Abstract
Objective
Zika virus (ZIKV) infection is a vector-borne disease that can be transmitted sexually and vertically. The vertical transmission of the virus may lead to congenital Zika syndrome in infants. The aim of this study is to conduct a systematic review and meta-analysis of published reports documenting the prevalence of congenital Zika-related disorders in infants of mothers infected with ZIKV during pregnancy.
Methods
We conducted a comprehensive search in Ovid MEDLINE, Ovid MEDLINE (R) Epub ahead of print, Embase, Embase Classic and Web of Science databases to identify human studies reporting prevalence of congenital disorders in infants of ZIKV-infected mothers.
Results
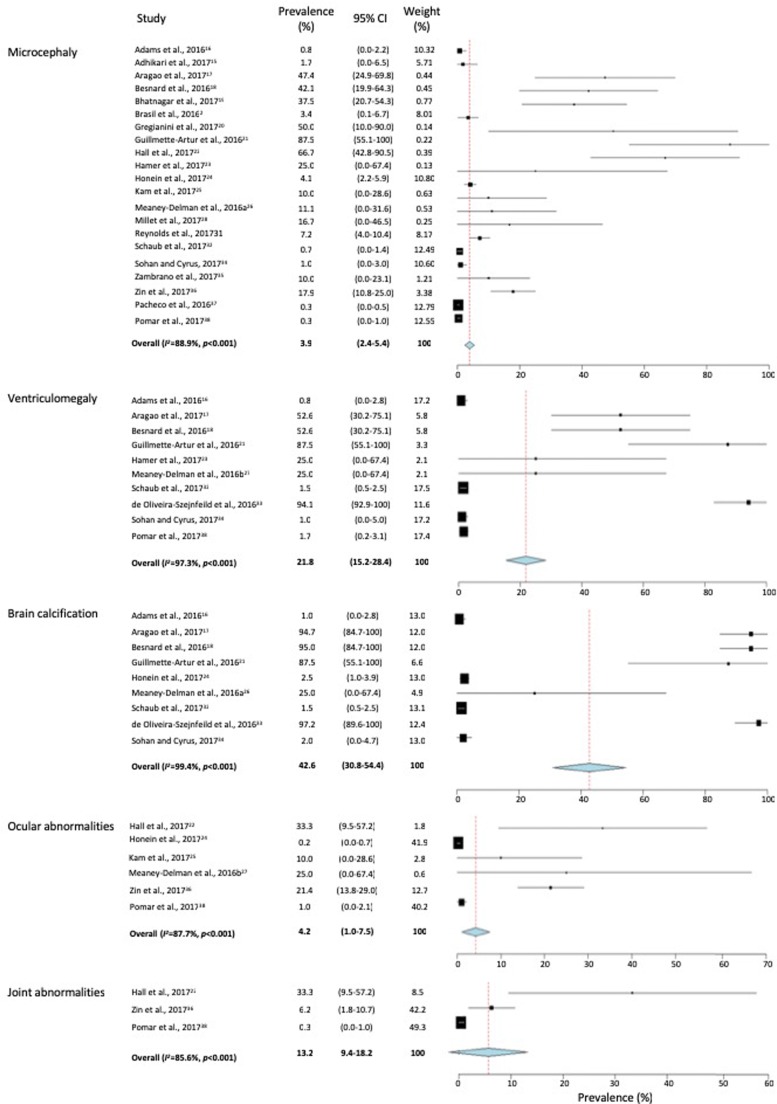
We identified 25 reports selected for inclusion in the current study (n = 4683 subjects). The majority of the studies were from South American high-risk countries. Only one third of the identified studies were conducted in the United States. Clinical maternal symptoms included maculopapular rash (76.9%), arthralgia (46.4%), fever (45.5%) and headache (31.8%) with myalgia and conjunctivitis only presented in 25% of the cases. The most prevalent congenital disorder in the newborns was brain calcifications (42.6; 95% CI, 30.8–54.4), followed by ventriculomegaly (21.8; 95% CI, 15.2–28.4), joint abnormalities (13.2; 95% CI, 9.4–18.2), ocular abnormalities (4.2; 95% CI, 1.0–7.5) and microcephaly (3.9; 95% CI, 2.4–5.4).
Conclusion
The current study highlights the high prevalence of a range of congenital disorders in newborns of mothers infected with ZIKV. It warrants developing studies to further clarify the mechanisms by which each of these disorders occurs in response to the viral infection during pregnancy and its vertical transmission to the infants.
Electronic supplementary material
The online version of this article (10.17269/s41997-019-00215-2) contains supplementary material, which is available to authorized users.
Keywords: Zika virus, Microcephaly, Congenital disease, Brain calcification, Systematic review
Résumé
Objectif
L'infection par le virus Zika (ZIKV) est une maladie à vecteur pouvant être transmise sexuellement et verticalement. La transmission verticale du virus peut entraîner un syndrome congénital de Zika chez les nourrissons. Le but de cette étude est de réaliser une revue systématique et une méta-analyse de rapports publiés documentant la prévalence de troubles congénitaux liés au Zika chez les nourrissons de mères infectées par le ZIKV pendant la grossesse.
Méthodes
Nous avons effectué une recherche exhaustive dans les bases de données Ovid MEDLINE, Ovid MEDLINE (R) avant impression, Embase, Embase Classic et Web of Science afin d'identifier des études humaines rapportant la prévalence de troubles congénitaux chez les nourrissons de mères infectées par le ZIKV.
Résultats
Nous avons identifié 25 rapports sélectionnés pour inclusion dans la présente étude (n = 4 683 sujets). La majorité des études ont été réalisées dans des pays d'Amérique du Sud présentant un risque élevé. Seulement un tiers des études identifiées ont été menées aux États-Unis. Les symptômes maternels cliniques incluaient une éruption maculo-papuleuse (76,9 %), une arthralgie (46,4 %), une fièvre (45,5 %) et des maux de tête (31,8 %) avec myalgie et conjonctivite ne se présentant que dans 25 % des cas. Les troubles congénitaux les plus fréquents chez les nouveau-nés étaient les calcifications cérébrales (42,6, IC à 95% : 30,8–54,4), suivies par la ventriculomégalie (21,8, IC à 95% : 15,2–28,4), les anomalies articulaires (13,2, IC à 95% : 9,4–18,2), des anomalies oculaires (4,2, IC à 95% : 1,0–7,5) et une microcéphalie (3,9, IC à 95% : 2,4–5,4).
Conclusion
La présente étude met en évidence la prévalence élevée d'une gamme de troubles congénitaux chez les nouveau-nés de mères infectées par le ZIKV. Cela justifie de développer des études pour préciser davantage les mécanismes par lesquels chacun de ces troubles se produit en réponse à l’infection virale pendant la grossesse et à sa transmission verticale aux nourrissons.
Mots-clés: Virus Zika, Microcéphalie, Maladie congénitale, Calcification du cerveau, Revue systématique
Introduction
Zika virus (ZIKV) is an arbovirus belonging to the flaviviridae family, as dengue, West Nile and chikungunya viruses, and is transmitted by arthropod vectors, mainly Aedes genus (e.g., Ae. aegypti) and can also spread through bodily fluid contact, blood transfusions, sexual transmission and vertical transmission (Wikan and Smith 2016). In 2007, Zika virus caused an outbreak on the island of Yap, Micronesia affecting ~ 75% of the local population (Wikan and Smith 2016). Following the suspected transmission from French Polynesia, autochthonous cases of ZIKV were identified in Brazil in May 2015 to spread to over 80 countries or territories across the globe (Wikan and Smith 2016; Brasil et al. 2016).
Identifying a patient with ZIKV infection can be challenging as the virus is asymptomatic in many cases. In symptomatic cases, infection may cause symptoms such as fever, lymphadenopathy and a number of Zika-specific symptoms such as maculopapular rash, and conjunctival injection (Brasil et al. 2016). Still, these symptoms may not be specific to ZIKV infection—one of the main obstacles to detecting the disease appropriately. Of the most alarming consequences is the ability of the virus to attack neuronal cells and cause neurological disorders in both developed and developing neurons (Hu et al. 2017). This may lead to severe consequences, particularly in uterus development. Vertical transmission of the virus with potential to cause neurological defects in fetuses was evident when ZIKV RNA was detected in the placenta, amniotic fluid and even fetal tissue (Noronha Ld et al. 2016).
A study reporting the prevalence of microcephaly in ZIKV-infected regions of Brazil (Kleber de Oliveira et al. 2016) reported a significant increase in the incidence of microcephaly immediately subsequent to the emergence of the virus in many areas where infected pregnant women were identified. In addition to severe microcephaly, there is an array of other congenital ZIKV-associated disorders that were reported in the fetuses of infected pregnant women and are unique to congenital ZIKV infection (Moore et al. 2017). These include cerebral cortices with subcortical calcifications, macular scarring and focal pigmentary retinal mottling, early hypertonia and symptoms of extrapyramidal involvement (Moore et al. 2017). Such widespread irreversible development disorders in newborns and the breadth of the ZIKV global spread, together with the related implications on healthcare, economy and overall well-being, all have led the World Health Organization to declare the ZIKV infection in February 2016 as a public health emergency of concern (Gulland 2016).
The current study was undertaken to conduct a systematic review and meta-analysis of published literature to describe the prevalence of a range of congenital diseases most associated with ZIKV infection. This will permit exploring the possible contribution of the infection in the burden of congenital disorders in infants and may facilitate developing public health measures and actions to curb their incidence.
Methods
Literature search
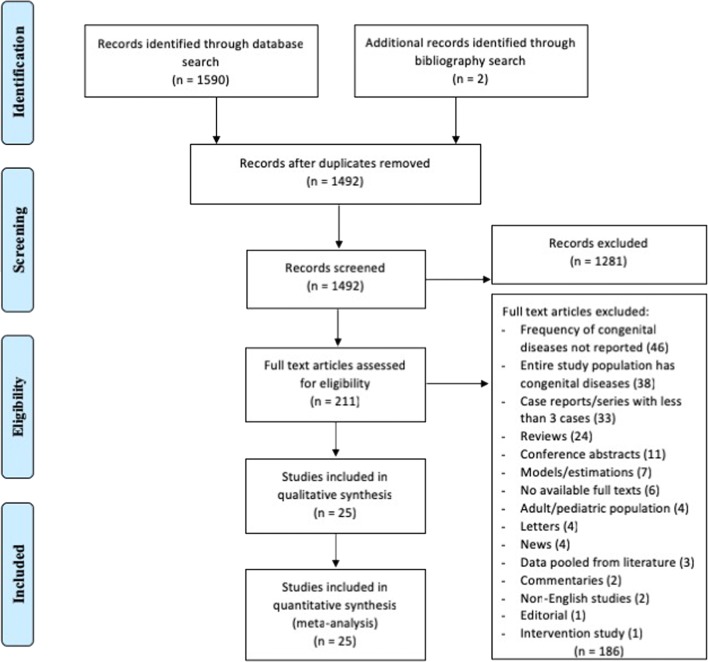
A systematic review in compliance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analysis) framework (Supplementary Table 1, Fig. 1) was carried out (Liberati et al. 2009). The literature search was conducted in Ovid MEDLINE, Ovid MEDLINE (R) Epub Ahead of Print, Embase and Embase Classic using the search terms (MeSH): “Zika” and/or “ZIKV” AND the congenital disease of interest combined with OR (Supplementary Tables 2 and 3). The time period of the search was from the inception of the databases to the end of October 2017. Only English-language articles on human subjects were included. Review papers, letters to the editor, vaccine trials, case reports, animal studies, conference abstracts and duplicated studies were excluded. Studies were only included if they contained primary data on the prevalence of any congenital disorder of interest in infants of pregnant women infected with Zika virus. Also, we conducted a similar search in the “Latin American and Caribbean Center on Health Science Information”—LILACs, using the same search terms and time period. We identified 34 English-language articles, of which 31 papers were not relevant to the study objective or fell within our exclusion criteria. Only two reports were relevant to this study: evaluating the frequencies of epileptic seizures (Alves et al. 2016) and ophthalmological findings (Ventura et al. 2016). Since these were the only reports on such conditions, there was not enough information to include either in our meta-analysis.
Fig. 1.
Flowchart of study selection and systematic literature review process. The flow diagram describes the systematic review of literature evaluating the prevalence of congenital disorders in infants of mothers infected with the Zika virus during pregnancy. Full texts of 211 studies were examined and 25 unique reports were identified to be included in the quantitative assessment and analysis
Inter-reviewer agreement
Two reviewers (SFN and SGR) independently reviewed the abstracts yielded from the search to determine those eligible for full-article review and inclusion in the study. Disagreements regarding study inclusion were resolved by an arbitrator (AB). Percentage agreement and Cohen’s Kappa (κ) score were calculated (Alves et al. 2016) and interpreted as described before (Badawi et al. 2018). The agreement between the two reviewers was excellent with a κ of 0.89 (95% CI, 0.82–0.97) (Ventura et al. 2016).
Data extraction and analysis
Data extracted from the selected studies included author’s name, year of publication, country of study, dates of patient recruitment, study population and the frequency of maternal symptoms such as rash, fever, conjunctivitis, arthralgia, headache and myalgia (%). The primary outcome measure of the current study was the prevalence of congenital disorders in infants (%) and data were extracted for conditions such as microcephaly, hydranencephaly, calcifications, ventriculomegaly, craniofacial disproportion, brainstem dysfunction, holoprosencephaly, craniosynostosis, encephalocele, meningoencephalitis, paraplegia, Blake’s pouch cyst, dyspnea, dysphagia, cerebral hyperechogenicity, and abnormalities in the cortices, corpus callosum, pons, optic nerve, cataracts, ears, joints and/or muscles. Of these conditions, only microcephaly, ventriculomegaly, brain calcification and ocular and joint abnormalities were the congenital disorders reported in at least three of the selected studies and were further evaluated for their prevalence. Meta-analysis of proportions (and 95% CI) was calculated for the clinical maternal symptoms and for a range of the selected congenital disorders—where sufficient set of data was available from the selected studies—using OpenMeta Analyst version 10.10, a free, cross-platform, open-source program (Wallace et al. 2012). We used binary random effects model as described before (Badawi et al. 2018), assuming that the proportion of the congenital diseases in infants of ZIKV-infected mothers is varied across populations. To assess whether there is true heterogeneity among the selected studies and that all the studies are evaluating the same effect within the same congenital diseases, we used the Q test that informs about the presence versus the absence of heterogeneity but does not report on the extent of such heterogeneity. Therefore, we calculated the I2 index (%) to complement the Q test and quantify the degree of heterogeneity among studies (Higgins and Thompson 2002). Given the limited power of Q test to detect true heterogeneity among a small number of studies, we also quantified the true heterogeneity by estimating the between-study variance in the random effects model (τ2), as previously described (DerSimonian and Laird 1986). P < 0.05 was considered to be statistically significant. Forest plots were used to illustrate the prevalence of a range of congenital diseases in infants of ZIKV-infected mothers from the selected studies and to inspect the heterogeneity of the individual findings. Publication bias was evaluated by regression test (Egger’s test) for Funnel plot asymmetry (Supplementary Table 4). Egger’s test assesses the tendency for the effects estimated from small sample size studies to differ from those estimated in larger studies.
Results
Search results
In the current study, the initial database and bibliography search yielded 1592 records which met the search inclusion/exclusion criteria (Supplementary Table 2). Following the removal of duplicate studies, 1492 records were screened for abstract review. Of those, 211 abstracts were eligible for full-text review. Based on the inclusion/exclusion criteria, 186 full-text articles were excluded (Fig. 1). Briefly, 46 did not report on the frequency of congenital disorders, 38 had the entire study population with congenital disorders, 33 were case reports/series with less than 3 cases, 24 were reviews, 4 were on an adult/paediatric population, 4 were letters, 3 had data pooled from literature, 2 were commentaries, 2 were non-English studies and 1 was an intervention study. In total, 25 studies examining the prevalence of congenital disorders of infants born to ZIKV-infected mothers were selected for inclusion in the current review (Table 1) (Brasil et al. 2016; Adhikari et al. 2017; Adams et al. 2016; Aragao et al. 2017; Besnard et al. 2016; Bhatnagar et al. 2017; Gregianini et al. 2017; Guillemette-Artur et al. 2016; Hall et al. 2017; Hamer et al. 2017; Honein et al. 2017; Kam et al. 2017; Meaney-Delman et al. 2016; Meaney-Delman et al. 2016; Millet et al. 2017; Rao et al. 2017; Reagan-Steiner et al. 2017; Reynolds et al. 2017; Schaub et al. 2017; Soares de Oliveira-Szejnfeld et al. 2016; Sohan and Cyrus 2017; Zambrano et al. 2017; Zin et al. 2017; Pacheco et al. 2016; Pomar et al. 2017).
Table 1.
Characteristics of selected studies
| Study ID | Country | Dates of recruitment | Design | Population | Number of mothers (n = 4683) | Objective |
|---|---|---|---|---|---|---|
| Adams et al., 2016 | Puerto Rico | 11.15–07.16 | Epidemiological report | Pregnant women in Puerto Rico | 672 | Update on the prevalence of Zika virus infection among pregnant women. |
| Adhikari et al., 2017 | USA | 03.16–10.16 | Prospective, observational | Pregnant Hispanic women receiving prenatal care for travel to area with active Zika transmission | 29 | Report the baseline prevalence of travel-associated Zika infection in pregnant women, determine travel characteristics of women with evidence of Zika infection and evaluate the maternal and neonatal outcomes. |
| Aragao et al., 2017 | Brazil | 12.15–11.16 | Retrospective study | Infants with congenital Zika syndrome 1 year of age or younger | 19 | Review neuroimaging of infants to detect cases without microcephaly and compare them with those with microcephaly. |
| Besnard et al., 2016 | French Polynesia | 10.13–03.14 | Retrospective, case series | Infants of pregnancies beginning between June 2013 and August 2014 | 19 | Detecting congenital cerebral malformations and dysfunction in fetuses and newborns. |
| Bhatnagar et al., 2017 | USA, Brazil, Colombia | 12.15–07.16 | Case series, tissue analysis | Pregnant women suspected with Zika virus infection | 32 | Investigating the mechanisms of Zika virus intrauterine transmission, replication and tropism and persistence. |
| Brasil et al., 2016 | Brazil | 09.15–05.16 | Prospective, cohort study | Pregnant women presented to acute febrile illness or rash | 116 | Characterizing the spectrum of ZIKV disease in pregnant women and infants. |
| Gregianini et al., 2017 | Brazil | 10.15–12.16 | Epidemiological report | Pregnant women with suspected ZIKV infection | 6 | Surveillance of Zika infection and microcephaly associated with congenital infection. |
| Guillmette-Artur et al., 2016 | French Polynesia | 06.13–05.14 | Retrospective, case series | Fetuses with intrauterine Zika virus infection | 3 | Analyzing the MRI cerebral findings in fetuses with intrauterine Zika virus infection. |
| Hall et al., 2017 | USA | 01.16–07.17 | Epidemiological report | Pregnant women with confirmed ZIKV infection | 15 | Surveillance of the Zika virus-associated neonatal birth defects. |
| Hamer et al., 2017 | Americas | 01.13–02.16 | Case series | Recently travelled pregnant women with confirmed, probable or clinically suspected Zika infection | 4 | Describing the clinical manifestations and epidemiology of Zika infection in exposed travellers. |
| Honein et al., 201724 | USA | 01.15–09.16 | Prospective, cohort study | Completed pregnancies with maternal, fetal or infant evidence of possible Zika infection | 442 | Estimating the proportion of fetuses or infants with birth defects after maternal Zika virus infection by trimester of infection and maternal symptoms. |
| Kam et al., 2017 | Brazil | 02.16–08.16 | Prospective, cohort study | Acute ZIKV-infected adult patients | 6 | Investigating the clinical and immunological response in acute ZIKV-infected patients. |
| Meaney-Delman et al., 2016a | USA | 08.15–02.16 | Epidemiological report, case series | Pregnant travellers with laboratory-confirmed Zika disease | 9 | Update on the prevalence of Zika virus infection among pregnant women. |
| Meaney-Delman et al., 2016b | USA | N/R | Case series | Pregnant women who had travelled to or lived in area with active Zika transmission | 5 | Investigating the clinical and laboratory characteristics of pregnant women with Zika virus RNA in serum. |
| Millet et al., 2017 | Spain | 01.16–12.16 | Cross-sectional, population-based | Imported pregnant ZIKV cases reported to Public Health Agency of Barcelona | 6 | Describe the diagnosed ZIKV cases and analyze the surveillance, prevention and control measures. |
| Rao et al., 2017 | USA | 01.16–08.16 | Longitudinal, observational study | Pregnant women who had travelled to or had sexual contact with a person who travelled to an area with active Zika transmission | 5 | Evaluating pregnant patients with potential exposure to Zika virus infection. |
| Raegan-Steiner et al., 201730 | USA | 01.16–12.16 | Epidemiological report | Completed pregnancies reported to US Zika Pregnancy Registry | 449 | Examining placental and fetal tissue specimens for Zika virus infection. |
| Reynolds et al., 2017 | USA | 01.16–12.16 | Epidemiological report | Completed pregnancies with laboratory-confirmed Zika virus infection reported to US Zika Pregnancy Registry | 250 | Analysis of completed pregnancies with laboratory evidence of possible Zika virus infection reported in 2016. |
| Schaub et al., 2017 | Martinique | 01.16–11.16 | Prospective, case series | Pregnant women with suspected Zika infection or any fetal abnormalities at ultrasound examination | 551 | Prospective analysis of fetal and maternal fluids and tissues in fetuses with confirmed Zika virus infection cases. |
| de Oliveira-Szejnfeld et al., 2016 | Brazil | 06.15–05.16 | Retrospective, review | Patients with rash occurring during pregnancy or suspected fetal CNS abnormality | 16 | Document the imaging findings associated with congenital Zika virus infection. |
| Sohan and Cyrus, 2017 | Trinidad and Tobago | 03.16–09.16 | Observational, case series | Pregnant women infected with Zika in limited-resource setting | 100 | Evaluating the fetal brain in pregnant women infected with Zika virus in a limited-resource setting. |
| Zambrano et al., 2017 | Ecuador | 06.16–07.16 | Case-control study | Pregnant women in third trimester of pregnancy | 32 | Examining the presence of ZIKV in both plasma and cervical cytology of pregnant women. |
| Zin et al., 2017 | Brazil | 01.16–10.16 | Descriptive, case series | Mother-infant pairs with PCR-confirmed Zika virus infection in maternal specimens | 112 | Evaluating eye findings in infants with confirmed Zika virus infection during pregnancy. |
| Pacheco et al., 2016 | Colombia | 08.15–04.16 | Epidemiological report | Pregnant women with clinical symptoms of Zika disease | 1484 | Clinically assessing patients and evaluated infant microcephaly. |
| Pomar et al., 2017 | French Guiana | 01.16–07.16 | Prospective, cohort study | Pregnant women with biologically confirmed ZIKV infection | 301 | Establishing the incidence of fetal central nervous system anomalies, congenital infection and fetal loss in pregnant women infected with ZIKV. |
Prevalence of congenital disorders
Systematic analysis of the selected studies reporting on the prevalence of the congenital diseases of interest had a total of 4683 mothers infected with the ZIKV during pregnancy. The majority of the studies were in South American countries, including Brazil (Brasil et al. 2016; Aragao et al. 2017; Gregianini et al. 2017; Kam et al. 2017; Soares de Oliveira-Szejnfeld et al. 2016; Zin et al. 2017), Colombia (Bhatnagar et al. 2017; Pacheco et al. 2016), Puerto Rico (Adams et al. 2016), French Polynesia (Guillemette-Artur et al. 2016), Martinique (Schaub et al. 2017), Trinidad and Tobago (Sohan and Cyrus 2017), French Guiana (Pomar et al. 2017) and Ecuador (Zambrano et al. 2017) (Table 1). Eight studies were conducted in the US and tended to focus on women who had recently travelled to South American or other Zika-infected countries (Adhikari et al. 2017; Hall et al. 2017; Honein et al. 2017; Meaney-Delman et al. 2016; Meaney-Delman et al. 2016; Rao et al. 2017; Reagan-Steiner et al. 2017; Reynolds et al. 2017). One study was conducted in Spain (Millet et al. 2017). The number of cases in the selected studies varied by approximately 371-fold as it ranged from 4 to 1484 cases. As shown in Table 2, the most prevalent clinical symptom (%) in mothers infected with ZIKV was maculopapular rash (76.9; 95% CI, 48.5–105.3), followed by arthralgia (46.4; 95% CI, 32.9–60.0), fever (45.5; 95% CI, 23.4–67.7) and headache (31.8; 95% CI, 10.9–52.6). Myalgia and conjunctivitis were only prevalent in about 25% of the cases. There was, however, a significant heterogeneity in these estimates among the selected studies as shown by the I2 index values that were varied from 68.8% to 99.6% (Table 2). Except in studies reporting the rates of ventriculomegaly, Egger’s test analysis of publication bias (Supplementary Table 4) demonstrated a non-symmetrical distribution for the effect size of each study within the individual congenital disease on either side of the pooled estimates. Egger’s test was only non-significant (p = 0.061) for studies on ventriculomegaly but significant (p < 0.001) for studies on microcephaly, brain calcification and ocular and joint abnormalities. This suggests evidence of publication bias in the studies reporting rates of microcephaly, brain calcification, ocular abnormalities and joint abnormalities in infants from ZIKV-infected mothers.
Table 2.
Prevalence of the maternal symptoms of Zika virus infection
| Study ID | Prevalence (%) | |||||
|---|---|---|---|---|---|---|
| Maculopapular rash | Fever | Conjunctivitis | Arthralgia | Headache | Myalgia | |
| Adhikari et al., 2017 | 80 | 40 | 40 | 40 | ||
| Aragao et al., 2017 | 58 | |||||
| Bhatnagar et al., 2017 | 94 | 59 | 13 | 28 | 19 | |
| Brasil et al., 2016 | 89.9 | 27.4 | 57.5 | 62.3 | 40.8 | |
| Gregianini et al., 2017 | 83 | 65 | 18 | 43 | 38 | |
| Hamer et al., 2017 | 100 | 50 | 25 | 50 | 75 | 25 |
| Kam et al., 2017 | 66.7 | 33.3 | 33.3 | 66.7 | 16.7 | |
| Meaney-Delman et al., 2016a | 100 | 77.8 | 75 | 50 | 50 | |
| Meaney-Delman et al., 2016b | 80 | 60 | 40 | 20 | 20 | |
| Rao et al., 201729 | 5.4 | 4.9 | 1.6 | 2.7 | 2.7 | |
| de Oliveira-Szejnfeld et al., 2016 | 81.3 | |||||
| Sohan and Cyrus, 2017 | 100 | |||||
| Overall prevalence ± SE (%) | 76.9 ± 14.5 | 45.5 ± 11.3 | 24.6 ± 10.9 | 46.4 ± 6.9 | 31.8 ± 10.6 | 26.6 ± 10.5 |
| 95% CI | 48.5–105.3 | 23.4–67.7 | 3.2–45.9 | 32.9–60.0 | 10.9–52.6 | 6.1–47.1 |
| τ 2 | 0.24 | 0.09 | 0.06 | 0.02 | 0.04 | 0.06 |
| Q | 2739 | 215 | 173 | 22 | 31 | 120 |
| I 2 | 99.6 | 96.3 | 97.1 | 68.8 | 84.0 | 95.0 |
Microcephaly, ventriculomegaly, brain calcification and ocular and joint abnormalities were the congenital disorders reported in at least three of the selected studies. The prevalence of microcephaly, however, was reported in 21 of the 25 selected studies (84%) whereas joint abnormalities were only reported in 3 studies (12%), as shown in Fig. 2. Meta-analysis of the prevalence (%) of congenital disorders in infants of ZIKV-infected mothers (Fig. 2) showed that the most prevalent congenital disorder was brain calcifications (42.6; 95% CI, 30.8–54.4), followed by ventriculomegaly (21.8; 95% CI, 15.2–28.4), joint abnormalities (13.2; 95% CI, 9.4–18.2), ocular abnormalities (4.2; 95% CI, 1.0–7.5) and microcephaly (3.9; 95% CI, 2.4–5.4). The proportions of the evaluated congenital disorders were varied by approximately 100-fold among the identified studies with brain calcifications showing the highest among-studies variability (167-fold). This wide variation in the proportion of congenital disorders from one study to the other may have resulted in the significant among-studies heterogeneity where I2 index ranged from 85.6–99.4% (p < 0.001).
Fig. 2.
Meta-analysis of the proportion of congenital disorders in infants born to Zika virus-infected mothers. Weights were calculated from binary random effects model analysis. Values represent proportions of microcephaly, ventriculomegaly, brain calcifications, ocular abnormalities and joint abnormalities with 95% confidence intervals.
Discussion
This study evaluates the prevalence of a number of congenital disorders in infants of mothers infected with ZIKV during pregnancy. Analysis of the selected reports has shown that brain calcification (42.6%), ventriculomegaly (21.8%), joint abnormalities (13.2%), ocular abnormalities (4.2%) and microcephaly (3.9%) are the most prevalent congenital diseases in infants of ZIKV-infected mothers.
Various pathways were proposed for such a vertical transmission by which the virus crosses the placental barrier and enters the fetal neural tissue. These include the viral invasion to the local dendritic cells and eventually the placenta (Krause et al. 2017) causing damage to the placental barrier (Quicke et al. 2016), the viral entry into the neural progenitor cells via the AXL receptors (Faizan et al. 2016), and/or the viral spread from basal and parietal decidua to chorionic villi and amniochorionic membranes to attack fetal tissues (Tabata et al. 2016), and altering the methylome of neural genes to damage the developing brain (Janssens et al. 2018).
The prevalence of microcephaly (3.9%) appears to be low despite being a well-known sequelae of the maternal ZIKV infection. However, when comparing this prevalence with that in the general population, the difference is significant. A study in Europe examining the prevalence of microcephaly in the general population reported a prevalence of only 1.53 per 10,000 births in 2012 (Morris et al. 2016). This study, however, concluded that there is a significant increase in the prevalence of microcephaly due to the Zika virus of a similar magnitude to those observed in endemic regions such as Brazil (Morris et al. 2016). It may also be worth noting that we observed a significant among-studies variation in the prevalence of microcephaly. This may be attributed to the clustering of observations around the trimesters of maternal infection. When the mother is infected in the third trimester, there are fewer congenital disorders in the infant as most of the development already occurred. As such, Zika risk for birth defects was recently reported to drop for each trimester (Reynolds et al. 2017). Similarly to microcephaly, the global prevalence of fetal ventriculomegaly was estimated to be approximately 0.06% (Isaacs et al. 2018), a figure markedly lower than 21.8% reported here in infants of ZIKV-infected mothers. Idiopathic basal ganglia calcification (Fahr’s disease or simply brain calcification) is a genetic condition characterized by calcification in the basal ganglia, especially the globus pallidus, and with occasional involvement of the internal capsule, thalamus and cerebral white matter (Manyam 2005). Although a rare disorder in the general population (Kıroğlu et al. 2010), a recent study reported that among the most common findings in fetuses and infants of laboratory-confirmed ZIKV mothers, brain calcification was present in 92% of the cases (Sanz Cortes et al. 2018). This figure is comparable with many of the reports assessed here, i.e., prevalence of 88–97% (Aragao et al. 2017; Besnard et al. 2016; Guillemette-Artur et al. 2016; Soares de Oliveira-Szejnfeld et al. 2016). A recent review reported that central nervous system calcifications (92.9%), ventriculomegaly (63.1%), microcephaly (39.7%) and ocular findings (2.7%) are among the most common congenital disorders in infants from ZIKV-infected mothers (Marques et al. 2019). These estimates, although in agreement with the order of prevalence of condition in our finding, are markedly higher than those reported here. That study did not conduct meta-analysis to generate these estimates but used overall percentages of a given condition from the total number of assessed cases.
The current study has several limitations. The identified reports had a wide among-studies variance in the proportion of the congenital disorders, which may have contributed to the observed significant heterogeneity. Sources of heterogeneity may also be due to the large variation among studies in the sample size and the different study designs, objectives and outcome measures. Also, the different methodologies used to define how exposure and outcome in mothers and infants were confirmed may have contributed to the large variation and publication bias observed here. This may have led to overestimating the prevalence of the reported congenital disorders. Another limitation is that most of the selected studies were epidemiologic reports that only presented the frequency of the congenital disorders of interest. Furthermore, including only English-language articles might have influenced the publication bias and may have led to missing important data relative to the prevalence of congenital disorders in infants born from infected mothers. Although the search was done only to the end of October 2017, not to include articles published in 2018 might not have compromised the meta-analysis given the different number of cases reported in these years with lower cases in 2018. The selected studies did not provide much insight into biochemical or immunological evidence that could have potentially provided valuable information on the vertical transmission. However, various mechanisms were separately proposed (Krause et al. 2017; Quicke et al. 2016; Faizan et al. 2016; Tabata et al. 2016; Janssens et al. 2018) as mentioned above to include the invasion to the local dendritic cells and placenta (Krause et al. 2017; Quicke et al. 2016), influence on the AXL receptors (Faizan et al. 2016) and spreading to chorionic villi and amniochorionic membranes to the fetal tissues and the developing brain (Tabata et al. 2016; Janssens et al. 2018). Additionally, the congenital disorders examined here are not comprehensive as there was not enough information in the selected studies to assess other diseases (e.g., meningoencephalitis, paraplegia, hyperechogenicity, epileptic seizures (Alves et al. 2016), ophthalmological findings (Ventura et al. 2016)) and provide estimates with any meaningful conclusion. Furthermore, our study did not evaluate the developmental delays since the recruited infants were born after 2015 and the timeline is small to diagnose this condition.
Conclusion
The current study highlights the high prevalence of a range of congenital disorders in newborns of mothers infected with ZIKV. It is critical to continue to follow up on those infants for further evaluation and future studies. Longitudinal assessments will allow for understanding of the vertical pathogenies of ZIKV infection. Studies can also be conducted to clarify the mechanisms by which congenital disorders occur in response to the viral infection during pregnancy and its vertical transmission to investigate the prevalence of viremia in the newborns as it may shed some light on their predisposition to these conditions.
Electronic supplementary material
(DOCX 22 kb)
Acknowledgements
This work was supported by the Public Health Agency of Canada (AB). The authors thank Mr. S.G. Ryoo for assistance with abstract selection, full-text screening and data analysis.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adams L, Bello-Pagan M, Lozier M, Ryff KR, Espinet C, Torres J, et al. Update: ongoing Zika virus transmission - Puerto Rico, November 1, 2015-July 7, 2016. Morbidity and Mortality Weekly Report. 2016;65:774–779. doi: 10.15585/mmwr.mm6530e1. [DOI] [PubMed] [Google Scholar]
- Adhikari EH, Nelson DB, Johnson KA, Jacobs S, Rogers VL, Roberts SW, et al. Infant outcomes among women with Zika virus infection during pregnancy: results of a large prenatal Zika screening program. American Journal of Obstetrics and Gynecology. 2017;216:292.e1–292.e8. doi: 10.1016/j.ajog.2017.01.018. [DOI] [PubMed] [Google Scholar]
- Alves, L. V., Cruz, D. D-C. S., van der Linden, A. M. C., Falbo, A. R., de Mello, M. J. G., Paredes, C. E., et al. (2016). Epileptic seizures in children with congenital Zika virus syndrome. Revista Brasileira de Saude Materno Infantil, 16(suppl 1). 10.1580/1806-9304201600s100003.
- Aragao MFVV, Holanda AC, Brainer-Lima AM, Petribu NCL, Castillo M, van der Linden V, et al. Nonmicrocephalic infants with congenital Zika syndrome suspected only after neuroimaging evaluation compared with those with microcephaly at birth and postnatally: how large is the Zika virus “iceberg”? American Journal of Neuroradiology. 2017;38:1427–1434. doi: 10.3174/ajnr.A5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawi A, Velummailum R, Ryoo SG, Senthinathan A, Yaghoubi S, Vasileva D, et al. Prevalence of chronic comorbidities in dengue fever and West Nile virus: a systematic review and meta-analysis. PLoS ONE. 2018;13:e0200200. doi: 10.1371/journal.pone.0200200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besnard, M., Eyrolle-Guignot, D., Guillemette-Artur, P., Lastère, S., Bost-Bezeaud, F., Marcelis, L., et al. (2016). Congenital cerebral malformations and dysfunction in fetuses and newborns following the 2013 to 2014 Zika virus epidemic in French Polynesia. Euro Surveillance, 21. 10.2807/1560-7917.ES.2016.21.13.30181. [DOI] [PubMed]
- Bhatnagar J, Rabeneck DB, Martines RB, Reagan-Steiner S, Ermias Y, Estetter LBC, et al. Zika virus RNA replication and persistence in brain and placental tissue. Emerging Infectious Diseases. 2017;23:405–414. doi: 10.3201/eid2303.161499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasil P, Pereira JP, Moreira ME, Ribeiro Nogueira RM, Damasceno L, Wakimoto M, et al. Zika virus infection in pregnant women in Rio de Janeiro. The New England Journal of Medicine. 2016;375:2321–2334. doi: 10.1056/NEJMoa1602412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Faizan MI, Abdullah M, Ali S, Naqvi IH, Ahmed A, Parveen S. Zika virus-induced microcephaly and its possible molecular mechanism. Intervirology. 2016;59:152–158. doi: 10.1159/000452950. [DOI] [PubMed] [Google Scholar]
- Gregianini TS, Ranieri T, Favreto C, Nunes ZMA, Tumioto Giannini GL, Sanberg ND, et al. Emerging arboviruses in Rio Grande do Sul, Brazil: chikungunya and Zika outbreaks, 2014-2016. Reviews in Medical Virology. 2017;27:e1943. doi: 10.1002/rmv.1943. [DOI] [PubMed] [Google Scholar]
- Guillemette-Artur P, Besnard M, Eyrolle-Guignot D, Jouannic J-M, Garel C. Prenatal brain MRI of fetuses with Zika virus infection. Pediatric Radiology. 2016;46:1032–1039. doi: 10.1007/s00247-016-3619-6. [DOI] [PubMed] [Google Scholar]
- Gulland A. Zika virus is a global public health emergency, declares WHO. BMJ. 2016;352:i657. doi: 10.1136/bmj.i657. [DOI] [PubMed] [Google Scholar]
- Hall NB, Broussard K, Evert N, Canfield M. Notes from the field: Zika virus-associated neonatal birth defects surveillance — Texas, January 2016–July 2017. Morbidity and Mortality Weekly Report. 2017;66:835–836. doi: 10.15585/mmwr.mm6631a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer DH, Barbre KA, Chen LH, Grobusch MP, Schlagenhauf P, Goorhuis A, et al. Travel-associated Zika virus disease acquired in the Americas through February 2016: A geosentinel analysis. Annals of Internal Medicine. 2017;166:99–108. doi: 10.7326/M16-1842. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Honein MA, Dawson AL, Petersen EE, Jones AM, Lee EH, Yazdy MM, et al. Birth defects among fetuses and infants of US women with evidence of possible Zika virus infection during pregnancy. JAMA. 2017;317:59–68. doi: 10.1001/jama.2016.19006. [DOI] [PubMed] [Google Scholar]
- Hu B, Huo Y, Yang L, Chen G, Luo M, Yang J, et al. ZIKV infection effects changes in gene splicing, isoform composition and lncRNA expression in human neural progenitor cells. Virology Journal. 2017;14:217. doi: 10.1186/s12985-017-0882-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs AM, Riva-Cambrin J, Yavin D, Hockley A, Pringsheim TM, Jette N, et al. Age-specific global epidemiology of hydrocephalus: systematic review, metanalysis and global birth surveillance. PLoS ONE. 2018;13(10):e0204926. doi: 10.1371/journal.pone.0204926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens S, Schotsaert M, Karnik R, Balasubramaniam V, Dejosez M, Meissner A, et al. Zika virus alters DNA methylation of neural genes in an organoid model of the developing human brain. mSystems. 2018;3:e00219–e00217. doi: 10.1128/mSystems.00219-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam YW, Leite JA, Lum FM, Tan JJL, Lee B, Judice CC, et al. Specific biomarkers associated with neurological complications and congenital central nervous system abnormalities from Zika virus–infected patients in Brazil. The Journal of Infectious Diseases. 2017;216:172–181. doi: 10.1093/infdis/jix261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kıroğlu Y, Çalli C, Karabulut N, Öncel C. Intracranial calcifications on CT. Diagnostic and Interventional Radiology. 2010;16:263–269. doi: 10.4261/1305-3825.DIR.2626-09.1. [DOI] [PubMed] [Google Scholar]
- Kleber de Oliveira W, Cortez-Escalante J, De Oliveira WT, Ikeda do Carmo GM, Henriques CMP, Coelho GE, et al. Increase in reported prevalence of microcephaly in infants born to women living in areas with confirmed Zika virus transmission during the first trimester of pregnancy -Brazil, 2015. Morbidity and Mortality Weekly Report. 2016;65:242–247. doi: 10.15585/mmwr.mm6509e2. [DOI] [PubMed] [Google Scholar]
- Krause KK, Azouz F, Shin OS, Kumar M. Understanding the pathogenesis of Zika virus infection using animal models. Immune Netw. 2017;17:287–297. doi: 10.4110/in.2017.17.5.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Murlow C, Gotzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of Clinical Epidemiology. 2009;62:e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Manyam BV. What is and what is not ‘Fahr’s disease. Parkinsonism & Related Disorders. 2005;11:73–80. doi: 10.1016/j.parkreldis.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Marques VM, Santos CS, Santiago IG, Marques SM, Nunes Brasil MDG, Lima TT, et al. Neurological complications of congenital Zika virus infection. Pediatric Neurology. 2019;91:3–10. doi: 10.1016/j.pediatrneurol.2018.11.003. [DOI] [PubMed] [Google Scholar]
- Meaney-Delman D, Hills SL, Williams C, Galang RR, Iyengar P, Hennenfent AK, et al. Zika virus infection among U.S. pregnant travelers - August 2015-February 2016. Morbidity and Mortality Weekly Report. 2016;65:211–214. doi: 10.15585/mmwr.mm6508e1. [DOI] [PubMed] [Google Scholar]
- Meaney-Delman D, Oduyebo T, Polen KND, White JL, Bingham AM, Slavinski SA, et al. Prolonged detection of Zika virus RNA in pregnant women. Obstetrics and Gynecology. 2016;128:724–730. doi: 10.1097/AOG.0000000000001625. [DOI] [PubMed] [Google Scholar]
- Millet J-P, Montalvo T, Bueno-Marí R, Romero-Tamarit A, Prats-Uribe A, Fernández L, et al. Imported Zika virus in a European city: how to prevent local transmission? Frontiers in Microbiology. 2017;8:1319. doi: 10.3389/fmicb.2017.01319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CA, Staples JE, Dobyns WB, Pessoa A, Ventura CV, da Fonseca EB, et al. Characterizing the pattern of anomalies in congenital Zika syndrome for pediatric clinicians. JAMA Pediatrics. 2017;171:288–295. doi: 10.1001/jamapediatrics.2016.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JK, Rankin J, Garne E, Loane M, Greenlees R, Addor M-C, et al. Prevalence of microcephaly in Europe: population based study. BMJ. 2016;354:i4721. doi: 10.1136/bmj.i4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noronha Ld ZC, Azevedo ML, Luz KG, Santos CN. Zika virus damages the human placental barrier and presents marked fetal neurotropism. Memórias do Instituto Oswaldo Cruz. 2016;111:287–293. doi: 10.1590/0074-02760160085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco, O., Beltrán, M., Nelson, C. A., Valencia, D., Tolosa, N., Farr, S. L., et al. (2016). Zika virus disease in Colombia - preliminary report. The New England Journal of Medicine. 10.1056/NEJMoa1604037. [DOI] [PubMed]
- Pomar L, Malinger G, Benoist G, Carles G, Ville Y, Rousset D, et al. Association between Zika virus and fetopathy: a prospective cohort study in French Guiana. Ultrasound in Obstetrics & Gynecology. 2017;49:729–736. doi: 10.1002/uog.17404. [DOI] [PubMed] [Google Scholar]
- Quicke KM, Bowen JR, Johnson EL, McDonald CE, Ma H, O’Neal JT, et al. Zika virus infects human placental macrophages. Cell Host & Microbe. 2016;20:83–90. doi: 10.1016/j.chom.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao R, Gaw SL, Han CS, Platt LD, Silverman NS. Zika risk and pregnancy in clinical practice: ongoing experience as the outbreak evolves. Obstetrics and Gynecology. 2017;129:1098–1103. doi: 10.1097/AOG.0000000000002049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagan-Steiner S, Simeone R, Simon E, Bhatnagar J, Oduyebo T, Free R, et al. Evaluation of placental and fetal tissue specimens for Zika virus infection - 50 states and District of Columbia, January-December, 2016. Morbidity and Mortality Weekly Report. 2017;66:636–643. doi: 10.15585/mmwr.mm6624a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds MR, Jones AM, Petersen EE, Lee EH, Rice ME, Bingham A, et al. Vital signs: update on Zika virus–associated birth defects and evaluation of all U.S. infants with congenital Zika virus exposure - U.S. Zika Pregnancy Registry, 2016. Morbidity and Mortality Weekly Report. 2017;66:366–373. doi: 10.15585/mmwr.mm6613e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz Cortes M, Rivera AM, Yepez M, Guimaraes CV, Diaz Yunes I, Zarutskie A, et al. Clinical assessment and brain findings in a cohort of mothers, fetuses and infants infected with ZIKA virus. American Journal of Obstetrics and Gynecology. 2018;218:440.e1–440.e36. doi: 10.1016/j.ajog.2018.01.012. [DOI] [PubMed] [Google Scholar]
- Schaub B, Vouga M, Najioullah F, Gueneret M, Monthieux A, Harte C, et al. Analysis of blood from Zika virus-infected fetuses: a prospective case series. The Lancet Infectious Diseases. 2017;17:520–527. doi: 10.1016/S1473-3099(17)30102-0. [DOI] [PubMed] [Google Scholar]
- Soares de Oliveira-Szejnfeld P, Levine D, Melo AS, Amorim MM, Batista AG, Chimelli L, et al. Congenital brain abnormalities and Zika virus: what the radiologist can expect to see prenatally and postnatally. Radiology. 2016;281:203–218. doi: 10.1148/radiol.2016161584. [DOI] [PubMed] [Google Scholar]
- Sohan K, Cyrus CA. Ultrasonographic observations of the fetal brain in the first 100 pregnant women with Zika virus infection in Trinidad and Tobago. International Journal of Gynaecology and Obstetrics. 2017;139:278–283. doi: 10.1002/ijgo.12313. [DOI] [PubMed] [Google Scholar]
- Tabata T, Petitt M, Puerta-Guardo H, Michlmayr D, Wang C, Fang-Hoover J, et al. Zika virus targets different primary human placental cells, suggesting two routes for vertical transmission. Cell Host & Microbe. 2016;20:155–166. doi: 10.1016/j.chom.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura, C. V., Maia, M., Ventura, B. V., van der Linden, V., Araujo, E. B., Ramos, R. C., et al. (2016). Ophthalmological findings in infants with microcephaly and presumable intra-uterus Zika virus infection. Arquivos Brasileiros de Oftalmologia, 79. 10.5935/0004-2749.20160002. [DOI] [PubMed]
- Wallace BC, Dahabreh IJ, Trikalinos TA, Lau J, Trow P, Schmid CH. Closing the gap between methodologists and end-users: R as a computational back-end. Journal of Statistical Software. 2012;49:1–15. doi: 10.18637/jss.v049.i05. [DOI] [Google Scholar]
- Wikan N, Smith DR. Zika virus: history of a newly emerging arbovirus. The Lancet Infectious Diseases. 2016;16:e119–e126. doi: 10.1016/S1473-3099(16)30010-X. [DOI] [PubMed] [Google Scholar]
- Zambrano H, Waggoner J, León K, Pinsky B, Vera K, Schettino M, et al. High incidence of Zika virus infection detected in plasma and cervical cytology specimens from pregnant women in Guayaquil, Ecuador. American Journal of Reproductive Immunology. 2017;77:e12630. doi: 10.1111/aji.12630. [DOI] [PubMed] [Google Scholar]
- Zin AA, Tsui I, Rossetto J, Vasconcelos Z, Adachi K, Valderramos S, et al. Screening criteria for ophthalmic manifestations of congenital Zika virus infection. JAMA Pediatrics. 2017;171:847–854. doi: 10.1001/jamapediatrics.2017.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 22 kb)