Abstract
Highly accurate quantitative detection of heavy metals is essential for environmental pollution monitoring and health safety. Here, for selective detection of Cu2+ ions with high sensitivity, thiacalix[4]arene (TCA) immobilized on graphene field-effect transistors (G-FETs) are developed. Our proposed TCA-immobilized G-FETs are successfully used to detect Cu2+ ions at concentrations ranging from 1 μM to 1 mM via changes in their transfer characteristics. Moreover, the measured transfer characteristics clearly shift only when Cu2+ ions are introduced in the buffer solution despite it containing other metal ions, including those of Na+, Mg2+, Ni2+, and Cd2+; this selective detection of Cu2+ ions is attributed to the planar arrangement of TCA on graphene. Therefore, TCA-immobilized G-FETs selectively detect Cu2+ with high sensitivity.
Introduction
Detection of heavy metal ions is necessary for environmental monitoring and health safety.1 In the same vein, identification and quantification of heavy metal ions is crucial for determining water quality.2−7 Currently, conventional analytical approaches such as atomic absorption spectrometry and inductively coupled plasma–mass spectrometry have been utilized in laboratory settings to identify and quantify heavy metal ions in environmental and biological samples.8−12 However, these approaches are not suitable for onsite analysis of heavy metal concentrations owing to the size of the equipment used for them. In addition, heavy metal analysis is not straightforward owing to the complexity of the analytical processes and relatively long measurement times. Considering this, portable devices that can be used for highly sensitive onsite detection of heavy metal ions are needed. Thus, in this study, we developed graphene-based devices to achieve this.
Graphene is a one-atom-thick two-dimensional carbon sheet characterized by high carrier mobility and chemical stability, which can also be used for device miniaturization.13,14 Owing to these properties, in recent times, graphene has attracted significant attention as a sensor material in sensor devices. An example of such a device is the graphene field-effect transistor (G-FET).15−19 In particular, when charged molecules are adsorbed on graphene channels in G-FETs, the adsorbed molecules induce carriers on the graphene channels, resulting a shift in the charge neutrality point of G-FETs.20 In addition, because of the high carrier mobility of graphene,13,14 these shifts lead to significant changes in the drain current. Consequently, G-FETs can be used to detect molecules with high sensitivity. However, graphene alone cannot be used for selective detection of different target molecules. Therefore, to obtain selectivity, different types of receptors, such as antibodies, aptamers, enzymes, DNA, and supramolecules, immobilized on graphene have been used in previous studies.20−29
In this work, to achieve selectivity, we study the use of thiacalix[4]arene (TCA) as a receptor. TCA is composed of benzene rings linked via sulphide bridges;30−33 it is known to form complexes with various heavy metal ions owing to its different conformations and the presence of bridging sulfur atoms.34−37 The coordination between TCA and different heavy metal ions occurs through three-dimensional coordinated structures.38−40 In particular, owing to its three-dimensional coordinated structure, TCA adsorbs several different heavy metal ions without selectivity. However, for selective detection of specific heavy metal ions, the coordination structure of metal ions needs to be modulated.
In our work, to realize selective detection of Cu2+, planar-coordinated structures between TCA and Cu2+ were formed by immobilizing TCA on the surface of graphene. This immobilization occurs because of π–π stacking between TCA and graphene, which limits the coordination forms of TCA.41,42 Our analysis results revealed that TCA-immobilized G-FETs electrically responded to Cu2+ ions over a wide concentration range, thus demonstrating their potential utility for monitoring Cu2+ ion concentrations, despite the presence of various other metal ions in solutions.
Results and Discussion
Detection of Cu2+ Ions Using TCA-Immobilized G-FETs
In this study, we demonstrated the detection of Cu2+ ions using TCA-immobilized G-FETs. Figure 1 shows the transfer characteristics of TCA-immobilized G-FETs before and after introducing Cu2+ ions at concentrations of 1, 10, 30, 100, and 300 μM. Bipolar characteristics were observed for the buffer solutions at all Cu2+ concentrations. Because the leakage current of G-FETs is 1000 times smaller than the drain current, the leakage current is negligible for detection of Cu2+. The results revealed that the transfer characteristics shifted in the positive gate-voltage direction when Cu2+ ions are introduced, indicating that G-FETs can be used to detect Cu2+ ions based on these electrical measurement changes. Furthermore, the shifts in the transfer curves increased with increasing Cu2+ concentration; in particular, the shift at a Cu2+ concentration of 300 μM was as large as ∼200 mV. Also, the average of the shifts at 300 μM was 186 ± 20 mV; the results indicated that G-FETs have repeatability for detection of Cu2+ ions. The observed positive shifts in Figure 1 were different from the results in a previous study that used G-FET-based sensors, wherein the accumulation of positive charges on graphene channels in G-FETs led to negative shifts in transfer curves.43 In contrast, our results shown in Figure 1 imply that the charge distribution in TCA changed because of the formation of complexes between Cu2+ and TCA, as shown in Figure 2;44−46 in turn, this change in the charge distribution induced potential changes in the graphene channel, resulting in the positive shift in transfer characteristics.
Figure 1.
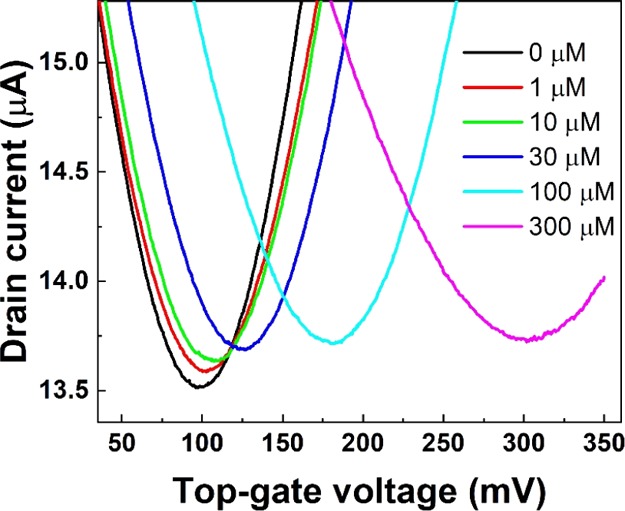
Transfer characteristics of the TCA-immobilized G-FET at different Cu2+ concentrations.
Figure 2.
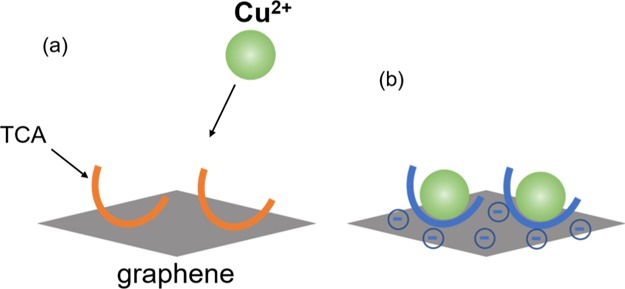
Schematic images of Cu2+ ions (a) before and (b) after coordinating with TCA.
Cu2+ Concentration Dependence
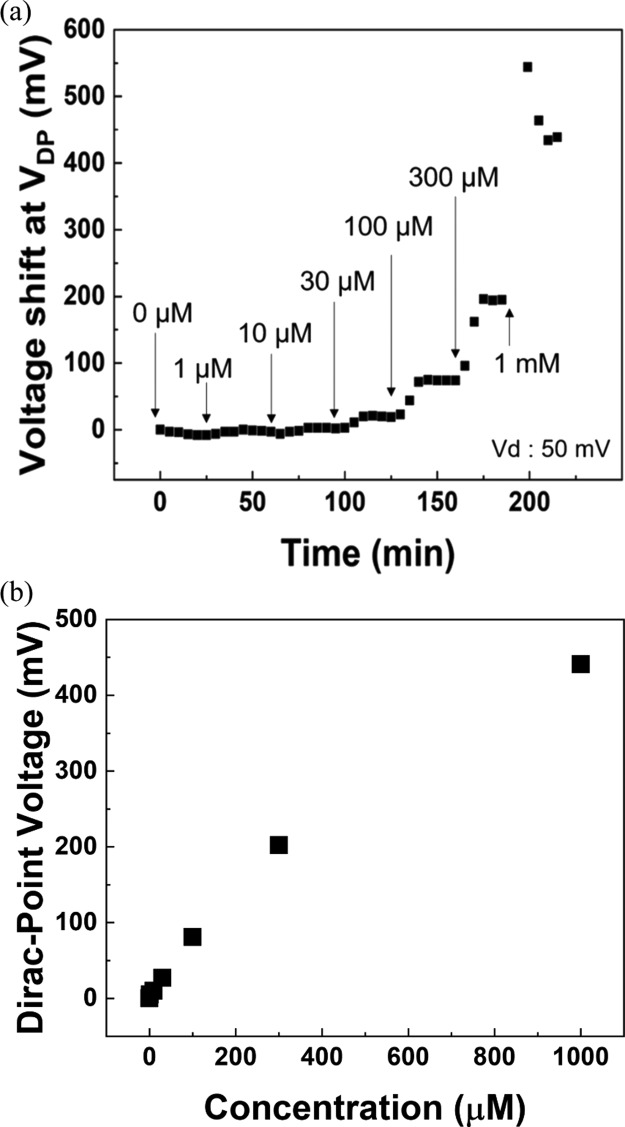
Next, the dependence of Cu2+ ion concentration on voltage characteristics of TCA-immobilized G-FETs was investigated. The transfer characteristics were measured every 5 min, and the Dirac-point voltage (VDP) was plotted. Figure 3a shows the shifts in VDP with time for various ion concentrations of Cu2+ ranging from 0 to 1 mM; the results shown in the figure reveal that the shift in VDP increased with increasing Cu2+ ion concentration. Furthermore, change in VDP almost stopped within 15 min at each Cu2+ concentration. This occurs because the adsorption of Cu2+ ions on TCA attains an equilibrium state. Therefore, G-FETs with TCA can be used to detect Cu2+ ions in a concentration range of 1 μM to 1 mM within 15 min. Figure 3b shows the amount of VDP shift as a function of concentration of Cu2+ ranging from 0 to 1 mM. The result reveals that the VDP shift increases linearly with the concentration of Cu2+ ranging from 0 to 300 μM. On the other hand, the VDP shift at 1 mM is slightly deviated from the linearity, indicating that the VDP shift gradually saturated over 300 μM.
Figure 3.
(a) Shifts in VDP with time for various ion concentrations of Cu2+ and (b) shift in VDP shift as a function of concentration of Cu2+ ranging from 0 to 1 mM.
Selectivity of TCA-Immobilized G-FETs for Cu2+ Ions
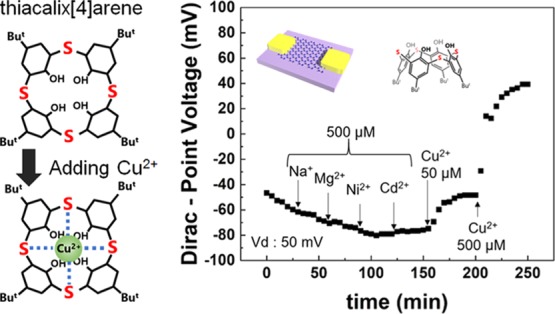
To investigate the selectivity of TCA-immobilized G-FETs for Cu2+, the shifts in VDP were measured in Tris-HCl buffer containing other metal ions (Na+, Mg2+, Ni2+, and Cd2+). Figure 4a shows these shifts in VDP when Na+, Mg2+, Ni2+, and Cd2+ ions at concentrations of 500 μM each were sequentially added to the solution, after which Cu2+ ions at concentrations of 50 and 500 μM were added; the results show that VDP did not clearly shift when Na+, Mg2+, Ni2+, and Cd2+ ions were introduced in the solution. In contrast, a clear VDP shift was observed when Cu2+ ions were introduced in the solution. Therefore, TCA-immobilized G-FETs showed selective detection of Cu2+ ions even in a solution containing other metal ions. Figure 4b shows the changes in VDP for each metal ion (Na+, Mg2+, Ni2+, Cd2+, and Cu2+) at concentrations of 500 μM in the form of a bar graph. In particular, the VDP shift at 500 μM was observed to be over 100 mV for Cu2+ ions, which was more than 10 times larger than those recorded when Na+, Mg2+, Ni2+, and Cd2+ ions were introduced. This selectivity for Cu2+ ions results from the immobilization of TCA on the graphene channel. The recognition of Cu2+ ions using TCA immobilized on graphene can be explained using the hard and soft acids and bases (HSAB) theory and the ligand field theory.47,48 According to the HSAB theory, soft metal ions (Cu2+, Ni2+, and Cd2+) tend to easily coordinate with sulfur atoms. Results from previous works indicate that TCA dispersed in solution captures not only Cu2+ ions, but also Ni2+ and Cd2+ ions through the liquid–liquid extraction method.35,49 However, it is interesting to note that, compared with these previous works, our results indicate that TCA on the graphene surfaces capture only Cu2+ ions, which, in turn, implies that the space degrees of freedom of TCA were limited by immobilizing it on graphene surfaces. In particular, in the case of the liquid–liquid extraction method, the orientation and position of TCA changes relatively freely in solutions; consequently, several different heavy metal ions are surrounded by three TCA molecules leading to their adsorption.35,49 However, when immobilized on G-FETs, TCA was planarly placed on the graphene surfaces; therefore, TCA cannot surround and adsorb several types of heavy metal ions when it is immobilized so. The coordination between Cu2+ ions and G-FETs occurs via the host–guest interaction. As depicted in Figure 5, Cu2+ ions are generally coordinated in a square shape, as are the bridging sulfur atoms in TCA leading to the coordination of the bridging sulfur atoms with Cu2+ ions and their subsequent adsorption.50 On the contrary, coordination forms of the other heavy metal ions are not of square type, but instead are of tetrahedron and octahedron types, which is why they do not coordinate with TCA immobilized on graphene.51 In summary, the selectivity of G-FETs for Cu2+ ions is attributed to the immobilization of TCA on graphene surfaces in G-FETs. Therefore, G-FETs can be successfully used in the selective detection of Cu2+.
Figure 4.
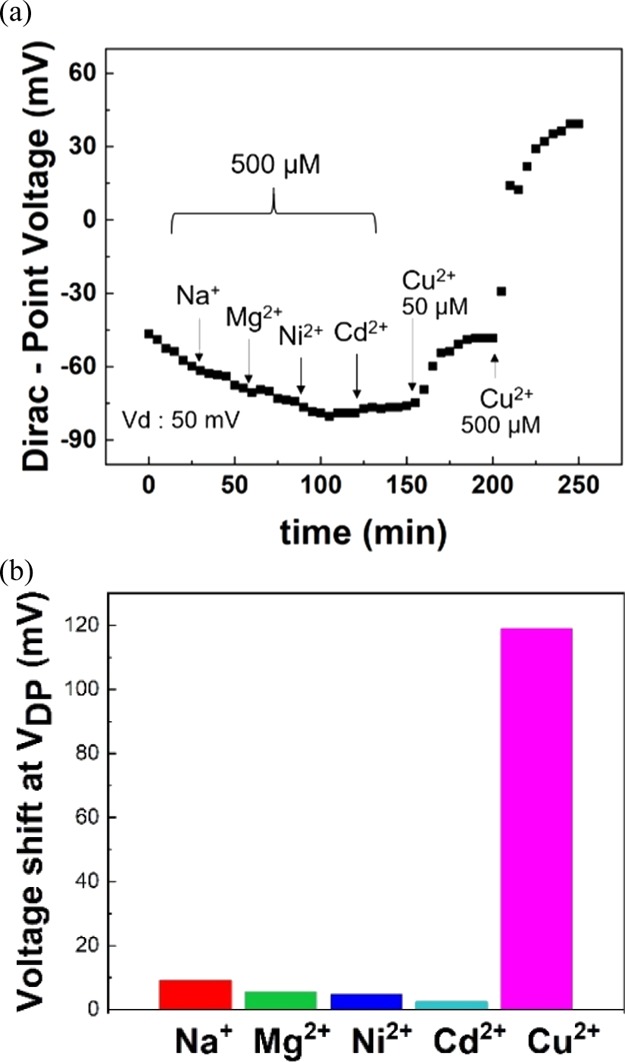
(a) VDP as a function of time with other metal ions (Na+, Mg2+, Ni2+, and Cd2+) at 500 μM and Cu2+ at 50 and 500 μM. (b) Shifts in VDP due to different metal ions (Na+, Mg2+, Ni2+, Cd2+, and Cu2+) at 500 μM.
Figure 5.
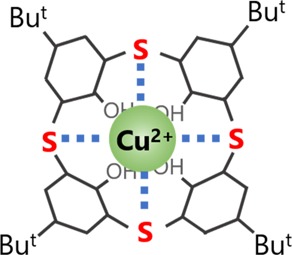
Coordination of TCA with Cu2+ ions.
Conclusions
In this study, we fabricated Cu2+ sensors using TCA-immobilized G-FETs. The transfer characteristics shifted in the positive gate-voltage direction on introducing Cu2+ ions in the solution; in addition, the VDP shift increased as the Cu2+ concentration was increased from 1 μM to 1 mM, which indicates that G-FETs can be used for quantitative analysis of Cu2+. Furthermore, TCA-immobilized G-FETs were able to successfully detect Cu2+ ions selectively in a buffer solution containing several different metal ions, viz., Na+, Mg2+, Ni2+, and Cd2+ ions. In particular, the amount of VDP shift on introducing Cu2+ ions at a concentration of 500 μM was about 10 times larger than that on introducing Na+, Mg2+, Ni2+, and Cd2+ ions at the same concentration. Therefore, our results indicate that the TCA-immobilized G-FETs provide high sensitivity and selectivity for the detection of Cu2+ ions, making them promising candidates as portable Cu2+ sensors. Moreover, our research shows the importance of coordination structures between TCA and metal ions for the detection of specific metal ions using G-FETs. Moreover, in a manner similar to the approach followed in our study, other heavy metal ion sensors could be realized by adjusting the molecular structure to ensure the formation of a coordinate structure between the molecule and metal ions.
Experimental Methods
Fabrication Process for G-FETs
First, monolayer graphene was synthesized on Cu foil (JX Nippon Mining & Metals, HA) via chemical vapor deposition. Before use, the Cu foil was annealed under a flow of 3% H2 and 97% Ar at 500 sccm for 41 min to remove any native oxide so that Cu exhibits catalytic activity. Graphene was synthesized at 1035 °C by cracking CH4 under both 3% H2 and 97% Ar flow at 15 sccm, and 5% CH4 and 95% Ar flow at 1500 sccm for 35 min. Next, the graphene was transferred onto a Si/SiO2 substrate using poly(methyl methacrylate) (PMMA) and annealed at 330 °C for 1 h under a 3% H2 and 97% Ar flow to remove impurities such as residual PMMA. Subsequently, Ni(10 nm) and Au(30 nm) were deposited as sources and drain electrodes via a photolithography technique. The channel distance and width for the source and drain were approximately 5 and 15 μm, respectively.52
Adsorption of TCA on Graphene and Measurement Methods
After G-FETs ware fabricated, a 50 μM solution was prepared by dissolving TCA (Tokyo Chemical Industry Co.) in chloroform. Then, the G-FETs were immersed in this prepared solution to immobilize TCA on graphene through π–π interactions between them.41,42 These G-FETs were then taken out of the solution and dried to obtain the required graphene-based sensors. Next, a silicon rubber pool was attached to the G-FETs so that the graphene channel could be immersed in a Tris-HCl buffer (20 mM, pH 8.0). A saturated Ag/AgCl electrode was used as the reference electrode.53 NaCl, MgCl2, CdSO4, NiSO4, and CuSO4 solutions at various concentrations were added to the Tris-HCl buffer solution to increase the Na+, Mg2+, Cd2+, Ni2+, and Cu2+ concentrations in the reagent solution, respectively. The transfer characteristics of the G-FETs were measured by applying a drain voltage of 50 mV using a semiconductor parameter analyzer (Keysight Technologies, B2912).
Acknowledgments
This research was partly supported by the Uehara Memorial Foundation and the Environment Research and Technology Development Fund (5RF-1802) of the Environmental Restoration and Conservation Agency of Japan and Grants-in-Aid for Scientific Research (B) (JP19H02582) and Challenging Research (Exploratory) (JP19K21963) from JSPS.
The authors declare no competing financial interest.
References
- Singh A.; Sharma R. K.; Agrawal M.; Marshall F. M. Health risk assessment of heavy metals via dietary intake of foodstuffs from the wastewater irrigated site of a dry tropical area of India. Food Chem. Toxicol. 2010, 48, 611–619. 10.1016/j.fct.2009.11.041. [DOI] [PubMed] [Google Scholar]
- Jang A.; Seo Y.; Bishop P. L. The removal of heavy metals in urban runoff by sorption on mulch. Environ. Pollut. 2005, 133, 117–127. 10.1016/j.envpol.2004.05.020. [DOI] [PubMed] [Google Scholar]
- Turdean G. L. Design and Development of Biosensors for the Detection of Heavy Metal Toxicity. Int. J. Electrochem. 2011, 2011, 1. 10.4061/2011/343125. [DOI] [Google Scholar]
- Gao C.; Yu X.-Y.; Xiong S.-Q.; Liu J.-H.; Huang X.-J. Electrochemical Detection of Arsenic(III) Completely Free from Noble Metal: Fe3O4 Microspheres-Room Temperature Ionic Liquid Composite Showing Better Performance than Gold. Anal. Chem. 2013, 85, 2673–2680. 10.1021/ac303143x. [DOI] [PubMed] [Google Scholar]
- Valko M.; Morris H.; Cronin M. Metals, Toxicity and Oxidative Stress. Curr. Med. Chem. 2005, 12, 1161–1208. 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- Sumner E. R.; Shanmuganathan A.; Sideri T. C.; Willetts S. A.; Houghton J. E.; Avery S. V. Oxidative protein damage causes chromium toxicity in yeast. Microbiology 2005, 151, 1939–1948. 10.1099/mic.0.27945-0. [DOI] [PubMed] [Google Scholar]
- Li P.; Zhang D.; Wu J.; Cao Y.; Wu Z. Flexible integrated black phosphorus sensor arrays for high performance ion sensing. Sens. Actuators, B 2018, 273, 358–364. 10.1016/j.snb.2018.06.077. [DOI] [Google Scholar]
- Steinnes E. Atmospheric deposition of heavy metals in Norway studied by the analysis of moss samples using neutron activation analysis and atomic absorption spectrometry. J. Radioanal. Chem. 1980, 58, 387–391. 10.1007/bf02533811. [DOI] [Google Scholar]
- Daşbaşı T.; Saçmacı Ş.; Ülgen A.; Kartal Ş. A solid phase extraction procedure for the determination of Cd(II) and Pb(II) ions in food and water samples by flame atomic absorption spectrometry. Food Chem. 2015, 174, 591–596. 10.1016/j.foodchem.2014.11.049. [DOI] [PubMed] [Google Scholar]
- McComb J. Q.; Rogers C.; Han F. X.; Tchounwou P. B. Rapid Screening of Heavy Metals and Trace Elements in Environmental Samples Using Portable X-Ray Fluorescence Spectrometer, A Comparative Study. Water, Air, Soil Pollut. 2014, 225, 2169. 10.1007/s11270-014-2169-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.; Wang Z.-Y.; Yang X.; Zhao H.-T.; Zhang Y.-C.; Dong A.-J.; Jing J.; Wang J. Determination of free amino acids and 18 elements in freeze-dried strawberry and blueberry fruit using an Amino Acid Analyzer and ICP-MS with micro-wave digestion. Food Chem. 2014, 147, 189–194. 10.1016/j.foodchem.2013.09.118. [DOI] [PubMed] [Google Scholar]
- Veglio’ F.; Beolchini F. Removal of metals by biosorption: a review. Hydrometallurgy 1997, 44, 301–316. 10.1016/s0304-386x(96)00059-x. [DOI] [Google Scholar]
- Lee C.; Wei X.; Kysar J. W.; Hone J. Measurement of the Elastic Properties and Intrinsic Strength of Monolayer Graphene. Science 2008, 321, 385. 10.1126/science.1157996. [DOI] [PubMed] [Google Scholar]
- Balandin A. A. Thermal properties of graphene and nanostructured carbon materials. Nat. Mater. 2011, 10, 569. 10.1038/nmat3064. [DOI] [PubMed] [Google Scholar]
- Sakamoto Y.; Uemura K.; Ikuta T.; Maehashi K. Palladium configuration dependence of hydrogen detection sensitivity based on graphene FET for breath analysis. Jpn. J. Appl. Phys. 2018, 57, 04FP05. 10.7567/jjap.57.04fp05. [DOI] [Google Scholar]
- Nozaki R.; Ikuta T.; Ueno K.; Tsukakoshi K.; Ikebukuro K.; Maehashi K. Ethanol Detection at the Parts per Billion Level with Single-Stranded-DNA-Modified Graphene Field-Effect Transistors. Phys. Status Solidi B 2019, 0, 1900376. 10.1002/pssb.201900376. [DOI] [Google Scholar]
- Hu S.-K.; Lo F.-Y.; Hsieh C.-C.; Chao L. Sensing Ability and Formation Criterion of Fluid Supported Lipid Bilayer Coated Graphene Field-Effect Transistors. ACS Sens. 2019, 4, 892–899. 10.1021/acssensors.8b01623. [DOI] [PubMed] [Google Scholar]
- Okuda S.; Ono T.; Kanai Y.; Ikuta T.; Shimatani M.; Ogawa S.; Maehashi K.; Inoue K.; Matsumoto K. Graphene Surface Acoustic Wave Sensor for Simultaneous Detection of Charge and Mass. ACS Sens. 2018, 3, 200–204. 10.1021/acssensors.7b00851. [DOI] [PubMed] [Google Scholar]
- Li P.; Liu B.; Zhang D.; Sun Y. e.; Liu J. Graphene field-effect transistors with tunable sensitivity for high performance Hg (II) sensing. Appl. Phys. Lett. 2016, 109, 153101. 10.1063/1.4964347. [DOI] [Google Scholar]
- Ohno Y.; Maehashi K.; Inoue K.; Matsumoto K. Label-Free Aptamer-Based Immunoglobulin Sensors Using Graphene Field-Effect Transistors. Jpn. J. Appl. Phys. 2011, 50, 070120. 10.1143/jjap.50.070120. [DOI] [Google Scholar]
- Pumera M. Graphene in biosensing. Mater. Today 2011, 14, 308–315. 10.1016/s1369-7021(11)70160-2. [DOI] [Google Scholar]
- Tehrani Z.; Burwell G.; Azmi M. A. M.; Castaing A.; Rickman R.; Almarashi J.; Dunstan P.; Beigi A. M.; Doak S. H.; Guy O. J. Generic epitaxial graphene biosensors for ultrasensitive detection of cancer risk biomarker. 2D Materials 2014, 1, 025004. 10.1088/2053-1583/1/2/025004. [DOI] [Google Scholar]
- Teixeira S.; Conlan R. S.; Guy O. J.; Sales M. G. F. Label-free human chorionic gonadotropin detection at picogram levels using oriented antibodies bound to graphene screen-printed electrodes. J. Mater. Chem. B 2014, 2, 1852–1865. 10.1039/c3tb21235a. [DOI] [PubMed] [Google Scholar]
- Zeng Q.; Cheng J.; Tang L.; Liu X.; Liu Y.; Li J.; Jiang J. Self-Assembled Graphene-Enzyme Hierarchical Nanostructures for Electrochemical Biosensing. Adv. Funct. Mater. 2010, 20, 3366–3372. 10.1002/adfm.201000540. [DOI] [Google Scholar]
- Zeng G.; Xing Y.; Gao J.; Wang Z.; Zhang X. Unconventional Layer-by-Layer Assembly of Graphene Multilayer Films for Enzyme-Based Glucose and Maltose Biosensing. Langmuir 2010, 26, 15022–15026. 10.1021/la102806v. [DOI] [PubMed] [Google Scholar]
- Ohno Y.; Okamoto S.; Maehashi K.; Matsumoto K. Direct Electrical Detection of DNA Hybridization Based on Electrolyte-Gated Graphene Field-Effect Transistor. Jpn. J. Appl. Phys. 2013, 52, 110107. 10.7567/jjap.52.110107. [DOI] [Google Scholar]
- Guo Y.; Guo S.; Ren J.; Zhai Y.; Dong S.; Wang E. Cyclodextrin Functionalized Graphene Nanosheets with High Supramolecular Recognition Capability: Synthesis and Host–Guest Inclusion for Enhanced Electrochemical Performance. ACS Nano 2010, 4, 4001–4010. 10.1021/nn100939n. [DOI] [PubMed] [Google Scholar]
- Maehashi K.; Sofue Y.; Okamoto S.; Ohno Y.; Inoue K.; Matsumoto K. Selective ion sensors based on ionophore-modified graphene field-effect transistors. Sens. Actuators, B 2013, 187, 45–49. 10.1016/j.snb.2012.09.033. [DOI] [Google Scholar]
- Campos R.; Borme J.; Guerreiro J. R.; Machado G.; Cerqueira M. F.; Petrovykh D. Y.; Alpuim P. Attomolar Label-Free Detection of DNA Hybridization with Electrolyte-Gated Graphene Field-Effect Transistors. ACS Sens. 2019, 4, 286–293. 10.1021/acssensors.8b00344. [DOI] [PubMed] [Google Scholar]
- Morohashi N.; Narumi F.; Iki N.; Hattori T.; Miyano S. Thiacalixarenes. Chem. Rev. 2006, 106, 5291–5316. 10.1021/cr050565j. [DOI] [PubMed] [Google Scholar]
- Iki N.; Kabuto C.; Fukushima T.; Kumagai H.; Takeya H.; Miyanari S.; Miyashi T.; Miyano S. Synthesis of p-tert-Butylthiacalix[4]arene and its Inclusion Property. Tetrahedron 2000, 56, 1437–1443. 10.1016/s0040-4020(00)00030-2. [DOI] [Google Scholar]
- Shokova E. A.; Kovalev V. V. Thiacalixarenes-A New Class of Synthetic Receptors. Russ. J. Org. Chem. 2003, 39, 1–28. 10.1023/a:1023416409935. [DOI] [Google Scholar]
- Sone T.; Ohba Y.; Moriya K.; Kumada H.; Ito K. Synthesis and properties of sulfur-bridged analogs of p-tert-Butylcalix[4]arene. Tetrahedron 1997, 53, 10689–10698. 10.1016/s0040-4020(97)00700-x. [DOI] [Google Scholar]
- Lhoták P. Chemistry of Thiacalixarenes. Eur. J. Org. Chem. 2004, 2004, 1675–1692. 10.1002/ejoc.200300492. [DOI] [Google Scholar]
- Morohashi N.; Iki N.; Sugawara A.; Miyano S. Selective oxidation of thiacalix[4]arenes to the sulfinyl and sulfonyl counterparts and their complexation abilities toward metal ions as studied by solvent extraction. Tetrahedron 2001, 57, 5557–5563. 10.1016/s0040-4020(01)00482-3. [DOI] [Google Scholar]
- Iki N.; Morohashi N.; Narumi F.; Miyano S. High Complexation Ability of Thiacalixarene with Transition Metal Ions. The Effects of Replacing Methylene Bridges of Tetra(p-t-butyl)calix[4]arenetetrol by Epithio Groups. Bull. Chem. Soc. Jpn. 1998, 71, 1597–1603. 10.1246/bcsj.71.1597. [DOI] [Google Scholar]
- Yushkova E. A.; Stoikov I. I. p-tert-Butyl Thiacalix[4]arenes Functionalized with Amide and Hydrazide Groups at the Lower Rim inCone,Partial Cone, and1,3-AlternateConformations Are ″Smart″ Building Blocks for Constructing Nanosized Structures with Metal Cations ofs-,p-, andd-Elements in the Organic Phase. Langmuir 2009, 25, 4919–4928. 10.1021/la8040902. [DOI] [PubMed] [Google Scholar]
- Evtugyn G.; Stoikov I.; Belyakova S.; Stoikova E.; Shamagsumova R.; Zhukov A.; Antipin I.; Budnikov H. Selectivity of solid-contact Ag potentiometric sensors based on thiacalix[4]arene derivatives. Talanta 2008, 76, 441–447. 10.1016/j.talanta.2008.03.029. [DOI] [PubMed] [Google Scholar]
- Modi K.; Panchal U.; Dey S.; Patel C.; Kongor A.; Pandya H. A.; Jain V. K. Thiacalix[4]arene-tetra-(quinoline-8- sulfonate): a Sensitive and Selective Fluorescent Sensor for Co (II). J. Fluoresc. 2016, 26, 1729–1736. 10.1007/s10895-016-1864-6. [DOI] [PubMed] [Google Scholar]
- Kumar R.; Lee Y. O.; Bhalla V.; Kumar M.; Kim J. S. Recent developments of thiacalixarene based molecular motifs. Chem. Soc. Rev. 2014, 43, 4824–4870. 10.1039/c4cs00068d. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Podoprygorina G.; Brusko V.; Böhmer V.; Janshoff A. Functionalized Calix[8]arenes, Synthesis and Self-assembly on Graphite. Chem. Mater. 2005, 17, 2290–2297. 10.1021/cm047980x. [DOI] [Google Scholar]
- Eroglu E.; Zang W.; Eggers P. K.; Chen X.; Boulos R. A.; Wahid M. H.; Smith S. M.; Raston C. L. Nitrate uptake by p-phosphonic acid calix[8]arene stabilized graphene. Chem. Commun. 2013, 49, 8172–8174. 10.1039/c3cc44093a. [DOI] [PubMed] [Google Scholar]
- Sofue Y.; Ohno Y.; Maehashi K.; Inoue K.; Matsumoto K. Highly Sensitive Electrical Detection of Sodium Ions Based on Graphene Field-Effect Transistors. Jpn. J. Appl. Phys. 2011, 50, 06GE07. 10.7567/jjap.50.06ge07. [DOI] [Google Scholar]
- Yang L.; Zhao H.; Li Y.; Ran X.; Deng G.; Zhang Y.; Ye H.; Zhao G.; Li C.-P. Indicator displacement assay for cholesterol electrochemical sensing using a calix[6]arene functionalized graphene-modified electrode. Analyst 2016, 141, 270–278. 10.1039/c5an01843a. [DOI] [PubMed] [Google Scholar]
- Liu L.; Zhang K.; Wei Y. A simple strategy for the detection of Cu(ii), Cd(ii) and Pb(ii) in water by a voltammetric sensor on a TC4A modified electrode. New J. Chem. 2019, 43, 1544–1550. 10.1039/c8nj05089a. [DOI] [Google Scholar]
- Wang L.; Wang X.; Shi G.; Peng C.; Ding Y. Thiacalixarene Covalently Functionalized Multiwalled Carbon Nanotubes as Chemically Modified Electrode Material for Detection of Ultratrace Pb2+ Ions. Anal. Chem. 2012, 84, 10560–10567. 10.1021/ac302747f. [DOI] [PubMed] [Google Scholar]
- Pearson R. G. Hard and Soft Acids and Bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. 10.1021/ja00905a001. [DOI] [Google Scholar]
- Pearson R. G. Hard and soft acids and bases, HSAB, part 1: Fundamental principles. J. Chem. Educ. 1968, 45, 581. 10.1021/ed045p581. [DOI] [Google Scholar]
- Iki N.; Kumagai H.; Morohashi N.; Ejima K.; Hasegawa M.; Miyanari S.; Miyano S. Selective oxidation of thiacalix[4]arenes to the sulfinyl- and sulfonylcalix[4]arenes and their coordination ability to metal ions. Tetrahedron Lett. 1998, 39, 7559–7562. 10.1016/s0040-4039(98)01645-1. [DOI] [Google Scholar]
- Theophanides T.; Anastassopoulou J. Copper and carcinogenesis. Crit. Rev. Oncol.-Hematol. 2002, 42, 57–64. 10.1016/s1040-8428(02)00007-0. [DOI] [PubMed] [Google Scholar]
- Swenson D.; Baenziger N. C.; Coucouvanis D. Tetrahedral mercaptide complexes. Crystal and molecular structures of [(C6H5)4P]2M(SC6H5)4 complexes (M = cadmium(II), zinc(II), nickel(II), cobalt(II), and manganese(II)). J. Am. Chem. Soc. 1978, 100, 1932–1934. 10.1021/ja00474a053. [DOI] [Google Scholar]
- Uemura K.; Ikuta T.; Maehashi K. Turbostratic stacked CVD graphene for high-performance devices. Jpn. J. Appl. Phys. 2018, 57, 030311. 10.7567/jjap.57.030311. [DOI] [Google Scholar]
- Katsura T.; Yamamoto Y.; Maehashi K.; Ohno Y.; Matsumoto K. High-Performance Carbon Nanotube Field-Effect Transistors with Local Electrolyte Gates. Jpn. J. Appl. Phys. 2008, 47, 2060–2063. 10.1143/jjap.47.2060. [DOI] [Google Scholar]