Abstract
Objective
In 2002, a mass immunization campaign using the 23-valent pneumococcal polysaccharide vaccine (PPV23) was carried out in Nunavik to control an outbreak caused by a virulent clone of serotype 1 Streptococcus pneumoniae. At the same time, the 7-valent pneumococcal conjugate vaccine (PCV7) was introduced for routine immunization of infants, replaced by the 10-valent vaccine (PCV10) in 2009, and the 13-valent vaccine (PCV13) in 2011. The objective of this study was to describe the epidemiology of invasive pneumococcal disease (IPD) in relation to pneumococcal vaccine use.
Method
Retrospective analysis of IPD cases identified by the Quebec Public Health Laboratory during the period 1997–2016.
Results
One hundred thirty-two IPD cases were identified during the study period. In adults, serotype 1 incidence decreased following the 2002 PPV23 mass campaign, but breakthrough cases occurred. Following PCV use, the incidence of vaccine-type IPD decreased markedly in children and also in adults but serotypes not covered by conjugate vaccines increased. The overall IPD rate was 43/100,000 person-years in the 1997–1999 pre-vaccine era and 58/100,000 person-years in 2010–2016.
Conclusions
The 2002 PPV23 mass immunization campaign may have contributed to control the serotype 1 outbreaks in Nunavik, but its effect was short-lived as IPDs caused by serotypes included in this vaccine continued to occur after 2005. PCV use in children induced important modifications in the epidemiology of IPD, but most of the benefits were eroded by serotype replacement.
Keywords: Immunization, Epidemiology, Streptococcus pneumonia, Pediatric vaccine, Inuit
Résumé
Objectif
En 2002, une campagne de vaccination de masse avec le vaccin pneumococcique polysaccharidique 23-valent (VPPS23) eut lieu au Nunavik pour contrôler une épidémie due à un clone virulent de Streptococcus pneumoniae de serotype 1. Au même moment, le vaccin pneumococcique conjugué heptavalent (VPC7) était introduit au calendrier de vaccination des nourrissons, puis fût remplacé par un vaccin décavalent (VPC10) en 2009 et par un vaccin 13-valent (VPC13) en 2011. Le but de cette étude était de décrire l’épidémiologie des infections invasives à pneumocoques (IIP) en rapport avec l’utilisation des vaccins pneumococciques.
Méthode
Analyse rétrospective des cas d’IIP identifiés par le Laboratoire de Santé Publique du Québec (LSPQ) durant la période 1997-2017.
Résultats
132 cas d’IIP furent identifiés au cours de la période d’étude. Chez les adultes, l’incidence des IIP dues au sérotype 1 déclina suite à la campagne de vaccination de masse de 2002 mais plusieurs cas survinrent tout de même par la suite. Après l’introduction du VPC7, l’incidence des IIP causées par des sérotypes vaccinaux baissa fortement dans toute la population mais un remplacement de sérotypes fût observé. L’incidence globale des IIP était de 43/100 000 personne-années au cours de la période pré-vaccins 1997-1999 et de 58/100 000 personne-années au cours de la période 2010-2016.
Conclusions
La campagne de vaccination de masse de 2002 a probablement contribué à contrôler l’épidémie d’IIP dues au sérotype 1 mais son effet fût de courte durée puisque des IIP causées par des sérotypes couverts par ce vaccin ont continué à arriver après 2005. L’introduction du VPC chez les enfants a induit des changements importants dans l’épidémiologie des IIP mais la majeure partie des gains a été perdue à cause du remplacement de sérotypes.
Mots-clés: Immunisation, Épidémiologie, Streptococcus pneumonia, Vaccin pédiatrique, Inuit
Introduction
Nunavik is the most northerly region of the province of Quebec. Approximately 90% of its population (n ≈ 13,000 in 2016) is Inuit, living in 14 villages along the Hudson and Ungava bays, with no road between them and to the south. Respiratory infections constitute a major public health problem in the northern communities of Canada, including the Nunavik region of Quebec, and Streptococcus pneumoniae (Sp) is one of the most important pathogens (MacMillan et al. 1996; Degani et al. 2008). The very high invasive pneumococcal disease (IPD) rate in the Inuit population could be due to several causes, including the harsh climate, household overcrowding, active and passive smoking, alcohol consumption, and illegal substances use (MacMillan et al. 1996). Unfavourable living conditions in the Inuit population is in large part explained by the phenomenon of acculturation resulting from colonial and assimilation policies conducted first by European settlers and thereafter by Canadian-Quebec governmental authorities and non-governmental organizations (Bonesteel 2008).
In Nunavik, as in other regions of Quebec, one dose of the 23-valent pneumococcal polysaccharide vaccine (PPSV23, including serotypes 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F, and 33F) is recommended for the immunization of persons ≥ 5 years of age with conditions associated with high risk of IPD since 1999, and for all adults ≥ 65 years of age since 2000. For those who have received a previous pneumococcal vaccine dose because of a medical condition that places them at highest risk of IPD, an additional PPSV23 dose should be administered, as long as 5 years have passed since the previous dose (Protocole d’immunisation du Québec n.d.).
In 2000, outbreaks of severe pneumonia caused by a virulent clone of serotype 1 Sp emerged in several villages of Nunavut and Nunavik (Marcy et al. 2002; Proulx et al. 2002). Virulent clones of serotype 1 Sp have a propensity to cause outbreaks in crowded and socially disadvantaged populations (Hausdorff et al. 2005). The epidemic of severe pneumonia which started in the Nunavik region in 2000 triggered a mass immunization campaign using PPSV23, targeting all residents ≥ 5 years of age (Proulx et al. 2002). The campaign was implemented in the spring and summer of 2002 and coverage of the target population was 83.7% (Ndiaye et al. 2006). In April 2002, the 7-valent pneumococcal conjugate vaccine (PCV7, including serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F) was introduced for the routine immunization of Nunavik infants (four doses) with a catch-up for children up to age 4 years (Protocole d’immunisation du Québec n.d.). A study on PCV7 showed that 92% of children had received the recommended four PCV doses by age 5 years (Cléophat et al. 2014). The 10-valent pneumococcal conjugate vaccine (PCV10, including serotypes 4, 6B, 9V, 14, 18C, 19F, 23F, 1, 5, 7F) replaced PCV7 in the summer of 2009, and the 13-valent vaccine (PCV13, including serotypes 4, 6B, 9V, 14, 18C, 19F, 23F, 1, 5, 7F, 3, 6A, 19A) replaced PCV10 in January 2011, with no catch-up in both instances. The objective of this study was to describe the epidemiology of IPD in relation to pneumococcal vaccines use in the population of Nunavik during the period 1997 to 2016, and to assess the magnitude of serotype replacement, as partial serotype replacement among children was also observed in Quebec and in a majority of countries in which PCVs have been used (Lefebvre and Cote 2015; Feikin et al. 2013).
Material and methods
IPD is a notifiable disease in Quebec since 1997 and is defined as a clinical infection associated with the identification of Sp by culture or nucleic acid amplification test in a normally sterile body fluid or site. In Nunavik, laboratory facilities are available in the two regional hospitals (Ungava Tullatavik Health Centre in Kuujjuaq and Inuulitsivik Health Centre in Puvirnituq). Sp isolates from culture are forwarded to the Quebec Public Health Laboratory (“Laboratoire de Santé publique du Québec”) for characterization, including serotype identification (Douville-Fradet et al. 2011).
A list of IPD cases diagnosed during the 1997–2015 period was obtained from the Nunavik Public Health Directorate. The immunization status of patients was extracted from medical records. Population denominators were provided by the Quebec Statistics Institute (“Institut de la Statistique du Québec”). The study period was divided into five eras: (i) pre-epidemic era (1997–1999); (ii) serotype 1 epidemic era (2000–2001); (iii) mass PPV23 vaccination and PCV7 catch-up period (2002–2003); (iv) PCV7 era (2004–2010); and (v) PCV10-13 era (2010–2016). Rates were compared using an exact two-sided test assuming a Poisson distribution, with R software 2.12.1 (R Foundation for Statistical Computing, Vienna, Austria). As PCV7 provides cross-protection against serotype 6A, this serotype was included in PCV7-types (Whitney et al. 2006). The effectiveness of PPSV23 to prevent serotype 1 IPD was assessed during the 2.5-year period following the end of the mass campaign during which this serotype circulated. It was calculated as 1 minus the ratio of the incidence rate in the vaccinated fraction of the population to the rate in the unvaccinated fraction (Orenstein et al. 1985). The study was performed under the mandate of epidemiologic surveillance and program evaluation of the Public Health Directorate of the Nunavik Region. As a consequence, the approval of a research ethics committee was not needed.
Involvement of the community
The study was performed under a mandate of epidemiologic surveillance and program evaluation of the Public Health Directorate of the Nunavik Region. As a consequence, the approval of a research ethics committee was not needed. The Registry of Notifiable Diseases was used as the primary source of information. In Nunavik as in all other regions of Quebec, the surveillance of notifiable diseases is governed by the “Loi sur la santé publique” (Gouvernement du Québec, S-2.2) and is under the responsibility of the Nunavik Public Health Director who reports to the Executive Director of the Nunavik Regional Board of Health and Social Services, who reports to the Board of Directors of the Nunavik Regional Board of Health and Social Services. The Board of Directors includes, among others, one representative appointed by each northern village included in the territory. All activities related to infectious disease surveillance and immunization program evaluation are conducted by health professionals in the two Nunavik regional hospitals, the Quebec Public Health Laboratory in the Montreal area, and the Public Health Directorate offices in Kuujjuaq and Quebec City. The involvement of communities is minimal. Major changes in internal policies, rules, and procedures at the Public Health Directorate level would be needed to implement the Tricouncil recommendations for Indigenous community engagement which have been proposed for academic research (Canadian Institutes of Health Research, Natural Sciences and Engineering Research Council of Canada, and Social Sciences and Humanities Research Council of Canada 2010).
Results
During the 20-year study period, a total of 132 IPD cases were recorded in the Nunavik population representing 224,135 person-years of observation. All IPD cases were identified by culture: 128 from blood, 2 from cerebrospinal fluid, and 2 from trans-thoracic aspirate. The overall IPD rate was 59/100,000 person-years (p-y), being, respectively, 43/100,000 in 1997–1999, 116/100,000 in 2000–2001, 98/100,000 in 2002–2003, 39/100,000 in 2004–2009, and 58/100,000 in 2010–2016. The age-distribution of cases was as follows: < 5 years = 58 cases (44%); 5–19 years = 24 cases (18%); 20–59 years = 34 cases (26%); ≥ 60 years = 16 cases (12%). The male/female sex ratio was 0.94 (64/68). Age-specific IPD rates were < 5 years = 206/100,000 p-y; 5–19 years = 35/100,000 p-y; 20–59 years = 31/100,000 p-y; ≥ 60 years = 134/100,000 p-y.
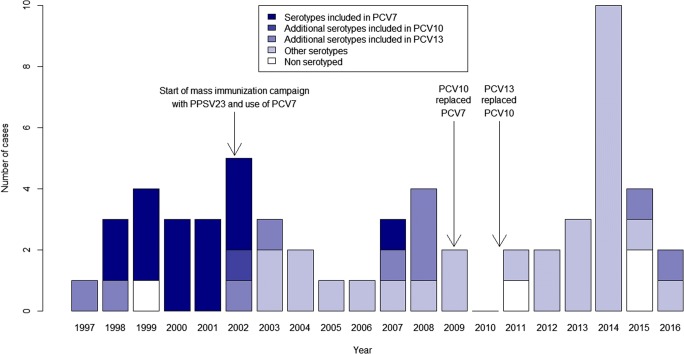
The distribution of IPD cases in children less than 5 years of age over the study period is shown in Fig. 1. Only one serotype 1 case was detected in this age group in November 2002. Following the introduction of PCV7 in 2002, a decrease in IPD frequency was seen, and only four cases were reported during the 2004–2006 period. Thereafter, the frequency of IPD cases rose again and during the years 2007–2009, four serotype 19A cases not covered by PCV7 were identified, out of a total of nine cases. Following the introduction of PCV10 in the summer of 2009, a new decrease in frequency was seen before it rose again during the years 2010–2016. During this latter period, 21 of 23 IPD cases were caused by serotypes not covered by PCVs, including 33F (5 cases), 10A, 20, 22A (3 cases each), 13, 15A, 15B (2 cases each), 6C, 9N, 10F, 21, 22F, and 33A (1 case each).
Fig. 1.
Number of cases of invasive pneumococcal disease in children less than 5 years of age in Nunavik, by year and serotype category, 1997–2016
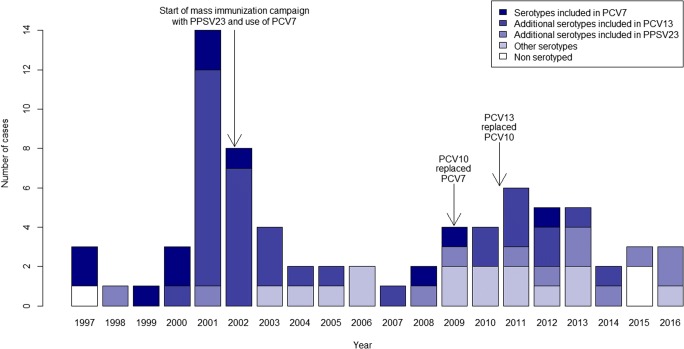
The distribution of IPD cases in the population ≥ 5 years of age is shown in Fig. 2. The outbreak caused by the virulent serotype 1 clone starting in 2000 is clearly seen. Following the mass immunization campaign in February–June 2002, nine serotype 1 cases were observed (five in 2002, three in 2003, and one in 2004). Since the introduction of PCV7 in children in 2002, only three PCV7-type cases were observed in persons ≥ 5 years of age. Following the introduction of PCV10 in children in 2009, and its replacement by PCV13 in 2011, nine PCV13-type cases were observed: six cases due to serotype 7F (also directly covered by PCV10), two cases due to serotype 19A (also covered by PCV10 via cross-protection), and one case due to serotype 3 (also covered by PPSV23).
Fig. 2.
Number of cases of invasive pneumococcal disease in persons ≥ 5 years of age in Nunavik, by year and serotype category, 1997–2016
IPD rates in children < 5 years of age by period and serotype category are shown in Table 1. A marked and statistically significant decrease in the incidence of IPD caused by serotypes included in PCV7 was observed following PCV7 use: 165/100,000 p-y in 1997–2001 vs. 12/100,000 p-y in 2004–2009 (p < 0.01). Non-PCV7 7 types, mainly 7F and 19A, emerged during this period but almost disappeared following PCV10 introduction in 2009 and PCV13 in 2011. During the latter period, almost all cases were caused by serotypes not covered by PCV10 and PCV13. The overall IPD rate in 2010–2016 (212/100,000 p-y) was not different from the rate in 1997–1999 (203/100,000 p-y) although lower than the rate in 2000–2002 (350/100,000 p-y).
Table 1.
Incidence rate of invasive pneumococcal disease per 100,000 person-years (and number of cases) in children less than 5 years of age in Nunavik, Quebec, by period and serotype category, 1997–2015
| Period | |||||
|---|---|---|---|---|---|
| 1997–1999 | 2000–2001 | 2002–2003 | 2004–2009 | 2010–2016 | |
| All serotypes | 203 (8) | 350 (6) | 295 (8) | 154 (13) | 212 (23) |
| PCV7 + 6A serotypes | 127 (5) | 350 (6) | 111 (3) | 12 (1) | 0 |
| Additional PCV10 serotypes | 0 | 0 | 74 (2) | 0 | 0 |
| Additional PCV13 serotypes | 51 (2) | 0 | 111 (3) | 47 (4) | 18 (2*) |
| Other serotypes | 0 | 0 | 0 | 95 (8) | 166 (18) |
| Serotype unknown | 25 (1) | 0 | 0 | 0 | 28 (3) |
*One serotype 19A-case and one serotype 3-case in vaccinated children
In persons ≥ 5 years of age (Table 2), the incidence of serotype 1 IPD was higher before than after the mass immunization campaign using the 23-valent polysaccharide vaccine: 65/100,000 p-y in 2000–2001 vs. 1.8/100,000 p-y in 2004–2009 (p < 0.01). The incidence of infections caused by the seven serotypes included in PCV7 also decreased following PCV use in children, although this was not statistically significant: 15/100,000 p-y in 1997–2001 vs. 3.5/100,000 p-y in 2004–2009 (p = 0.07). Serotype replacement was observed, and non-vaccine serotypes accounted for 29% (11/38) of all cases.
Table 2.
Incidence rate of invasive pneumococcal disease per 100,000 person-years (and number of cases) in persons ≥ 5 years of age in Nunavik, Quebec, by period and serotype category, 1997–2015
| Period | |||||
|---|---|---|---|---|---|
| 1997–1999 | 2000–2001 | 2002–2003 | 2004–2009 | 2010–2016 | |
| All serotypes | 16 (4) | 100 (17) | 68 (12) | 22 (13) | 36 (28) |
| PCV13 serotypes | 8 (2) | 94 (16) | 62 (11) | 8.5 (5) | 13 (10) |
| PCV7 + 6A serotypes | 8 (2) | 4 (23.5) | 1 (6) | 2 (3.5) | 1 (1) |
| Additional PCV13 serotypes | 0 | 70.5 (12) | 56 (10) | 5.5 (3) | 12 (9) |
| Serotype 1 | 0 | 65 (11) | 56 (10) | 1.8 (1) | 0 |
| Additional PPSV23 serotypes | 4 (1) | 6 (1) | 0 | 3.5 (2) | 9 (7) |
| Other serotypes | 0 | 0 | 6 (1) | 8.5 (5) | 9 (7) |
| Serotype unknown | 4 (1) | 0 | 0 | 1.5 (1) | 3 (2) |
In the period following the PPSV23 mass vaccination campaign (from mid-2002 to 2016), 21 PPSV23 breakthrough cases were observed in persons ≥ 5 years of age (Table 3). The six breakthrough cases caused by serotype 1 occurred within 2.5 years after vaccination. The estimated effectiveness of PPS23 against serotype 1 IPD in the population ≥ 5 years of age was 61% (6 cases in 18,183 vaccinated person-years vs. 3 cases in 3541 unvaccinated person-years) (95% confidence interval, − 56% to 90%). No vaccine failure was observed for PCV7 or PCV10 in children. There were two vaccine failures for PCV13: one 19A IPD case in a fully vaccinated child and a serotype 3 case in a 15-month-old child who had received two PCV13 doses, respectively at 6 and 12 months of age.
Table 3.
Cases of invasive pneumococcal disease in Nunavik, Quebec, consecutive to vaccine failure
| Year of case | Vaccine | Age at vaccination in years | Serotype | Delay between last vaccine dose and disease onset in years |
|---|---|---|---|---|
| 2002 | PPSV23 | 76 | 1 | 1.75 |
| PPSV23 | 65 | 1 | 2.0 | |
| PPSV23 | 33 | 1 | 0.8 | |
| PPSV23 | 5 | 1 | 0.7 | |
| 2003 | PPSV23 | 5 | 1 | 1.4 |
| 2004 | PPSV23 | 4 | 1 | 2.5 |
| 2009 | PPSV23 | 58 | 20 | 12.7 |
| PPSV23 | 10 | 4 | 7.6 | |
| 2010 | PPSV23 | 54 | 19A | 8.6 |
| 2011 | PPSV23 | 29 | 3 | 9.4 |
| PPSV23 | 37 | 10A | 9.6 | |
| 2012 | PPSV23 | 63 | 7F | 10 |
| PPSV23 | 41 | 10A | 10.1 | |
| PPSV23 | 35 | 4 | 10.2 | |
| 2013 | PPSV23 | 26 | 19A | 11 |
| PPSV23 | 55 | 22F | 11.2 | |
| PPSV23 | 21 | 17F | 11.1 | |
| 2014 | PPSV23 | 53 | 9N | 12.7 |
| 2015 | PCV13 | 2, 4, 6, and 14 months | 19A | 0.2 |
| PPSV23 | 22 | 12F | 13.5 | |
| 2016 | PCV13 | 6 and 12 months | 3 | 0.25 |
| PPSV23 | 59 | 10A | 14.7 | |
| PPSV23 | 43 | 22F | 14 |
Discussion
Laboratory surveillance of IPD in the Arctic region of Nunavik is particularly challenging. Antibiotics are rapidly prescribed to all patients with clinical signs of acute infections, and always before any airlift. Blood cultures can be performed in the two regional hospitals only but are not systematically prescribed. It is thus likely that IPD rates reported in our study are gross underestimates of their real frequency. Nevertheless, the overall reported IPD rate in the Nunavik region (61/100,000 p-y) is six times higher than the rate reported in the general population of Quebec (11/100,000 in 2014) (Feikin et al. 2013). Ethnic disparity in IPD rates has also been observed between Alaska Caucasian and Native children (Wenger et al. 2010) and in Northern Canada (Li et al. 2016).
The introduction of pneumococcal conjugate vaccines for children was associated with herd effect (indirect protection) and serotype replacement. This was observed with a variable magnitude in all countries (Feikin et al. 2013). This was also observed in the Nunavik population and replacement was of much higher magnitude than in other populations (Hausdorff and Hanage 2016) and in high-risk groups including Indigenous populations of Alaska and Northern Canada (Wenger et al. 2010; Li et al. 2016).
In children < 5 years of age, the low IPD rate recorded in 1997–1999 (203/100,000 p-y) could be due to a low level of awareness and testing. The rate recorded in 2000–2001 (350/100,000 p-y) may be more representative of the situation prevailing before any PCV use. In 2010–2016, the overall IPD rate (212/100,000 p-y) had decreased by 39% when compared to the 2000–2001 rate, suggesting that the almost complete disappearance of PCV13 serotypes was not entirely taken over by non-PCV13 serotypes, as observed in Quebec and in a majority of countries in which PCVs have been used (Lefebvre and Cote 2015; Feikin et al. 2013).
Following the PPSV23 mass campaign in the spring of 2002, the overall rate of serotype 1 IPD decreased in persons ≥ 5 years of age, 84% of whom had been vaccinated (Ndiaye et al. 2006). However, vaccine failures were observed and the mass campaign did not prevent the emergence of previously uncommon serotypes several years later. In a meta-analysis of randomized controlled trials, the estimate of the short-term efficacy of polysaccharide pneumococcal vaccines to prevent IPD cases caused by homologous serotypes in adults was 74% (Moberley et al. 2013). The same level of protection was measured in an observational study among Alaska Native adults (Singleton et al. 2007). In our study, the estimate of the effectiveness of PPSV23 against serotype 1 IPD is 71% (95% CI, − 75% to 95%) in adults ≥ 18 years of age. This value is in line with the 61% effectiveness of pneumococcal polysaccharide vaccines observed among immunocompetent adults in the US (Shapiro et al. 1991). Immunity conferred by PPSV23 against vaccine-type IPD wanes with time but the exact duration of protection is not known (Moberley et al. 2013). Contrarily to what was observed for pneumococcal conjugate vaccines, plain polysaccharide vaccines are poorly immunogenic in young children and do not induce a herd effect in slowing down the transmission of Sp in the population (Durando et al. 2013). Results of our study suggest that the mass PPSV23 campaign played a role in controlling the serotype 1 Sp outbreak in the Nunavik population, but natural disappearance of the virulent clone and the herd effect generated by the introduction of PCV10 and PCV13, both of which contain the serotype 1 antigen, for the immunization of children may have contributed to the disappearance of serotype 1 IPD since 2005.
There was no computerized immunization registry in Nunavik during the study period, and given the small number of cases, comparison of trends over time as well as estimation of vaccine effectiveness should be interpreted with care. The acceptability of vaccines is generally high in the population of Nunavik. The target population of 83.7% was vaccinated during the 2002 mass immunization campaign with PPSV23 (Ndiaye et al. 2006), and 92% of children born between 2002 and 2005 had received the recommended 4 PCV7 doses by age 5 years (Cléophat et al. 2014). Conditions are thus favourable for the use of vaccines to control pneumococcal infections, and the real question is how to improve the impact of the program.
For children, PCV13 is the vaccine with the largest coverage in terms of serotypes and four doses are given. It would be difficult to do more.
In persons ≥ 5 years of age, results of our study showed that approximately 90% of IPD cases were caused by serotypes included in PPSV23 in 2010–2016. A possible approach would be a systematic revaccination of high-risk individuals and elderly adults after or every 10 years. In a recent study among elderly adults in Japan, revaccination with PPSV23 more than 5 years after a first dose was well tolerated and associated with increases in protective antibodies to levels generally comparable to those measured after a primovaccination (Kawakami et al. 2016).
An alternative approach would be vaccination with PCV13 in combination with PPSV23, as recommended for high-risk and elderly adults in the US (Tomczyk et al. 2014). Because of PCV13 use in children, however, IPD caused by serogroups included in this vaccine have decreased markedly in Nunavik (15% of adult cases in 2010–2015). This downward trend also observed in other parts of the world is likely to continue, questioning the usefulness of PCV13 for IPD prevention in adults (Moore et al. 2015; Waight et al. 2015). It may be that the serotype distribution among non-bacteremic pneumococcal pneumonia is different and more diverse than the distribution among IPD cases as shown in Ontario (Shigayeva et al. 2016). For this reason, more studies on the etiology of non-bacteremic pneumonia in the Arctic regions of Canada are needed to define the best preventive strategy and the optimal use of available viral and bacterial vaccines for this highly vulnerable population.
Conclusion
The mass immunization campaign using the PPSV23 in 2002 and the introduction of PCVs for the routine immunization of infants induced important modifications in the epidemiology of IPD in the Nunavik region. After a decline in IPD rates during the 2000s, the rate rose again and is now back to a similar level than before 2002. Benefits of PCVs have been eroded by the emergence of non-vaccines serotypes. Currently, IPD rates in Nunavik remain much higher than that in the southern part of the province, in both children and adults. More effective pneumococcal vaccines and socio-environmental strategies are needed to eliminate geographic disparities in IPD risk.
Acknowledgements
The authors thank all the health professionals in Nunavik who participated in the data collection.
Funding
The study was performed thanks to the financial support of the Quebec Ministry of Health and Social Services, GlaxoSmithKline, and Pfizer. Sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of data; and preparation of the manuscript.
Compliance with ethical standards
Conflict of interest
Philippe De Wals received research grants, honoraria, and reimbursements of travel expenses from vaccine manufacturers, including GlaxoSmithKline, Novartis, Pfizer, and Sanofi Pasteur. The remaining authors declare no conflict of interest.
References
- Bonesteel S. Canada’s relationship with Inuit: a history of policy and program development. Ottawa: Indian and Northern Affairs; 2008. [Google Scholar]
- Canadian Institutes of Health Research, Natural Sciences and Engineering Research Council of Canada, and Social Sciences and Humanities Research Council of Canada. (2010). Tri-council policy statement: ethical conduct for research involving humans, Chapter 9. Available at: http://www.pre.ethics.gc.ca/pdf/eng/tcps2/TCPS_2_FINAL_Web.pdf. Last access: July 2018.
- Cléophat JE, Le Meur JB, Proulx JF, De Wals P. Uptake of pneumococcal vaccines in the Nordic region of Nunavik, province of Quebec, Canada. Canadian Journal of Public Health. 2014;105(4):e268–e272. doi: 10.17269/cjph.105.4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degani N, Navarro C, Deeks SL, Lovgren M. Invasive bacterial diseases in northern Canada. Emerging Infectious Diseases. 2008;14:34–40. doi: 10.3201/eid1401.061522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douville-Fradet, M., Amini, R., Boulianne, N., Khuc, N.H., De Wals, P., Fortin, E. et al. (2011). Impact du programme d’immunisation par le vaccin pneumococcique conjugué heptavalent (VPC-7) au Québec. Québec: Institut national de santé publique du Québec, 99p. Available at: http://www.inspq.qc.ca/pdf/publications/1313_ImpactProgImmuVaccinVP7.pdf.
- Durando P, Faust SN, Fletcher M, Krizova P, Torres A, Welte T. Experience with pneumococcal polysaccharide conjugate vaccine (conjugated to CRM197 carrier protein) in children and adults. Clinical Microbiology and Infection. 2013;19(S1):1–9. doi: 10.1111/1469-0691.12320. [DOI] [PubMed] [Google Scholar]
- Gouvernement du Québec. S-2.2. Loi sur la Santé publique. Chapitre VIII. Intoxications, infections et maladies à déclaration obligatoire. Available at : http://legisquebec.gouv.qc.ca/fr/ShowDoc/cs/S-2.2. Accessed 14 October 2018.
- Feikin DR, Kagucia EW, Loo JD, Link-Gelles R, Puhan MA, et al. Serotype-specific changes in invasive pneumococcal disease after pneumococcal conjugate vaccine introduction: a pooled analysis of multiple surveillance sites. PLoS Medicine. 2013;10(9):e1001517. doi: 10.1371/journal.pmed.1001517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausdorff WP, Hanage WP. Interim results of an ecological experiment — conjugate vaccination against the pneumococcus and serotype replacement. Human Vaccines & Immunotherapeutics. 2016;12(2):358–374. doi: 10.1080/21645515.2015.1118593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausdorff WP, Feikin DR, Klugman KP. Epidemiological differences among pneumococcal serotypes. The Lancet Infectious Diseases. 2005;5(2):83–93. doi: 10.1016/S1473-3099(05)70083-9. [DOI] [PubMed] [Google Scholar]
- Kawakami K, Kishino H, Kanazu S, Toshimizu N, Takahashi K, Sterling T, Wang M, Musey L. Revaccination with 23-valent pneumococcal polysaccharide vaccine in the Japanese elderly is well tolerated and elicits immune responses. Vaccine. 2016;34:3875–3881. doi: 10.1016/j.vaccine.2016.05.052. [DOI] [PubMed] [Google Scholar]
- Lefebvre, B., Cote, J. (2015). Programme de surveillance du pneumocoque: Rapport 2014. Québec: Institut national de santé publique du Québec. Available at : https://www.inspq.qc.ca/sites/default/files/publications/2081_surveillance_pneumocoque.pdf.
- Li YA, Martin I, Tsang R, Squires SG, Demczuk W, Desai S. Invasive bacterial diseases in northern Canada, 2006–2013. Canada Communicable Disease Report. 2016;42:74–80. doi: 10.14745/ccdr.v42i04a01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMillan HI, MacMillan AB, Offord DR, Dingle JL. Aboriginal health. CMAJ. 1996;155:1569–1576. [PMC free article] [PubMed] [Google Scholar]
- Marcy JF, Roberts A, Lior L, Tam TWS, VanCaeseele P. Outbreak of community-acquired pneumonia in Nunavut, October and November 2000. Canada Communicable Disease Report. 2002;28:131–138. [PubMed] [Google Scholar]
- Moberley, S., Holden, J., Tatham, D. P., & Andrews, R. M. (2013). Vaccines for preventing pneumococcal infection in adults (Review). The Cochrane Database of Systematic Reviews, (1) Available at: http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD000422.pub3/pdf. [DOI] [PMC free article] [PubMed]
- Moore MR, Link-Gelles R, Schaffner W, et al. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance. The Lancet Infectious Diseases. 2015;15(3):301–309. doi: 10.1016/S1473-3099(14)71081-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndiaye AA, De Wals P, Proulx JF, Ouakki M, Jetté L, Déry S. Impact of a mass immunization campaign to control an outbreak of severe respiratory infections in Nunavik, northern Canada. International Journal of Circumpolar Health. 2006;65:297–304. doi: 10.3402/ijch.v65i4.18120. [DOI] [PubMed] [Google Scholar]
- Orenstein WA, Bernier RH, Dondero TJ, Hinman AR, Marks JS, Bart KJ, Sirotkin B. Field evaluation of vaccine efficacy. Bulletin of the World Health Organization. 1985;63(6):1055–1068. [PMC free article] [PubMed] [Google Scholar]
- Protocole d’immunisation du Québec. Édition 6. Ministère de la santé et des services Sociaux du Québec. Available at: http://publications.msss.gouv.qc.ca/msss/document-000105/.
- Proulx JF, Déry S, Jetté L, Ismaël J, Libman M, De Wals P. Pneumonia epidemic caused by a virulent strain of Streptococcus pneumoniae serotype 1 in Nunavik, Quebec. Canada Communicable Disease Report. 2002;28:129–131. [PubMed] [Google Scholar]
- Shapiro ED, Berg AT, Austrian R, Schroeder D, Parcells V, Margolis A, et al. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. The New England Journal of Medicine. 1991;325:1453–1460. doi: 10.1056/NEJM199111213252101. [DOI] [PubMed] [Google Scholar]
- Shigayeva A, Rudnick W, Green K, Tyrrell G, Demczuk WH, Gold WL, Gubbay J, Jamieson F, Plevneshi A, Pong-Porter S, Richardson S, McGeer A, Toronto Invasive Bacterial Diseases Network Association of serotype with respiratory presentations of pneumococcal infection, Ontario, Canada, 2003-2011. Vaccine. 2016;34(6):846–853. doi: 10.1016/j.vaccine.2015.11.021. [DOI] [PubMed] [Google Scholar]
- Singleton RJ, Butler JC, Bulkow LR, Hurlburt D, O’Brien KL, Doan W, et al. Invasive pneumococcal disease epidemiology and effectiveness of 23-valent pneumococcal polysaccharide vaccine in Alaska Native adults. Vaccine. 2007;25:2288–2295. doi: 10.1016/j.vaccine.2006.11.065. [DOI] [PubMed] [Google Scholar]
- Tomczyk S, Bennett NM, Stoecker C, Gierke R, Moore MR, Whitney CG, Hadler S, Pilishvili T. Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR. Morbidity and Mortality Weekly Report. 2014;63(37):822–825. [PMC free article] [PubMed] [Google Scholar]
- Waight PA, Andrews NJ, Ladhani NJ, et al. Effect of the 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease in England and Wales 4 years after its introduction: an observational cohort study. The Lancet Infectious Diseases. 2015;15(6):629. doi: 10.1016/S1473-3099(15)00030-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenger J, Zulz T, Bruden D, Singleton R, Bruce MG, Bulkow L, et al. Invasive pneumococcal disease in Alaskan children. The Pediatric Infectious Disease Journal. 2010;29(3):251–256. doi: 10.1097/INF.0b013e3181bdbed5. [DOI] [PubMed] [Google Scholar]
- Whitney CG, Pilishvili T, Farley MM, Schaffner W, Craig AS, Lynfield R, et al. Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: a matched case-control study. Lancet. 2006;368(9546):1495–1502. doi: 10.1016/S0140-6736(06)69637-2. [DOI] [PubMed] [Google Scholar]