Abstract
Objective
To examine the feasibility and preliminary impact of a home-based obesity prevention intervention among Canadian families.
Methods
Families with children 1.5–5 years of age were randomized to one of three groups: (1) four home visits (HV) with a health educator, emails, and mailed incentives (4HV; n = 17); (2) two HV, emails, and mailed incentives (2HV; n = 14); or (3) general health advice through emails (control; n = 13). Parents randomized to the 2HV and 4HV groups completed post-intervention satisfaction surveys. At baseline and post-intervention, parents reported frequency of family meals and their children’s fruit, vegetable, and sugar-sweetened beverage (SSB) intake. We assessed the children’s physical activity, sedentary behaviour, and sleep using accelerometers and their % fat mass using bioelectrical impedance analysis. Differences in outcomes at post-intervention, controlling for baseline, were examined using generalized estimating equations.
Results
Of the 44 families enrolled, 42 (96%) had 6-month outcome data. Satisfaction with the intervention was high; 80% were “very satisfied” and 20% were “satisfied.” At post-intervention, children randomized to the 4HV and 2HV groups had significantly higher fruit intake and children randomized to the 2HV group had significantly lower percentage of fat mass, as compared to the control. No significant intervention effect was found for frequency of family meals, the children’s vegetable or SSB intake, physical activity, sedentary behaviour, or sleep.
Conclusions
Our results suggest that the delivery of a home-based intervention is feasible among Canadian families and may lead to improved diet and weight outcomes among children. A full-scale trial is needed to test the effectiveness of this home-based intervention.
Clinical trials registration number
Keywords: Obesity, Family, Health behaviour, Randomized controlled trial
Résumé
Objectif
Examiner la faisabilité et l’impact préliminaire d’une intervention de prévention de l’obésité à domicile menée auprès de familles canadiennes.
Méthode
Des familles avec enfants de 1,5 à 5 ans ont été affectées aléatoirement à l’un de trois groupes: (1) 4 visites à domicile (VAD) par un/e éducateur/trice sanitaire, messages par courriel et récompenses par la poste (4VAD; n = 17); (2) 2 VAD, messages par courriel et récompenses par la poste (2VAD; n = 14); ou (3) conseils de santé généraux par courriel (groupe témoin; n = 13). Les parents affectés aux groupes 2VAD et 4VAD ont rempli un questionnaire sur leur satisfaction après l’intervention. Au départ et après l’intervention, les parents ont fait état de la fréquence de leurs repas en famille et de la consommation de fruits, de légumes et de boissons édulcorées au sucre (BÉS) de leurs enfants. Nous avons évalué l’activité physique, le comportement sédentaire et le sommeil des enfants à l’aide d’accéléromètres, et leur pourcentage de masse adipeuse par analyse d’impédance bioélectrique. Les différences des résultats après l’intervention, après avoir apporté des ajustements en fonction des données de départ, ont été examinées à l’aide d’équations d’estimation généralisées.
Résultats
Sur les 44 familles inscrites, 42 (96 %) ont produit des données sur 6 mois. La satisfaction par rapport à l’intervention a été élevée; 80 % des familles étaient « très satisfaites » et 20 % étaient « satisfaites ». Après l’intervention, les enfants affectés aléatoirement aux groupes 4VAD et 2VAD avaient une consommation de fruits significativement plus élevée et les enfants affectés aléatoirement au groupe 2VAD un pourcentage de masse adipeuse significativement inférieur à ceux du groupe témoin. Aucun effet significatif de l’intervention n’a été observé pour ce qui est de la fréquence des repas en famille, ni de la consommation de légumes ou de BÉS, de l’activité physique, du comportement sédentaire ou du sommeil des enfants.
Conclusions
Nos résultats indiquent qu’il est faisable de mener des interventions à domicile auprès des familles canadiennes, et que cela peut améliorer le régime et les problèmes de poids des enfants. Un essai en vraie grandeur est nécessaire pour tester l’efficacité de cette intervention à domicile.
Mots-clés: Obésité, Famille, Comportement en matière de santé, Essai contrôlé randomisé
Introduction
In Canada, the high rate of overweight or obesity among young children is an important public health issue (Shields and Tremblay 2010). Dietary intake, screen time, and sleep duration are key modifiable behaviours that impact the young children’s obesity risk (de Ruyter et al. 2012; Poitras et al. 2017; Miller et al. 2015). National data suggest that many Canadian children are not meeting recommendations for these weight-related behaviours (Garriguet 2004; Garriguet et al. 2016; Chaput and Janssen 2016). Interventions to prevent obesity need to begin early in life when many eating, screen time, and sleep habits are established (Singer et al. 1995; Moore et al. 1995). However, changing weight-related behaviours of young children requires engaging and changing the behaviours of their caregivers, in particular their parents (Hingle et al. 2010).
Interventions delivered within the home setting are well suited to engage the entire family unit in behaviour change efforts. Home-based interventions also allow for tailoring of behaviour change strategies to each family’s particular home context, e.g., their own resources for food preparation or media home environment. Based on evidence from obesity prevention trials in Australia and the USA (Wen et al. 2012; Haines et al. 2013; Savage et al. 2016), a 2016 Institute of Medicine report identified home-based interventions as one of the most promising strategies for the prevention of childhood obesity (National Academies of Sciences, Engineering, and Medicine 2016). Research examining such an approach in Canada is limited; Anand and colleagues conducted a 6-month-long randomized controlled trial (RCT) among 57 Aboriginal families with school-aged children and found that families who received home visits from an Aboriginal Health Counsellor significantly improved their dietary intake, but no significant improvements were found for physical activity, sedentary behaviour, or weight (Anand et al. 2007). There are no published studies that have tested a home-based approach for obesity prevention among families with preschool-aged children in Canada.
Research by members of our team demonstrated that a home-based intervention, Healthy Habits, Happy Homes, can improve weight-related behaviours and outcomes among American families with young children. In a 6-month-long RCT among 121 families, we found that preschool-aged children whose families received 4 home visits with a health educator, phone calls and texts, and mailed incentives that targeted family meals, sleep, and screen time increased their sleep duration, decreased their screen time, and decreased their BMI by 0.4 kg/m2 (95% CI, − 0.8, 0.0) compared to children in the control group (Haines et al. 2013). While the results demonstrate the strong potential of this home-based intervention to prevent excess weight gain, the trial participants were primarily African-American and Latino families in the USA. Given that parenting practices and cultural norms regarding the children’s weight-related behaviours have been found to differ across cultures (Patrick et al. 2005; Giannotti and Cortesi 2009; Mindell et al. 2010; Hamilton et al. 2013; Barkin et al. 2006), it is unknown whether this home-based obesity prevention intervention is feasible or effective among Canadian families.
The primary aim of this pilot RCT was to test, among Canadian families, the feasibility and acceptability of the Guelph Family Health Study intervention, a home-based obesity prevention intervention based on the Healthy Habits, Happy Homes intervention. Our secondary aim was to examine the preliminary impact of the Guelph Family Health Study intervention, compared to a minimum-attention control, on frequency of family meals; the children’s fruit, vegetable, and sugar-sweetened beverage (SSB) intake; the children’s physical activity, sedentary behaviour, and sleep assessed using accelerometers; and the children’s adiposity (% fat mass) assessed by bioelectrical impedance analysis. A detailed analysis of the impact of the intervention on the children’s dietary intake has been previously published (Mirotta et al. 2018). This study expands upon the previous analyses by examining the preliminary impact of intervention on the children’s adiposity, physical activity, sedentary behaviour, sleep, as well as our key dietary outcomes: family meal frequency and the children’s fruit, vegetable, and SSB intake. The long-term goal of this research is to inform a full-scale trial of this home-based intervention in Canada.
Methods
Study design and participants
The Guelph Family Health Study intervention pilot is an external pilot randomized controlled trial. Between August 2014 and January 2015, we enrolled 44 families with children between the ages of 1.5 and 5 years (Fig. 1). Recruitment strategies included posters and rack cards at agencies that provide services for families with young children, i.e., Family Health Team, Community Health Centre and Ontario Early Years Centre, and postings on the Ontario Early Years Centre Facebook page and the University of Guelph webpage. Exclusion criteria were if families (1) planned to move within 1 year, (2) had children outside of the target age range, or (3) were non-English speaking. Fifty-one families completed an eligibility survey either via an online survey (78%) or via phone with the study coordinator (22%); of the 51 families screened for eligibility, 44 met eligibility and completed the baseline assessment (Fig. 1). All parents provided written consent for themselves and their children and when possible, children provided verbal assent for themselves. All participating families (control and intervention) received grocery gift cards as a thank you for completing the baseline and follow-up assessments. Once the baseline assessment was complete, the study coordinator used a pseudo-random number generator to randomly assign each family to a study group. The study protocol was approved by the University of Guelph Research Ethics Board (REB14AP008 and REB14AP009).
Fig. 1.
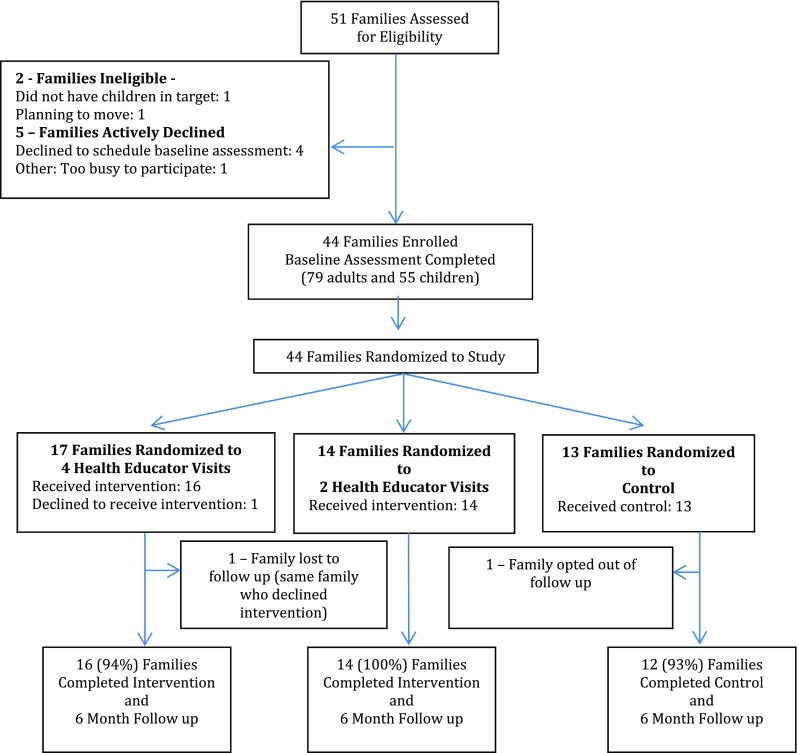
Study design and participant flow of the Guelph Family Health Study Pilot RCT
Adaptations to Healthy Habits, Happy Homes intervention
Before testing the Guelph Family Health Study intervention within Canada, we conducted focus groups with 28 Canadian parents of preschool-aged children to identify the parents’ preferences with regard to intervention content and delivery mode (O’Kane et al. 2018). Parents identified that they preferred home visits and emails rather than phone calls or text messages to support behaviour change. Based on this feedback, we adapted the Healthy Habits, Happy Homes intervention by removing phone calls and text messages and adding mobile-friendly emails. We also included behaviour change messages related to decreasing intake of sugar-sweetened beverages and increasing physical activity (Moore et al. 1995; DeBoer et al. 2013). To examine the feasibility of testing various doses of home visits by the health educators, we added a second intervention arm to the study that included two home visits with a health educator.
Study groups
Families (n = 44) were randomized to one of three groups: four home visits with a health educator, tailored emails, and mailed incentives (4HV; n = 17); two home visits with a health educator, tailored emails, and mailed incentives (2HV; n = 14); or general health advice through emails (control; n = 13).
Theoretical framework of the intervention
Our intervention is informed by both the Family Systems Theory (FST) and the Self-Determination Theory (SDT). The FST posits that families are systems of interconnected and interdependent individuals who must be understood (and intervened upon) as a system rather than as individuals (Ackerman 1958). Our intervention proposes to influence household routines using a whole family, home-based approach. The SDT posits that, as compared to behaviour change motivated by external factors, autonomously motivated (or self-determined) behaviour change is more likely to result in lasting change (Ryan 2000). As described by SDT, there are three psychological needs that influence level of autonomous motivation: (1) autonomy (feeling like there is a choice), (2) competence (feeling like they have the ability to make the change); and (3) relatedness (feeling connected to others). To promote autonomous motivation for behaviour change, our intervention uses motivational interviewing (MI), which is a collaborative, client-centered, counseling style that has been shown to foster these psychological needs (Resnicow and McMaster 2012).
Guelph Family Health Study intervention: 4HV and 2HV
Home visits were conducted by four health educators; all of the health educators were graduate students and Registered Dietitians who had 1 to 2 years of counseling experience. The health educators received a 2-day MI training from MI experts at the Monarch System™ and completed two evaluated practice MI sessions before leading home visits. Initial home visits typically lasted 1 h and began with health educators briefly describing the structure of the home visits. Health educators described behavioural goals of the study (e.g., limiting SSB consumption, engaging in family physical activity, establishing sleep routines to increase child sleep duration, limiting the children’s sedentary time, and having more family meals). Families then rated their current routines and behaviours from 1 (very unsatisfied) to 10 (very satisfied) using a health behaviour wheel that listed each of the target health behaviour goals. Health educators then asked families if they wanted to set any behaviour change goals. If families identified a behaviour change goal, health educators used MI techniques to work with families to identify specific steps required to implement the desired change and to discuss possible challenges and potential workarounds to address the identified challenges. Families were also asked if they wanted an accountability process for the behaviour change, i.e., to have the health educators email to check on progress. To facilitate self-monitoring of behaviour, families were provided a paper family routine tracker on which they could record their behaviour and identify possible facilitators or barriers to their behaviour change. If families did not choose to set a behaviour change goal, health educators acknowledged that families were not ready to make a change at that time and asked if families wanted any additional information or support regarding their family’s health behaviours.
Follow-up home visits typically lasted 30 to 60 minutes. For families that set a behaviour goal at the previous visit, follow-up visits involved health educators reviewing and discussing progress towards the goals that were previously set by the families and possible ideas for solutions to identified challenges. Families were asked if they wanted to set a new behaviour change goal or revise a previously established goal. If families did not set a goal at the previous home visit, the health educator had families rate their satisfaction with their current routines and behaviours again using the health behaviour wheel. Health educators then asked families if they wanted to set any behaviour change goals. Although timing varied somewhat due to the family’s schedules, home visits in both the 4HV and the 2HV groups were scheduled approximately 4–6 weeks apart.
Families were sent weekly emails that were tailored to the behaviour change goal set by the family. These emails included strategies to support behaviour change, e.g., indoor games to increase the children’s physical activity. Each month, families were mailed supports for behaviour change goals, e.g., the children’s book for bedtime. If families did not set a health behaviour goal, the weekly emails and mailed supports were sent per a standardized schedule.
Minimal-attention control
Families randomized to control received monthly emails containing publicly available handouts on general child health, e.g., current Canadian physical activity guidelines.
Measures
Our primary objective was to measure the feasibility and acceptability of the Guelph Family Health Study intervention and the RCT design within the Canadian context. The process evaluation measures used to assess this objective are described in Table 1.
Table 1.
Feasibility and acceptability measures and results for the Guelph Family Health Study pilot RCT study design and intervention
| Purpose | Variable | Assessment method | Results |
|---|---|---|---|
| Feasibility of study design | Recruitment | Recruitment activity log* | Promotion on the University website recruited the highest percentage of the participants (27%), followed by social media posts by our study partners (22%) and word of mouth (14%). |
| Outcome assessment | Study records* | All but two families (96% of the families) completed post-intervention health assessment visits and questionnaires. One family was lost to follow-up, and the other family opted out of the assessment due to family stressors. | |
| Acceptability of study design | Randomization | Comparison of follow-up rates in intervention and control* | The retention rate for families randomized to the 4HV intervention was 94% (16/17 families) and to the 2HV intervention was 100% (14/14 families). Retention in the control was 92% (12/13 families). |
| Feasibility of GFHS intervention | Implementation of health educator training |
Attendance log for trainings* MI evaluation of health educators† |
All health educators (n = 4) completed the 2-day motivational interviewing (MI) training provided by the Monarch System™. All health educators were evaluated on their motivational interviewing at the end of the 2-day training. All health educators were found to be competent in using motivational interviewing at the end of the training. |
| Fidelity of intervention |
Home visit log‡ Fidelity to motivational interviewing at home visits† Report of delivery of weekly emails from email software* Delivery of monthly mailouts* |
Health educators administered all home visits per study protocol within the 6-month intervention. MI experts, who were not involved in the intervention, reviewed audio recordings of 10% (n = 15) of the home visits and used the Motivational Interviewing Treatment Integrity (MITI) version 4.2.1 to assess MI fidelity (Hamilton et al. 2013). MI adherence was found to be 85.2%. All emails were delivered as per study protocol. All mailouts were delivered as per study protocol. |
|
| Implementation of intervention |
Completion of home visits log‡ Receipt of weekly emails from email software* Receipt of mailed incentives* |
All but one family (96%) received their home visits with a health educator as per the study protocol; the family was randomized to the 4HV group and declined all home visits. Sixty percent of the weekly emails were opened by intervention families. One hundred percent of the weekly mailings were received by intervention families. |
|
| Acceptability of GFHS intervention | Parent satisfaction | Post-intervention survey completed by families randomized to interventions (4HV and 2HV)§ |
One hundred percent of the families were satisfied with the study. One hundred percent of the families would recommend the GFHS to a friend or relative. Families reported that the health educator visits were the most helpful component of the GFHS in helping them create healthier routines (88% found the home visits useful or very useful). Incentives (mailed the children’s activities) were the second most helpful (66%), followed by the weekly emails (63%). Eleven families responded to the open-ended question about ways to improve the Guelph Family Health Study intervention. Four families suggested improving the emails by providing additional research and information in the emails. Sample quote: “Better emails. Maybe add a section at bottom ‘to take it further’ or something. Some link to study or scholarly research.” Three families requested more feedback on their families’ health behaviours. Sample quote: “I would be interested in feedback about monitoring/testing results.” Two families suggested improving the quality and seasonal appropriateness of the mailed incentives. Sample quote: “Fewer, higher quality ‘incentives’ mailed out.” One family identified that 2HV was too few to make an impact. Quote: “It would have been nice to have more than 2 home visits to discuss what was/wasn’t working.” One family suggested that the intervention should include ways to engage reluctant family members into the intervention. |
Completed by *study staff, †Monarch System™ staff, ‡GFHS health educator, and §parent participant
Table 2 summarizes the outcome measures used to examine the preliminary impact of the Guelph Family Health Study intervention on frequency of family meals; the children’s intake of fruit, vegetables, and SSBs; physical activity; sedentary behaviour; sleep; and adiposity. To minimize risk of bias, the staff who conducted measurements of weight-related behaviours and outcomes were not involved in the delivery of the intervention.
Table 2.
Description of the outcome measures used in the Guelph Family Health Pilot Study
| Outcome | Description of measure |
|---|---|
| Child percentage of fat mass |
Child body composition was measured before and after the intervention using whole-body, single-frequency, 50-kHz bioelectrical impedance analysis (BIA, Quantum IV—Body Composition Analyzer™, RJL Systems, Clinton Township, MI) per manufacturer protocol. Preparation for BIA required the children to have fasted and avoided vigorous physical activity for 30 min prior to the test and removed any clothing with metal pieces (e.g., pants with zipper). Children lay supine on a hospital bed, with head on a pillow, and with arms and legs abducted 30° from midline. Children were instructed to lie still until the test was complete (approx. 1 min). The equation of Kushner et al. was used to calculate TBW in litres as follows, where H is height in centimetres (cm), R is resistance in ohms (Ω), and W is weight in kilograms (kg) (Fomon et al. 1982): TBW = 0.593H2/R + 0.065W + 0.04 TBW was then divided by an age- and gender-specific hydration constant to determine the percentage of fat mass (Kushner et al. 1992; Frisancho 2004). |
|
Child dietary intake: Fruit, vegetable, and sugar-sweetened beverage |
The primary parent (the first parent to sign up for the study) completed 3-day food records (Burrows et al. 2010; Gibson 2005) for their children before and after the intervention. Parents were instructed on how to complete food records by the study coordinator. Parents were instructed to provide as much detail as possible in describing foods and beverages consumed by their children. Parents recorded information on timing, eating occasion, description, and amounts of foods and beverages consumed. Food record data were inputted into ESHA, The Food Processor version 11.0.110. (ESHA Research, Salem, OR, USA, 2016) using standard operating procedures for data input, checking, and export to ensure accuracy of reported data. Food records were analyzed using ESHA Food Processor (version 11.0.110) for 3-day average intakes of food group servings (vegetables and fruit). Research assistants reviewed food records to calculate 3-day average intakes of sugar-sweetened beverages (SSBs), including juice. |
| Physical activity and sedentary behaviour—accelerometry |
For assessment of physical activity (PA) and sedentary behaviour, ActiGraph GT3X accelerometers were used (Actigraph 2015). Parents were instructed to keep the accelerometer on the non-dominant wrist of their children for 24 h a day for 3–7 days, only removing it if the child was in water for a long period of time (e.g., swimming lessons). The ActiLife software was used as a data analysis platform. Within the ActiLife software, the accelerometer data was downloaded in 1-sec epochs. The Choi et al. (2007) algorithm was used to identify and remove any periods of non-wear time. Only subjects with a minimum of 360 min (6 h) of valid wear time for a minimum of 3 days were included in the analysis. Wear time was then divided into the different PA intensities (SED, LPA, and MVPA) using age-dependent cut points. For toddlers (less than 3 years old), the Trost et al. (2012) cut points were applied, and for preschoolers (3 years old or greater), the Butte et al. (2014) cut points were used. Data were presented in the form of percentage of wear time spent in each intensity (SED, LPA, MVPA) to account for variable wear times. Physical activity and sedentary behaviour variables include - Percent of wear time spent in sedentary (SED) - Percent of wear time spent in light PA (LPA) - Percent of wear time spent in moderate to vigorous PA (MVPA) |
| Sleep quantity |
Parents were instructed to keep the accelerometers on the non-dominant wrist of their children for 3–7 days, only removing it if the child was in water for a long period of time (e.g., swimming lessons). To determine sleep periods, the Sadeh and Tudor-Locke algorithms were applied to the raw sleep data in 1-min epochs. Participants were included if they had at least two consecutive nights of sleep data. To measure sleep duration, the software provides a “total sleep time” variable for each night and these values were averaged for each participant to provide average nighttime sleep duration. This value represents the total time the participant spends sleeping (in minutes), excluding the time they spend awake in bed (Buysse et al. 1989). |
| Family meal frequency | The primary parent reported frequency of family meals with a single item on the baseline and 6-month post-intervention questionnaire that assessed frequency that either parent ate a meal with their children over the past week. Response options were one to two times, three to four times, five to six times, and seven or more times. Due to high level of responses towards the upper end of the response options, we dichotomized this variable as ≥ 7 times per week and ≤ 6 times per week. |
Statistical analyses
To assess the feasibility and acceptability of the intervention and RCT design, we calculated frequencies for the attendance and recruitment data and for the close-ended questions from the parent post-intervention satisfaction surveys. To assess the preliminary impact of the intervention, we first performed univariate analyses of variables of interest to examine baseline distributions of characteristics by study group. In intent-to-treat complete case analyses, we used generalized estimating equation (GEE) models to examine differences in post-intervention weight-related behaviours and outcomes between the study groups after controlling for baseline (Liang and Zeger 1986). We used GEE to account for dependent observations within the families. For continuous measures, we used linear regression, and for dichotomous outcomes (family meals ≥ 7 times per week), we used logistic regression models. Analyses examining the intervention effect on child percentage of fat mass were adjusted for child age and sex. We performed all analyses using SAS University version 3.6 (Basic Edition).
Results
We randomized 44 families to the three study groups. Follow-up rates for the 4HV, the 2HV, and the control arms were 94, 100, and 93%, respectively (Fig. 1). Of the families who completed baseline and 6-month assessment (n = 42), 34 (81%) had both mothers and fathers participate (75% 4HV, 79% 2HV, 93% control; Table 3). The majority of parents were married or living with a partner (94% 4HV, 93% 2HV, 100% control) and identified as white (82% 4HV, 76% 2HV, 70% control). Approximately 30% of the families reported total household incomes of less than US$59,999/year. Nearly 50% of the mothers and 70% of the fathers were classified as overweight or obese (BMI ≥ 25 kg/m2). The mean age of the children was 3.0 (1.2) years, and 32% of the children were classified as overweight (6%) or at risk of overweight (26%) based on World Health Organization criteria (de Onis et al. 2006). None of the child participants were classified as obese.
Table 3.
Baseline characteristics of participants in the Guelph Family Health Study Pilot overall and by the study group
| Overall, 42 families n (%) |
Intervention 4HV, 16 families n (%) |
Intervention 2HV, 14 families n (%) |
Control, 12 families n (%) |
|
|---|---|---|---|---|
| Relation to child, n = 76 | ||||
| Mother | 42 (100.0) | 16 (100.0) | 14 (100.0) | 12 (100.0) |
| Father | 34 (81.0) | 12 (75.0) | 11 (78.6) | 11 (92.7) |
| Maternal marital status, n = 42 | ||||
| Married or living with partner | 40 (95.2) | 15 (93.7) | 13 (92.8) | 12 (100.0) |
| Parental race/ethnicity, n = 76 | ||||
| White | 58 (73.4) | 23 (82.1) | 19 (76.0) | 16 (69.6) |
| Other: Chinese; Latin American; South Asian | 18 (23.7) | 5 (17.9) | 6 (24.0) | 7 (30.4) |
| Mother weight status, n = 42 | ||||
| Normal weight, BMI 18.5 to < 25.0 | 20 (47.6) | 6 (37.5) | 9 (64.3) | 5 (41.7) |
| Overweight, BMI 25.0 to < 30.0 | 11 (26.2) | 4 (25.0) | 3 (21.4) | 4 (33.3) |
| Obese, BMI ≥ 30.0 | 9 (21.4) | 5 (31.3) | 2 (14.3) | 2 (16.7) |
| Father weight status, n = 33** | ||||
| Normal weight, BMI 18.5 to < 25.0 | 9 (26.5) | 2 (16.7) | 4 (36.4) | 3 (27.3) |
| Overweight, BMI 25.0 to < 30.0 | 11 (29.4) | 4 (33.3) | 3 (27.3) | 4 (36.4) |
| Obese, BMI ≥ 30.0 | 13 (41.2) | 5 (41.7) | 4 (36.4) | 4 (36.4) |
| Total household income | ||||
| Less than US$59,999 | 12 (28.6) | 4 (25.0) | 4 (28.6) | 4 (33.3) |
| US$60,000 to US$99,999 | 13 (31.0) | 5 (31.3) | 5 (35.7) | 3 (25.0) |
| Greater or equal to US$100,000 | 16 (38.1) | 6 (37.5) | 5 (35.7) | 5 (41.7) |
| Child characteristics, n = 53 | ||||
| Sex, female | 28 (52.8) | 10 (52.6) | 10 (58.8) | 8 (47.1) |
| Age, mean years (SD) | 3.00 (1.2) | 2.70 (1.1) | 3.29 (1.1) | 3.06 (1.2) |
| Child weight status per World Health Organization criteria (Mindell et al. 2010), n = 52** | ||||
| Normal weight | 35 (66.0) | 14 (73.7) | 13 (76.5) | 8 (47.1) |
| Risk of overweight/overweight* | 17 (32.6) | 5 (26.3) | 4 (23.5) | 8 (47.1) |
*Risk of overweight and overweight categories combined due to small numbers in some cells
**Numbers vary slightly due to missing data in the outcome measures
Feasibility and acceptability
Table 1 outlines feasibility and acceptability outcomes for the study design and the Guelph Family Health Study intervention. We enrolled 44 families into the study with promotion on the University website (recruited 27% of the participants) and social media posts by our study partner, the Ontario Early Years Centre (recruited 22% of the participants) being the most successful strategies for recruitment. The retention rates were high among families randomized to the intervention arms (94% 4HV and 100% 2HV) and the control arm (92%), suggesting that the randomization process was acceptable to participants. The completion rate for the evaluation procedure was similarly high, suggesting feasibility of the evaluation protocol.
All health educators (n = 4) attended the 2-day motivational interview training and were found to be competent at MI techniques by the end of the training. The health educators’ fidelity to MI at the home visits was 85.2%, based on assessment using the Motivational Interviewing Treatment Integrity (MITI) version 4.2.1 (Moyers et al. 2014).
Among the 31 families randomized to the intervention arms (17 to 4HV and 14 to 2HV), all but 1 family completed all home visits as per study protocol; 1 family who was randomized to receive 4HV declined all home visits. All families received the weekly mailings and 60% of the intervention emails were opened by the recipient.
Twenty-seven parents randomized to the intervention groups completed the post-intervention survey. All of these parents reported that they would recommend the study to a friend or family member. The home visits were rated the most useful aspect of the intervention with 88% of the respondents reporting the home visits were useful in helping them create healthier family routines. Sixty-six percent of the families identified that the mailed incentives were useful and 63% identified that the emails were useful in helping them create healthier routines. Eleven families provided recommendations to improve the intervention, including the emails and incentives, in the open-ended questions on the post-intervention survey (Table 1).
Preliminary impact
Table 4 shows the children’s behavioural and body composition outcomes by study group at baseline and 6-month follow-up. Family meal frequency by the study group at baseline and 6-month follow-up is shown in Table 5. At 6 months, we observed an intervention effect for child adiposity (% fat mass) among children in the 2HV group compared to the control (− 3.54%; 95% CI − 6.11, − 0.97). We also observed significant intervention effects for fruit intake among children in the 4HV (0.92 cups/day; 95% CI 0.33, 1.51) and the 2HV (0.68 cups/day; 95% CI 0.18, 1.17) group compared to the control. There was no significant intervention effect for frequency of family meals, the children’s vegetable or SSB intake, the children’s physical activity, sedentary behaviour, and sleep duration.
Table 4.
Intervention effect on body fat and behavioural outcomes among children participating in the Guelph Family Health Pilot Study (n = 53)
| Baseline mean (SD) | 6 months mean (SD) | Change mean (SD) | Effect β (95% CI) | P value | |
|---|---|---|---|---|---|
| Fat mass (%) | |||||
| 4HV | 31.72 (3.67) | 28.82 (3.44) | − 2.90 (3.24) | − 1.30 (− 3.76, 1.17) | 0.30 |
| 2HV | 29.16 (4.13) | 24.20 (4.47) | − 4.96 (2.58) | − 3.54 (− 6.11, − 0.97) | 0.01 |
| Control | 31.50 (4.65) | 28.68 (5.74) | − 2.82 (3.35) | ||
| Fruit intake (cups/day) | |||||
| 4HV | 1.89 (1.29) | 2.41 (1.40) | 0.52 (1.09) | 0.92 (0.33, 1.51) | 0.00 |
| 2HV | 1.80 (1.46) | 2.12 (0.92) | 0.32 (1.09) | 0.68 (0.18, 1.17) | 0.01 |
| Control | 1.74 (0.93) | 1.41 (0.66) | − 0.33 (0.79) | ||
| Vegetable intake (cups/day) | |||||
| 4HV | 0.61(0.70) | 1.63 (1.59) | 0.88 (1.93) | − 0.31 (− 1.77, 1.16) | 0.68 |
| 2HV | 0.84 (1.16) | 2.00 (3.19) | 1.83 (2.81) | 0.36 (− 1.34, 2.06) | 0.68 |
| Control | 0.62 (0.70) | 1.75 (2.07) | 1.14 (1.86) | ||
| SSB and juice (servings/day) | |||||
| 4HV | 1.94 (1.68) | 2.53 (2.10) | 0.59 (2.69) | 0.20 (− 1.57, 1.97) | 0.30 |
| 2HV | 1.87 (1.60) | 1.53 (1.46) | − 0.33 (1.50) | − 0.75 (− 2.17, 0.67) | 0.82 |
| Control | 2.93 (3.79) | 2.93 (4.16) | 0.00 (3.04) | ||
| Sleep duration (min/night) | |||||
| 4HV | 422.92 (97.97) | 417.64 (112.03) | − 5.28 (151.24) | − 19.42 (− 121.94, 83.10) | 0.71 |
| 2HV | 450.91 (125.58) | 470.16 (157.78) | 19.24 (127.29) | 22.21 (− 79.54, 123.96) | 0.67 |
| Control | 376.16 (74.83) | 418.88 (159.11) | 42.72 (175.91) | ||
| MVPA (% of the total daily activity) | |||||
| 4HV | 13.89 (5.41) | 13.10 (2.81) | − 0.79 (5.77) | 0.65 (− 2.08, 3.38) | 0.64 |
| 2HV | 13.50 (3.20) | 12.98 (4.42) | − 0.52 (3.51) | 0.64 (− 2.51, 3.80) | 0.69 |
| Control | 14.84 (4.95) | 12.75 (3.87) | − 2.09 (3.94) | ||
| LPA (% of the total daily activity) | |||||
| 4HV | 15.74 (7.65) | 21.49 (7.13) | 5.74 (7.76) | 1.06 (− 4.31, 6.43) | 0.70 |
| 2HV | 21.76 (7.63) | 22.46 (7.11) | 0.70 (4.93) | − 1.33 (− 6.07, 3.41) | 0.58 |
| Control | 18.25 (8.25) | 21.83 (6.46) | 3.58 (6.16) | ||
| Sedentary time (% daily of total activity) | |||||
| 4HV | 69.16 (5.45) | 65.41 (5.58) | − 3.76 (6.24) | − 1.46 (− 4.85, 1.94) | 0.40 |
| 2HV | 64.74 (5.43) | 64.21 (6.31) | − 0.53 (3.58) | 0.17 (− 2.90, 3.24) | 0.91 |
| Control | 66.91 (5.58) | 65.43 (4.15) | − 1.49 (3.39) | ||
Numbers vary slightly due to missing data in the outcome measures. Estimates are italicized when the confidence interval does not include zero. Adjusted for child sex and age
Table 5.
Intervention effect on the frequency of family meals among families participating in the Guelph Family Health Pilot Study (n = 42)
| Baseline n (%) |
6 months n (%) |
Odds ratio OR (95% CI) |
P value | ||
|---|---|---|---|---|---|
| Frequency of family meals | |||||
| 4 HV | 7 or more | 9 (64.29) | 8 (57.14) | 0.36 (0.04, 3.13) | 0.66 |
| 2HV | 7 or more | 7 (50) | 6 (42.86) | 0.28 (0.03, 2.32) | 0.38 |
| Control | 7 or more | 4 (36.36) | 6 (54.55) | ||
Numbers vary slightly due to missing data in the outcome measures
Discussion
In this 6-month pilot RCT, we found that a home-based intervention using tailored family-level motivational coaching focused on improving household routines related to the children’s eating, physical activity, sedentary time, and sleep was feasible for implementation and acceptable to Canadian families with young children. Our preliminary impact results suggest that this home-based intervention approach may also improve the children’s dietary intake and adiposity.
We enrolled 44 families to participate in this RCT and found that online strategies were the most successful, contributing to 49% of the recruited families. Our results are similar to those from an Australian-based RCT of an mHealth infant feeding intervention targeting expectant mothers and mothers with infants, which found that, compared to practitioner referral and in-person recruitment, online recruitment methods, in particular advertisements on parenting-related Facebook pages, were the quickest, least expensive, and most effective recruitment method (Laws et al. 2016). Survey data from Canada and the USA suggest that use of social media sites, in particular Facebook, is high among parents (Consumer Lifestyle Marketing and Promotion Market Research 2015; Duggan et al. 2015). Thus, social media platforms are an important and cost-effective avenue for engaging parents in future health promotion interventions.
Our results indicate that our home-based intervention approach was feasible to implement with all but one family completing all home visits per the study protocol. This level of engagement is higher than typically found in family-based obesity-prevention interventions delivered in community settings (Ostbye et al. 2012; Haines et al. 2016). By meeting parents in their home, our intervention circumvents many of the logistical issues that parents identify as barriers to attendance at community-based sessions, including transportation, childcare, time constraints, and competing demands (Heinrichs et al. 2005). Our results suggest this home-based approach was feasible and well accepted by families. Approximately 88% of the families in both the 4HV and the 2HV intervention groups reported on the post-intervention survey that the home visits were useful or very useful in supporting behaviour change, suggesting that both “doses” of home visits were well accepted. However, one family who received 2HV commented that more visits would have been beneficial. Identifying the ideal “dose,” with regard to both acceptability and cost-effectiveness, is an important goal for future research examining this home-based approach.
While the completion rate for home visits was high, the open-rate for the intervention emails was only 60%. On the post-intervention survey, 63% of the intervention parents rated the emails as useful or very useful in helping them create healthier home routines. Feedback on the emails suggests that parents felt the emails did not provide sufficient information to be useful and that some parents were interested to know the research behind the information provided. We designed the emails to be visually appealing, mobile-friendly, and brief in response to findings that many parents of young children report having numerous competing demands for their time (Heinrichs et al. 2005). To balance the parents’ expressed desire for more information with time constraints associated with this stage of life, future emails could include a “want to know more” section for those parents keen to do a more in-depth exploration of a particular topic.
While this pilot was not designed as a fully powered study, our results provide some preliminary evidence that, compared to a minimal-attention control, our home-based intervention may lead to improvements in the children’s adiposity and dietary intake. We found a significant intervention effect among children in the 2HV group compared to control. This finding is counter to the majority of intervention research, which suggests that a higher intervention dose results in improved outcomes (Hansen et al. 2002; Jørgensen et al. 2005). Given our small sample size, it is possible that this difference in the percentage of fat mass across groups is due to random error. An adequately powered trial is needed to test the effectiveness of various doses of this home-based approach. The difference in sleep duration across study groups may also explain this difference in the percentage of fat mass; at 6-month follow-up, children randomized to the 2HV group were sleeping 1 h more per night, on average, than those randomized to the other two study groups. An additional hour of sleep during the preschool years has been shown to be associated with lower levels of body fat cross-sectionally and in later childhood (Carter et al. 2011). Our results support the existing evidence of the important impact sleep may have on the children’s obesity risk and underscore the need to identify effective strategies to support healthy routines at home to increase the children’s sleep duration.
Our results suggest our home-based intervention may improve the children’s dietary intake, in particular, their fruit intake, but had little impact on the children’s physical activity or sedentary behaviour. These results may suggest that the families’ motivation for change was higher for dietary behaviours as compared to their children’s physical activity and sedentary behaviour. It is also possible that our intervention was not intensive enough to effect change across all of these behaviours. The Healthy Habits, Happy Homes intervention, which focused on three key behaviours-sleep, family meals, and sedentary behaviour-did significantly impact the children’s sleep and the children’s weekend screen time, as assessed by parent-report (Haines et al. 2013). The addition of physical activity and SSBs as target behaviours in this intervention may have diluted the behaviour change messages. While not possible with the small sample size in the current study, a larger trial of this tailored family-level motivational coaching approach could examine intervention outcomes by specific behaviour change goals set by the family to help elucidate if particular behaviour targets lead to improved outcomes for families.
Limitations should be considered when interpreting our results. First, our self-selection recruitment method may have led to systematic differences between participants and non-participants. Thus, it is unclear how our results would generalize to those who did not take up our intervention. Second, it is also unclear how our results would generalize to racial/ethnic minority populations as the majority of participants in our sample identified as being white. However, previous studies suggest this home-based approach is feasible among Aboriginal families in Canada and African-American and Latino families in the USA (Haines et al. 2013; Anand et al. 2007). Finally, the small sample size was insufficient to test the impact of the intervention on child behavioural and weight outcomes; a full-scale trial is needed to test the effectiveness of this home-based approach.
Conclusion
Our home-based intervention and the RCT design were feasible and acceptable to Canadian families with young children. Our home-based intervention significantly increased the children’s fruit intake and decreased their percentage of fat mass immediately post-intervention. A full-scale trial that includes a longer follow-up period is needed to determine the long-term impact of this home-based intervention approach. Future research should also examine the costs and cost-effectiveness of this home-based approach.
Compliance with ethical standards
The study protocol was approved by the University of Guelph Research Ethics Board (REB14AP008 and REB14AP009).
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Ackerman NW. The psychodynamics of family life. New York: Basic Books; 1958. [Google Scholar]
- Actigraph. (2015). Actilife 6. Retrieved from: http://www.actigraphcorp.com/products/actilife-6/.
- Anand SS, Davis AD, Ahmed R, Xie C, Hill A, Sowden J, et al. A family-based intervention to promote healthy lifestyles in an aboriginal community in Canada. Canadian Journal of Public Health. 2007;98(6):447–452. doi: 10.1007/BF03405436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkin S, Ip E, Richardson I, Klinepeter S, Finch S, Krcmar M. Parental media mediation styles for children aged 2 to 11 years. Archives of Pediatrics & Adolescent Medicine. 2006;160(4):395–401. doi: 10.1001/archpedi.160.4.395. [DOI] [PubMed] [Google Scholar]
- Burrows TL, Martin RJ, Collins CE. A systematic review of the validity of dietary assessment methods in children when compared with the method of doubly labeled water. Journal of the American Dietetic Association. 2010;110(10):1501–1510. doi: 10.1016/j.jada.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Butte NF, Wong WW, Lee JS, Adolph AL, Puyau MR, Zakeri IF. Prediction of energy expenditure and physical activity in preschoolers. Medicine and Science in Sports and Exercise. 2014;46(6):1216–1226. doi: 10.1249/MSS.0000000000000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Research. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Carter PJ, Taylor BJ, Williams SM, Taylor RW. Longitudinal analysis of sleep in relation to BMI and body fat in children: the FLAME study. BMJ. 2011;342:d2712. doi: 10.1136/bmj.d2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaput JP, Janssen I. Sleep duration estimates of Canadian children and adolescents. Journal of Sleep Research. 2016;25(5):541–548. doi: 10.1111/jsr.12410. [DOI] [PubMed] [Google Scholar]
- Choi L, Capen Ward S, Schnelle JF, Buchowski MS. Assessment of wear/nonwear time classification algorithms for triaxial accelerometer. Medicine and Science in Sports and Exercise. 2007;44(10):2009–2016. doi: 10.1249/MSS.0b013e318258cb36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consumer Lifestyle Marketing and Promotion Market Research. (2015). Social Media Trends—Canada—June 2015. London, England: Mintel.
- de Onis, M., Garza, C., Onyango, A. W., & Martorell, R. (Eds.). (2006). WHO child growth standards. Acta Paediatrica, 95(suppl.450), 5–101.
- de Ruyter JC, Olthof MR, Seidell JC, Katan MB. A trial of sugar-free or sugar-sweetened beverages and body weight in children. The New England Journal of Medicine. 2012;367(15):1397–1406. doi: 10.1056/NEJMoa1203034. [DOI] [PubMed] [Google Scholar]
- DeBoer MD, Scharf RJ, Demmer RT. Sugar-sweetened beverages and weight gain in 2- to 5-year-old children. Pediatrics. 2013;132(3):413–420. doi: 10.1542/peds.2013-0570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan, M., Lenhart, A., Lamp, C., Ellison, N., & Pew Research Centre. (2015). [2016-02-23]. Parents and social media http://www.pewinternet.org/2015/07/16/parents-and-social-media/webcite.
- Fomon SJ, Haschke F, Ziegler EE, Nelson SE. Body composition of reference children from birth to age 10 years. The American Journal of Clinical Nutrition. 1982;35:1169–1175. doi: 10.1093/ajcn/35.5.1169. [DOI] [PubMed] [Google Scholar]
- Frisancho AR. Anthropometric standards for the assessment of growth and nutritional status. Ann Arbor: The University of Michigan Press; 2004. [Google Scholar]
- Garriguet D. Nutrition: findings from the Canadian Community Health Survey—overview of Canadians’ eating habits. Health Statistics Division: Ottawa; 2004. [Google Scholar]
- Garriguet D, Carson V, Colley RC, Janssen I, Timmons BW, Tremblay MS. Physical activity and sedentary behaviour of Canadian children aged 3 to 5. Health Reports. 2016;27:14–23. [PubMed] [Google Scholar]
- Giannotti F, Cortesi F. Family and cultural influences on sleep development. Child and Adolescent Psychiatric Clinics of North America. 2009;18(4):849–861. doi: 10.1016/j.chc.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Gibson RS. Principles of nutritional assessment. 2. New York: Oxford University Press; 2005. [Google Scholar]
- Haines J, McDonald J, O’Brien A, Sherry B, Bottino CJ, Schmidt ME, Taveras EM. Healthy habits, happy homes: randomized controlled trial to improve household routines for obesity prevention among preschool aged children. JAMA Pediatrics. 2013;167(11):1072–1079. doi: 10.1001/jamapediatrics.2013.2356. [DOI] [PubMed] [Google Scholar]
- Haines J, Rifas-Shiman SL, Gross D, McDonals J, Kleinman K, Gillman MW. Randomized trial of an obesity prevention intervention that embeds weight-related messages within a general parenting program. Obesity (Silver Spring) 2016;24(1):191–199. doi: 10.1002/oby.21314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton K, Thomson CE, White KM. Promoting active lifestyles in young children: investigating mothers’ decisions about their child’s physical activity and screen time behaviours. Maternal and Child Health Journal. 2013;17(5):968–976. doi: 10.1007/s10995-012-1081-0. [DOI] [PubMed] [Google Scholar]
- Hansen NB, Lambert MJ, Forman EM. The psychotherapy dose-response effect and its implications for treatment delivery services. Clinical Psychology: Science and Practice. 2002;9(3):329–343. [Google Scholar]
- Heinrichs N, Bertram H, Kuschel A, Hahlweg K. Parent recruitment and retention in a universal prevention program for child behavior and emotional problems: barriers to research and program participation. Prevention Science. 2005;6(4):275–286. doi: 10.1007/s11121-005-0006-1. [DOI] [PubMed] [Google Scholar]
- Hingle M, O'Connor T, Dave J, Baranowski T. Parental involvement in interventions to improve child dietary intake: a systematic review. Preventive Medicine. 2010;51(2):103–111. doi: 10.1016/j.ypmed.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen TS, Rasmussen M, Aarestrup AK, Ersbøll AK, Jørgensen SE, Goodman E, et al. The role of curriculum dose for the promotion of fruit and vegetable intake among adolescents: results from the Boost intervention. BMC Public Health. 2005;15(536):1–12. doi: 10.1186/s12889-015-1840-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner RF, Schoeller DA, Fjeld CR, Danford L. Is the impedance index (ht2/R) significant in predicting total body water? The American Journal of Clinical Nutrition. 1992;56:835–839. doi: 10.1093/ajcn/56.5.835. [DOI] [PubMed] [Google Scholar]
- Laws RA, Litterbach EK, Denney-Wilson EA, Russell CG, Taki S, Ong KL, et al. A comparison of recruitment methods for an mhealth intervention targeting mothers: lessons from the growing healthy program. Journal of Medical Internet Research. 2016;18(9):e248. doi: 10.2196/jmir.5691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13–22. doi: 10.1093/biomet/73.1.13. [DOI] [Google Scholar]
- Miller AL, Lumeng JC, LeBourgeois MK. Sleep patterns and obesity in childhood. Current Opinion in Endocrinology, Diabetes, and Obesity. 2015;22(1):41–47. doi: 10.1097/MED.0000000000000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindell JA, Sadeh A, Kohyama J, Howd TH. Parental behaviors and sleep outcomes in infants and toddlers: a cross-cultural comparison. Sleep Medicine. 2010;11(4):393–399. doi: 10.1016/j.sleep.2009.11.011. [DOI] [PubMed] [Google Scholar]
- Mirotta, J. A., Darlinton, G., Haines, J., Ma, D. W. L., & Duncan, A. M. (2018). Health study’s home-based obesity prevention intervention increases fibre and fruit intake in preschool-aged children. Canadian Journal of Dietetic Practice and Research. [DOI] [PubMed]
- Moore LL, Nguyen US, Rothman KJ, Cupples LA, Ellison RC. Preschool physical activity level and change in body fatness in young children: the Framingham Children’s Study. American Journal of Epidemiology. 1995;142(9):982–988. doi: 10.1093/oxfordjournals.aje.a117747. [DOI] [PubMed] [Google Scholar]
- Moyers, T. B., Manuel, J. K., & Ernst, D. (2014). Motivational interviewing treatment integrity coding manual 4.2.1. Unpublished manual. Retrieved from http://casaa.unm.edu/download/MITI4_2.pdf.
- O’Kane Carley, Wallace Angela, Wilson Laura, Annis Angela, Ma David W.L., Haines Jess. Family-Based Obesity Prevention: Perceptions of Canadian Parents of Preschool-Age Children. Canadian Journal of Dietetic Practice and Research. 2018;79(1):13–17. doi: 10.3148/cjdpr-2017-027. [DOI] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine (2016). Obesity in the early childhood years: state of the science and implementation of promising solutions: Workshop Summary. Washington: The National Academies Press. [PubMed]
- Ostbye T, Krause KM, Stroo M, Lovelady CA, Evenson KR, Peterson BL. Parent-focused change to prevent obesity in preschoolers: results from the KAN-DO study. Preventive Medicine. 2012;55(3):188–195. doi: 10.1016/j.ypmed.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick H, Nicklas TA, Hughes SO, Morales M. The benefits of authoritative feeding style: caregiver feeding styles and children's food consumption patterns. Appetite. 2005;44(2):243–249. doi: 10.1016/j.appet.2002.07.001. [DOI] [PubMed] [Google Scholar]
- Poitras VJ, Gray CE, Janssen X, Aubert S, Carson V, Faulkner G, Tremblay MS. Systematic review of the relationships between sedentary behaviour and health indicators in the early years (0–4 years) BMC Public Health. 2017;17(Suppl 5):868. doi: 10.1186/s12889-017-4849-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnicow K, McMaster F. Motivational interviewing: moving from why to how with autonomy support. International Journal of Behavioral Nutrition and Physical Activity. 2012;9:19. doi: 10.1186/1479-5868-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan RM. Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being. The American Psychologist. 2000;55(1):68–78. doi: 10.1037/0003-066X.55.1.68. [DOI] [PubMed] [Google Scholar]
- Savage JS, Birch LL, Marini M, Anzman-Frasca S, Paul IM. Effect of the INSIGHT responsive parenting intervention on rapid infant weight gain and overweight status at age 1 year: a randomized clinical trial. JAMA Pediatrics. 2016;170(8):742–749. doi: 10.1001/jamapediatrics.2016.0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields M, Tremblay MS. Canadian childhood obesity estimates based on WHO, IOTF and CDC cut-points. International Journal of Pediatric Obesity. 2010;5(3):265–273. doi: 10.3109/17477160903268282. [DOI] [PubMed] [Google Scholar]
- Singer MR, Moore LL, Garrahie EJ, Ellison RC. The tracking of nutrient intake in young children: the Framingham Children’s Study. American Journal of Public Health. 1995;85(12):1673–1677. doi: 10.2105/AJPH.85.12.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trost SG, Fees BS, Haar SJ, Murray AD, Crowe LK. Identification and validity of accelerometer cut-points for toddlers. Obesity. 2012;20(11):2317–2319. doi: 10.1038/oby.2011.364. [DOI] [PubMed] [Google Scholar]
- Wen LM, Baur LA, Simpson JM, Rissel C, Wardle K, Flood VM. Effectiveness of home based early intervention on children’s BMI at age 2: randomised controlled trial. BMJ. 2012;344:e3732. doi: 10.1136/bmj.e3732. [DOI] [PMC free article] [PubMed] [Google Scholar]


