Sir,
Rapid eye movement (REM) behavior disorder (RBD) is a parasomnia featured by vivid dream enactment which manifests with vocalizations and motor behaviors.[1] It is mostly seen in elderly males and often precedes a neurodegenerative disorder.[2] Fahr's syndrome (idiopathic striopallidodentate calcinosis) is a rare condition with unknown etiology, which can present with involuntary movements, along with cognitive deficits leading to dementia in due course. It is also found to be associated with obsessive–compulsive disorder, personality change, and psychosis.[3] Their coexistence is rare, but the presentation of either of these in old age could herald a neurodegenerative process.[2] We report a case presenting with RBD along with an incidental neuroimaging finding of bilateral basal ganglia calcification while discussing the possible etiological role of this radiological finding to the symptom presentation and prognostic significance.
A 65-year-old male patient with no significant past and personal history presented with an 8-month history of illness featured by initial and middle insomnia, sleep-locked episodes usually in the later part of sleep featured by kicking, sudden confusional arousal, jumping from bed and walking resulting in fall and minor injuries. Such episodes would occur approximately once a week without any known precipitating factor and would last for few minutes before getting aborted. He would awaken suddenly with a faint recollection of the dream content and would return to sleep again after the termination of the episode on its own. He also complained of intermittently feeling low with paroxysms of anxiety. There was no history of hypersomnia or narcolepsy. His neurological examination, including the current higher mental functions, was normal; however, we planned to look for any evolving neurocognitive deficits in the course.
A possibility of RBD versus complex partial seizure was kept. In polysomnography, this behavior was found to be time locked to REM sleep with the absence of any epileptiform activity, and the electromyographic channel suggested the absence of atonia in the chin and leg [Figure 1]. Computed tomogram of the brain revealed incidental finding of bilateral basal ganglia calcification suggestive of Fahr's disease [Figure 2]. Routine blood investigations showed normal complete hemogram, serum electrolytes, liver, thyroid, and renal function tests. There was no history of any recent infections or head trauma. This coupled with absence of any haematological and metabolic derangements rules out any secondary cause for this brain imaging finding.
Figure 1.
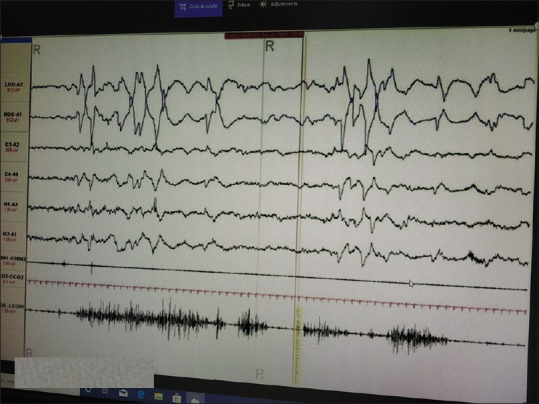
Polysomnographic finding of rapid eye movement sleep which depicted rapid eye movement-behavior disorder. rapid eye movement sleep is evidenced by ocular movements in electrooculography. Note lower limb muscle movement in rapid eye movement behavior disorder
Figure 2.
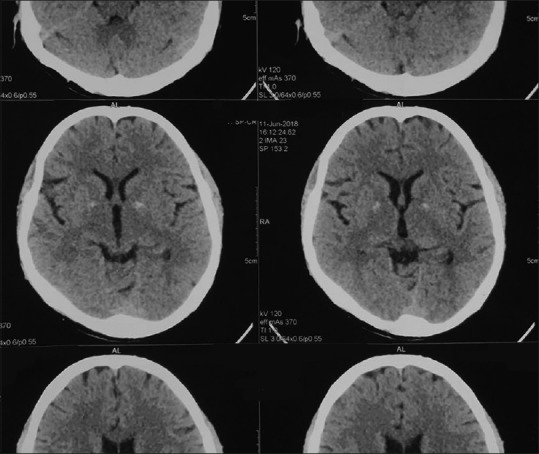
Computed tomography scan of the brain showing bilateral basal ganglia calcification
The patient was diagnosed with mixed anxiety and depression, new-onset idiopathic RBD (in view of the absence of any obvious neurological features) along with Fahr's disease. He was started on escitalopram 10 mg/day, clonazepam 0.5 mg/day and followed after 10 days. There was a remarkable reduction in the frequency, duration, and intensity of the RBD episodes alongside the improvement in mood symptoms.
RBD is a parasomnia in which there is a loss of skeletal muscular atonia that should normally occur during REM sleep resulting in dream enactment. It has a male preponderance and usually seen in the age group of 40–70 years and the onset before 40 years being usually associated with narcolepsy. It is manifested with dream mentation and motor behavior that mirrors the dream content occurring in the later half of sleep. The risks associated are injury to self and the bed partner consequent to the dream enactment behavior.[1] It is often considered to be a harbinger of neurodegenerative disorders such as Parkinson's disease (PD), multiple system atrophy (MSA), or progressive dementia such as Lewy body dementia (LBD).[4] RBD is often considered a step in the manifestation of neurodegeneration occurring in the brain stem before spreading to other central nervous system regions with a 5-year conversion rate ranging from 8% to 45%.[2]
Fahr's disease is a genetically dominant disorder characterized by the abnormal deposits of calcium, predominantly in the lenticular nucleus and internal globus pallidus. It usually presents with motor abnormalities, headache, dementia, seizures, dysarthria, spasticity, visual impairments, and athetosis.[3] Mutations in the SLC20A2 gene which encodes a phosphate transporter protein is thought to be responsible for this condition. This dominant genetic inheritance is thought to lead to disorders of calcium metabolism occasionally associated with it.[5]
RBD associated with Fahr's disease has been rarely cited in literature. It is important to probe this association because of the common threads in their causality and the increased chances for progressive neurodegeneration with their co-occurrence. RBD has been postulated to be a marker of synucleiopathies such as PD, LBD, and MSA.[4] Familial Fahr's disease has also been proposed to be linked to alpha-synucleiopathy.[6] Both the conditions being markers of synucleiopathies, their togetherness can be proposed to increase the risk further. Furthermore, the research into the pathophysiology of RBD revealed dysfunctional cholinergic-dopaminergic substrates involving the pedunculopontine nucleus, laterodorsal nucleus, and nigrostriatal system.[2] Fahr's disease results in basal ganglia calcification, possibly affecting the similar substrates through disruption of the thalamo-cortico-striatal circuit.[3] Changes in the pedunculopontine nucleus and nigrostriatal system are also found in neurodegenerative conditions (for example, PD). This explains a common etiological link between RBD, Fahr's disease, and neurodegenerative disorders.
The use of benzodiazepines can produce dramatic improvement in RBD symptoms, as seen in our case, but in the long run, they can alter the NREM-REM sleep architecture. Antidepressants such as selective serotonin reuptake inhibitors and tricyclic antidepressant are reported to worsen RBD.[1] In our case, escitalopram produced minimal improvement in his depressive and anxiety symptoms without any significant bearing on the RBD symptoms. In view of this rare association of RBD and Fahr's disease in our case, we would be vigilant for any evolving neurocognitive disorders in the course. To conclude, the current report points to the rare association of RBD and Fahr's disease which is probably the second case reported worldwide. We propose that clinicians should be vigilant that he/she might be dealing with a neurodegenerative patient when such a copresentation in an elderly comes to his clinical attention.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of inerest.
REFERENCES
- 1.Boeve BF. REM sleep behavior disorder: Updated review of the core features, the REM sleep behavior disorder-neurodegenerative disease association, evolving concepts, controversies, and future directions. Ann N Y Acad Sci. 2010;1184:15–54. doi: 10.1111/j.1749-6632.2009.05115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gagnon JF, Postuma RB, Mazza S, Doyon J, Montplaisir J. Rapid-eye-movement sleep behaviour disorder and neurodegenerative diseases. Lancet Neurol. 2006;5:424–32. doi: 10.1016/S1474-4422(06)70441-0. [DOI] [PubMed] [Google Scholar]
- 3.Demir AB, Güneş A, Özyurtlu D, Bora I. Togetherness of REM Behavior Disorder and Fahr Disease: A Case Report. J Sleep Disord Treat Care. 2012;1:1–2. [Google Scholar]
- 4.Barone DA, Henchcliffe C. Rapid eye movement sleep behavior disorder and the link to alpha-synucleinopathies. Clin Neurophysiol. 2018;129:1551–64. doi: 10.1016/j.clinph.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hozumi I, Kurita H, Ozawa K, Furuta N, Inden M, Sekine SI, et al. Inorganic phosphorus (Pi) in CSF is a biomarker for SLC20A2-associated idiopathic basal ganglia calcification (IBGC1) J Neurol Sci. 2018;388:150–4. doi: 10.1016/j.jns.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 6.Lhatoo SD, Perunovic B, Love S, Houlden H, Campbell MJ. Familial idiopathic brain calcification-a new and familial alpha-synucleinopathy. Eur Neurol. 2003;49:223–6. doi: 10.1159/000070189. [DOI] [PubMed] [Google Scholar]


