Abstract
Sensing of metal contaminants at ultralow concentrations in aqueous environments is vital in today’s overpopulated world, with an extremely stringent limit (<5 ppb) for Cd2+ ions in drinking water. Here, we utilize sonochemically exfoliated molybdenum disulfide (MoS2) nanosheets functionalized with l-cysteine (Cys) as highly sensitive and selective two-dimensional (2D) materials for solid-state chemiresistors. We specifically targeted Cd2+ ions due to their high toxicity at low concentrations. MoS2-Cys nanosheets are fabricated using an ad hoc, low-complexity, one-pot synthesis method. Porous MoS2–Cys thin films with a high surface area are assembled from these nanosheets. Two-terminal chemiresistors incorporating MoS2–Cys films are demonstrated to be preferentially sensitive to Cd2+ ions at neutral pH, irrespective of other metal ions present in water flowing through the device. A 5 ppb concentration of the Cd2+ ions in the water stream increases the device resistivity by 20 times. Our devices operate at broad (1–500 ppb) range and fast (∼1 s) response times. Cd2+ is selectively detected because of preferential, size-driven adsorption at the interstitials between l-cysteine functional groups, combined with pH-controlled charge transfer that removes electronic gap states from MoS2. MoS2–Cys-based chemiresistors can be deployed in-line to detect metal ions without any need for additional offline measurements.
Introduction
Contamination of global water resources has increased substantially in recent years due to increasing human activity and population. To meet the drinking water requirements of today’s world, sophisticated diagnostics of clean water and wastewater streams is vital, especially for concerns of metal-ion contamination.1 Contaminants commonly found in drinking water include transition metals such as Mn, Fe, Co, Cu, Mo, Zn, Cu, Cd, and Hg, as well as post-transition metals such as Pb, As, and B. All of these contaminants, and others, are globally regulated to levels in the ppb or sub-ppb range.1 In many cases, metal ions can cause harmful health effects if consumed by humans or animals, which can have acute or chronic consequences due to bioaccumulation. Specifically, the Environmental Protection Agency (EPA) mandated that the limit of Cd in drinking water should be as low as 5 ppb concentration. Comparison of this limit with the significantly higher (50 ppb) EPA limit for manganese, not considered to be damaging to health, is indicative of the importance of high-sensitivity detection of cadmium in water, where it normally restructures as Cd2+ ions.
State-of-the-art detection methods for metal ions in water include (i) electrochemical techniques such as anodic stripping voltammetry, which can access the ppb and sub-ppb concentration ranges;2−9 (ii) optical detection techniques (including fluorescence, luminescence, or colorimetric detection10−16) and (iii) electrical transduction sensing via chemiresistor or field-effect transistor architectures.17−25 Of these detection categories, electrical transduction devices are preferred due to reduced complexity, as they do not require additional offline measurements (such as with optical methods) and are not complicated by the need for high-maintenance cost reference electrodes (as in the case of electrochemical methods).1 Three-terminal field-effect transistors based on molybdenum disulfide (MoS2) and diselenide (MoSe2) are known to exhibit high-accuracy and low-detection threshold for gas sensing,20−27 but equivalent sensors for detection of metal ions in water have not yet been demonstrated. Furthermore, a two-terminal chemiresistor is highly desirable over transistor (triode) architectures in terms of reduced device complexity.
A chemiresistor detects contaminants as they act as dopants or impurities that induce changes in the device’s electrical conductivity, even when they are present only at the ppb level.17−25 This is different from electrochemical sensors in which redox processes must occur at the electrode surface. In chemiresistors, thickness and surface area of the active layer are directly related to the sensor response time and sensitivity, with thinner films and higher surface areas leading to better performance. Molybdenum disulfide (MoS2) is an excellent candidate material for chemiresistor sensing as well as other environmental applications.28,29 Two-dimensional (2D) MoS2 nanosheets exhibit p-type semiconducting properties with electrical transport sensitive to doping by charge transfer.29 Few-layer 2D MoS2 can be readily exfoliated by solution-processing techniques28,30 and restacked into thin films by common thin-film fabrication methods, including drop casting, spin coating, and vacuum filtration. Solution-processed MoS2 nanosheets are uniquely positioned as robust sensing platforms for metal detection because they can be easily decorated with carboxylated groups28 in a one-pot methodology that favorably compares, in terms of cost competitiveness and low complexity, to the preparation of reduced graphene oxide and epitaxial growth of 2D MoS2 on graphene or SiC.18,29 Functionalization of carboxylated MoS2 (MoS2–COOH) with amino acids may add receptors that are sensitive to specific metal ions. l-Cysteine and small molecules such as glutathione are known to have a high affinity for divalent metal ions such as Cd and Hg, respectively,28,31−33 and can be similarly used to sensitize MoS2 toward these elements.
Here, MoS2 nanosheets functionalized with l-cysteine (MoS2–Cys) will be examined as candidate materials for chemiresistors showing high selectivity and preferential sensitivity for Cd2+ ions in water at neutral pH in the 1–500 ppb range. These sensors will be prepared from MoS2–COOH by a simple, low-cost method to display a low detection limit for Cd2+. Chemiresistors based on our technology have been demonstrated to operate irrespective of the presence of counterions or other metal ions in water. Compared to similar devices comprising an active layer of epitaxially grown SiC,18 MoS2–Cys will have lower fabrication costs, and will be shown to have a lower detection limit for Cd2+. MoS2–Cys is anticipated to display selective and tunable sensitivity to different metal ions depending on the pH of the metal ion solution used, where a sensitivity enhancement of 1–2 orders of magnitude can be achieved for Cd2+ sensing.
Results and Discussion
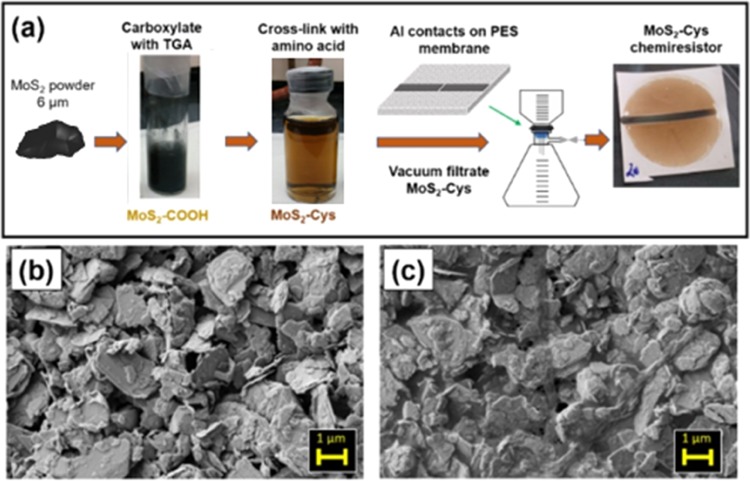
Preparation of our in-line chemiresistor devices utilizes thin films of material deposited on a poly(ether)sulfone (PES) porous support backing to form a high-surface area sensor. The fabrication protocol is summarized in Figure 1a, where the active sensing layer is comprised of MoS2–Cys. The chemiresistor sensitivity is controlled by defining a sensing area between two metal electrodes, Al in this case, deposited prior to the vacuum filtration of MoS2 solution. Pores in the PES are preserved when the Al thickness is on the order of 100 nm. In this way, both MoS2–Cys films and Al electrodes are porous and do not disturb the water flow through the device during the sensing process.
Figure 1.
(a) MoS2–Cys preparation using thioglycolic acid (TGA) to add carboxyl groups followed by amide cross-linking for Cys functionalization and fabrication of two-terminal chemiresistors. MoS2–Cys thin films can be prepared by vacuum filtration and incorporated in these devices. 2-keV scanning electron microscopy (SEM) images of (b) MoS2–COOH and (c) MoS2–Cys (1:20 MoS2/Cys mass ratio), showing Cys functionalization as a network of filaments on the surface of MoS2 aggregates. Photographs are the authors’ own work.
Figure 1b,c shows the SEM images of MoS2-COOH and MoS2–Cys thin films, respectively. In both panels (b) and (c), it is evident that 2D MoS2 nanosheets tend to stack in aggregates from 100 nm to 2 μm thickness. Although individual nanoscale platelets are not readily visible by SEM, numerous nanoscale platelets mixed with micron-size pieces have been detected and investigated via atomic force microscopy. The number of layers present in the flakes ranges from 5 to 10 layers for nanoscale platelets to hundreds of layers for micron-size platelets.28
Comparison of the two panels demonstrates the effects of cysteine on the thin-film morphology as a cross-linker between MoS2 platelets. MoS2–COOH prepared by the same method used here has been previously shown to be mainly arranged in few-layer nanosheets.28 On the contrary, thicker aggregates are visible in Figure 1b from thin films obtained by vacuum filtration, and a more continuous film is noticeable in panel (c) due to Cys-induced cross-linking. We thus infer that functionalization converts MoS2–COOH films made of disconnected platelets into cross-linked networks of MoS2 flakes interconnected by Cys filaments, with the ability to preferentially retain ions with which they exhibit affinity.
Figure 2a presents the SEM micrograph of a MoS2–Cys chemiresistor channel, along with elemental maps obtained by energy-dispersive X-ray (EDX). Magnification of the SEM image is lower than previously reported in Figure 1b,c. EDX maps show the distribution of N, Mo, and S, as well as Al from the gap-cell contacts. Nitrogen is uniformly distributed, also indicating a uniform presence of Cys filaments, as the only nitrogen source in this system is the amine from these functionals. Mo and S are distributed throughout as expected, with some large particles of MoS2 visible on the surface. Examination of sulfur atomic percentages determined from EDX show larger amounts of S than expected for stoichiometric MoS2 alone, confirming that Cys functionals (also containing S) are present.
Figure 2.
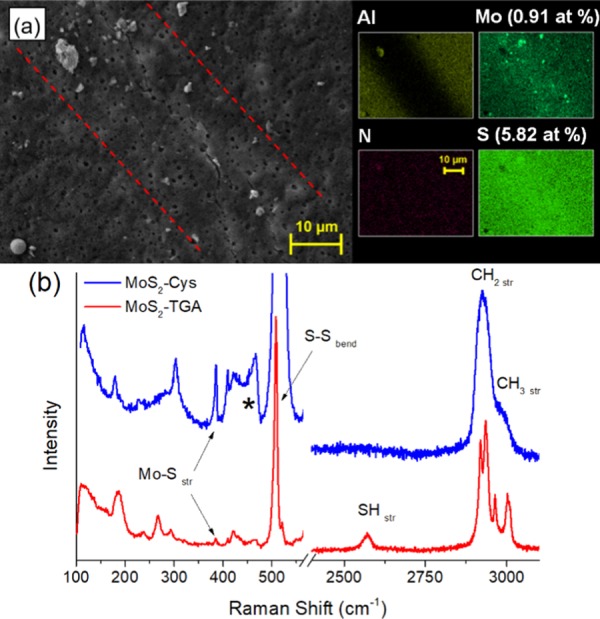
(a) Ten kiloelectron Volts SEM/EDX images of 1:10 mass ratio MoS2:Cys chemiresistor channel. MoS2 flakes are visible in the device channel between red dotted lines. Region outside the channel is occupied by Al contacts. The porous nature of the chemiresistor is evidenced by minimally covered 0.8 μm pores (black dots) from the underlying PES membrane. EDX S, Mo, N, and Al maps are also reported. (b) Raman spectra of MoS2–COOH (blue) and MoS2–Cys (red) casted on Si wafers. Before Cys functionalization, Raman S–S and SH stretching bands representing residual TGA are clearly visible. After functionalization, a new feature (*) is the fingerprint of Cys attachment to MoS2.
Figure 2b compares the Raman spectra of MoS2–COOH and MoS2–Cys. MoS2–COOH was measured before being washed to remove unreacted TGA that has not combined with MoS2. Raman-active S–S bending, S–H stretching, and hydrocarbon stretching modes from TGA are clearly visible, along with the stretching modes characteristic of MoS2, at 409 and 385 cm–1.28 After functionalization, the sharp S–H stretching peak of TGA is replaced by a significantly broader counterpart from cysteine, which is centered at ∼2650 cm–1. Hydrocarbon modes are also significantly broadened because of the structural disorder introduced by the solid-state nature of these peaks, as opposed to their molecular counterparts in TGA.28 The most important Raman feature for our work, marked with (*) in Figure 2b, is distinctive of cysteine bonding with the chalcogenide backbone. Assignment of this peak to Cys-bonded MoS2 has been widely corroborated in ref (28) with additional characterization, which shows a clear upshift of the 409 cm–1 Raman peak of MoS2 in the presence of stable and irreversible l-cysteine functionalization.
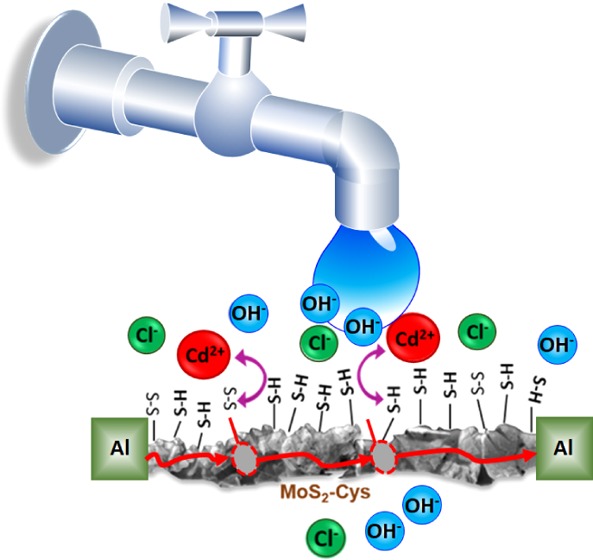
We anticipate that stable Cys bonding to MoS2 is critical toward MoS2–Cys sensing performance. Figure 3a shows the response of a MoS2–Cys chemiresistor upon injection with a deionized (DI) water stream contaminated with increasing concentrations of Cd2+ ions, from 1 to 500 ppb. The chemiresistors operate by measuring the relative resistivity changes (δρ/ρ) over time when impurities are added to water flowing in-line through two-terminal porous MoS2–Cys gap cells. We anticipate that δρ is normally positive (i.e., MoS2 resistivity increases with increasing metal-ion concentrations in the filtrated water stream). Thus, our sensing process is different from the decrease in water ionic resistance due to impurities.
Figure 3.
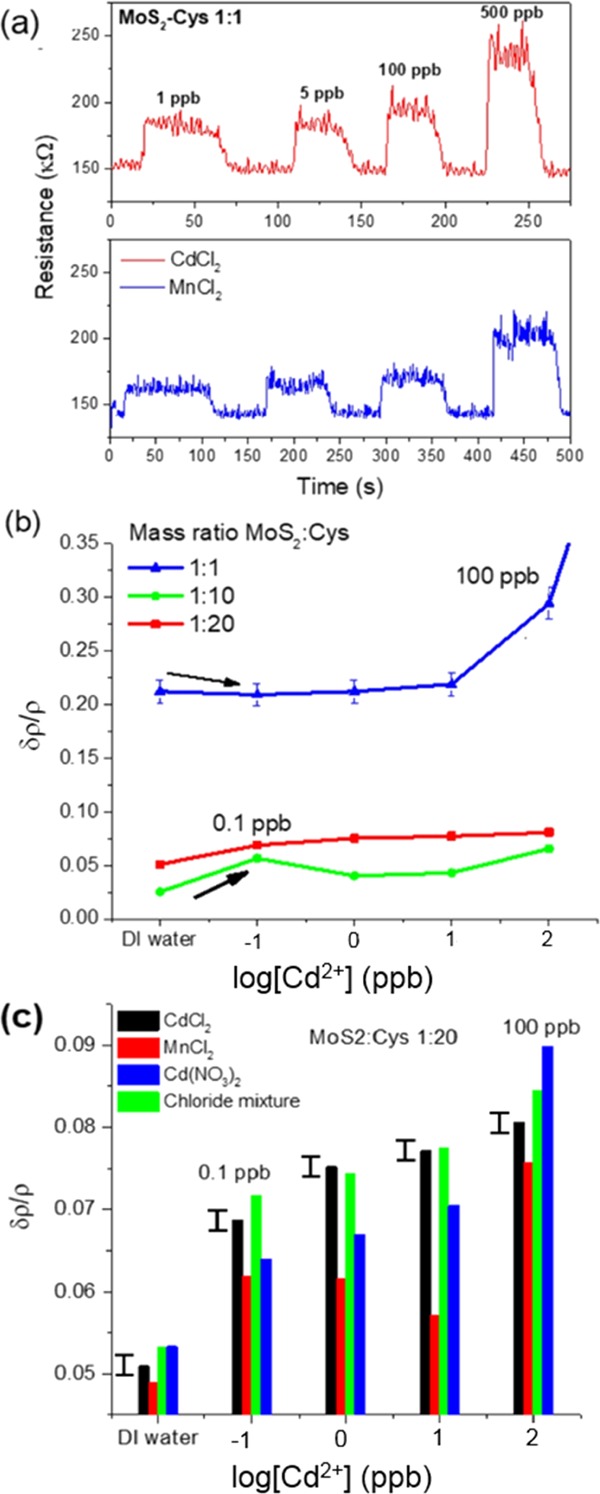
(a) Time domain sensing performance of MoS2–Cys chemiresistor (MoS2/Cys = 1:1) for as-prepared CdCl2 and MnCl2 water-containing solutions at 1–500 ppb concentrations. Metal ions are detected by the resulting increase in the resistance (δρ/ρ) (b) δρ/ρ of chemiresistors at different MoS2/Cys mass ratios at different Cd2+ concentrations in the presence of Cl– counterions. (c) δρ/ρ of 1:20 MoS2/Cys chemiresistor challenged with permeation from dilute CdCl2, Cd(NO3)2, and MnCl2, solutions, as well as a 1:1 mixture of CdCl2 and MnCl2. At a very low concentration range, the sensor is selective for Cd2+ independent of the counterion present, with the highest sensitivity and lowest interference at 10 ppb.
The response in terms of resistivity increase is nearly immediate (<1 s). However, at MoS2/Cys = 1:1 mass ratio, differentiation between Mn2+ and Cd2+ ions is minimal, with both ions appearing to increase the sensor resistance due to charge transfer between MoS2–Cys and the coordinating metal ions, which remove electronic states (acting as electron donors) from the band gap of MoS2.28 Furthermore, the sensitivity of 1:1 mass ratio MoS2/Cys sensors is below the EPA-mandated level1 of 5 ppb for Cd in drinking water. Chemiresistor performance reported in Figure 3a needed to be improved both in terms of sensitivity and selectivity, which required to adjust the MoS2/Cys ratio in the active layer and the pH of the water stream, respectively.
To increase the sensitivity of MoS2–Cys chemiresistors to lower concentrations of Cd2+ ions, larger amounts of l-cysteine were used in the thin-film fabrication process. Figure 3b reports δρ/ρ from chemiresistors prepared from MoS2–Cys thin films at 1:1, 1:10, and 1:20 MoS2/Cys mass ratios and their comparison with analytical standards of water at varying concentrations of Cd2+ ions. Differently from the 1:1 MoS2/Cys device, both 1:10 and 1:20 active layers show improved sensitivity to Cd2+ due to significantly smaller error bars, which enable a linear response in the 0.1–10 ppb range. However, the price of higher sensitivity to lower concentrations is paid in terms of lower overall magnitude of δρ/ρ, as displayed by sensors with a lower MoS2/Cys ratio. This happens because ρ, the dry base resistance, significantly increases from 140 ± 10 kΩ at 1:1 mass ratio to 250 ± 10 kΩ at 1:20 (see Supporting Information Figure S1). This demonstrates that Cys bonding is a necessary ingredient to improve the electrical conductivity of MoS2 thin films. As l-cysteine is an electrical insulator, increase in electrical conductivity at higher Cys contents could be assigned to the addition of states in the band gap of MoS2 via electronic charge transfer.27
Consistency of Cd2+ sensing performance in the presence of different counterions was also examined. Figure 3c shows the values of δρ/ρ of a 1:20 MoS2/Cys chemiresistor challenged with (i) Cd2+ from CdCl2 and Cd(NO3)2 (pH = 5.0), (ii) Mn2+ (pH = 6.0) from MnCl2 and (iii) a mixture of CdCl2 and MnCl2 (pH = 5.6) leading to interference of Mn2+ and Cd2+ ions. At the lowest concentrations, this chemiresistor is preferentially selective to Cd2+, with the highest sensitivity and lowest interference observed at 10 ppb. For a single ion–counterion pair, the sensors shown here are capable of quantitatively indicating the concentration in the 1–100 ppb range. When a mixture with different counterions is used, the sensors continue to function qualitatively to determine if Cd is present at very low concentrations, but the observed difference between Cd concentration values decreases, making the concentration more difficult to determine. This effect results from the sensitivity to different ions at different concentrations depending on the MoS2/Cys ratio used in the fabrication process. However, because of the strong difference in the acidity of the water streams used in Figure 3c, detailed understanding of the optimal performance in terms of pH is required.
To examine the effect of pH on the chemiresistor performance, 10 ppb solutions of Cd2+ and Mn2+ ions were tested at buffered pH from 3 to 10. Figure 4a shows δρ/ρ for 1:20 MoS2/Cys sensors at varying pH. At the lowest (pH < 5) and highest (pH > 9) values, little selectivity can be noticed between metal ions at similar hydrodynamic radii, such as Cd2+ and Mn2+. Chemiresistors are “shorted” by the high ionic conductivity of water, with low resistances in the 10–1000 Ω range (Figure 2S, Supporting Information). This limits the useful sensing window to pH = 5–9, which is well within the range for drinking water. Selectivity to different ions still varies dramatically in this range. However, at a neutral pH of 6–7 (i.e., the values expected for drinking water), Figure 4a also shows that the sensor is highly sensitive to Cd2+ regardless of the presence of chloride or nitrate counterions. Interference from Mn2+ ions is also negligible in this range by comparison. This indicates that optimized MoS2–Cys chemiresistors fabricated at 1:20 constituent ratios satisfy the detection requirements for Cd2+ ions, both in terms of sensitivity and selectivity from metal ions of comparable radii. Of course, additional selectivity from metal ions at different hydrodynamic radii can always be obtained by preliminary filtration of the water stream.
Figure 4.
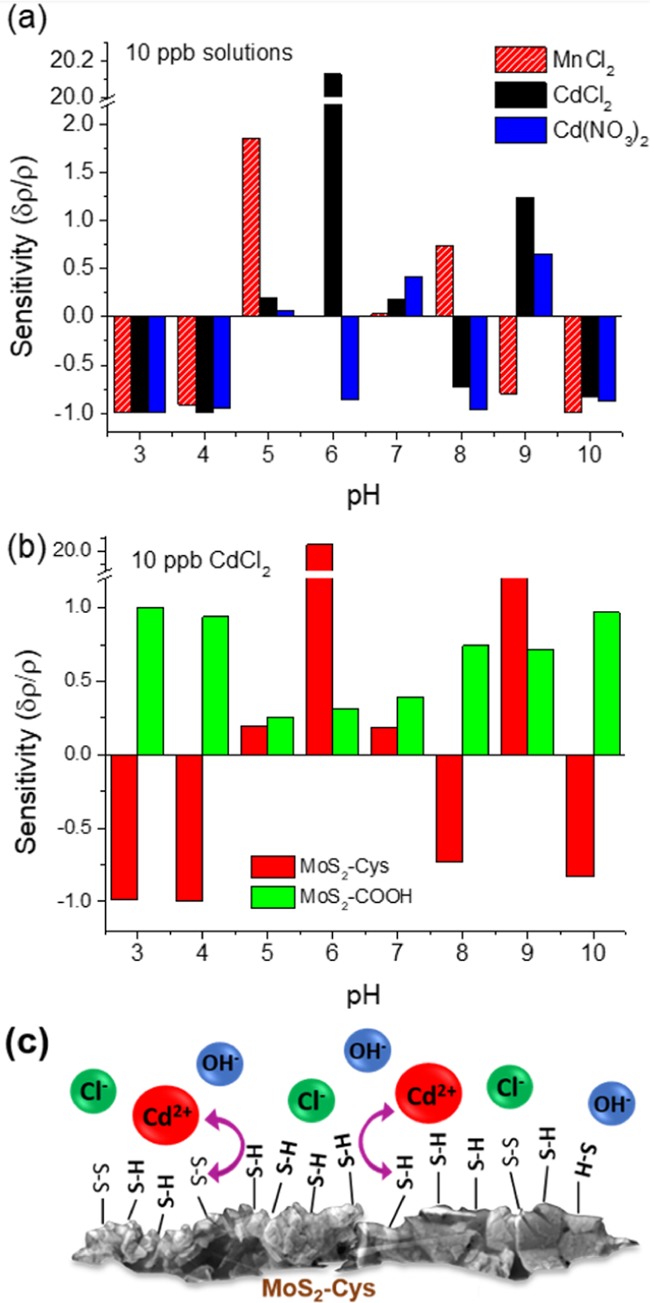
Bar graphs demonstrating the sensitivity of (a) MoS2–Cys sensors to different metal ions depending on the pH and metal ion used and (b) MoS2–COOH compared to MoS2–Cys using a 10 ppb solution at varying pH. (c) Charge transfer at the nanostructured surface of MoS2–Cys films. The high surface area facilitates charge transfer between dilute metal ions and the thiol and disulfide groups that modulate the conductivity across the MoS2 backbone of the chemiresistor.
From a fundamental standpoint, it is worth noting that sensing of Cd2+ is also enhanced at pH = 9.0 due to the formation of Cd(OH)2 groups that may form in basic environments. The formation of these groups is independent of the counterions present, and our results indicate that MoS2–Cys chemiresistors are strongly sensitive to them. Although Cd2+ sensing is dramatically enhanced at pH = 9, this effect is completely overshadowed by the tremendous sensitivity increase (∼20) at neutral pH. At this pH level, CdCl+ complexes are present, as Cd is not completely dissociated from Cl ions. MoS2–Cys is found to be uniquely sensitive to this compound, perhaps due strong interaction with S–H and S–S ligands present on cysteine and cystine in the MoS2–Cys layer. Additionally, at this pH level, the sensor shows a very strong but negative (i.e., δρ < 0) sensitivity for Cd(NO3)2, allowing for not only selectivity for Cd2+ at the appropriate pH but also for the type of counterion present in this specific case. While MoS2–Cys provides minimal sensitivity to Mn at neutral pH, Figure 2a shows that sensitivity to Mn2+ can be obtained at different pH windows, and specifically at pH = 5 and 8 when MnCl2 and MnOH+ complexes are present, respectively. These results indicate that MoS2–Cys chemiresistors can be modified to detect different metal ions by modifying the pH of the water stream.
To investigate the role of cysteine in the sensing mechanism, chemiresistors from MoS2–COOH were also constructed, and their performance is shown in Figure 2b under the same testing conditions as that of the MoS2–Cys device in panel (a). It can be readily observed that, at pH = 6−7, δρ/ρ ≈ 0.3 for the MoS2–COOH device, to be compared with δρ/ρ ≈ 20 for MoS2–Cys. We thus infer that sensitivity to Cd2+ ions is clearly due to cysteine and is not a native property of MoS2. Furthermore, although MoS2–COOH appears to be sensitive to Cd2+, δρ/ρ has been found to be independent of the specific metal ion in such chemiresistors and is a sole consequence of changes in pH. This can also be inferred from the approximately linear increase in δρ/ρ from pH = 5 to 10. Along with the morphology noticeable from Figure 2b,c, this result strongly suggests that preferential sensitivity of MoS2–Cys to Cd2+ is not the result of metal-ion intercalation between MoS2 layers but due to Cd2+ adsorption at cysteine functionals, which modifies the charge transfer processes between cysteine-based functional groups and the MoS2 backbone. As shown in Figure 4c, the high surface area and thinness of the MoS2–Cys film facilitates charge transfer between the metal ions and solution over a large area, which is sufficient to affect the conductivity of the film backbone, even at very dilute ion concentrations in water. Electrical transport in solids is extremely sensitive to the presence of gap states. We thus suspect that Cd2+ is selectively detected because of its size-driven affinity to l-cysteine, combined with charge transfer that removes electronic states from the electronic band gap of MoS2. The degree of charge transfer depends on the number and type of ions present in the solution and can be increased or decreased by tuning the pH of the incoming solution.
Conclusions
MoS2–Cys thin films have been shown to sense dilute Cd2+ ions in water at neutral pH and in the 1–10 ppb range, with high selectivity and low interference from other metal ions. MoS2–Cys porous chemiresistors are shown to have tunable sensitivity to different metal ions depending on the pH of filtrated water. Cd2+ is preferentially adsorbed by the Cys groups (consistently with significantly lower effects in MoS2–COOH), and ions are sensed through charge transfer at the surface of MoS2–Cys films with a high density of thiol and disulfide groups that modulate their conductivity. MoS2–Cys is a chemiresistor that can be easily produced in large quantities using readily available raw materials and low-complexity fabrication methods. The chemiresistive devices described in this paper are naturally porous and permeated by water, so they can be used to provide in-line and real-time sensing information about metal-ion concentration, with high reusability. Their response time is ∼1 s, or less. The lower detection limit is in the sub-ppb range, and sensing can be accomplished without any need for offline measurements or additional equipment, as in the case of electrochemical or optical detection methods. Our work provides a benchmark for chemiresistor design using functionalized MoS2 to sense metal ions in water.
Materials and Methods
Preparation of MoS2–Cys Suspensions
A detailed description of the methodology used for the preparation of MoS2–Cys is reported by Bazylewski et al.28,31 Briefly, 7.6 g of microcrystalline MoS2 powder (Sigma Aldrich, CAS 1317-33-5; 6 μm average grain size, 40 μm grain size max) was mixed with 38 mL of thioglycolic acid (TGA, Sigma Aldrich, CAS 68-11-1) and stirred for 24 h at room temperature. Four hundred milliliters of distilled water was added to the mixture that was ultrasonicated for 2 h to produce a stable suspension of carboxylated MoS2 (MoS2–COOH) nanosheets at 1.5 g/L concentration. Functionalization with l-cysteine (Sigma Aldrich, CAS 59-90-4) was performed by amide cross-linking. To this end, N-(3-dimethyl-aminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC, Sigma Aldrich, CAS 25952-53-8) and N-hydroxy-succinimide (NHS, Sigma Aldrich, CAS 6066-82-6) were added to the suspension at 1:1 and 1:1.5 (respectively) mass ratios over MoS2 and stirred for 15 min. Afterward, MoS2/Cys is obtained by mixing an l-cysteine solution in distilled water to these suspensions.28,31l-Cysteine solutions can be prepared at varying Cys concentrations to adjust the MoS2/Cys mass ratio at the desired level, where MoS2/Cys mass ratios of 1:20 or less have been found to remain stable in water over periods longer than one month. Specific characterization protocols are used to determine the cysteine bonding regime in MoS2–Cys.28
Chemiresistor Fabrication and Measurement
Thin films of MoS2–Cys were prepared from 50 mL of solution deposited on poly(ether)sulfone (PES, 3M Co.) membranes with 0.8 μm pore size accordingly to the vacuum filtration method originally developed by Eda et al.34 for graphene oxide thin films. These films are subsequently dried in air for 4 h at room temperature prior to further processing. A gap cell consisting of two aluminum electrodes (1 mm width, 100 nm thickness, 25 μm gap between) was evaporated on these films at base pressures better than 10−6 mTorr in a Kurt–Lesker thermal evaporator setup, with the Al thickness measured in situ by a Sycom STM-2 quartz crystal monitor. Electrodes are defined using a shadow mask.
Chemiresistor fabrication was completed by integrating the two-terminal gap cell described above into a custom-built transducing apparatus reported in detail by Van Middelkoop et al.35 In this setup, Al electrodes are contacted with a bridge circuit interfaced with a digital–analog converter (Emant 300) that is computer-controlled by a Matlab routine for continuous monitoring of the device resistivity over time. This apparatus includes a 100 mL capacity funnel on top of the Al/MoS2–Cys/PES gap cell membrane and a fritted glass support connected to a pump underneath. Initially, the device resistivity is primed by filtrating 100 mL of high-resistivity (18 MΩ cm) deionized (DI) water through the porous chemiresistor, during which baseline resistivity (ρ) is measured by applying, at each time interval, an array of currents in the 0–600 μA range at a 40 μA step. Resulting voltages are measured, and ρ is determined through the slope of the I–V curves. As this is significantly lower than water resistivity, ρ is proportional the resistance of the MoS2–Cys gap cell.
Tests were performed by adding to 5–8 mL of water with dissolved metal ions into the 100 mL DI water funnel, while δρ is continuously monitored by the transducing circuit of the chemiresistor. In a variety of tests, calibrated water solutions at metal ion concentrations from 0.1 to 500 ppb were poured through the apparatus. Addition of ion-containing water to the filtration stream is repeated with high reproducibility and low δρ/ρ drift over time. Metal-containing solutions were prepared using CdCl2 (Sigma Aldrich, CAS 10108-64-2) and Cd(NO3)2·4H2O (CAS 10022-68-1) measured by a high-accuracy Denver PI314 scale and dissolved in deionized water by considering the concentration of metal ions (either Mn2+ or Cd2+) in the range of 0.1–500 ppb. Preferential sensitivity to Cd2+ is verified in the presence of different counterions (i.e., Cl– and NO3–, from CdCl2 and Cd(NO3)2·4H2O, respectively) and with metal ions (e.g., Mn2–) at similar hydrodynamic radius as that of Cd2+. Control experiments with MoS2–COOH active layers in lieu of MoS2–Cys have also been performed. Throughout the course of standard testing, each sensor (of 0.0252 mm size over a 25 mm2 filtration disk) is primed with a minimum of 10 L of water passed through it before metal contaminants are tested.
Thin Film Microscopic Characterization
A Zeiss scanning electron microscope (LEO 1540XB) equipped with an energy-dispersive X-ray (EDX) spectrometer with imaging capabilities was used to characterize MoS2–COOH and MoS2–Cys thin films and map the distribution of Mo and S (from MoS2) and N (from Cys). SEM samples were prepared by vacuum filtration onto polycarbonate membranes (pore size 0.5 μm). Samples were coated with 1 nm layer of osmium prior to measurement to increase the surface conductivity using an Os plasma coater (Flogen, OPC80T). Samples of sensor devices for EDX analysis were measured as prepared, on PES membranes. Extensive additional structural and spectroscopic characterization is reported elsewhere.28,31
Raman Characterization
Unpolarized Raman spectra were recorded on a Renishaw InVia microspectrometer in a backscattering arrangement. A 633 nm helium–neon laser with a maximum output power of 17 mW was used as the excitation source. The power at the sample surface was 0.02 mW/μm2 across an 8 μm2 area. Special attention was paid to avoid damage of the sample by heating by shuttering the laser when not actively measuring. Raman measurements were performed on MoS2–COOH and MoS2–Cys samples cast from solution onto (110) silicon wafers. Samples were dried at 50 °C in air, then washed with distilled water, and dried again at 50 °C for 30 min.
Acknowledgments
P.B. acknowledges an Ontario Centres of Excellence (OCE) Talent Edge Postdoctoral Fellowship. G.F. acknowledges a Canada Research Chair in Carbon-based nanomaterials and Nano-optoelectronics. Funding from the Canada Foundation for Innovation (award no. 212442) and the Discovery Grant program of the Natural Sciences and Engineering Research Council of Canada (RGPIN-2015-06004) are also gratefully acknowledged. Part of this work was carried out at the Western University Nanofabrication Facility.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.9b03246.
Additional graphics and tables on baseline resistances and performance of MoS2 chemiresistors (PDF)
Author Contributions
The manuscript was written through contributions of all the authors. All the authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Kruse P. Review on water quality sensors. J. Phys. D: Appl. Phys. 2018, 51, 203002 10.1088/1361-6463/aabb93. [DOI] [Google Scholar]
- Zhao D.; Guo X.; Wang T.; Alvarez N. T.; Shanov V. N.; Heineman W. R. Simultaneous Detection of Heavy Metals by Anodic Stripping Voltammetry Using Carbon Nanotube Thread. Electroanalysis 2014, 26, 488–496. 10.1002/elan.201300511. [DOI] [Google Scholar]
- Gao W.; Nyein H. H. Y.; Shahpa Z.; Fahad H. M.; Chen K.; Emaminejad S.; Gao Y.; Tai L.-C.; Ota H.; Wu E.; Bullock J.; Zeng Y.; Lien D.-H.; Javey A. Wearable Microsensor Array for Multiplexed Heavy Metal Monitoring of Body Fluids. ACS Sens. 2016, 1, 866–874. 10.1021/acssensors.6b00287. [DOI] [Google Scholar]
- Ruecha N.; Rodthongkum N.; Cate D. M.; Volckens J.; Chailapakul O.; Henry C. S. Sensitive electrochemical sensor using a graphene-polyaniline nanocomposite for simultaneous detection of Zn(II), Cd(II), and Pb(II). Anal. Chim. Acta 2015, 874, 40–48. 10.1016/j.aca.2015.02.064. [DOI] [PubMed] [Google Scholar]
- Kang W.; Pei X.; Rusinek C. A.; Bange A.; Haynes E. N.; Heineman W. R.; Papautsky I. Determination of Lead with a Copper-Based Electrochemical Sensor. Anal. Chem. 2017, 89, 3345–3352. 10.1021/acs.analchem.6b03894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan X.; Park J. Y. A miniaturized and flexible cadmium and lead ion detection sensor based on micro-patterned reduced graphene oxide/carbon nanotube/bismuth composite electrodes. Sens. Actuators, B 2018, 255, 1220–1227. 10.1016/j.snb.2017.08.046. [DOI] [Google Scholar]
- Simpson A.; Pandey R. R.; Chusuei C. C.; Ghosh K.; Patel R.; Wanekaya A. K. Fabrication characterization and potential applications of carbon nanoparticles in the detection of heavy metal ions in aqueous media. Carbon 2018, 127, 122–130. 10.1016/j.carbon.2017.10.086. [DOI] [Google Scholar]
- Abdul W.; Muhammad M.; Nisar U. Periodic-Table-Style Paper Device for Monitoring Heavy Metals in Water. Trends Anal. Chem. 2018, 105, 37–51. 10.1016/j.trac.2018.04.012. [DOI] [PubMed] [Google Scholar]
- Li L.; Liu D.; Shi A.; You T. Simultaneous stripping determination of cadmium and lead ions based on the N-doped carbon quantum dots-graphene oxide hybrid. Sens. Actuators, B 2018, 255, 1762–1770. 10.1016/j.snb.2017.08.190. [DOI] [Google Scholar]
- Zhang L.; Huang X.; Cao Y.; Xin Y.; Ding L. Fluorescent binary ensemble based on pyrene derivative and sodium dodecyl sulfate assemblies as a chemical tongue for discriminating metal ions and brand water. ACS Sens. 2017, 2, 1821–1830. 10.1021/acssensors.7b00634. [DOI] [PubMed] [Google Scholar]
- Liu S.; Qin X.; Tian J.; Wang L.; Sun X. Photochemical preparation of fluorescent 2,3-diaminophenazine nanoparticles for sensitive and selective detection of Hg(II) ions. Sens. Actuators, B 2012, 171–172, 886–890. 10.1016/j.snb.2012.05.089. [DOI] [Google Scholar]
- Ullah N.; Mansha M.; Khan I.; Qurashi A. Nanomaterial-based optical chemical sensors for the detection of heavy metals in water. Trends Anal. Chem. 2018, 100, 155–166. 10.1016/j.trac.2018.01.002. [DOI] [Google Scholar]
- Rana M.; Chowdhury P. L-glutathione capped CdSeS/ZnS quantum dot sensor for the detection of environmentally hazardous metal ions. J. Lumin. 2019, 206, 105–112. 10.1016/j.jlumin.2018.10.060. [DOI] [Google Scholar]
- Kim S.; Gwon S.; Bae J. A highly selective ratiometric chemosensor for Hg2+ based on 1,2-diaminoanthraquinone. Sen’i Gakkaishi 2014, 70, 254–257. 10.2115/fiber.70.254. [DOI] [Google Scholar]
- Sasaki Y.; Minamiki T.; Tokito S.; Minami T. A molecular self-assembled colourimetric chemosensor array for simultaneous detection of metal ions in water. Chem. Commun. 2017, 53, 6561–6564. 10.1039/C7CC03218H. [DOI] [PubMed] [Google Scholar]
- Sareen D.; Kaur P.; Singh K. Strategies in detection of metal ions using dyes. Coord. Chem. Rev. 2014, 265, 125–154. 10.1016/j.ccr.2014.01.015. [DOI] [Google Scholar]
- Gong J.-L.; Sarkar T.; Badhulika S.; Mulchandani A. Label-free chemiresistive biosensor for mercury (II) based on single-walled carbon nanotubes and structure-switching DNA. Appl. Phys. Lett. 2013, 102, 013701 10.1063/1.4773569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santangelo M. F.; Shtepliuk I.; Filippini D.; Ivanov I. G.; Yakimova R.; Eriksson J. Real-time sensing of lead with epitaxial graphene-integrated microfluidic devices. Sens. Actuators, B 2019, 288, 425–431. 10.1016/j.snb.2019.03.021. [DOI] [Google Scholar]
- Varghese S. S.; Varghese S. H.; Swaminathan S.; Singh K. K.; Mittal V. Two-dimensional materials for sensing: graphene and beyond. Electronics 2015, 4, 651–687. 10.3390/electronics4030651. [DOI] [Google Scholar]
- Late D. J.; Doneux T.; Bougouma M. Single-layer MoSe2 based NH3 gas sensor. Appl. Phys. Lett. 2014, 105, 233103 10.1063/1.4903358. [DOI] [Google Scholar]
- He Q.; Zeng Z.; Yin Z.; Li H.; Wu S.; Huang X.; Zhang H. Fabrication of Flexible MoS2 Thin-Film Transistor Arrays for Practical Gas-Sensing Applications. Small 2012, 8, 2994–2999. 10.1002/smll.201201224. [DOI] [PubMed] [Google Scholar]
- Li H.; Yin Z.; He Q.; Li H.; Huang X.; Lu G.; Fam D. W. H.; Tok A. L. Y.; Zhang Q.; Zhang H. Fabrication of Single- and Multilayer MoS2 Film-Based Field-Effect Transistors for Sensing NO at Room Temperature. Small 2012, 8, 63–67. 10.1002/smll.201101016. [DOI] [PubMed] [Google Scholar]
- Perkins F. K.; Friedman A. L.; Cobas E.; Campbell P. M.; Jernigan G.; Jonker B. T. Chemical Vapor Sensing with Monolayer MoS2. Nano Lett. 2013, 13, 668–673. 10.1021/nl3043079. [DOI] [PubMed] [Google Scholar]
- Late D. J.; Huang Y.-K.; Liu B.; Acharya J.; Shirodkar S. N.; Luo J.; Yan A.; Charles D.; Waghmare U. V.; Dravid V. P.; Rao C. N. R. Sensing Behavior of Atomically Thin-Layered MoS2 Transistors. ACS Nano 2013, 7, 4879–4891. 10.1021/nn400026u. [DOI] [PubMed] [Google Scholar]
- Liu B.; Chen L.; Liu G.; Abbas A. N.; Fathi M.; Zhou C. ACS Nano 2014, 8, 5304–5314. 10.1021/nn5015215. [DOI] [PubMed] [Google Scholar]
- Saravanan A.; Huang B.-R.; Chu J. P.; Prasannan A.; Tsai H.-C. High-Performance Chemical Sensing Using Schottky-Contacted Chemical Vapor Deposition Grown Monolayer MoS2 Transistors. Sens. Actuators, B 2019, 292, 70–79. 10.1016/j.snb.2019.04.108. [DOI] [Google Scholar]
- Wang Z.; Mi B. Environmental Applications of 2D Molybdenum Disulfide Nanosheets. Environ. Sci. Technol. 2017, 51, 8229–8244. 10.1021/acs.est.7b01466. [DOI] [PubMed] [Google Scholar]
- Bazylewski P.; Van Middelkoop S.; Divigalpitiya R.; Fanchini G. Few-layer molybdenum disulfide nanosheets functionalized with l-cysteine for selective capture of Cd2+ ions. Flat Chem. 2018, 11, 15–23. 10.1016/j.flatc.2018.11.001. [DOI] [Google Scholar]
- Santhana Krishna Kumar A.; Jiang S.-J. Preparation and characterization of exfoliated graphene oxide–L-cystine as an effective adsorbent of Hg (II) adsorption. RSC Adv. 2015, 5, 6294. 10.1039/C4RA12564A. [DOI] [Google Scholar]
- Ries L.; Petit E.; Michel T.; Diogo C. C.; Gervais C.; Salameh C.; Behelany M.; Balme S.; Miele P.; Onofrio N.; Voiry D. Enhanced sieving from exfoliated MoS2 membranes via covalent functionalization. Nat. Mater. 2019, 18, 1112. 10.1038/s41563-019-0464-7. [DOI] [PubMed] [Google Scholar]
- Bazylewski P.; Divigalpitiya R.; Fanchini G. In situ Raman spectroscopy distinguishes between reversible and irreversible thiol modifications in l-cysteine. RSC Adv. 2017, 7, 2964–2970. 10.1039/C6RA25879D. [DOI] [Google Scholar]
- Zhang J.; Sun X.; Wu J. Heavy Metal Ion Detection Platforms Based on a Glutathione Probe: A Mini Review. Appl. Sci. 2019, 9, 489 10.3390/app9030489. [DOI] [Google Scholar]
- Muthivhi R.; Parani S.; May B.; Oluwafemi O. S. Green synthesis of gelatin-noble metal polymer nanocomposites for sensing of Hg2+ ions in aqueous media. Nano-Struct. Nano-Objects 2018, 13, 132–138. 10.1016/j.nanoso.2017.12.008. [DOI] [Google Scholar]
- Eda G.; Fanchini G.; Chhowalla M. Large-area ultrathin films of reduced graphene oxide as a transparent and flexible electronic material. Nat. Nanotechnol. 2008, 3, 270–274. 10.1038/nnano.2008.83. [DOI] [PubMed] [Google Scholar]
- Van Middelkoop S.; Cen A.; Bauld R.; Fanchini G. In-line chemiresistors based on multilayer graphene for cadmium dication sensing in water. FlatChem 2019, 17, 100118 10.1016/j.flatc.2019.100118. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.