, 65.64; H, 7.22; O, 27.14; found: C, 65.67; H, 7.24;
O, 27.16.
Compound 11iii
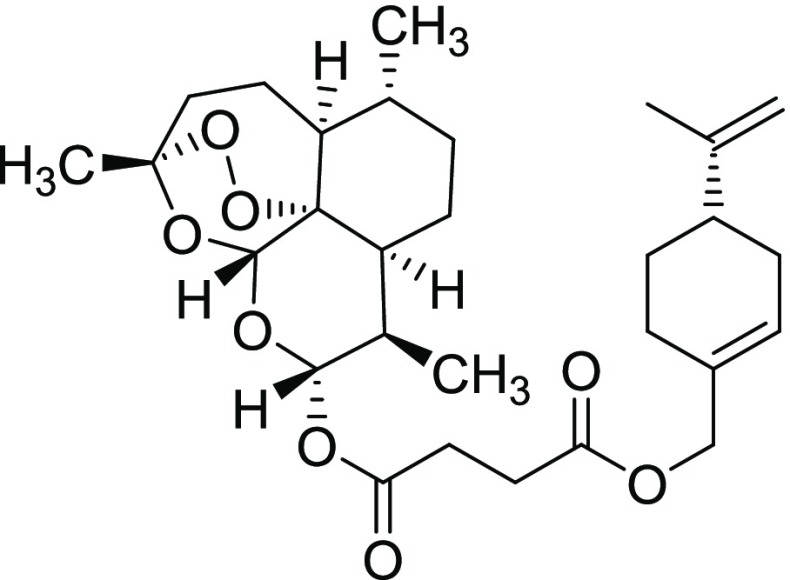 Yield = 82%. Rf = 0.67 (hexane/EtOAc 4:1, molybdato phosphate). 1H NMR
(CDCl3, 200 MHz): δ = 5.75 (d, 1H, J = 9.8 Hz), 5.72–5.71 (m, 1H), 5.39 (s, 1H), 4.68–4.66
(m, 2H), 4.43 (s, 2H), 2.71–2.61 (m, 4H), 2.60–2.41
(m, 1H), 2.39–2.25 (m, 1H), 2.12–1.76 (m, 10H), 1.69
(s, 3H), 1.63–1.41 (m, 3H), 1.38 (s, 3H), 1.35–1.17
(m, 3H), 1.16–0.94 (m, 1H), 0.92 (d, 3H, J = 5.6 Hz), 0.81 (d, 3H, J = 7.2 Hz) ppm. 13C NMR (CDCl3, 50 MHz): δ = 171.9, 171.0, 149.5,
132.4, 125.9, 108.7, 104.4, 92.1, 91.5, 80.0, 68.7, 51.5, 45.2, 40.7,
37.2, 34.1, 31.8, 30.4, 29.2, 28.9, 27.2, 26.3, 26.1, 25.8, 24.5,
21.9, 20.7, 20.2, 12.0 ppm. MS (ESI): m/z for [C29H43O8]+: 519.
Anal. calcd for C29H42O8: C, 67.16;
H, 8.16; O, 24.68; found: C, 67.17; H, 8.14; O, 24.66.
Yield = 82%. Rf = 0.67 (hexane/EtOAc 4:1, molybdato phosphate). 1H NMR
(CDCl3, 200 MHz): δ = 5.75 (d, 1H, J = 9.8 Hz), 5.72–5.71 (m, 1H), 5.39 (s, 1H), 4.68–4.66
(m, 2H), 4.43 (s, 2H), 2.71–2.61 (m, 4H), 2.60–2.41
(m, 1H), 2.39–2.25 (m, 1H), 2.12–1.76 (m, 10H), 1.69
(s, 3H), 1.63–1.41 (m, 3H), 1.38 (s, 3H), 1.35–1.17
(m, 3H), 1.16–0.94 (m, 1H), 0.92 (d, 3H, J = 5.6 Hz), 0.81 (d, 3H, J = 7.2 Hz) ppm. 13C NMR (CDCl3, 50 MHz): δ = 171.9, 171.0, 149.5,
132.4, 125.9, 108.7, 104.4, 92.1, 91.5, 80.0, 68.7, 51.5, 45.2, 40.7,
37.2, 34.1, 31.8, 30.4, 29.2, 28.9, 27.2, 26.3, 26.1, 25.8, 24.5,
21.9, 20.7, 20.2, 12.0 ppm. MS (ESI): m/z for [C29H43O8]+: 519.
Anal. calcd for C29H42O8: C, 67.16;
H, 8.16; O, 24.68; found: C, 67.17; H, 8.14; O, 24.66.
Compound 11iv
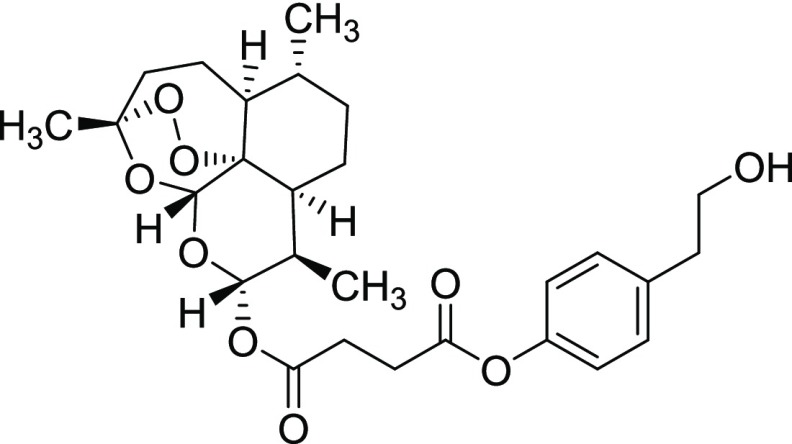 Yield = 53%. Rf = 0.50 (hexane/EtOAc 4:1, molybdato phosphate). 1H NMR
(CDCl3, 200 MHz): δ = 7.17 (d, 2H, J = 8.6 Hz), 6.98 (d, 1H, J = 8.6 Hz), 5.78 (d, 1H, J = 10.0 Hz), 5.40 (s, 1H), 3.76 (t, 2H, J = 6.6 Hz), 2.85–2.75 (m, 6H), 2.55–2.51 (m, 1H), 2.34–2.25
(m, 1H), 2.02–1.41 (m, 6H), 1.41 (s, 3H), 1.35–0.99
(m, 4H), 0.91 (d, 3H, J = 5.4 Hz), 0.80 (d, 3H, J = 7.2 Hz) ppm. 13C NMR (CDCl3, 50
MHz): δ = 170.9, 170.8, 149.1, 136.3, 129.9, 121.5, 104.5, 92.3,
91.5, 80.1, 63.5, 51.5, 45.2, 38.5, 37.2, 36.2, 34.1, 31.8, 29.2,
29.0, 25.8, 24.6, 21.9, 20.2, 14.2, 12.0 ppm. MS (ESI): m/z for [C27H36O9Na]+: 528. Anal. calcd for C27H36O9: C, 64.27; H, 7.19; O, 28.54; found: C, 64.28; H, 7.20;
O, 28.55.
Yield = 53%. Rf = 0.50 (hexane/EtOAc 4:1, molybdato phosphate). 1H NMR
(CDCl3, 200 MHz): δ = 7.17 (d, 2H, J = 8.6 Hz), 6.98 (d, 1H, J = 8.6 Hz), 5.78 (d, 1H, J = 10.0 Hz), 5.40 (s, 1H), 3.76 (t, 2H, J = 6.6 Hz), 2.85–2.75 (m, 6H), 2.55–2.51 (m, 1H), 2.34–2.25
(m, 1H), 2.02–1.41 (m, 6H), 1.41 (s, 3H), 1.35–0.99
(m, 4H), 0.91 (d, 3H, J = 5.4 Hz), 0.80 (d, 3H, J = 7.2 Hz) ppm. 13C NMR (CDCl3, 50
MHz): δ = 170.9, 170.8, 149.1, 136.3, 129.9, 121.5, 104.5, 92.3,
91.5, 80.1, 63.5, 51.5, 45.2, 38.5, 37.2, 36.2, 34.1, 31.8, 29.2,
29.0, 25.8, 24.6, 21.9, 20.2, 14.2, 12.0 ppm. MS (ESI): m/z for [C27H36O9Na]+: 528. Anal. calcd for C27H36O9: C, 64.27; H, 7.19; O, 28.54; found: C, 64.28; H, 7.20;
O, 28.55.
Compound 11v
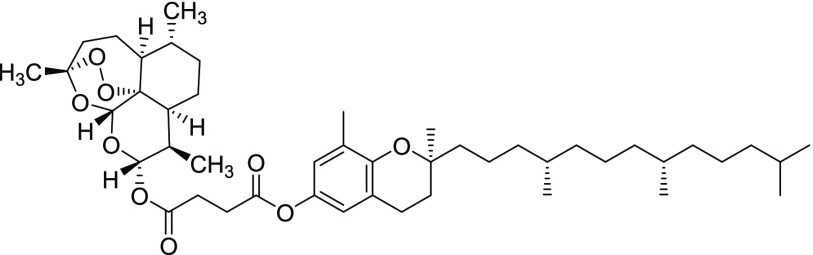 Yield = 72%. Rf = 0.19 (hexane/EtOAc 9:1, molybdato phosphate). 1H NMR
(CDCl3, 200 MHz): δ = 6.65–6.58 (m, 2H), 5.80
(d, 1H, J = 9.8 Hz), 5.42 (s, 1H), 2.91–2.60
(m, 8H), 2.58–2.45 (m, 1H), 2.36–2.21 (m, 1H), 2.04
(s, 3H), 2.02–1.05 (m, 25H), 1.41 (s, 3H), 1.23 (s, 3H), 0.94
(d, 3H, J = 5.8 Hz), 0.89–0.80 (m, 18H) ppm. 13C NMR (CDCl3, 50 MHz): δ = 171.3, 170.9,
149.7, 142.3, 127.2, 121.0, 120.8, 118.9, 104.4, 92.2, 91.5, 80.0,
51.5, 45.2, 40.1, 39.3, 37.4, 37.2, 34.1, 32.7, 32.6, 31.7, 29.2,
28.9, 25.9, 24.6, 24.4, 24.2, 22.7, 22.6, 22.4, 22.0, 21.0, 20.2,
19.7, 19.6, 16.0, 12.4 ppm. MS (ESI): m/z for [C46H73O9]+: 769.
Anal. calcd for C46H72O9: C, 71.84;
H, 9.44; O, 18.72; found: C, 71.87; H, 9.43; O, 18.75.
Yield = 72%. Rf = 0.19 (hexane/EtOAc 9:1, molybdato phosphate). 1H NMR
(CDCl3, 200 MHz): δ = 6.65–6.58 (m, 2H), 5.80
(d, 1H, J = 9.8 Hz), 5.42 (s, 1H), 2.91–2.60
(m, 8H), 2.58–2.45 (m, 1H), 2.36–2.21 (m, 1H), 2.04
(s, 3H), 2.02–1.05 (m, 25H), 1.41 (s, 3H), 1.23 (s, 3H), 0.94
(d, 3H, J = 5.8 Hz), 0.89–0.80 (m, 18H) ppm. 13C NMR (CDCl3, 50 MHz): δ = 171.3, 170.9,
149.7, 142.3, 127.2, 121.0, 120.8, 118.9, 104.4, 92.2, 91.5, 80.0,
51.5, 45.2, 40.1, 39.3, 37.4, 37.2, 34.1, 32.7, 32.6, 31.7, 29.2,
28.9, 25.9, 24.6, 24.4, 24.2, 22.7, 22.6, 22.4, 22.0, 21.0, 20.2,
19.7, 19.6, 16.0, 12.4 ppm. MS (ESI): m/z for [C46H73O9]+: 769.
Anal. calcd for C46H72O9: C, 71.84;
H, 9.44; O, 18.72; found: C, 71.87; H, 9.43; O, 18.75.
Compound 11vi
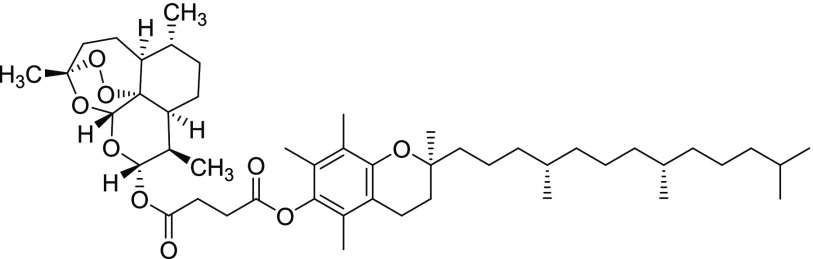 Yield = 60%. Rf = 0.27 (hexane/EtOAc 9:1, molybdato phosphate). 1H NMR
(CDCl3, 200 MHz): δ = 5.80 (d, 1H, J = 9.8 Hz), 5.42 (s, 1H), 2.96–2.70 (m, 4H), 2.56–2.49
(m, 3H), 2.45–2.21 (m, 1H), 2.36–2.21 (m, 1H), 2.02
(s, 3H), 1.99 (s, 3H), 1.95 (s, 3H), 1.87–1.01 (m, 8H), 0.95–0.81
(m, 18H) ppm. 13C NMR (CDCl3, 50 MHz): δ
= 170.9, 170.7, 149.4, 140.5, 126.7, 124.9, 122.9, 117.3, 104.4, 92.3,
91.5, 80.0, 75.0, 51.6, 45.2, 39.4, 37.4, 37.3, 37.2, 36.2, 34.1,
32.8, 32.7, 31.8, 29.2, 27.9, 25.9, 24.8, 24.4, 22.7, 22.6, 22.0,
21.0, 20.2, 19.8, 19.7, 12.9, 12.1, 11.8 ppm. MS (ESI): m/z for [C48H76O9]+: 784. Anal. calcd for C46H72O9: C, 72.33; H, 9.61; O, 18.06; found: C, 72.31; H, 9.62; O,
18.05.
Yield = 60%. Rf = 0.27 (hexane/EtOAc 9:1, molybdato phosphate). 1H NMR
(CDCl3, 200 MHz): δ = 5.80 (d, 1H, J = 9.8 Hz), 5.42 (s, 1H), 2.96–2.70 (m, 4H), 2.56–2.49
(m, 3H), 2.45–2.21 (m, 1H), 2.36–2.21 (m, 1H), 2.02
(s, 3H), 1.99 (s, 3H), 1.95 (s, 3H), 1.87–1.01 (m, 8H), 0.95–0.81
(m, 18H) ppm. 13C NMR (CDCl3, 50 MHz): δ
= 170.9, 170.7, 149.4, 140.5, 126.7, 124.9, 122.9, 117.3, 104.4, 92.3,
91.5, 80.0, 75.0, 51.6, 45.2, 39.4, 37.4, 37.3, 37.2, 36.2, 34.1,
32.8, 32.7, 31.8, 29.2, 27.9, 25.9, 24.8, 24.4, 22.7, 22.6, 22.0,
21.0, 20.2, 19.8, 19.7, 12.9, 12.1, 11.8 ppm. MS (ESI): m/z for [C48H76O9]+: 784. Anal. calcd for C46H72O9: C, 72.33; H, 9.61; O, 18.06; found: C, 72.31; H, 9.62; O,
18.05.
General Procedure for the Synthesis of Derivatives 11vii and 11viii:
To a solution of artesunate (0.52 mmol, 1.0 equiv) in dry CH2Cl2 (3.0 mL), DCC (1.04 mmol, 2.0 equiv) and DMAP (0.32 mmol, 60 mol %) were added at room temperature. After the addition of the opportune phytochemical (1.04 mmol, 2.0 equiv), the reaction mixture was slowly stirred overnight (18 h) under a N2 atmosphere. The precipitated dicyclohexylurea was removed by filtration, and the solvent was removed under reduced pressure. The residue was purified by gradient column chromatography (hexane/EtOAc 1:1, 1:2).
Compound 11vii
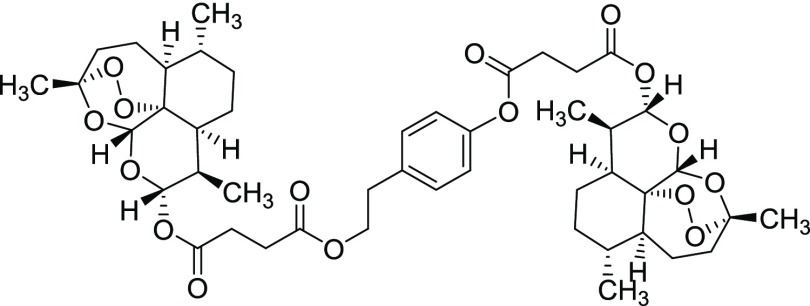 Yield = 47%. Rf = 0.50 (hexane/EtOAc 9:1, molybdato phosphate). 1H NMR
(CDCl3, 200 MHz): δ = 7.00 (d, 2H, J = 8.6 Hz), 6.81 (d, 2H, J = 8.6 Hz), 5.59 (d, 1H, J = 6.4 Hz), 5.54 (d, 1H, J = 6.4 Hz),
5.24 (s, 1H), 5.22 (s, 1H), 4.05 (t, 2H, J = 7.0
Hz), 2.74–2.57 (m, 6H), 2.50–2.25 (m, 6H), 2.20–2.06
(m, 2H), 1.84–1.19 (m, 12H), 1.18 (s, 3H), 1.17 (s, 3H), 1.11–0.82
(m, 8H), 0.74 (d, 6H, J = 5.8 Hz), 0.64–0.60
(m, 6H) ppm. 13C NMR (CDCl3, 50 MHz): δ
= 171.7, 170.8, 170.7, 170.5, 149.16, 135.2, 129.7, 129.6, 121.4,
104.1, 92.1, 92.0, 91.3, 79.9, 64.8, 51.4, 48.4, 45.0, 37.0, 36.0,
34.2, 33.9, 33.7, 31.6, 30.6, 30.3, 29.8, 29.4, 29.0, 28.9, 28.8,
28.6, 25.6, 24.8, 24.4, 21.7, 20.0, 11.8, 11.7 ppm. MS (ESI): m/z calcd for [C45H61O16]+: 856. Anal. calcd for C45H60O16: C, 63.07; H, 7.06; O, 29.87; found: C, 63.08;
H, 7.05; O, 29.85.
Yield = 47%. Rf = 0.50 (hexane/EtOAc 9:1, molybdato phosphate). 1H NMR
(CDCl3, 200 MHz): δ = 7.00 (d, 2H, J = 8.6 Hz), 6.81 (d, 2H, J = 8.6 Hz), 5.59 (d, 1H, J = 6.4 Hz), 5.54 (d, 1H, J = 6.4 Hz),
5.24 (s, 1H), 5.22 (s, 1H), 4.05 (t, 2H, J = 7.0
Hz), 2.74–2.57 (m, 6H), 2.50–2.25 (m, 6H), 2.20–2.06
(m, 2H), 1.84–1.19 (m, 12H), 1.18 (s, 3H), 1.17 (s, 3H), 1.11–0.82
(m, 8H), 0.74 (d, 6H, J = 5.8 Hz), 0.64–0.60
(m, 6H) ppm. 13C NMR (CDCl3, 50 MHz): δ
= 171.7, 170.8, 170.7, 170.5, 149.16, 135.2, 129.7, 129.6, 121.4,
104.1, 92.1, 92.0, 91.3, 79.9, 64.8, 51.4, 48.4, 45.0, 37.0, 36.0,
34.2, 33.9, 33.7, 31.6, 30.6, 30.3, 29.8, 29.4, 29.0, 28.9, 28.8,
28.6, 25.6, 24.8, 24.4, 21.7, 20.0, 11.8, 11.7 ppm. MS (ESI): m/z calcd for [C45H61O16]+: 856. Anal. calcd for C45H60O16: C, 63.07; H, 7.06; O, 29.87; found: C, 63.08;
H, 7.05; O, 29.85.
Compound 11viii
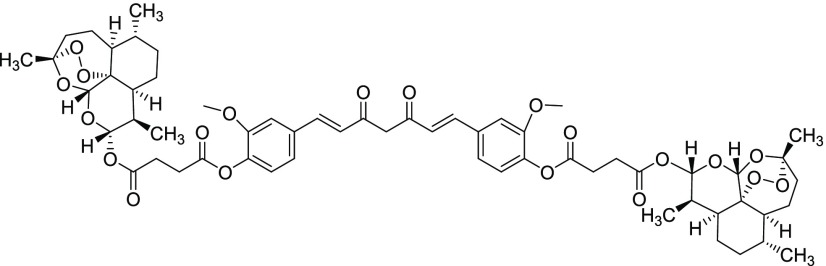 Yield = 59%. Rf = 0.39 (hexane/EtOAc 9:1, molybdato phosphate). 1H NMR
(CDCl3, 200 MHz): δ = 7.57 (d, 2H, J = 15.8 Hz), 7.13–7.02 (m, 6H), 6.52 (d, 2H, J = 15.8 Hz), 5.83–5.76 (m, 2H), 5.40 (s, 2H), 3.82 (s, 6H),
2.97–2.76 (m, 8H), 2.59–2.48 (m, 2H), 2.42–2.26
(m, 2H), 2.12–1.50 (m, 12H), 1.46 (s, 6H), 1.39–0.99
(m, 8H), 0.91 (d, 6H, J = 5.4 Hz), 0.74 (d, 6H, J = 18.2 Hz) ppm. 13C NMR (CDCl3,
50 MHz): δ = 183.1, 170.8, 170.1, 151.3, 141.2, 139.9, 134.0,
124.3, 123.3, 121.1, 111.4, 104.4, 101.8, 92.3, 91.5, 80.1 60.3, 55.9,
51.5, 45.2, 37.2, 36.2, 34.1, 31.8, 29.2, 28.7, 25.9, 25.0, 24.6,
22.0, 20.2, 14.2, 12.0 ppm.MS (ESI): m/z for [C59H73O20]+: 1101.
Anal. calcd for C59H72O20: C, 64.35;
H, 6.59; O, 29.06; found: C, 64.31; H, 6.62; O, 29.05.
Yield = 59%. Rf = 0.39 (hexane/EtOAc 9:1, molybdato phosphate). 1H NMR
(CDCl3, 200 MHz): δ = 7.57 (d, 2H, J = 15.8 Hz), 7.13–7.02 (m, 6H), 6.52 (d, 2H, J = 15.8 Hz), 5.83–5.76 (m, 2H), 5.40 (s, 2H), 3.82 (s, 6H),
2.97–2.76 (m, 8H), 2.59–2.48 (m, 2H), 2.42–2.26
(m, 2H), 2.12–1.50 (m, 12H), 1.46 (s, 6H), 1.39–0.99
(m, 8H), 0.91 (d, 6H, J = 5.4 Hz), 0.74 (d, 6H, J = 18.2 Hz) ppm. 13C NMR (CDCl3,
50 MHz): δ = 183.1, 170.8, 170.1, 151.3, 141.2, 139.9, 134.0,
124.3, 123.3, 121.1, 111.4, 104.4, 101.8, 92.3, 91.5, 80.1 60.3, 55.9,
51.5, 45.2, 37.2, 36.2, 34.1, 31.8, 29.2, 28.7, 25.9, 25.0, 24.6,
22.0, 20.2, 14.2, 12.0 ppm.MS (ESI): m/z for [C59H73O20]+: 1101.
Anal. calcd for C59H72O20: C, 64.35;
H, 6.59; O, 29.06; found: C, 64.31; H, 6.62; O, 29.05.
Procedure for the Synthesis of Derivative 11ix
To a solution of artesunic acid (0.52 mmol, 1.0 equiv) in dry DMF (3.0 mL), K2CO3 (1.04 mmol, 2.0 equiv) and 4-hydroxyphenethyl bromide (0.52 mmol, 1.0 equiv) were added at room temperature. The reaction mixture was warmed at 90 °C and stirred overnight (18 h) under a N2 atmosphere. After this time, the precipitate was removed by filtration, and the solution was diluted with H2O (20 mL) and extracted with EtOAc (3 × 25 mL). The organic phase was washed with brine dried over Na2SO4, filtered, and concentrated under reduced pressure. The crude product was purified by column chromatography (hexane/EtOAc 1:1).
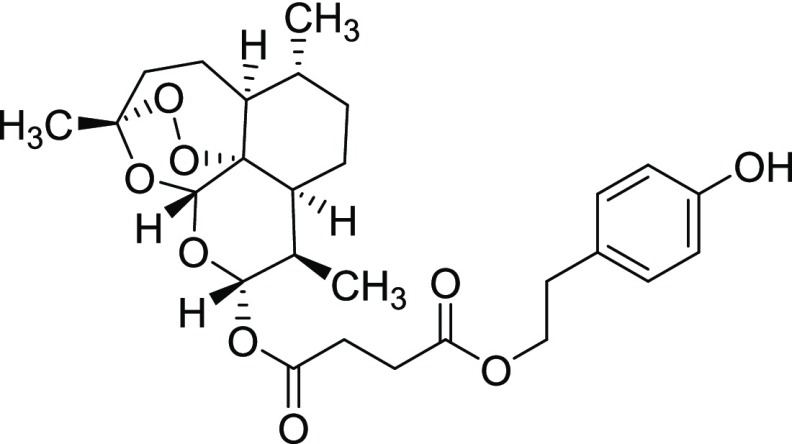 Yield = 46%. Rf = 0.33 (hexane/EtOAc 4:1, molybdato phosphate). 1H NMR
(CDCl3, 200 MHz): δ = 7.04 (d, 2H, J = 8.4 Hz), 6.76 (d, 2H, J = 8.4 Hz), 5.75 (d, 2H, J = 9.8 Hz), 5.42 (s, 2H), 4.22 (t, 2H, J = 7.0 Hz), 2.83 (d, 2H, J = 7.0 Hz), 2.63–2.48
(m, 4H), 2.35–2.28 (m, 2H), 1.97–1.42 (m, 6H), 1.41
(s, 3H), 1.27–0.99 (m, 4H), 0.91 (d, 3H, J = 3.8 Hz), 0.80 (d, 3H, J = 7.0 Hz) ppm. 13C NMR (CDCl3, 50 MHz): δ = 172.0, 171.1, 151.2,
130.0, 129.6, 115.5, 104. 6, 92.2, 91.5, 80.2, 65.4, 51.5, 45.2, 37.3,
36.2, 34.1, 34.0, 33.2, 31.8, 29.7, 29.1, 28.9, 28.0, 25.9, 24.6,
22.7, 22.0, 21.0, 20.2, 14.2, 13.2, 12.0 ppm. MS (ESI): m/z for [C27H35O9]−: 503. Anal. calcd for C27H36O9: C, 64.27; H, 7.19; O, 28.54; found: C, 64.29; H, 7.18;
O, 28.53.
Yield = 46%. Rf = 0.33 (hexane/EtOAc 4:1, molybdato phosphate). 1H NMR
(CDCl3, 200 MHz): δ = 7.04 (d, 2H, J = 8.4 Hz), 6.76 (d, 2H, J = 8.4 Hz), 5.75 (d, 2H, J = 9.8 Hz), 5.42 (s, 2H), 4.22 (t, 2H, J = 7.0 Hz), 2.83 (d, 2H, J = 7.0 Hz), 2.63–2.48
(m, 4H), 2.35–2.28 (m, 2H), 1.97–1.42 (m, 6H), 1.41
(s, 3H), 1.27–0.99 (m, 4H), 0.91 (d, 3H, J = 3.8 Hz), 0.80 (d, 3H, J = 7.0 Hz) ppm. 13C NMR (CDCl3, 50 MHz): δ = 172.0, 171.1, 151.2,
130.0, 129.6, 115.5, 104. 6, 92.2, 91.5, 80.2, 65.4, 51.5, 45.2, 37.3,
36.2, 34.1, 34.0, 33.2, 31.8, 29.7, 29.1, 28.9, 28.0, 25.9, 24.6,
22.7, 22.0, 21.0, 20.2, 14.2, 13.2, 12.0 ppm. MS (ESI): m/z for [C27H35O9]−: 503. Anal. calcd for C27H36O9: C, 64.27; H, 7.19; O, 28.54; found: C, 64.29; H, 7.18;
O, 28.53.
Procedure for the Synthesis of Derivative 11x
To a solution of dihydroartemisinin (0.52 mmol, 1.0 equiv) in dry DMF (3.0 mL), K2CO3 (1.04 mmol, 2.0 equiv) and 4-hydroxyphenethyl bromide (0.52 mmol, 1.0 equiv) were added at room temperature. The reaction mixture was warmed at 90 °C and stirred overnight (18 h) under a N2 atmosphere. After this time, the precipitate was removed by filtration, and the solution was diluted with H2O (20 mL) and extracted with EtOAc (3 × 25 mL). The organic phase was washed with brine dried over Na2SO4, filtered, and concentrated under reduced pressure. The crude product was purified by column chromatography (hexane/EtOAc 1:1).
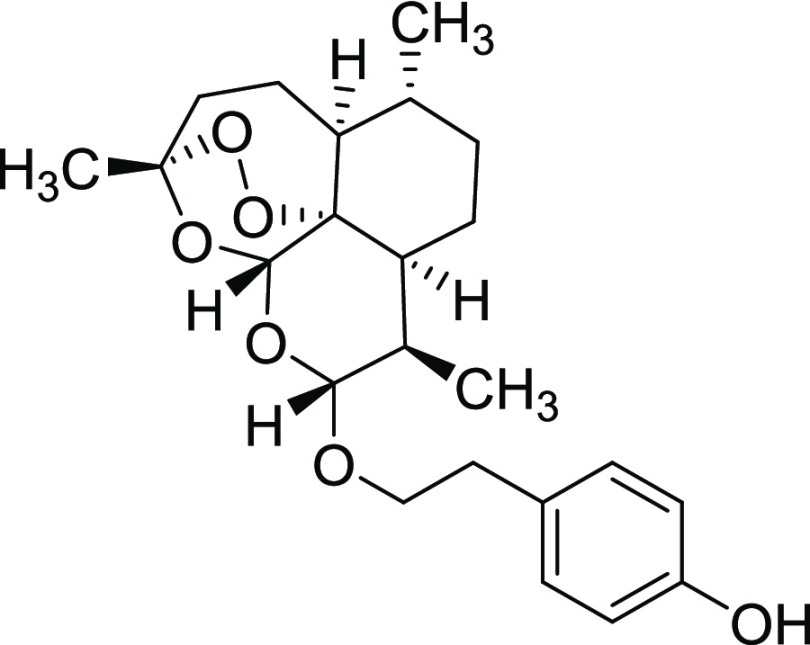 Yield = 53% (a mixture of α and β isomers). Rf = 0.27 (hexane/EtOAc 9:1,
molybdato phosphate). 1H NMR (CDCl3, 200 MHz):
δ = 7.06–7.00 (m, 2H), 6.76–6.69 (m, 2H), 5.11
(s, 1H), 4.74 (d, J = 3.4 Hz), 4.04–3.92 (m,
1H), 3.57–3.46 (m, 1H), 2.80–2.72 (m, 2H), 2.56–2.51
(m, 1H), 2.37–2.30 (m, 1H), 2.29–1.41 (m, 6H), 1.39
(s, 3H), 1.37–0.91 (m, 4H), 0.90–0.85 (m, 3H), 0.82
(d, 3H, J = 7.4 Hz) ppm. 13C NMR (CDCl3, 50 MHz): δ = 154.2, 131.2, 130.9, 130.0, 115.1, 115.0,
104.1, 103.2, 102.8, 101.7, 88.8, 87.9, 81.1, 69.5, 69.1, 52.4, 51.9,
46.2, 44.2, 39.4, 37.2, 37.1, 36.5, 36.4, 35.4, 34.6, 34.4, 31.4,
30.9, 26.1, 25.9, 24.6, 24.2, 20.3, 20.0, 19.5, 12.9 ppm. MS (ESI): m/z for [C23H31O6]−: 403. Anal. calcd for C23H32O6: C, 68.29; H, 7.97; O, 23.73; found: C, 68.230;
H, 7.99; O, 23.74.
Yield = 53% (a mixture of α and β isomers). Rf = 0.27 (hexane/EtOAc 9:1,
molybdato phosphate). 1H NMR (CDCl3, 200 MHz):
δ = 7.06–7.00 (m, 2H), 6.76–6.69 (m, 2H), 5.11
(s, 1H), 4.74 (d, J = 3.4 Hz), 4.04–3.92 (m,
1H), 3.57–3.46 (m, 1H), 2.80–2.72 (m, 2H), 2.56–2.51
(m, 1H), 2.37–2.30 (m, 1H), 2.29–1.41 (m, 6H), 1.39
(s, 3H), 1.37–0.91 (m, 4H), 0.90–0.85 (m, 3H), 0.82
(d, 3H, J = 7.4 Hz) ppm. 13C NMR (CDCl3, 50 MHz): δ = 154.2, 131.2, 130.9, 130.0, 115.1, 115.0,
104.1, 103.2, 102.8, 101.7, 88.8, 87.9, 81.1, 69.5, 69.1, 52.4, 51.9,
46.2, 44.2, 39.4, 37.2, 37.1, 36.5, 36.4, 35.4, 34.6, 34.4, 31.4,
30.9, 26.1, 25.9, 24.6, 24.2, 20.3, 20.0, 19.5, 12.9 ppm. MS (ESI): m/z for [C23H31O6]−: 403. Anal. calcd for C23H32O6: C, 68.29; H, 7.97; O, 23.73; found: C, 68.230;
H, 7.99; O, 23.74.
Biological Part
Cell Culture Condition
The C3PV cell line is a primary human fibroblast grown according to Botta et al.31 SK-MEL3, SK-MEL24 and RPMI-7951 are three different metastatic melanoma cancer cell lines. SK-MEL3 was grown in McCoy’s medium supplemented with 15% fetal bovine serum (FBS), penicillin (100 U/mL), and streptomycin (1 mg/mL). SK-MEL24 and RPMI-7951 were grown in Eagle’s minimum essential medium containing 15 and 10% FBS, respectively, penicillin (100 U/mL), and streptomycin (1 mg/mL). All cell lines were maintained at 37 °C in a humidified atmosphere (95%) and 5% CO2.
Treatment Protocol: General Procedure
To study the effect of artemisinin and its derivatives on cell viability, the C3PV, SK-MEL3, SK-MEL24, and RPMI-7951 cell lines were seeded in 96-well plates (6000 cells/well in 100 μL of medium) and incubated overnight to allow cell adherence. Afterward, the medium was replaced with fresh medium containing the appropriate dose of compounds. Artemisinin and its derivatives were used in a range of 0.01 to 1 μM for 24 h. The analyses of cell viability were done at the end of treatment. The assays were performed in quadruplicate for both treatments.
Treatment Protocol for DFO Assay
To study the mechanism of action of artemisinin and compounds 11iii, 11iv, 11viii, and 11ix, the SK-MEL3, SK-MEL24 and RPMI-7951 cell lines were seeded in 96-well plates (6000 cells/well in 100 μL of medium) and incubated overnight to allow cell adherence. Afterward, the medium was replaced with fresh medium containing DFO (20 μM) for 1 h. Then the appropriate dose of artemisinin and its compounds 11iii, 11iv, and 11viii were added for 24 h. The analyses of cell viability were done at the end of treatment. The assays were performed in quadruplicate for both treatments.
Cell Viability Assay
Cell viability was evaluated using MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide] cell proliferation assay. Briefly, after incubation for 3 h at 37 °C with MTT (0.5 mg/mL), the supernatant was replaced with 100 μL of analysis solution containing 10% sodium dodecyl sulfate and 0.6% acetic acid in dimethyl sulfoxide to dissolve the formazan crystals. Optical density measurements were performed with a scanning spectrophotometer DTX880 Multimode Detector (Beckman Coulter) using a 630 nm (background) and a 570 nm filter.
Statistical Analysis
The IC50 values were determined by nonlinear regression using the program, graphpad prism 6. The SI values were calculated using the formula
Acknowledgments
This article is dedicated to Dr. Luigi Villanova, ex-President of Lachifarma, for his pioneering studies on artemisinin as an antimelanoma agent. The article is published with the contribution of MIUR Ministero dell’Istruzione, dell’Università della Ricerca Italiano, project PRIN 2017, ORIGINALE CHEMIAE in Antiviral Strategy—Origin and Modernization of Multi-Component Chemistry as a Source of Innovative Broad Spectrum Antiviral Strategy, cod. 2017BMK8JR.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.9b02600.
Stability of compound 11iv by TLC and HPLC analyses; HPLC analysis of compound 11iv (Figure S1); HPLC analysis of tyrosol 8 (Figure S2); HPLC analysis of compound 11iv after 48 h treatment with cell culture medium at room temperature (Figure S3); EPR procedure; EPR spectra of compounds 11iv, 11iii, 11ix, artesunate and 11viii (Figures S4−S8).
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Tu Y. The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nat. Med. 2011, 17, 1217–1220. 10.1038/nm.2471. [DOI] [PubMed] [Google Scholar]
- Posner G. H. Antimalarial peroxides in the qinghaosu (artemisinin) and yingzhaosu families. Expert Opin. Ther. Pat. 1998, 8, 1487–1493. 10.1517/13543776.8.11.1487. [DOI] [Google Scholar]
- Li Y.; Wu Y. L. An over four millennium story behind Qinghaosu (artemisinin): a fantastic antimalarial drug from a traditional Chinese herb. Curr. Med. Chem. 2003, 10, 2197–2230. 10.2174/0929867033456710. [DOI] [PubMed] [Google Scholar]
- Dhingra V.; Vishweshwar R. K.; Lakshmi N. M. Current status of artemisinin and its derivatives as antimalarial drugs. Life Sci. 2000, 66, 279–300. 10.1016/S0024-3205(99)00356-2. [DOI] [PubMed] [Google Scholar]
- Lai H. C.; Singh N. P.; Sasaki T. Development of artemisinin compounds for cancer treatment. Invest. New Drugs 2013, 31, 230–246. 10.1007/s10637-012-9873-z. [DOI] [PubMed] [Google Scholar]
- Efferth T.; Dunstan H.; Sauerbrey A.; Miyachi H.; Chitambar C. R. The anti-malarial artesunate is also active against cancer. Int. J. Oncol. 2001, 18, 767–773. 10.3892/ijo.18.4.767. [DOI] [PubMed] [Google Scholar]
- Efferth T. Mechanistic perspectives for 1,2,4-trioxanes in anticancer therapy. Drug Resist. Updates 2005, 8, 85–97. 10.1016/j.drup.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Kelter G.; Steinbach D.; Konkimalla V. B.; Tahara T.; Taketani S.; Fiebig H. H.; Efferth T. Role of transferrin receptor and ABC transporters ABCB6 and ABCB7 for resistence and differentiation of tumors cells towards artesunate. PLoS One 2007, 2, e798 10.1371/journal.pone.0000798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler A. R.; Gilbert B. C.; Hulme P.; Irvine L. R.; Renton L.; Whitwood A. C. EPR Evidence for the Involvement of Free Radicals in the Iron-Catalysed Decomposition of Qinghaosu (Artemisinin) and Some Derivatives; Antimalarial Action of Some Polycyclic Endoperoxides. Free Radical Res. 1998, 28, 471–476. 10.3109/10715769809066884. [DOI] [PubMed] [Google Scholar]
- Francis S. E.; Sullivan D. J. Jr.; Goldberg D. E. Hemoglobin metabolism in the malaria parasite Plasmodium falciparum. Annu. Rev. Microbiol. 1997, 51, 97–123. 10.1146/annurev.micro.51.1.97. [DOI] [PubMed] [Google Scholar]
- a Tietze L. F.; Bell H. P.; Chandrasekhar S. Natural Product Hybrids as New Leads for Drug Discovery. Angew. Chem., Int. Ed. 2003, 42, 3996–4028. 10.1002/anie.200200553. [DOI] [PubMed] [Google Scholar]; b Zhang N.; Yu Z.; Yang X.; Hu P.; He Y. Synthesis of novel ring-contracted artemisinin dimers with potent anticancer activities. Eur. J. Med. Chem. 2018, 150, 829–840. 10.1016/j.ejmech.2018.03.010. [DOI] [PubMed] [Google Scholar]; c Letis A. S.; Seo E. J.; Nikolaropoulos S. S.; Efferth T.; Giannis A.; Fousteris M. A. Synthesis and cytotoxic activity of new artemisinin hybrid molecules against human leukemia cells. Bioorg. Med. Chem. 2017, 25, 3357–3367. 10.1016/j.bmc.2017.04.021. [DOI] [PubMed] [Google Scholar]; d Singh N. P.; Lai H. C.; Park J. S.; Gerhardt T. E.; Kim B. J.; Wang S.; Sasaki T. Effects of artemisinin dimers on rat breast cancer cells in vitro and in vivo. Anticancer Res. 2011, 31, 4111–4114. [PubMed] [Google Scholar]
- Fröhlich T.; Reiter C.; Saeed M. E. M.; Hutterer C.; Hahn F.; Leidenberger M.; Friedrich O.; Kappes B.; Marschall M.; Efferth T.; Tsogoeva S. B. Synthesis of Thymoquinone–Artemisinin Hybrids: New Potent Antileukemia, Antiviral, and Antimalarial Agents. ACS Med. Chem. Lett. 2018, 9, 534–539. 10.1021/acsmedchemlett.7b00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artemisinin derivatives for the treatment of melanoma WO2009/043538.
- Surh Y. J. Cancer Chemoprevention with dietary phytochemicals. Nat. Rev. Cancer 2003, 3, 768–780. 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- Garbe C.; Eigentler T. K.; Keilholz U.; Hauschild A.; Kirkwood J. M. Systematic review of medical treatment in melanoma: current status and future prospects. Oncologist 2011, 16, 5–24. 10.1634/theoncologist.2010-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzaei H.; Naseri G.; Rezaee R.; Mohammadi M.; Bakazemi Z.; Mirzaei H. R.; Salehi H.; Peyvandi M.; Pawelek J. M.; Sahebkar A. Curcumin: A new candidate for melanoma therapy?. Int. J. Cancer 2016, 139, 1683–1695. 10.1002/ijc.30224. [DOI] [PubMed] [Google Scholar]
- Ghosh R.; Nadiminty N.; Fitzpatrick J. E.; Alworth W. L.; Slaga T. J.; Kumar A. P. Eugenol Causes Melanoma Growth Suppression through Inhibition of E2F1 Transcriptional Activity. J. Biol. Chem. 2005, 280, 5812–5819. 10.1074/jbc.M411429200. [DOI] [PubMed] [Google Scholar]
- Ottino P.; Duncan J. R. Effect of α-Tocopherol Succinate on Free Radical and Lipid Peroxidation Levels in BL6 Melanoma Cells Free Radical Biology and Medicine. Free Radical Biol. Med. 1997, 22, 1145–1151. 10.1016/S0891-5849(96)00529-1. [DOI] [PubMed] [Google Scholar]
- Mijatovic S. A.; Timotijevic G. S.; Miljkovic D. M.; Radovic J. M.; Maksimovic-Ivanic D. D.; Dekanski D. P.; Stosic-Grujicic S. D. Multiple antimelanoma potential of dry olive leaf extract. Int. J. Cancer 2011, 128, 1955–1965. 10.1002/ijc.25526. [DOI] [PubMed] [Google Scholar]
- Rasheed S. A.; Efferth T.; Asangani I. A.; Allgayer H. First evidence that the antimalarial drug artesunate inhibits invasion and in vivo metastatis in lung cancer by targeting essential extracellular proteases. Int. J. Cancer 2010, 127, 1475–1485. 10.1002/ijc.25315. [DOI] [PubMed] [Google Scholar]
- Soomro S.; Langenberg T.; Mahringer A.; Konkimalla V. B.; Horwedel C.; Holenya P.; Brand A.; Cetin C.; Fricker G.; Dewerchin M.; Carmeliet P.; Conway E. M.; Jansen H.; Efferth T. Design of novel artemisinin-like derivates with cytotoxic and antiangiogenic properties. J. Cell. Mol. Med. 2011, 15, 1122–1135. 10.1111/j.1582-4934.2010.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkimalla V. B.; McCubrey J. A.; Efferth T. The role of downstream signalling pathways of the epidermal growth factor receptors for Artesunate’s activity in cancer cells. Curr. Cancer Drug Targets 2009, 9, 72–80. 10.2174/156800909787314020. [DOI] [PubMed] [Google Scholar]
- Houben R.; Becker J. C.; Kappel A.; Terheyden P.; Brocker E. B.; Goetz R.; Rapp U. R. Constitutive activation of the Ras-Raf signaling pathway in metastatic melanoma is associated with poor prognosis. J. Carcinog. 2004, 3, 6. 10.1186/1477-3163-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Olivier M.; Hollstein M.; Hainaut P. TP53 Mutations in Human Cancers: Origins, Consequences, and Clinical Use. Cold Spring Harbor Perspect. Biol. 2010, 2, a001008 10.1101/cshperspect.a001008. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Petitjean A.; Achatz M. I. W.; Borresen-Dale A. L.; Hainaut P.; Olivier M. TP53 mutations in human cancers: functional selection and impact on cancer prognosis and outcomes. Oncogene 2007, 26, 2157–2165. 10.1038/sj.onc.1210302. [DOI] [PubMed] [Google Scholar]
- a Halachmi S.; Gilcherest B. A. Update on genetic events in the pathogenesis of melanoma. Curr. Opin. Oncol. 2001, 13, 129–136. 10.1097/00001622-200103000-00008. [DOI] [PubMed] [Google Scholar]; b Pollock P. M.; Trent J. M. The genetics of cutaneous melanoma. Clin. Lab. Med. 2000, 20, 667–690. [PubMed] [Google Scholar]
- a Sharma A.; Trivedi N. R.; Zimmerman M. A.; Tuevasan D. A.; Smith C. D.; Robertson G. P. Mutant V599E B-Raf regulates growth and vascular, development of malignant melanoma tumors. Cancer Res. 2005, 65, 2412–2421. 10.1158/0008-5472.CAN-04-2423. [DOI] [PubMed] [Google Scholar]; b Smalley K. S. M.; Brafford P.; Haass N. K.; Brandner J. M.; Brown E.; Herlyn M. Up-regulated expression of zonula occludens protein-1 in human melanoma associates with N-cadherin and contributes to invasion and adhesion. Am. J. Pathol. 2005, 166, 1541–1554. 10.1016/S0002-9440(10)62370-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuma N. H.; Smit F. J.; de Kock C.; Combrinck J.; Smith P. J.; N’Da D. D. Synthesis and biological evaluation of a series of non-hemiacetal ester derivatives of artemisinin. Eur. J. Med. Chem. 2016, 122, 635–646. 10.1016/j.ejmech.2016.07.027. [DOI] [PubMed] [Google Scholar]
- Ooko E.; Saeed M. E.; Kadioglu O.; Sarvi S.; Colak M.; Elmasaoudi K.; Janah R.; Greten H. J.; Efferth T. Artemisinin derivatives induce iron-dependent cell death (ferroptosis) in tumor cells. Phytomedicine 2015, 22, 1045–1054. 10.1016/j.phymed.2015.08.002. [DOI] [PubMed] [Google Scholar]
- Posner G. H.; Wang D.; Cumming J. N.; Oh C. H.; French A. N.; Bodley A. L.; Shapiro T. A. Further evidence supporting the importance of and the restrictions on a carbon-centered radical for high antimalarial activity of 1,2,4-trioxanes like artemisinin. J. Med. Chem. 1995, 38, 2273–2275. 10.1021/jm00013a001. [DOI] [PubMed] [Google Scholar]
- Alberti A.; Macciantelli D.. Spin Trapping. In Electron Paramagnetic Resonance – a Practitioner’s Toolkit; Brustolon M.; Giamello E., Eds.; Wiley: Hoboken, 2009; pp 287–323. [Google Scholar]
- Botta L.; Filippi S.; Bizzarri B. M.; Meschini R.; Caputo M.; Proietti-De-Santis L.; Iside C.; Nebbioso A.; Gualandi G.; Saladino R. Oxidative nucleophilic substitution selectively produces cambinol derivatives with antiproliferative activity on bladder cancer cell lines. Bioorg. Med. Chem. Lett. 2019, 29, 78–82. 10.1016/j.bmcl.2018.11.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.