Abstract
Orexins/hypocretins and their receptors (OXRs) are ubiquitously distributed throughout the nervous system and peripheral tissues. Recently, various reports have indicated that orexins play regulatory roles in numerous physiological processes involved in obesity, energy homeostasis, sleep–wake cycle, analgesia, alcoholism, learning, and memory. This review aims to outline recent progress in the research and development of orexins used in biochemical signaling pathways, secretion pathways, and the regulation of energy metabolism/adipose tissue development. Orexins regulate a variety of physiological functions in the body by activating phospholipase C/protein kinase C and AC/cAMP/PKA pathways, through receptors coupled to Gq and Gi/Gs, respectively. The secretion of orexins is modulated by blood glucose, blood lipids, hormones, and neuropeptides. Orexins have critical functions in energy metabolism, regulating both feeding behavior and energy expenditure. Increasing the sensitivity of orexin-coupled hypothalamic neurons concurrently enhances spontaneous physical activity, non-exercise activity thermogenesis, white adipose tissue lipolysis, and brown adipose tissue thermogenesis. With this comprehensive review of the current literature on the subject, we hope to provide an integrated perspective for the prevention/treatment of obesity.
Background
Obesity is closely associated with dysregulation of appetite, and the hypothalamus is a key site for neuronal regulation of food intake. The hypothalamic nucleus is connected by and interdependent on receiving, integrating, and sending hunger signals to regulate appetite.1 The central nervous system releases appetite-stimulating factors, including neuropeptide Y (NPY), agouti-related protein (AgRP), orexins, melanin-concentrating hormone, β-endorphin, γ-aminobutyric acid, and norepinephrine (NE). It also secretes antiappetite factors such as α-melanocyte-stimulating hormone (a proopiomelanocortin derivative), cocaine- and amphetamine-regulated transcript (CART), corticotrophin-releasing hormone, and glucagon-like peptide-1. Ghrelin is the only appetite-stimulating hormonal signal from the periphery, and peripheral antiappetite signals include leptin, insulin, cholecystokinin (CCK), obestatin, and adiponectin.2 Furthermore, the hypothalamus is the most important site for central regulation of glycolipid metabolism and energy homeostasis,3 and several hormones and neuropeptides in the hypothalamus modulate or participate in these biological processes.3,4
Orexins are also known as hypocretins, and were originally identified as appetite-stimulating peptides produced in the lateral hypothalamus (LH).5 Subsequent research has indicated that orexin is extensively involved in the physiological processes of the sleep–wake cycle, analgesia, alcoholism, learning, and memory.6 The neurons responsible for producing orexin rapidly perceive the body’s nutritional status by responding to metabolic signals such as peripheral blood glucose, as well as leptin and ghrelin levels.7 In previous studies, both mice deficient in orexin-producing neurons and orexin-knockout mice demonstrated symptoms of paroxysmal narcolepsy and delayed obesity, despite reductions in food intake.8 Kakizaki et al. also implied that orexin neurons are involved in body weight gain via the interactive effects of exercise and diet, with each orexin receptor playing a unique role.9 These studies suggest that orexin functions as a regulator of obesity. It is well known that obesity is a result of an imbalance between caloric intake and energy expenditure, manifesting as excess adipose tissue development, decreased lipolysis, and high fat storage.9 This review will provide an integrated perspective for the prevention of obesity, by explaining the physiological effects of orexins on energy metabolism and adipose tissue development.
Characteristics and Tissue Distribution of Orexin
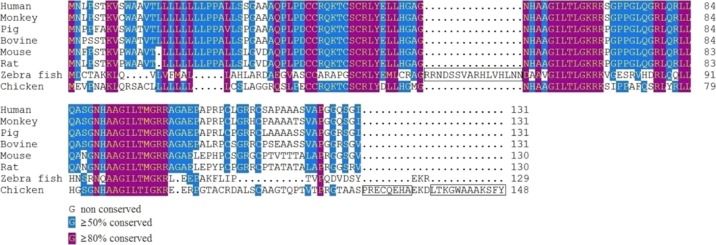
The precursor peptide of orexin (prepro-orexin) gives rise to two neuropeptides of different lengths: orexin-A and orexin-B. The human prepro-orexin gene consists of 1432 base-pairs. It contains two exons and one intron, and it encodes 131 amino-acid (AA) residues. One prepro-orexin generates one orexin-A and one orexin-B that are 33 AAs and 28 AA peptides, respectively, sharing 13 common AAs.5c,10 Homology analysis using a comparison of the AA sequences of prepro-orexin from eight different animal species (Figure 1) revealed a high degree of homology in mammals (Table 1). Zebrafish and humans share only 29.5% homology in their prepro-orexin AA sequence, whereas chickens and humans share 48.9% homology. The AA sequence of orexin-A is identical, however, between humans, oxen, pigs, and rodents. The AA sequence of orexin-B is identical in mice and rats, and it differs from the human sequence by only two residues.11 In addition, the AA sequences of chicken orexin-A and -B showed about 85 and 65% similarities with their human counterparts.12 The high conservation of orexin-A and orexin-B among species implies that the proteins serve fundamental and critical biological functions.
Figure 1.
Sequence alignment of prepro-orexin AAs in eight species, using the Clustal method (MEGALIGN, DNASTAR software). One insertion in zebra fish and two insertions in chicken are boxed. Genbank accession numbers were as follows, NP_001515.1 (human), NP_001181361.1 (monkey), NP_999321.1 (pig), NP_001159992.1 (bovine), NP_034540.1 (mouse), NP_037311.1 (rat), NP_001070860.1 (zebra fish), and NP_989516.1 (chicken).
Table 1. Estimates of Prepro-Orexin Sequence Identity (%, above Diagonal) and Evolutionary Divergence (%, below Diagonal) among Eight Speciesa.
| human | monkey | pig | bovine | mouse | rat | zebra fish | chicken | |
|---|---|---|---|---|---|---|---|---|
| human | 95.4 | 90.1 | 90.1 | 80.0 | 81.5 | 29.5 | 48.9 | |
| monkey | 4.7 | 90.1 | 90.1 | 81.5 | 83.1 | 29.5 | 48.9 | |
| pig | 10.7 | 10.7 | 91.6 | 76.9 | 79.2 | 30.2 | 48.1 | |
| bovine | 10.7 | 10.7 | 8.9 | 80.8 | 80.8 | 30.2 | 46.6 | |
| mouse | 20.2 | 18.2 | 24.4 | 19.2 | 94.6 | 29.5 | 49.2 | |
| rat | 18.2 | 16.3 | 21.2 | 19.2 | 5.6 | 30.2 | 48.5 | |
| zebra fish | 101.7 | 101.7 | 98.5 | 95.4 | 97.1 | 94.0 | 31.0 | |
| chicken | 60.6 | 60.6 | 62.4 | 67.8 | 63.1 | 63.1 | 114.2 |
Genbank accession numbers were as follows, NP_001515.1 (human), NP_001181361.1 (monkey), NP_999321.1 (pig), NP_001159992.1 (bovine), NP_034540.1 (mouse), NP_037311.1 (rat), NP_001070860.1 (zebra fish), and NP_989516.1 (chicken).
In the central nervous systems of rats, orexin-containing neurons project to the hypothalamus, thalamus, midbrain, pons, medulla oblongata, cerebral cortex, and limbic system.5b,13 In the LH, ventromedial hypothalamic nucleus (VMN), paraventricular thalamus, dorsal raphe nuclei, locus coeruleus, and periaqueductal central gray, high orexin contents (orexin-A and orexin-B peptides) have been found in high concentrations. Moderate orexin concentrations have been found in the suprachiasmatic nucleus (SChN), median eminence, paraventricular hypothalamic nucleus (PVN), arcuate nucleus (ARC), supraoptic nucleus, the nucleus of the solitary tract, and the substantia nigra. Orexin-B expression in these tissues have been found to be 2.1–3.0-fold the orexin-A concentrations found in rats.13 In the peripheral nervous system, orexins have widespread distribution in the sympathetic nerves, testes, heart, adrenal glands, kidneys, pancreas, placenta, stomach, intestines, and in adipose tissues.14
Characteristics and Tissue Distribution of Orexin Receptors
The orexin system contains two G-protein-coupled receptors: the orexin-1 receptor (OX1R) and the orexin-2 receptor (OX2R).15 In humans, the two OXR mRNAs consist of 1564 and 1843 base pairs, and these encode 425 and 444 AAs, respectively. Rat OXR mRNAs consist of 2469 and 3114 base pairs, and they are translated into 416 and 460 AAs, respectively. Humans and rats share 94 and 95% sequence identity for OX1R and OX2R, respectively. This indicates that the OXR genes are highly conserved among species. Furthermore, the AA sequences of human OX1R and OX2R have 64% homology between them. Both receptors can bind both orexin-A and orexin-B, but they bind with different affinities. OX2R is a nonselective receptor, so it has the same affinity for both orexins. OX1R, however, is a selective receptor, with a far greater affinity for orexin-A over orexin-B.5c,11
The distribution of OXRs is similar to the projection sites of orexin-neurons, but in situ hybridization results indicate that OX1R and OX2R mRNAs have complementary distributions. OX1R has widespread distribution in many brain regions involving the VMN, the prefrontal and infralimbic cortex, the paraventricular thalamic nucleus, the dorsal raphe nucleus, the locus coeruleus, and the hippocampus. OX2R has been observed in a complementary distribution to this, throughout the septal nuclei, the medial thalamic groups, the raphe nuclei, the cerebral cortex, and in many hypothalamic nuclei related to the PVN, the ventral premammillary nucleus, the tuberomammillary nucleus, and the dorsomedial nucleus (DMN).16 Genes expression results have shown that OX1R mRNA is predominantly expressed in the VMN, whereas high levels of mRNA are present in the locus coeruleus, dorsal raphe, tenia tecta, and the hippocampal formation. OX2R mRNA has been shown to be most abundant in the PVN, and is also mainly expressed in the paraventricular thalamic nuclei, subthalamus, nucleus accumbens, anterior pretectal nucleus, and in the cerebral cortex, in rats.17 The high expression levels of OXRs in the hypothalamus support their proposed role in appetite regulation. In the periphery, OX1R mRNA has been found in the pituitary gland, kidneys, adrenal glands, thyroid, testes, ovaries, and the jejunum. OX2R mRNA has been detected in the pituitary gland, adrenal glands, and in the lungs, and particularly high levels of OX2R mRNA have been found in the adrenal glands of rats.14b
Signal Transduction of Orexins
In neuronal cells, both OX1R and OX2R are coupled to the Gq class of G-proteins, with similar signal transductions, resulting in the activation of phospholipase C (PLC) and the subsequent elevation of intracellular calcium concentration, leading to neuron excitation.18 Orexins also inhibit K+ channels or activate nonselective cation channels, resulting in the depolarization of neurons,19 which can in turn lead to increased excitabilities and firing rates of the neurons.20 Kukkonen and Åkerman inferred that orexins increased the electrical activities of neurons via the PLC-PIP2(Phosphatidylinositol 4,5-bisphosphate) pathway, eliciting both presynaptic (e.g., increased Ca2+ influx) and postsynaptic (e.g., inhibited K+ channels) activated nonselective cation channel responses or Na+/Ca2+ exchanges.21
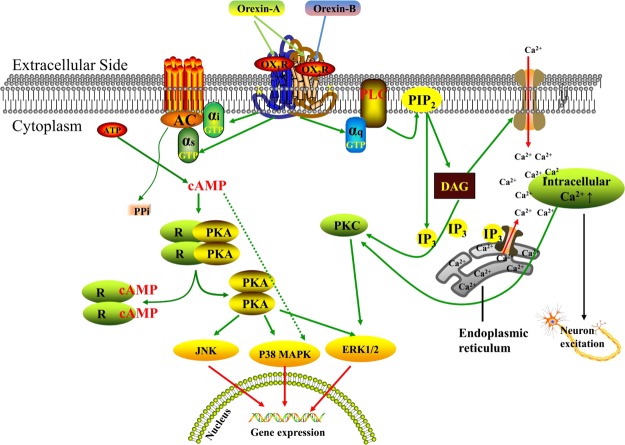
Recent studies have shown that OXRs modulate physiological activities by regulating PLC-activated Ca2+ channels through either the Gq-coupled receptor or through intracellular cyclic Adenosine Monophosphate (cAMP) levels via a Gi/Gs-coupled receptor pathway.22 Adenylate cyclase (AC) and PLC are key enzymes in the G-protein signaling pathway, and they are both located on the cell membrane. AC transforms adenosine monophosphate (AMP) into cAMP, thereby activating protein kinase A (PKA). PLC converts PIP2 to inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG), which stimulates Ca2+ release and activates protein kinase C (PKC). PKA and PKC are important intracellular kinases that transmit signals to the nucleus, thereby regulating gene and protein expression and playing key roles in multiple physiological and pathological processes.21,23 Shen et al.24 and Gorojankina et al.25 demonstrated that orexin-A activates extracellular signal-regulated kinase (ERK1/2), p38 mitogen-activated protein kinase (p38 MAPK), Jun N-terminal kinase (JNK), AC, and PLC, and induces a significant rise in cAMP, IP3, and intracellular calcium. OX2R-activated phosphorylation of ERK via the Gq/PLC/PKC, Gi, and Gs/AC/cAMP/PKA pathways is more potent than OX1R-induced ERK phosphorylation through the modulation of PKC, PI3K, Ras, and Src. Moreover, OX2R can activate the p38MAPK pathway through AC/cAMP, which is downstream of Gi/Gs.26 OXRs have also been shown to connect to multiple Ca2+ signaling mechanisms in all native and recombinant cell types. Briefly, OXRs, at high orexin concentrations, activate the typical Gαq → PLC → IP3 → Ca2+ system (Ca2+ release from stores), and at lower orexin concentrations, primarily activate the receptor-operated Ca2+ influx pathway (Gαq → PLC → DAG → Ca2+ influx from the extracellular side)22b (Figure 2). Thus, orexins always regulate a variety of physiological functions in the body by activating PLC/PKC and AC/cAMP/PKA pathways through receptors coupled to Gq and Gi/Gs, respectively.
Figure 2.
Downstream pathway of orexin. Orexin-A bound with both orexin receptors, whereas orexin-B only bound with OX2R. OX1R and OX2R mediated downstream pathways via coupling Gq, Gs, and Gi class of G-proteins. Abbreviations: OX1R: orexin receptor 1; OX2R: orexin receptor 2; PLC: phospholipase C; cAMP: cyclic adenosine monophosphate; AC: adenylate cyclase; AMP: adenosine monophosphate; PIP2: phosphatidylinositol 4,5-bisphosphate; PKA: protein kinase A; IP3: inositol 1,4,5-trisphosphate; DAG: diacylglycerol; PKC: protein kinase; ERK: extracellular signal-regulated kinase; p38 MAPK: p38 mitogen-activated protein kinase; JNK: Jun N-terminal kinase.
Furthermore, some reports have indicated that OX1R may form homodimers or heterodimers with other G-protein-coupled receptors. For example, OX1R exists predominantly as a homodimer, in order to perform potential regulation of receptor organization in the basal state.27 OX1R can also dimerize with the cholecystokinin A receptor (CCK1R), however, and this heterodimer decreases G-protein-dependent signaling and opposes the migration of human colon cancer cells.28 Xue et al. determined that ghrelin, through an OX1R and growth hormone secretagogue receptor 1a (GHSR1a) heterodimer, upregulated the Gas-cAMP-cAMP-responsive element signaling pathway and promoted neuroblastoma cell proliferation in vitro.29
Orexins Regulate Food Intake
Previous studies have shown that the ARC, VMN, DMN, PVN, SChN, and LH all affect feeding behavior, and together constitute the “core” of the appetite-regulating network in the hypothalamus. The LH is a key site of regulation of food intake and energy balance, as evidenced by the fact that injuries of the LH region result in reductions in food intake and weight loss.30 A distribution analysis of orexins and their receptors has revealed that orexin signals cover the encephalic regions involved in appetite, satiety, and energy homeostasis.13,16 Intracerebroventricular injections of orexin-A and orexin-B have confirmed that orexins promote food intake in a dose-dependent manner.5c Orexin-A increased daytime feeding in rats, in one study, but nocturnal feeding was reduced and there was no change in 24 h food intake on days two and eight under an 8-day intracerebroventricular infusion with orexin-A (18 nmol/day). Increased feeding in satiated rats and prolonged feeding in fasted rats which had been administered a single intracerebroventricular injection of orexin-A (23.4 nmol) 3 or 6 h into the light phase was also observed.31 These observations suggest that orexins may regulate feeding in some particular cases, such as when circadian rhythms influence feeding patterns and under conditions of hypoglycemia. The duration of orexin-A- and orexin-B-stimulated feedings have also been found to differ. Orexin-A has been found to induce feedings that last for about 4 h, but orexin-B-induced feedings only last about 2 h.5c The variance in orexin-A- and orexin-B-stimulated feeding duration may be attributed to the high lipophilicity of orexin-A, which permits the rapid passage of orexin-A through the blood–brain barrier (BBB) by simple diffusion. Conversely, orexin-B has low lipophilicity and cannot pass through the BBB.32 Moreover, intracerebroventricular injection of orexin-A has promoted behaviors related to feeding, but orexin-B has not been shown to have similar effects in rats.33 In this context, many studies have suggested that orexin-A plays a major role in multiple orexin-regulated appetite subsystems. In addition, central injection of mammalian orexin-A or -B did not significantly stimulate food intake in chicks, in one study.34 Possible reasons for this may be substantial differences in the peptide sequences and structures of OXRs and their receptors between mammalian and avian species.12,35
SB-334867, a selective OX1R antagonist, was found to inhibit feeding behavior and food intake, elevate the onset of behavioral satiety, and decrease post-treatment weight gain in one study.36 Rodgers et al.37 found that SB-334867 increased behavioral satiety and suppressed the hyperphagic effect of orexin-A in rats, suggesting that orexin-A stimulates feeding chiefly through OX1R. Orexins promote appetite by stimulating NPY/AgRP neurons and simultaneously inhibiting the antiappetitive POMC (proopiomelanocortin)/CART neurons. Evidence in support of this mechanism is that a NPY Y1/5 antagonist completely blocks orexin-A-stimulated food intake in rats.38 Ida et al. found that the regulatory action of orexin-A on feeding is also related to corticotropin releasing factor (CRF). Intracerebroventricular injection of CRF suppresses orexin expression and inhibits orexin-A-stimulated feeding, whereas intracerebroventricular injection of a CRF antagonist or anti-CRF serum markedly enhances orexin-A-stimulated feeding. Moreover, an NPY Y1 receptor antagonist abolishes the increased food intake caused by CRF antagonist injection with orexin-A in rats.39 The opioid pathway may be also involved in orexin-stimulated feeding, because a nonselective antagonist of opioid receptors blocks orexin-stimulated feeding. This is probably due to an effect on the central pleasure and reward mechanism of feeding behavior.40 Sheng et al. provided direct evidence that the glucose sensitivities of LH orexin-GI (glucose-inhibited) neurons influenced reward-based feeding in mice.41 Additionally, intracerebroventricular injection of orexins stimulates gastric acid secretion by activating the vagus nerve and promoting the cephalic phase of eating.42 However, intraperitoneal injection of orexin-A does not promote gastric acid secretion in rats.43 Feeding-induced stomach dilation, stomach fulfillment, and release of cholecystokinin-8 rapidly reduce the expression of orexins in rats.44 Therefore, orexins primarily modulate acute feeding behavior, integrate multiple metabolic signals, and adapt to the energy demands of the body by altering wakefulness and feeding behaviors.
Orexins Regulate Energy Expenditure
Orexins not only modulate appetite regulation, but they also control energy expenditure. The balance between food intake and energy expenditure determines long-term energy homeostasis. Daily energy expenditure in animal always includes exercise, nonexercise activity thermogenesis (NEAT), thermoregulation, thermic effects of food, and basal metabolic rate (BMR).45 Lubkin and Stricker-Krongrad reported that injection of orexin-A into the third ventricles of mice brains increased the mice’s BMRs independent of physical activity, but that orexin-B did not show such an effect.46 In fact, orexins are critical central neuropeptides for the regulation of NEAT. High-fat diets decreased spontaneous physical activity (SPA) and NEAT in mice, and the activation of orexin neurons abolished this decrease and produced an increase in NEAT.47 Orexin-A was microinjected through an intraventricular cannula into the hypothalamic paraventricular nuclei of diet-induced obese (DIO) or diet-resistant (DR) rats in one study, and energy expenditure was measured for 2 h. The results revealed that NEAT was significantly increased in the DR rats as compared to DIO rats.48 The probable cause was that the DR rats had higher sensitivities to orexin-A, which promoted SPA, along with a subsequent increase of oxygen consumption and CO2 production, which in turn increased NEAT. Conversely, the DIO rats lacked NEAT, and their obesities caused reductions in their sensitivities to orexin-A. Similar results have shown that dual orexin receptor antagonists reduce orexin-A-induced increases in SPA, total energy expenditure, and NEAT during SPA, wake, rest, and sleep in male Sprague-Dawley (SD) rats.49 In addition to increasing energy expenditure by enhancing NEAT, orexins also regulate brown adipose tissue (BAT) thermogenesis.50 Injection of orexins into the rat rostral raphe pallidus has been shown to increase BAT, CO2 production, and thermogenesis.51 Mice that have undergone ablation of orexin neurons present with BAT dysfunction and have increased fat masses, which can be modulated through intracerebroventricular injections of orexin-A to activate BAT thermogenesis, increasing their body temperatures and reducing adiposity.52 Yoshimichi et al. also reported an increase in body temperature caused by a central injection of orexin-A in rats under anesthesia, with no feeding or physical activity.53 These studies show that orexins can increase energy expenditure by producing increases in BMR, NEAT, and thermoregulation, and that orexin-A plays a dominant role in this process. Furthermore, the orexin-activated GAD65 (glutamic acid decarboxylase 65 cells) network in the LH has been shown to maintain healthy levels of physical activity in mice.54
In fact, the neuromodulation of energy metabolism by orexins is a complicated and integrated process that involves several brain regions. Following the injection of orexins into the rostral LH, tuberomammillary nucleus, substantia nigra, dorsal raphe nucleus, locus coeruleus, nucleus accumbens, and hypothalamic paraventricular nucleus, orexins have been found to improve SPA and NEAT. Meanwhile, numerous brain regions involved in regulatory networks of food intake also participate in the NEAT network or in other aspects of energy expenditure.17,48,51,55 These instances, and the widespread distribution of orexins and OXRs throughout the brain, indicate that the orexin systems of different brain regions may control some of the same functions, and that the outcomes of the orexin system result from activation of OXRs in corresponsive brain regions via projections of orexins.
Central Orexins Affect Adipose Development
As is well known, two types of adipose tissue—white adipose tissue (WAT) and BAT—exist in mammals.56 WAT is mainly responsible for metabolic energy storage, whereas BAT maintains body temperature through its thermogenic activity, by dissipating energy.57 Orexin-A knockout mice show late-onset obesity despite no significant change in food intake.58 Transgenic mice that synthesize orexin-A during growth are resistant to diet-induced obesity.59 Perez-Leighton et al.60 demonstrated that the injection of orexin-A into the lateral thalamus of SD rats for 10 consecutive days reduced diet-induced obesity without affecting food intake. During these processes, orexin-A regulates WAT lipolysis and energy utilization by increasing SPA60 and sympathetic innervation.61 Shen et al. injected orexin-A into rat ventricles in two doses, and found that high doses of orexin-A promoted WAT lipolysis, whereas low doses reduced WAT lipolysis.55a The reason for this is that high doses of orexin are more likely to affect relevant brain sites, increasing sympathetic stimulation of WAT and thereby increasing lipolysis, whereas lower doses of orexin-A reduce lipolysis by suppressing the activity of the sympathetic nervous system (SNS). Shen et al.55a subsequently reported that higher doses of orexin-A stimulated histamine secretion, by stimulating the histaminergic neurons in the tuberomammillary nucleus in order to promote lipolysis. These findings suggest that central orexins accelerate lipolysis and energy utilization in WAT. Another recent study also discovered that orexin neurons in the LH are functional and integrative factors that regulate the browning of WAT.62
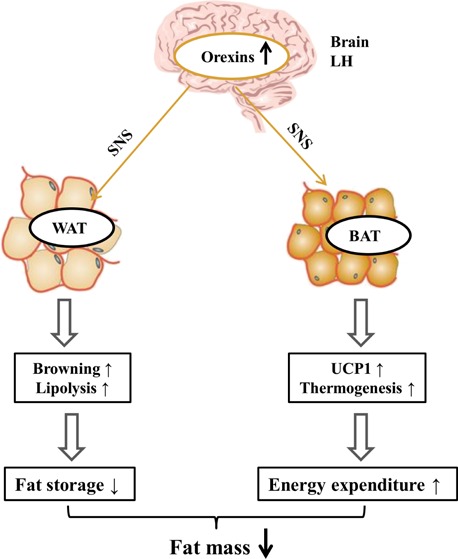
Central orexins play integral roles in adaptive thermogenesis and body weight regulation, via effects on BAT differentiation and function. Sellayah et al.63 demonstrated that orexins and their receptors are indispensable in the development of BAT, because they induced brown adipose cell differentiation through the activation of P38-MAPK in mice. In a subsequent study by Sellayah and Sikder,64 orexin-knockout mice rapidly increased in body weight and showed markedly lower triglyceride accumulation in BAT tissue compared to wild-type mice. Meanwhile, Sellayah et al. found that both OX1R and OX2R knockout mice also had increased body weights and reduced triglyceride accumulations, and that OX1R knockout showed the most pronounced effects.64 This is due to the inhibition of thermogenesis, resulting from orexin-induced BAT dysfunction and the abnormal accumulation of energy stores in the body from late-onset pathological obesity.8a Therefore, regular secretion of orexins and their receptors in BAT prevent obesity caused by abnormal thermogenesis. Recent studies have shown that BAT is also densely innervated by the SNS, and can release NE, stimulating thermogenesis via mitochondrial uncoupling protein 1 (UCP1).57b,63,65 From the findings of these studies, we composed a possible model for the action of central orexins on adipose development, shown in Figure 3. This model indicates that central orexins activate the SNS and promote browning and lipolysis in WAT, and improved UCP1 expression and thermogenesis in BAT, thus decreasing fat mass.
Figure 3.
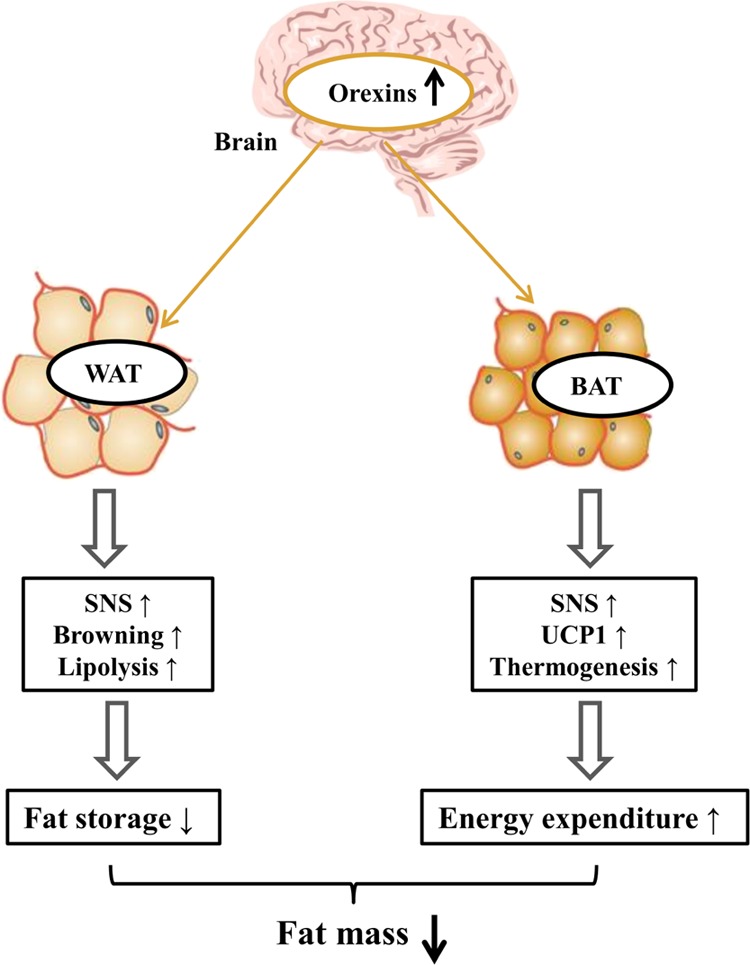
Proposed model for action of central orexin on adipose tissue. Abbreviations: WAT: white adipose tissue; BAT: brown adipose tissue; SNS: sympathetic nervous system; UCP1: uncoupling protein 1.
Peripheral Orexins Affect Adipose Tissues
Compared to the in vivo effects of orexins, in vitro treatment of adipose tissue with orexins shows different functions. Digby et al.66 first discovered orexins and their receptors, OX1R and OX2R, in human adipose tissues. They also found that treatment with orexin-A and orexin-B significantly affected the expression of hormone-sensitive lipase, and glycerol release. These results suggested that orexins play an important role in lipolysis and adipogenesis. Subsequently, Skrzypski et al.67 demonstrated that orexin-A promoted glucose uptake and triacylglycerol content, and decreased the release of glycerol in cultured rat adipocytes. Peripheral orexins have similar effects on the adipose tissues of pigs. OXRs are expressed in both porcine preadipocytes and adipocytes.68 Orexins promote the proliferation and differentiation of porcine preadipocytes through increasing lipid accumulation, suppressing glycerol release and upregulating the expression of proadipogenic genes.68a However, orexin-A (but not orexin-B) regulates lipid metabolism, including suppressing glycerol release and increasing glucose uptake, in porcine adipocytes.68b These findings indicate that orexins promote fat deposition by reducing lipolysis and improving adipogenesis and that orexin-A fully participates in these biological processes in vitro.
As glucose supports a related source for adipogenesis, Skrzypski et al.67 cultured isolated rat adipocytes using orexin-A and discovered that orexin-A further enhanced glucose uptake, with time- or concentration-dependent increases in glucose uptake by 3T3-L1 adipocytes exposed to orexin-A. LY294002, an inhibitor of PI3K, blocked orexin-A-stimulated glucose uptake in two kinds of adipocytes. Orexin-A also increased the phosphorylation of protein kinase B (AKT) which is a PI3K downstream effector protein. Glucose transporter 4 (GLUT4), a distinct glucose transport protein, always translocates from the cytoplasm into the plasma membrane when activated. To further verify the role of PI3K-AKT, using immunofluorescence, Skrzypski et al.67 found significant GLUT4 translocation from the cytoplasm into the cell periphery in adipocytes exposed to orexin-A, but also found that LY294002 prevented this GLUT4 translocation. Orexin-A also induced increased phosphorylation of AS160, which is downstream of AKT, and stimulated GLUT4 translocation in its phosphorylated state. In addition, LY294002 inhibited orexin-A-induced adipogenesis, including increased triacylglycerol content and the conversion of glucose into nonesterified fatty acid (NEFA), and orexin-A-inhibited lipolysis instance glycerol release in 3T3-L1 adipocytes.67 Peroxisome proliferator-activated receptor γ (PPARγ) plays a key role in triacylglycerol accumulation and the regulation of adipocyte-specific genes, with PPARγ2 being the major isoform in adipocytes.69 After incubation of human adipocytes with 100 nmol of orexin-A, Digby et al.66 detected a significant increase in PPARγ2 mRNA expression of 1.5-fold. Orexin-A also enhanced PPARγ2 levels in isolated rat adipocytes and 3T3-L1 adipocytes, along with stimulation of adiponectin secretion and cellular triacylglycerol accumulation. The PPARγ2 antagonist BADGE (bisphenol A diglycidyl ether) and knocking out of PPARγ2 both blocked these effects.67 These studies suggest that orexin-A stimulates cellular triacylglycerol accumulation mainly by mediating PI3K-AKT and PPARγ2 in adipocytes.
Factors that Influence the Secretion of Orexins
The orexin neurons are glucose-sensitive neurons. Their functions are suppressed by elevated blood glucose levels, whereas reduced blood glucose levels excite these neurons. In cultured rat hypothalamic neurons undergoing a reduction in glucose concentration from 8.3 to 2.8 mmol/L, 41% showed significant increases in Ca2+ influx. These results indicate that orexin neurons are glucose-sensitive and that they can translate energy status into neural signals.70 Fasting causes low blood glucose, upregulates the expression of orexin mRNA in the hypothalamus, and increases the phosphorylation of cAMP-response element binding protein in orexin-responsive neurons. This suggests that these neurons have functional responses to feeding status. Intraperitoneal injections of insulin and 2-DG (2-deoxy-d-glucose) cause hypoglycemia in animals and lower glucose levels in cells; these two starving stimuli can both induce the expression of orexin-A. The function of orexin-A is closely associated with the type of stimulation. For example, acute stimulation, such as intraperitoneal injection of 2-DG, is suitable for studying neuronal activation, whereas chronic stimulation, such as fasting, is suitable for studying changes in orexin-A expression after neuronal activation.33 However, Iqbal et al.71 demonstrated that long-term feeding restrictions in sheep did not affect the expression of prepro-orexin. This finding suggests that orexin neurons may have increased sensitivities to reduced blood glucose, but are insensitive to chronic hypoglycemia.
A high-fat diet stimulates the expression of orexins in the perifornical hypothalamus, and this is highly correlated with increases in the concentrations of triglycerides in the blood.72 Intravenous injection of triglycerides in normal rats with fixed levels of leptin, blood glucose, and insulin stimulates the expression of orexin mRNA. This result demonstrates that orexin neurons are sensitive to triglycerides.72 Orexins may explain high-fat diet-induced eating, and the consequent development of obesity. Orexins are stimulated by increased circulating lipids during a positive energy balance, and are also stimulated by decreased blood glucose levels during a negative energy balance.
Leptin is a polypeptide hormone secreted by adipose tissues that reduces energy intake and increases energy metabolism. It alleviates symptoms of obesity by enhancing the secretion of endogenous insulin and lowering blood glucose levels.73 Orexin neurons express the leptin receptor, and exogenous leptin can downregulate the level of orexins in rat LHs, which suggests that leptin inhibits the expression of orexins. However, increased levels of leptin are often accompanied by an upregulation of orexins in high-fat DIO rats.72 Thus, it is possible that leptin is not the key factor regulating the expression of orexins. Ghrelin is an acylated polypeptide secreted by both the hypothalamus and the stomach, which regulates appetite. It has been shown that ghrelin enhances the expression of orexins via NPY and AgRP.74 Additionally, exercise also stimulates the activities of orexin neurons in mice.75
Conclusions
In summary, orexins, important neuropeptides in the hypothalamus for regulating appetite and energy homeostasis efficiently, are involved in multiple physiological functions, such as sleep–wake cycle and analgesia, to name a few, through the widespread distribution of their receptors. In addition, orexins slow the onset of diet-induced obesity by enhancing the sensitivity of orexin-coupled hypothalamic neurons and simultaneously increasing NEFA, WAT lipolysis, and BAT thermogenesis. A selective antagonist of the orexin receptor OX1R, SB-334867, suppresses food intake and feeding behaviors, leading to weight loss. It is suggested that a potential approach for the effective treatment of obesity may involve an OX1R antagonist. Orexins stimulate complex peripheral signaling pathways, and their involvements as neuropeptides in the regulation of neural activities are also complicated. However, further studies on orexins should focus on their intracellular signal transduction mechanisms and their interactions with other neuropeptides, in order to provide insights regarding the prevention of metabolic disorders such as anorexia, obesity, and diabetes.
Acknowledgments
This study was financially supported by the Natural Science Foundation of Chongqing (cstc2019jcyj-msxmX0513), the Fundamental Research Funds for the Central Universities (SWU118071, XDJK2017A003), the National Natural Science Foundation of China (no. 31772564), and the Innovation Team Building Program in Chongqing universities (CXTDG201602004).
The authors declare no competing financial interest.
References
- Kalra S. P.; Dube M. G.; Pu S.; Xu B.; Horvath T. L.; Kalra P. S. Interacting appetite-regulating pathways in the hypothalamic regulation of body weight 1. Endocr. Rev. 1999, 20, 68–100. 10.1210/edrv.20.1.0357. [DOI] [PubMed] [Google Scholar]
- Kmiec Z. Central regulation of food intake in ageing. J. Physiol. Pharmacol. 2006, 57, 7–16. [PubMed] [Google Scholar]
- García-Cáceres C.; Yi C.-X.; Tschöp M. H. Hypothalamic astrocytes in obesity. Endocrinol Metab. Clin. N. Am. 2013, 42, 57–66. 10.1016/j.ecl.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Karnani M.; Burdakov D. Multiple hypothalamic circuits sense and regulate glucose levels. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R47–R55. 10.1152/ajpregu.00527.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Gautvik K. M.; de Lecea L.; Gautvik V. T.; Danielson P. E.; Tranque P.; Dopazo A.; et al. Overview of the most prevalent hypothalamusspecific mRNAs, as identified by directional tag PCR subtraction. Proc. Natl. Acad. Sci. U.S.A. 1996, 93, 8733–8738. 10.1073/pnas.93.16.8733. [DOI] [PMC free article] [PubMed] [Google Scholar]; b de Lecea L.; Kilduff T. S.; Peyron C.; Gao X. B.; Foye P. E.; Danielson P. E.; Fukuhara C.; Battenberg E. L. F.; Gautvik V. T.; Bartlett F. S.; Frankel W. N.; van den Pol A. N.; Bloom F. E.; Gautvik K. M.; Sutcliffe J. G. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc. Natl. Acad. Sci. U.S.A. 1998, 95, 322–327. 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Sakurai T.; Amemiya A.; Ishii M.; Matsuzaki I.; Chemelli R. M.; Tanaka H.; Williams S. C.; Richardson J. A.; Kozlowski G. P.; Wilson S.; Arch J. R. S.; Buckingham R. E.; Haynes A. C.; Carr S. A.; Annan R. S.; McNulty D. E.; Liu W.-S.; Terrett J. A.; Elshourbagy N. A.; Bergsma D. J.; Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 1998, 92, 573–585. 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- a Tanaka S.; Honda Y.; Takaku S.; Koike T.; Oe S.; Hirahara Y.; Yoshida T.; Takizawa N.; Takamori Y.; Kurokawa K. Involvement of PLAGL1/ZAC1 in hypocretin/orexin transcription. Int. J. Mol. Med. 2019, 43, 2164–2176. 10.3892/ijmm.2019.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Seifinejad A.; Li S.; Mikhail C.; Vassalli A.; Pradervand S.; Arribat Y.; Pezeshgi Modarres H.; Allen B.; John R. M.; Amati F.; Tafti M. Molecular codes and in vitro generation of hypocretin and melanin concentrating hormone neurons. Proc. Natl. Acad. Sci. U.S.A. 2019, 116, 17061–17070. 10.1073/pnas.1902148116. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Kortunay S.; Erken H. A.; Erken G.; Genç O.; Şahiner M.; Turgut S.; Turgut G. Orexins increase penicillin-induced epileptic activity. Peptides 2012, 34, 419–422. 10.1016/j.peptides.2012.02.013. [DOI] [PubMed] [Google Scholar]
- a Moriguchi T.; Sakurai T.; Nambu T.; Yanagisawa M.; Goto K. Neurons containing orexin in the lateral hypothalamic area of the adult rat brain are activated by insulin-induced acute hypoglycemia. Neurosci. Lett. 1999, 264, 101–104. 10.1016/s0304-3940(99)00177-9. [DOI] [PubMed] [Google Scholar]; b Yamanaka A.; Beuckmann C. T.; Willie J. T.; Hara J.; Tsujino N.; Mieda M.; Tominaga M.; Yagami K.-i.; Sugiyama F.; Goto K.; Yanagisawa M.; Sakurai T. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron 2003, 38, 701–713. 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]; c Burdakov D.; Gerasimenko O.; Verkhratsky A. Physiological changes in glucose differentially modulate the excitability of hypothalamic melanin-concentrating hormone and orexin neurons in situ. J. Neurosci. 2005, 25, 2429–2433. 10.1523/jneurosci.4925-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Chang X.; Suo L.; Xu N.; Zhao Y. Orexin-A Stimulates Insulin Secretion Through the Activation of the OX1 Receptor and Mammalian Target of Rapamycin in Rat Insulinoma Cells. Pancreas 2019, 48, 568–573. 10.1097/mpa.0000000000001280. [DOI] [PubMed] [Google Scholar]
- a Hara J.; Beuckmann C. T.; Nambu T.; Willie J. T.; Chemelli R. M.; Sinton C. M.; Sugiyama F.; Yagami K.-i.; Goto K.; Yanagisawa M.; Sakurai T. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron 2001, 30, 345–354. 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]; b Hara J.; Yanagisawa M.; Sakurai T. Difference in obesity phenotype between orexin-knockout mice and orexin neuron-deficient mice with same genetic background and environmental conditions. Neurosci. Lett. 2005, 380, 239–242. 10.1016/j.neulet.2005.01.046. [DOI] [PubMed] [Google Scholar]
- Kakizaki M.; Tsuneoka Y.; Takase K.; Kim S. J.; Choi J.; Ikkyu A.; Abe M.; Sakimura K.; Yanagisawa M.; Funato H. Differential Roles of Each Orexin Receptor Signaling in Obesity. iScience 2019, 20, 1–13. 10.1016/j.isci.2019.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons D. J.; Hellysaz A.; Ammari R.; Broberger C. Hypocretin/orexin peptides excite rat neuroendocrine dopamine neurons through orexin 2 receptor-mediated activation of a mixed cation current. Sci. Rep. 2017, 7, 41535. 10.1038/srep41535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T. Orexins and orexin receptors: implication in feeding behavior. Regul. Pept. 1999, 85, 25–30. 10.1016/s0167-0115(99)00076-2. [DOI] [PubMed] [Google Scholar]
- Ohkubo T.; Boswell T.; Lumineau S. Molecular cloning of chicken prepro-orexin cDNA and preferential expression in the chicken hypothalamus. Biochim. Biophys. Acta, Gene Struct. Expression 2002, 1577, 476–480. 10.1016/s0167-4781(02)00483-9. [DOI] [PubMed] [Google Scholar]
- Mondal M. S.; Nakazato M.; Date Y.; Murakami N.; Hanada R.; Sakata T.; Matsukura S. Characterization of orexin-A and orexin-B in the microdissected rat brain nuclei and their contents in two obese rat models. Neurosci. Lett. 1999, 273, 45–48. 10.1016/s0304-3940(99)00624-2. [DOI] [PubMed] [Google Scholar]
- a Perez-Leighton C. E.; Billington C. J.; Kotz C. M. Orexin modulation of adipose tissue. Biochim. Biophys. Acta, Mol. Basis Dis. 2014, 1842, 440–445. 10.1016/j.bbadis.2013.06.007. [DOI] [PubMed] [Google Scholar]; b Jöhren O.; Neidert S. J.; Kummer M.; Dendorfer A.; Dominiak P. Prepro-orexin and orexin receptor mRNAs are differentially expressed in peripheral tissues of male and female rats. Endocrinology 2001, 142, 3324–3331. 10.1210/endo.142.8.8299. [DOI] [PubMed] [Google Scholar]; c Nakabayashi M.; Suzuki T.; Takahashi K.; Totsune K.; Muramatsu Y.; Kaneko C.; Date F.; Takeyama J.; Darnel A. D.; Moriya T.; Sasano H. Orexin-A expression in human peripheral tissues. Mol. Cell. Endocrinol. 2003, 205, 43–50. 10.1016/s0303-7207(03)00206-5. [DOI] [PubMed] [Google Scholar]
- Preti A. Orexins (hypocretins): their role in appetite and arousal. Curr. Opin. Investig. Drugs 2002, 3, 1199–1206. [PubMed] [Google Scholar]
- Marcus J. N.; Aschkenasi C. J.; Lee C. E.; Chemelli R. M.; Saper C. B.; Yanagisawa M.; Elmquist J. K. Differential expression of orexin receptors 1 and 2 in the rat brain. J. Comp. Neurol. 2001, 435, 6–25. 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- Trivedi P.; Yu H.; MacNeil D. J.; Van der Ploeg L. H. T.; Guan X.-M. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett. 1998, 438, 71–75. 10.1016/s0014-5793(98)01266-6. [DOI] [PubMed] [Google Scholar]
- Park J.-H.; Shim H.-M.; Na A.-Y.; Bae J.-H.; Im S.-S.; Song D.-K. Orexin A regulates plasma insulin and leptin levels in a time-dependent manner following a glucose load in mice. Diabetologia 2015, 58, 1542–1550. 10.1007/s00125-015-3573-0. [DOI] [PubMed] [Google Scholar]
- Xu T.-R.; Yang Y.; Ward R.; Gao L.; Liu Y. Orexin receptors: multi-functional therapeutic targets for sleeping disorders, eating disorders, drug addiction, cancers and other physiological disorders. Cell. Signal. 2013, 25, 2413–2423. 10.1016/j.cellsig.2013.07.025. [DOI] [PubMed] [Google Scholar]
- a Liu R.-J.; Van Den Pol A. N.; Aghajanian G. K. Hypocretins (orexins) regulate serotonin neurons in the dorsal raphe nucleus by excitatory direct and inhibitory indirect actions. J. Neurosci. 2002, 22, 9453–9464. 10.1523/jneurosci.22-21-09453.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Arrigoni E.; Mochizuki T.; Scammell T. E. Activation of the basal forebrain by the orexin/hypocretin neurones. Acta Physiol. 2010, 198, 223–235. 10.1111/j.1748-1716.2009.02036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukkonen J. P.; Åkerman K. E.. Intracellular signal pathways utilized by the hypocretin/orexin receptors. Hypocretins; Springer, 2005; pp 221–231. [Google Scholar]
- a Smart D.; Jerman J. C.; Brough S. J.; Rushton S. L.; Murdock P. R.; Jewitt F.; Elshourbagy N. A.; Ellis C. E.; Middlemiss D. N.; Brown F. Characterization of recombinant human orexin receptor pharmacology in a Chinese hamster ovary cell-line using FLIPR. Br. J. Pharmacol. 1999, 128, 1–3. 10.1038/sj.bjp.0702780. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Turunen P. M.; Ekholm M. E.; Somerharju P.; Kukkonen J. P. Arachidonic acid release mediated by OX1 orexin receptors. Br. J. Pharmacol. 2010, 159, 212–221. 10.1111/j.1476-5381.2009.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekholm M. E.; Johansson L.; Kukkonen J. P. IP 3-independent signalling of OX 1 orexin/hypocretin receptors to Ca 2+ influx and ERK. Biochem. Biophys. Res. Commun. 2007, 353, 475–480. 10.1016/j.bbrc.2006.12.045. [DOI] [PubMed] [Google Scholar]
- Shen Y.; Zhao Y.; Zheng D.; Chang X.; Ju S.; Guo L. Effects of orexin A on GLUT4 expression and lipid content via MAPK signaling in 3T3-L1 adipocytes. J. Steroid Biochem. Mol. Biol. 2013, 138, 376–383. 10.1016/j.jsbmb.2013.07.005. [DOI] [PubMed] [Google Scholar]
- Gorojankina T.; Grébert D.; Salesse R.; Tanfin Z.; Caillol M. Study of orexins signal transduction pathways in rat olfactory mucosa and in olfactory sensory neurons-derived cell line Odora: multiple orexin signalling pathways. Regul. Pept. 2007, 141, 73–85. 10.1016/j.regpep.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Tang J.; Chen J.; Ramanjaneya M.; Punn A.; Conner A. C.; Randeva H. S. The signalling profile of recombinant human orexin-2 receptor. Cell. Signal. 2008, 20, 1651–1661. 10.1016/j.cellsig.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Xu T.-R.; Ward R. J.; Pediani J. D.; Milligan G. The orexin OX1 receptor exists predominantly as a homodimer in the basal state: potential regulation of receptor organization by both agonist and antagonist ligands. Biochem. J. 2011, 439, 171–183. 10.1042/bj20110230. [DOI] [PubMed] [Google Scholar]
- Bai B.; Chen X.; Zhang R.; Wang X.; Jiang Y.; Li D.; Wang Z.; Chen J. Dual-agonist occupancy of orexin receptor 1 and cholecystokinin A receptor heterodimers decreases G-protein–dependent signaling and migration in the human colon cancer cell line HT-29. Biochim. Biophys. Acta, Mol. Cell Res. 2017, 1864, 1153–1164. 10.1016/j.bbamcr.2017.03.003. [DOI] [PubMed] [Google Scholar]
- Xue Q.; Bai B.; Ji B.; Chen X.; Wang C.; Wang P.; Yang C.; Zhang R.; Jiang Y.; Pan Y. Ghrelin through GHSR1a and OX1R heterodimers reveals a Gαs–cAMP-cAMP response element binding protein signaling pathway in vitro. Front. Mol. Neurosci. 2018, 11, 245. 10.3389/fnmol.2018.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abizaid A.; Horvath T. L. Brain circuits regulating energy homeostasis. Regul. Pept. 2008, 149, 3–10. 10.1016/j.regpep.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes A. C.; Jackson B.; Overend P.; Buckingham R. E.; Wilson S.; Tadayyon M.; Arch J. R. S. Effects of single and chronic intracerebroventricular administration of the orexins on feeding in the rat. Peptides 1999, 20, 1099–1105. 10.1016/s0196-9781(99)00105-9. [DOI] [PubMed] [Google Scholar]
- Kastin A. J.; Akerstrom V. Orexin A but not orexin B rapidly enters brain from blood by simple diffusion. J. Pharmacol. Exp. Ther. 1999, 289, 219–223. [PubMed] [Google Scholar]
- Okumura T.; Takakusaki K. Role of orexin in central regulation of gastrointestinal functions. J. Gastroenterol. 2008, 43, 652–660. 10.1007/s00535-008-2218-1. [DOI] [PubMed] [Google Scholar]
- Furuse M.; Ando R.; Bungo T.; Shimojo M.; Masuda Y. Intracerebroventricular injection of orexins does not stimulate food intake in neonatal chicks. Br. Poult. Sci. 1999, 40, 698–700. 10.1080/00071669987115. [DOI] [PubMed] [Google Scholar]
- Ohkubo T.; Tsukada A.; Shamoto K. cDNA cloning of chicken orexin receptor and tissue distribution: sexually dimorphic expression in chicken gonads. J. Mol. Endocrinol. 2003, 31, 499–508. 10.1677/jme.0.0310499. [DOI] [PubMed] [Google Scholar]
- Ishii Y.; Blundell J. E.; Halford J. C. G.; Upton N.; Porter R.; Johns A.; Rodgers R. J. Satiety enhancement by selective orexin-1 receptor antagonist SB-334867: influence of test context and profile comparison with CCK-8S. Behav. Brain Res. 2005, 160, 11–24. 10.1016/j.bbr.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Rodgers R. J.; Halford J. C. G.; Nunes de Souza R. L.; Canto de Souza A. L.; Piper D. C.; Arch J. R. S.; Upton N.; Porter R. A.; Johns A.; Blundell J. E. SB-334867, a selective orexin-1 receptor antagonist, enhances behavioural satiety and blocks the hyperphagic effect of orexin-A in rats. Eur. J. Neurosci. 2001, 13, 1444–1452. 10.1046/j.0953-816x.2001.01518.x. [DOI] [PubMed] [Google Scholar]
- Dube M. G.; Horvath T. L.; Kalra P. S.; Kalra S. P. Evidence of NPY Y5 receptor involvement in food intake elicited by orexin A in sated rats. Peptides 2000, 21, 1557–1560. 10.1016/s0196-9781(00)00311-9. [DOI] [PubMed] [Google Scholar]
- Ida T.; Nakahara K.; Kuroiwa T.; Fukui K.; Nakazato M.; Murakami T.; Murakami N. Both corticotropin releasing factor and neuropeptide Y are involved in the effect of orexin (hypocretin) on the food intake in rats. Neurosci. Lett. 2000, 293, 119–122. 10.1016/s0304-3940(00)01498-1. [DOI] [PubMed] [Google Scholar]
- Sweet D. C.; Levine A. S.; Kotz C. M. Functional opioid pathways are necessary for hypocretin-1 (orexin-A)-induced feeding. Peptides 2004, 25, 307–314. 10.1016/j.peptides.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Sheng Z.; Santiago A. M.; Thomas M. P.; Routh V. H. Metabolic regulation of lateral hypothalamic glucose-inhibited orexin neurons may influence midbrain reward neurocircuitry. Mol. Cell. Neurosci. 2014, 62, 30–41. 10.1016/j.mcn.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabauskas G.; Moises H. C. Gastrointestinal-projecting neurones in the dorsal motor nucleus of the vagus exhibit direct and viscerotopically organized sensitivity to orexin. J. Physiol. 2003, 549, 37–56. 10.1113/jphysiol.2002.029546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H.; Takahashi N.; Tanno S.; Nagamine M.; Takakusaki K.; Okumura T. A selective orexin-1 receptor antagonist, SB334867, blocks 2-DG-induced gastric acid secretion in rats. Neurosci. Lett. 2005, 376, 137–142. 10.1016/j.neulet.2004.11.043. [DOI] [PubMed] [Google Scholar]
- Gallmann E.; Arsenijevic D.; Spengler M.; Williams G.; Langhans W. Effect of CCK-8 on insulin-induced hyperphagia and hypothalamic orexigenic neuropeptide expression in the rat. Peptides 2005, 26, 437–445. 10.1016/j.peptides.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Garland T.; Schutz H.; Chappell M. A.; Keeney B. K.; Meek T. H.; Copes L. E.; Acosta W.; Drenowatz C.; Maciel R. C.; Van Dijk G.; Kotz C. M.; Eisenmann J. C. The biological control of voluntary exercise, spontaneous physical activity and daily energy expenditure in relation to obesity: human and rodent perspectives. J. Exp. Biol. 2011, 214, 206–229. 10.1242/jeb.048397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubkin M.; Stricker-Krongrad A. Independent feeding and metabolic actions of orexins in mice. Biochem. Biophys. Res. Commun. 1998, 253, 241–245. 10.1006/bbrc.1998.9750. [DOI] [PubMed] [Google Scholar]
- Bunney P. E.; Zink A. N.; Holm A. A.; Billington C. J.; Kotz C. M. Orexin activation counteracts decreases in nonexercise activity thermogenesis (NEAT) caused by high-fat diet. Physiol. Behav. 2017, 176, 139–148. 10.1016/j.physbeh.2017.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak C. M.; Kotz C. M.; Levine J. A. Central orexin sensitivity, physical activity, and obesity in diet-induced obese and diet-resistant rats. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E396–E403. 10.1152/ajpendo.00293.2005. [DOI] [PubMed] [Google Scholar]
- Coborn J. E.; DePorter D. P.; Mavanji V.; Sinton C. M.; Kotz C. M.; Billington C. J.; Teske J. A. Role of orexin-A in the ventrolateral preoptic area on components of total energy expenditure. Int. J. Obes. 2017, 41, 1256. 10.1038/ijo.2017.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison S. F.; Madden C. J.; Tupone D. Central control of brown adipose tissue thermogenesis. Front. Endocrinol. 2012, 3, 5. 10.3389/fendo.2012.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupone D.; Madden C. J.; Cano G.; Morrison S. F. An orexinergic projection from perifornical hypothalamus to raphe pallidus increases rat brown adipose tissue thermogenesis. J. Neurosci. 2011, 31, 15944–15955. 10.1523/jneurosci.3909-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellayah D.; Sikder D. Orexin restores aging-related brown adipose tissue dysfunction in male mice. Endocrinology 2014, 155, 485–501. 10.1210/en.2013-1629. [DOI] [PubMed] [Google Scholar]
- Yoshimichi G.; Yoshimatsu H.; Masaki T.; Sakata T. Orexin-A regulates body temperature in coordination with arousal status. Exp. Biol. Med. 2001, 226, 468–476. 10.1177/153537020122600513. [DOI] [PubMed] [Google Scholar]
- Kosse C.; Schöne C.; Bracey E.; Burdakov D. Orexin-driven GAD65 network of the lateral hypothalamus sets physical activity in mice. Proc. Natl. Acad. Sci. U.S.A. 2017, 114, 4525–4530. 10.1073/pnas.1619700114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Shen J.; Tanida M.; Yao J.-f.; Niijima A.; Nagai K. Biphasic effects of orexin-A on autonomic nerve activity and lipolysis. Neurosci. Lett. 2008, 444, 166–171. 10.1016/j.neulet.2008.08.031. [DOI] [PubMed] [Google Scholar]; b Sperandeo R.; Maldonato M. N.; Messina A.; Cozzolino P.; Monda M.; Cerroni F.; Romano P.; Salerno M.; Maltese A.; Roccella M. Orexin system: network multi-tasking. Acta Med. Mediterr. 2018, 34, 349–356. 10.19193/0393-6384_2018_2_55. [DOI] [Google Scholar]; c Teske J. A.; Perez-Leighton C. E.; Billington C. J.; Kotz C. M. Role of the locus coeruleus in enhanced orexin A-induced spontaneous physical activity in obesity-resistant rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R1337–R1345. 10.1152/ajpregu.00229.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Messina G.; Monda V.; Moscatelli F.; Valenzano A. A.; Monda G.; Esposito T.; De Blasio S.; Messina A.; Tafuri D.; Barillari M. R. Role of orexin system in obesity. Biol. Med. 2015, 7, 248. 10.4172/0974-8369.1000248. [DOI] [Google Scholar]
- Liu L. B.; Chen X. D.; Zhou X. Y.; Zhu Q. The Wnt antagonist and secreted frizzled-related protein 5: implications on lipid metabolism, inflammation and type 2 diabetes mellitus. Biosci. Rep. 2018, 38, BSR20180011. 10.1042/BSR20180011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Zhang F.; Su H.; Song M.; Zheng J.; Liu F.; Yuan C.; Fu Q.; Chen S.; Zhu X.; Wang L. Calcium supplementation alleviates high-fat diet (HFD)-induced estrous cycle irregularity and subfertility associated with concomitantly enhanced thermogenesis of brown adipose tissue (BAT) and browning of white adipose tissue (WAT). J. Agric. Food Chem. 2019, 67, 7073. 10.1021/acs.jafc.9b02663. [DOI] [PubMed] [Google Scholar]; b Kawarasaki S.; Kuwata H.; Sawazaki H.; Sakamoto T.; Nitta T.; Kim C. S.; Jheng H. F.; Takahashi H.; Nomura W.; Ara T. A new mouse model for noninvasive fluorescence-based monitoring of mitochondrial UCP 1 expression. FEBS Lett. 2019, 593, 1201–1212. 10.1002/1873-3468.13430. [DOI] [PubMed] [Google Scholar]
- Tsuneki H.; Murata S.; Anzawa Y.; Soeda Y.; Tokai E.; Wada T.; Kimura I.; Yanagisawa M.; Sakurai T.; Sasaoka T. Age-related insulin resistance in hypothalamus and peripheral tissues of orexin knockout mice. Diabetologia 2008, 51, 657–667. 10.1007/s00125-008-0929-8. [DOI] [PubMed] [Google Scholar]
- Funato H.; Tsai A. L.; Willie J. T.; Kisanuki Y.; Williams S. C.; Sakurai T.; Yanagisawa M. Enhanced orexin receptor-2 signaling prevents diet-induced obesity and improves leptin sensitivity. Cell Metabol. 2009, 9, 64–76. 10.1016/j.cmet.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Leighton C. E.; Boland K.; Teske J. A.; Billington C.; Kotz C. M. Behavioral responses to orexin, orexin receptor gene expression, and spontaneous physical activity contribute to individual sensitivity to obesity. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E865–E874. 10.1152/ajpendo.00119.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley S.; Pinto S.; Segal J.; Perez C. A.; Viale A.; DeFalco J.; Cai X.; Heisler L. K.; Friedman J. M. Identification of neuronal subpopulations that project from hypothalamus to both liver and adipose tissue polysynaptically. Proc. Natl. Acad. Sci. U.S.A. 2010, 107, 7024–7029. 10.1073/pnas.1002790107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins L.; Seoane-Collazo P.; Contreras C.; González-García I.; Martínez-Sánchez N.; González F.; Zalvide J.; Gallego R.; Diéguez C.; Nogueiras R.; Tena-Sempere M.; López M. A Functional Link between AMPK and Orexin Mediates the Effect of BMP8B on Energy Balance. Cell Rep. 2016, 16, 2231–2242. 10.1016/j.celrep.2016.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellayah D.; Bharaj P.; Sikder D. Orexin is required for brown adipose tissue development, differentiation, and function. Cell Metabol. 2011, 14, 478–490. 10.1016/j.cmet.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Sellayah D.; Sikder D. Orexin receptor-1 mediates brown fat developmental differentiation. Adipocyte 2012, 1, 58–63. 10.4161/adip.18965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhanam P.; Treglia G.; Ahima R. S. Detection of brown adipose tissue by 18F- FDG PET/CT in pheochromocytoma/paraganglioma: A systematic review. J. Clin. Hypertens. 2018, 20, 615. 10.1111/jch.13228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digby J. E.; Chen J.; Tang J. Y.; Lehnert H.; Matthews R. N.; Randeva H. S. Orexin receptor expression in human adipose tissue: effects of orexin-A and orexin-B. J. Endocrinol. 2006, 191, 129–136. 10.1677/joe.1.06886. [DOI] [PubMed] [Google Scholar]
- Skrzypski M.; Le T.; Kaczmarek P.; Pruszynska-Oszmalek E.; Pietrzak P.; Szczepankiewicz D.; Kolodziejski P. A.; Sassek M.; Arafat A.; Wiedenmann B.; Nowak K. W.; Strowski M. Z. Orexin A stimulates glucose uptake, lipid accumulation and adiponectin secretion from 3T3-L1 adipocytes and isolated primary rat adipocytes. Diabetologia 2011, 54, 1841–1852. 10.1007/s00125-011-2152-2. [DOI] [PubMed] [Google Scholar]
- a Wojciechowicz T.; Skrzypski M.; Szczepankiewicz D.; Hertig I.; Kołodziejski P. A.; Billert M.; Strowski M. Z.; Nowak K. W. Orexins A and B stimulate proliferation and differentiation of porcine preadipocytes. Exp. Biol. Med. 2016, 241, 1786–1795. 10.1177/1535370216649261. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Pruszynska-Oszmalek E.; Kolodziejski P. A.; Kaczmarek P.; Sassek M.; Szczepankiewicz D.; Mikula R.; Nowak K. W. Orexin A but not orexin B regulates lipid metabolism and leptin secretion in isolated porcine adipocytes. Domest. Anim. Endocrinol. 2018, 63, 59–68. 10.1016/j.domaniend.2017.12.003. [DOI] [PubMed] [Google Scholar]
- Liu L.; Wang G.; Xiao Y.; Shipp S. L.; Siegel P. B.; Cline M. A.; Gilbert E. R. Peripheral neuropeptide Y differentially influences adipogenesis and lipolysis in chicks from lines selected for low or high body weight. Comp. Biochem. Physiol., Part A: Mol. Integr. Physiol. 2017, 213, 1–10. 10.1016/j.cbpa.2017.08.001. [DOI] [PubMed] [Google Scholar]
- Muroya S.; Uramura K.; Sakurai T.; Takigawa M.; Yada T. Lowering glucose concentrations increases cytosolic Ca 2+ in orexin neurons of the rat lateral hypothalamus. Neurosci. Lett. 2001, 309, 165–168. 10.1016/s0304-3940(01)02053-5. [DOI] [PubMed] [Google Scholar]
- Iqbal J.; Henry B. A.; Pompolo S.; Rao A.; Clarke I. J. Long-term alteration in bodyweight and food restriction does not affect the gene expression of either preproorexin or prodynorphin in the sheep. Neuroscience 2003, 118, 217–226. 10.1016/s0306-4522(02)00815-1. [DOI] [PubMed] [Google Scholar]
- Wortley K. E.; Chang G.-Q.; Davydova Z.; Leibowitz S. F. Orexin gene expression is increased during states of hypertriglyceridemia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R1454–R1465. 10.1152/ajpregu.00286.2002. [DOI] [PubMed] [Google Scholar]
- Benoit S. C.; Clegg D. J.; Seeley R. J.; Woods S. C. Insulin and leptin as adiposity signals. Recent Prog. Horm. Res. 2004, 59, 267–285. 10.1210/rp.59.1.267. [DOI] [PubMed] [Google Scholar]
- Toshinai K.; Date Y.; Murakami N.; Shimada M.; Mondal M. S.; Shimbara T.; Guan J.-L.; Wang Q.-P.; Funahashi H.; Sakurai T.; Shioda S.; Matsukura S.; Kangawa K.; Nakazato M. Ghrelin-induced food intake is mediated via the orexin pathway. Endocrinology 2003, 144, 1506–1512. 10.1210/en.2002-220788. [DOI] [PubMed] [Google Scholar]
- Shiuchi T.; Miyatake Y.; Otsuka A.; Chikahisa S.; Sakaue H.; Séi H. Role of orexin in exercise-induced leptin sensitivity in the mediobasal hypothalamus of mice. Biochem. Biophys. Res. Commun. 2019, 514, 166–172. 10.1016/j.bbrc.2019.04.145. [DOI] [PubMed] [Google Scholar]