Abstract
Objectives
TNM stage is the preeminent cancer staging system and a fundamental determinant of disease prognosis. Our goal was to evaluate the predictive power of TNM stage for gastric adenocarcinoma (GAC), in a low-incidence country.
Methods
A province-wide chart review of GAC patients diagnosed from April 1, 2005 to March 31, 2008 was conducted in Ontario and linked to routinely collected vital status data with a follow-up on March 31, 2012. TNM staging was classified using the sixth and seventh Union International for Cancer Control/American Joint Committee on Cancer editions. Kaplan-Meier and log-rank tests compared stage-stratified survival estimates. Discrimination was evaluated using Harrell’s C statistic.
Results
The cohort included 2366 patients. One- and 5-year survival was 43% and 17%. Using the sixth edition, 9% of patients had stage I disease, 5.4% stage II, 7.3% stage III, and 64% stage IV; 15% were not staged. Using the seventh edition, 9% were stage I, 7.7% stage II, 16% stage III, and 54% stage IV; 14% were not staged. Stage-stratified 5-year survival ranged from 68% to 7% with the sixth edition and from 70% to 4% with the seventh edition. Harrell’s C statistic was 0.64 (0.63–0.65) for the broad sixth edition staging categories and 0.68 (0.67–0.69) for the broad seventh edition. Discriminative power was similar for the refined stage categories and across multiple subgroup analyses; it was best in non-metastatic patients.
Conclusion
Existing staging systems for GAC used in North America predict individualized prognosis poorly. The creation of a more complex prediction tool is necessary to provide accurate and precise prognostication information to oncologists, patients, and their families.
Electronic supplementary material
The online version of this article (10.17269/s41997-018-0102-1) contains supplementary material, which is available to authorized users.
Keywords: Gastric adenocarcinoma, TNM stage, Prognosis
Résumé
Objectifs
La classification TNM est le principal système de stadification du cancer, en plus d’être un déterminant fondamental du pronostic de la maladie. Nous avons cherché à évaluer la capacité prédictive de la stadification TNM pour l’adénocarcinome gastrique (ACG) dans un pays à faible incidence.
Méthode
Un examen des dossiers médicaux des patients ayant reçu un diagnostic d’ACG entre le 1er avril 2005 et le 31 mars 2008 a été mené à l’échelle de l’Ontario et maillé aux données sur le statut vital systématiquement recueillies, avec suivi jusqu’au 31 mars 2012. Le stade TNM a été déterminé selon les 6e et 7e éditions de la classification de l’UICC/AJCC. Les estimations de survie stratifiées selon le stade ont été comparées à l’aide des tests de Kaplan-Meier et du log-rank. La discrimination a été évaluée à l’aide de l’indice C de Harrell.
Résultats
La cohorte était constituée de 2 366 patients. Le taux de survie après un et cinq ans était de 43 % et de 17 %, respectivement. Selon la 6e édition, 9 % des patients avaient un cancer de stade I, 5,4 % de stade II, 7,3 % de stade III et 64 % de stade IV; pour 15 %, le stade était indéterminé. Selon la 7e édition, 9 % des patients avaient un ACG de stade I, 7,7 % de stade II, 16 % de stade III et 54 % de stade IV; pour 14 %, le stade était indéterminé. La survie après cinq ans stratifiée selon le stade variait entre 68 % et 7 % selon la 6e édition et entre 70 % et 4 % selon la 7e édition. L’indice C de Harrell était de 0,64 (0,63-0,65) pour les catégories de stadification générales de la 6e édition et de 0,68 (0,67-0,69) pour les catégories générales de la 7e édition. La capacité de discrimination était semblable pour les catégories de stadification détaillées et selon de nombreuses analyses par sous-groupes; elle était la plus efficace pour les patients ayant un ACG non métastatique.
Conclusions
Le système de stadification existant de l’ACG utilisé en Amérique du Nord est inefficace pour offrir un pronostic individuel. Il est nécessaire de créer un outil de prédiction plus complexe pour fournir des pronostics exacts et précis aux oncologues, aux patients et aux familles.
Mots-clés: Adénocarcinome gastrique, Stade de la tumeur, Pronostic
Introduction
Gastric cancer, specifically adenocarcinoma, is one of the five most commonly diagnosed cancers in the world for both men and women and a leading cause of cancer-related mortality (Siegel et al. 2016; Canadian Cancer Society 2017). The highest incidence is recorded in Eastern Asia, Central and Eastern Europe, and South America, contrasting with a much lower incidence in North America (Siegel et al. 2016; Canadian Cancer Society 2017). Each year, it is responsible for over 700,000 deaths (Siegel et al. 2016; Canadian Cancer Society 2017). In North America, the UK, and other low-incidence countries, 40–60% of patients are diagnosed with metastatic disease at first presentation (Howlader et al. 2017; Coburn et al. 2010; National Cancer Registration and Analysis Service 2016; Northern Ireland Cancer Registry 2016; ISD Scotland 2016).
The tumor-node-metastasis (TNM) staging classification system for gastric adenocarcinoma is the most commonly used of two international staging systems and has undergone many revisions from the sixth to the seventh editions to the eighth edition (American Joint Committee on Cancer 2002, 2012, 2017). However, changes to the staging system may not reflect improvements in the prognostic capacity of TNM stage using appropriate performance metrics for prediction models (Collins et al. 2015). Validations of predictive performance lack the appropriate statistical approach and are performed in high-incidence countries or in small case series (Marrelli et al. 2012; Reim et al. 2013; Patel et al. 2013; McGhan et al. 2012; Zhang et al. 2014). Therefore, the purpose of this study was to evaluate the predictive performance of TNM stage for the first time in a large, population-based cohort of gastric adenocarcinoma (GAC) patients in Canada.
Methods
Study design and population
This was a retrospective cohort study of GAC patients diagnosed in Ontario, Canada. Ontario is Canada’s most populous province, with over 13 million inhabitants. Ontario administers healthcare through a universal, single-payer system. The Ontario Cancer Registry (OCR) was used to identify incident cases of gastric cancer registered between April 1, 2005 and March 31, 2008 (ICD-9 151). Patients were included if they had a valid Ontario Health Insurance Plan (OHIP) number, had an adenocarcinoma histology, were 18 and older, and had no other primary cancer recorded in the OCR and if their diagnosis was not based on autopsy or death certificate only. This methodology has been used to identify GAC cases elsewhere (Coburn et al. 2010). GAC patients were excluded if a corresponding hospital chart could not be located, or if following chart review we determined the tumour was located primarily in the esophagus, or the histology was a non-adenocarcinoma.
Data sources and collection
A province-wide chart review was conducted at over 100 institutions between November 2009 and November 2011 (Mahar et al. 2015). Chart review data were collected by a specially trained, single physician abstractor across all sites (JVR). Data were collected from more than one hospital for 40% of patients and included information from multiple endoscopy, radiology, and pathology reports per patient. Additional abstraction of data from operative reports for each cancer-directed surgery was completed by a surgical resident in 2013 (MD). Esophagoduodenoscopy pathology reports from biopsies provided histopathological information on the tumour outside of anatomic stage, such as grade and signet ring cell histology, used to characterize disease behaviour and estimate prognosis.
The chart review data were linked to vital status data in 2013. The staging algorithm was developed, tested, and refined between 2014 and 2016. Death clearance data in the Registered Persons Database (RPDB) is provided to the Institute for Clinical Evaluative Sciences (ICES) by the Ontario Registrar General (ORG), the Canadian Institute for Health Information Discharge Abstract & Same Day Surgery Databases, the National Ambulatory Care Reporting System, the Continuing Care Reporting System, and the National Rehabilitation System. Although the ORG is the gold standard, death clearance data held by the RPDB linked to ICES data closely approximate death data at the MOHLTC from the ORG supplemented by Statistics Canada (Iron et al. 2008). At study initiation, this data source was more up to date than the ORG or OCR data holdings at ICES, which are updated annually (Iron et al. 2008).
Staging variables
Stage data were collected in the 180 days prior to the diagnosis date registered in the OCR, and in the 180 days following diagnosis or until the date of surgical resection, if performed. The following sources of information were used to determine T, N, and M categories: esophagoduodenoscopy, abdominal ultrasound, computed tomography (chest and abdominal/pelvic), chest x-ray, magnetic resonance imaging, positron emission tomography, endoscopic biopsy pathology, surgical resection pathology, endoscopic mucosal resection pathology operative reports, clinical notes. T, N, and M classification variables were separately estimated according to the source of the staging information (radiology, pathology, operative, clinical). Staging was classified separately according to the sixth and seventh editions of the Union International for Cancer Control (UICC)/American Joint Committee on Cancer (AJCC) staging system, which is used to classify adenocarcinomas of the stomach (American Joint Committee on Cancer 2002, 2012). The eighth edition had not been released at the time of data analysis, and implementation has been delayed until January 1, 2018 (American Joint Committee on Cancer 2017). The Collaborative Staging approach was followed to assign TNM stage using the available information. A summary of the three staging system editions is provided in the Supplementary Appendix (Table S1). TNM stage was classified as both the broad categories (stages I, II, III, IV), as well as the refined categories (e.g., IA, IIb, IIIc) for the sixth and seventh editions.
Overall survival
The primary outcome of this study death due to any cause was considered an event. Time survived was measured from the date of diagnosis to the recorded death date or the date of last contact with the healthcare system—whichever came first—for a maximum of 7 years (maximum follow-up date March 31, 2013). Date of last contact is determined within ICES using available outpatient and inpatient records to identify a date after which an individual no longer uses services or accesses the health system. This is used in disease cohorts to identify when a patient may have left the province and is thus lost to follow-up. Patients were censored if they did not experience the event (death) within this time period. A record of death in any of these databases was considered an event.
Statistical analyses
Stage-specific survival estimates were reported. One- and 5-year survival estimates and corresponding 95% confidence intervals were calculated using Kaplan-Meier methods. Differences in survival across TNM stage groupings were compared using the log-rank test. P values of < 0.05 were considered statistically significant. The predictive performance of the TNM staging classification editions was evaluated by investigating discrimination. Discrimination refers to the ability of the prognostic model (TNM stage) to accurately predict the event of interest (death). Harrell’s C statistic was used to measure discrimination for time-to-event outcomes (Steyerberg 2009). A C statistic of 0.5 indicates the prognostic model performs not better than chance, while a C statistic of 1.0 indicates perfect discrimination. We also estimated the Akaike information criterion (AIC) for each model to understand model fit and to facilitate direct comparisons among the different models and subgroups. Harrell’s C statistic and AIC values were computed using the Cox proportional hazards regression with TNM stage as the only variable. TNM stage was operationalized as a categorical variable using both the refined categories (e.g., stages IA, IIIb) and as a categorical variable for the broad categories (stages I, II, III, IV). Four separate models computed predictive performance across the sixth and seventh editions.
Four sensitivity analyses were performed. The first excluded patients with unknown stage disease. The second was restricted to the cohort of patients with non-gastroesophageal junction (GEJ) tumours to decrease heterogeneity in the patient population. Patients with esophageal cancer may have erroneously been classified as gastric adenocarcinoma, if the tumour crossed the GEJ. In addition, the staging classification systems have changed staging GEJ tumours as both esophageal and gastric adenocarcinomas across iterations. The third excluded patients who did not undergo surgical resection, to increase the likelihood that staging was performed using pathological data. The fourth excluded patients with metastatic disease (stage IV), where there may be more heterogeneity in survival within the single TNM staging category. All analyses were performed using SAS 9.4 Copyright 2008 (Cary, NC, USA). Cell sizes containing < 6 patients were suppressed in accordance with the privacy and confidentiality regulations of ICES and the Ontario Privacy Commissioner.
Results
We identified 2516 registered cases of GAC during the study period that met the study criteria. Hospital charts were located for 2491 patients, and cases in which the primary site was not GC (n = 61) and those with non-adenocarcinoma histology (n = 83) were excluded. The final cohort consisted of 2366 GAC patients. The median follow-up time was 9 months for the entire cohort and 60 months in censored patients; 18% were censored. One-year overall survival was 43%, and 5-year overall survival was 17%. Table 1 describes patient demographics and pathological information. More than 50% of patients were 70 years or older at diagnosis, and almost two thirds were male. Distal tumours were most commonly diagnosed, followed by tumours of the gastroesophageal junction. Histopathological details of the tumour, such as tumour grade and signet ring cell, were rarely documented on pathology reports; missing data ranged from 40% to 84% across these variables.
Table 1.
Cohort demographics (n = 2366)
| Variable | %* |
|---|---|
| Age (years) | |
| < 50 | 9.0 |
| 50–54 | 6.5 |
| 55–59 | 8.8 |
| 60–64 | 10.4 |
| 65–69 | 12.6 |
| 70+ | 52.6 |
| Sex | |
| Male | 64.8 |
| Tumour location | |
| Distal | 37.7 |
| Entire | 7.7 |
| GEJ | 25.6 |
| Middle | 16.3 |
| Proximal | 8.5 |
| Unknown | 4.2 |
| Grade** | |
| Well differentiated | 2.9 |
| Moderately well differentiated | 17.3 |
| Poorly differentiated | 38.0 |
| Undifferentiated | 1.2 |
| Unknown | 40.5 |
| Metaplasia** | |
| No | 6.0 |
| Yes | 17.7 |
| Unknown | 76.2 |
| Dysplasia** | |
| No | 6.6 |
| Yes | 9.1 |
| Unknown | 84.3 |
| Signet ring cell** | |
| No | 1.1 |
| Yes | 23.5 |
| Unknown | 75.4 |
*Percentages may not add up to 100 due to rounding
**Pathology data from OGD biopsy reports
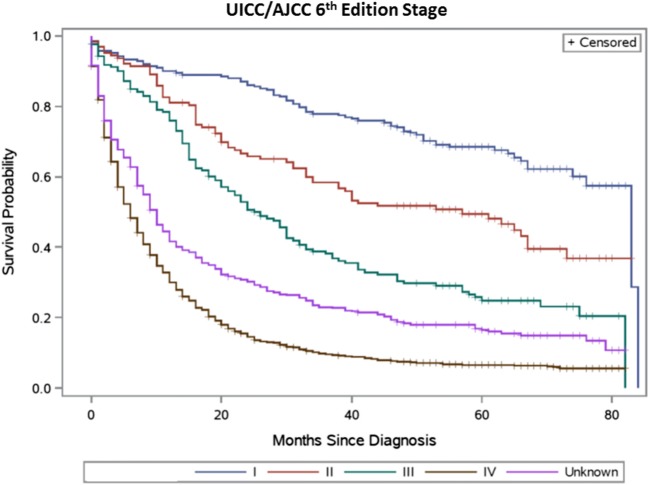
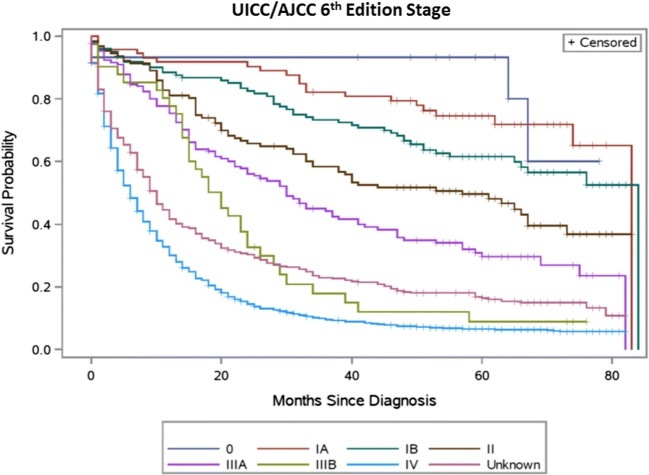
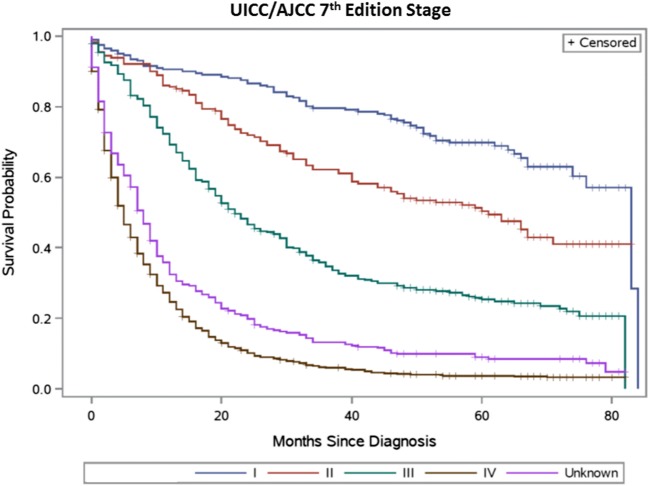
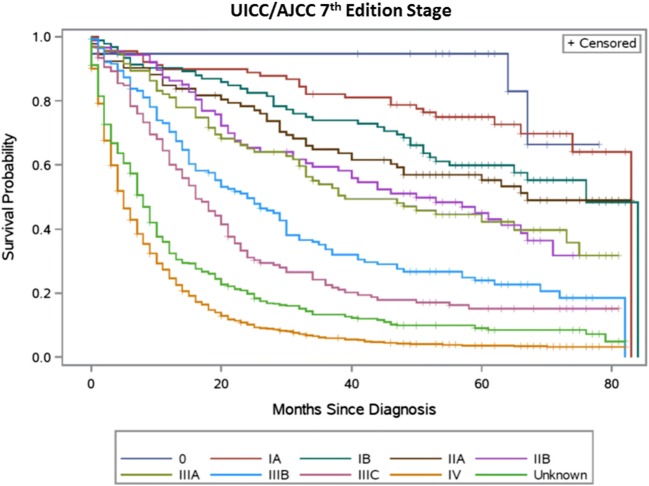
Table 2 summarizes the distribution of TNM stage groupings and observed survival for the sixth and seventh editions, respectively. Figures 1, 2, 3, and 4 present the survival distributions for each UICC/AJCC staging edition, respectively, for both the broad and refined stage categories. At any point in time, the probability of dying was significantly different across the refined and broad sixth edition TNM stage groupings (p < 0.0001) and both the refined and broad seventh edition stage groupings (p < 0.001). One- and 5-year survival decreased with increasing stage categories in both editions.
Table 2.
Distribution of TNM stage, 1- and 5-year overall survival probability by staging edition
| TNM stage* | % | 1-year survival | 5-year survival |
|---|---|---|---|
| Sixth edition refined | |||
| IA | 3.7 | 0.92 | 0.75 |
| IB | 5.2 | 0.88 | 0.62 |
| II | 5.4 | 0.81 | 0.50 |
| IIIA | 5.6 | 0.76 | 0.30 |
| IIIB | 1.7 | 0.78 | 0.09 |
| IV | 63.9 | 0.30 | 0.07 |
| Unknown | 14.5 | 0.42 | 0.16 |
| Sixth edition broad | |||
| I | 8.9 | 0.90 | 0.68 |
| II | 5.4 | 0.81 | 0.50 |
| III | 7.3 | 0.76 | 0.25 |
| IV | 63.9 | 0.30 | 0.07 |
| Unknown | 14.5 | 0.42 | 0.16 |
| Seventh edition refined | |||
| IA | 4.6 | 0.90 | 0.75 |
| IB | 3.9 | 0.90 | 0.60 |
| IIA | 3.9 | 0.85 | 0.55 |
| IIB | 3.7 | 0.86 | 0.45 |
| IIIA | 4.0 | 0.81 | 0.42 |
| IIIB | 6.1 | 0.70 | 0.24 |
| IIIC | 5.9 | 0.60 | 0.15 |
| IV | 54.3 | 0.25 | 0.04 |
| Unknown | 13.5 | 0.32 | 0.09 |
| Seventh edition broad | |||
| I | 8.5 | 0.91 | 0.70 |
| II | 7.7 | 0.86 | 0.50 |
| III | 16.0 | 0.69 | 0.25 |
| IV | 54.3 | 0.25 | 0.04 |
| Unknown | 13.5 | 0.32 | 0.09 |
*Patients diagnosed with stage 0 cancer (n = 15 sixth edition, n = 19 seventh edition) were not included in the table
Fig. 1.
UICC/AJCC sixth edition stage—broad stage categories (15 patients diagnosed with stage 0 cancer were excluded)
Fig. 2.
UICC/AJCC sixth edition TNM stage categories—refined
Fig. 3.
Seventh edition UICC/AJCC TNM stage categories—broad (19 patients diagnosed with stage 0 cancer were excluded)
Fig. 4.
UICC/AJCC seventh edition TNM stage—refined categories
Discrimination
Discrimination statistics are summarized in Table 3. Harrell’s C statistic for the broad sixth edition of TNM stage was 0.64 (0.63–0.65) in the entire cohort and 0.68 (0.67–0.69) in the broad seventh edition stage groupings. The concordance statistics did not change when the refined categories of TNM stage were evaluated. TNM prognostic ability did not improve when the cohort was restricted to patients with non-GEJ tumours or when patients missing TNM stage were excluded. We restricted the original cohort to patients who underwent surgical resection and therefore were most likely assigned a pathologic TNM stage. The concordance statistics for the sixth edition increased slightly and were relatively unchanged for the seventh edition. Over 50% of patients initially presented with stage IV disease and were missing detailed information on T and N stages. Subgroup analysis excluding these patients and examining the predictive performance of TNM stage among patients with exclusively locoregional disease was performed. The concordance statistics for both the sixth and seventh editions were significantly improved for the broad and refined stage classifications.
Table 3.
Predictive performance of TNM stage by edition, including sensitivity analyses
| Harrell’s C statistic (95% CI) | Akaike information criterion | |||
|---|---|---|---|---|
| Sixth edition | Seventh edition | Sixth edition | Seventh edition | |
| Entire cohort (n = 2366) | ||||
| TNM stage broad | 0.64 (0.63–0.65) | 0.68 (0.67–0.69) | 27,112 | 26,861 |
| TNM stage refined | 0.64 (0.63–0.65) | 0.68 (0.67–0.69) | 27,110 | 26,843 |
| Excluding unknown stage (n = 2046) | ||||
| TNM stage broad | 0.64 (0.63–0.65) | 0.69 (0.68–0.70) | 22,539 | 22,343 |
| TNM stage refined | 0.64 (0.63–0.65) | 0.69 (0.68–0.71) | 22,536 | 22,324 |
| Excluding GEJ tumours (n = 1761) | ||||
| TNM stage broad | 0.65 (0.64–0.66) | 0.69 (0.68–0.70) | 18,955 | 18,777 |
| TNM stage refined | 0.65 (0.64–0.66) | 0.69 (0.68–0.71) | 18,951 | 18,763 |
| Surgical resection (n = 944) | ||||
| TNM stage broad | 0.66 (0.64–0.68) | 0.68 (0.66–0.70) | 7793 | 7761 |
| TNM stage refined | 0.66 (0.64–0.69) | 0.69 (0.67–0.71) | 7789 | 7746 |
| Non-metastatic (n = 846) | ||||
| TNM stage broad | 0.72 (0.69–0.74) | 0.70 (0.68–0.72) | 6741 | 8006 |
| TNM stage refined | 0.72 (0.70–0.74) | 0.72 (0.70–0.74) | 6737 | 7984 |
TNM tumour, lymph node, metastasis; n sample size; CI confidence intervals
Akaike information criterion
AIC values are summarized in Table 3. Smaller values suggest better model fit.
Discussion
The results of this study suggest that TNM stage alone does not have adequate predictive power to provide individualized estimates of survival to all Canadian GAC patients. The findings in this study were consistent for both broad and refined TNM stage groupings. These observations persisted when the cohort was restricted to patients with known stage disease and to non-GEJ tumours. Predictive performance improved when used to prognosticate for those patients who underwent resection (with corresponding pathologic data available) and to those with non-metastatic disease. Providing accurate prognostication to patients for decision-making will require the consideration of additional prognostic factors which are not routinely collected for patients in Ontario. Our findings may be generalizable to low-incidence countries in North America and Europe.
Widespread agreement that TNM stage may not adequately prognosticate survival for individual GAC patients (Sano et al. 2017; Rausei et al. 2016; Shu et al. 2017; Lu et al. 2013; Kwon et al. 2016) has precipitated the development and validation of new staging systems and prognostic nomograms. These clinical prediction tools, if well developed and rigorously validated, add important information alongside TNM stage to provide accurate, personalized survival estimates (Collins et al. 2015; Kattan et al. 2016). A number of post-operative prediction tools for GAC have superior prognostic ability compared to TNM stage alone, both in development and in external validation studies (Ashfaq et al. 2015; Dikken et al. 2014; Han et al. 2012; Kattan et al. 2003; Novotny et al. 2006). However, these tools require detailed pathologic and operative data, such as surgical margin status and the number of lymph nodes harvested, or they include data not routinely collected or recommended for collection by surgeons or pathologists. Future studies incorporating variables such as age, sex, tumour location (Bringeland et al. 2017), perineural invasion (Postlewait et al. 2015), or the presence of signet ring cell histology (Pernot et al. 2015) may improve prediction by providing supplementary prognostic information. However, many histological prognostic factors such as tumour grade and signet ring cell were missing in 40% and 84% of our cases, respectively. Canadian pathology synoptic reporting systems may need to consider including additional clinicopathologic data to meet the demands of personalized medicine in oncology.
Existing staging systems and prediction tools do not provide prognostic information for the majority of patients diagnosed with metastatic disease in North America and Europe and who are not surgical candidates. Understanding heterogeneity in prognosis for this population is important, in addition to creating interventions targeting earlier diagnosis. Changes to the eighth edition may address the significant heterogeneity in survival outcomes within previous TNM staging classifications; the eighth edition includes a clinical versus pathologic stage system, which we were not able to apply with existing data (American Joint Committee on Cancer 2017). This important change recognizes that many GC patients are not candidates for surgical resection. Distinguishing pathologic versus clinical staging for these patients may result in better predictive performance of TNM staging classifications, although it does not seem likely to explain entirely the lack of discrimination demonstrated by data in the current study. However, the proposed changes do not address that over 50% of patients in low-incidence countries are diagnosed with metastatic disease and therefore fall within a single stage grouping. Among metastatic patients, there exists significant heterogeneity in survival (Dixon et al. 2016). It is important to elucidate prognostic factors within this category in order to identify patients who may benefit from more aggressive treatment, those who may respond to alternate treatment regimens, and to help target future drug development.
This study is limited by the collaborative staging-based algorithm used to assign TNM stage. If this staging algorithm was inaccurate, it may increase the heterogeneity in survival of patients within each stage category and decrease predictive performance. We believe misclassification most likely occurred across subdivisions of the refined stage categories, for example, cross-over between stage IIA and stage IIB, rather than between stages I and II. We have evaluated predictive performance both with the refined six/eight-category classification systems and with the broader four-category system and produced similar results. Misclassification in the seventh edition between the stage IV and other stage categories was also possible, as patients with any evidence of metastases were categorized conservatively as being stage IV. However, the results observed in this study were consistent among sensitivity analyses restricted to patients with the most accurate staging data and to non-metastatic patients, implying that any deficiencies in the staging algorithm employed do not entirely account for the poor predictive capacity of TNM stage. Simple adjustments to model building such as considering stage as a time-varying exposure may improve prognostic capacity; however, this was outside the scope of this study. Future research creating new tools should give careful consideration to model building. In addition, this study was based on a cohort diagnosed 10 years ago. It is possible that improvements in prognosis may have occurred in that time span, as the result of changes in practice. It is unlikely that those changes would impact the predictive accuracy of the TNM staging system designed for use during the study time frame. An evaluation of TNM stage in a contemporary cohort, following application of the new system, will be necessary.
Conclusion
TNM stage is often used to estimate personalized prognoses for GAC patients; however, these estimates may be inaccurate in low-incidence countries. Anatomic TNM stage revisions have not improved individualized predictive performance of this cornerstone prognostic factor, reflecting uncertainty in prognostication for these patients. The creation of a more complex prediction tool incorporating non-anatomic information is necessary to provide accurate and precise prognostic information to oncologists, patients, and their families.
Electronic supplementary material
(DOCX 17 kb)
References
- American Joint Committee on Cancer . AJCC cancer staging manual—6th Edition. Chicago: Springer-Verlag New York, Inc; 2002. [Google Scholar]
- American Joint Committee on Cancer . AJCC staging manual. 7th edtion. Chicago: Spring; 2012. [Google Scholar]
- American Joint Committee on Cancer (2017) AJCC cancer staging manual, 8th Edition, Springer International Publishing.
- Ashfaq A, Kidwell JT, McGhan LJ, et al. Validation of a gastric cancer nomogram using a cancer registry. Journal of Surgical Oncology. 2015;112:377–380. doi: 10.1002/jso.23999. [DOI] [PubMed] [Google Scholar]
- Bringeland EA, Wasmuth HH, Mjones P, et al. A population-based study on incidence rates, Lauren distribution, stage distribution, treatment, and long-term outcomes for gastric adenocarcinoma in Central Norway 2001–2011. Acta Oncologica. 2017;56:39–45. doi: 10.1080/0284186X.2016.1227086. [DOI] [PubMed] [Google Scholar]
- Canadian Cancer Society (2017) Canadian Cancer Statistics 2017.
- Coburn NG, Lourenco LG, Rossi SE, et al. Management of gastric cancer in Ontario. Journal of Surgical Oncology. 2010;102:54–63. doi: 10.1002/jso.21561. [DOI] [PubMed] [Google Scholar]
- Collins GS, Reitsma JB, Altman DG, et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ. 2015;350:g7594. doi: 10.1136/bmj.g7594. [DOI] [PubMed] [Google Scholar]
- Dikken JL, Coit DG, Baser RE, et al. Performance of a nomogram predicting disease-specific survival after an R0 resection for gastric cancer in patients receiving postoperative chemoradiation therapy. International Journal of Radiation Oncology, Biology, Physics. 2014;88:624–629. doi: 10.1016/j.ijrobp.2013.11.213. [DOI] [PubMed] [Google Scholar]
- Dixon M, Mahar AL, Helyer LK, et al. Prognostic factors in metastatic gastric cancer: results of a population-based, retrospective cohort study in Ontario. Gastric Cancer. 2016;19:150–159. doi: 10.1007/s10120-014-0442-3. [DOI] [PubMed] [Google Scholar]
- Han DS, Suh YS, Kong SH, et al. Nomogram predicting long-term survival after d2 gastrectomy for gastric cancer. Journal of Clinical Oncology. 2012;30:3834–3840. doi: 10.1200/JCO.2012.41.8343. [DOI] [PubMed] [Google Scholar]
- Howlader N, Noone AM, Krapcho M., et al. (2017). SEER Cancer Statistics Review, 1975-2014, based on November 2016 SEER data submission, Bethesda MD, National Cancer Institute.
- Iron K, Zagorski B, Sykora K, et al. Living and dying in Ontario: an opportunity for improved health information. Ontario: Institute for Clinical Evaluative Sciences; 2008. [Google Scholar]
- ISD Scotland . Detect cancer early staging data. Scotland: ISD; 2016. [Google Scholar]
- Kattan MW, Karpeh MS, Mazumdar M, et al. Postoperative nomogram for disease-specific survival after an R0 resection for gastric carcinoma. Journal of Clinical Oncology. 2003;21:3647–3650. doi: 10.1200/JCO.2003.01.240. [DOI] [PubMed] [Google Scholar]
- Kattan MW, Hess KR, Amin MB, et al. American Joint Committee on Cancer acceptance criteria for inclusion of risk models for individualized prognosis in the practice of precision medicine. CA: a Cancer Journal for Clinicians. 2016;66:370–374. doi: 10.3322/caac.21339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon OK, Kim SW, Chae HD, et al. Validation of the 7th AJCC/UICC staging system for gastric cancer and a proposal for a new TNM system based on a prognostic score: a retrospective multicenter study. Annals of Surgical Treatment and Research. 2016;91:295–302. doi: 10.4174/astr.2016.91.6.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Huang CM, Zheng CH, et al. Consideration of tumor size improves the accuracy of TNM predictions in patients with gastric cancer after curative gastrectomy. Surgical Oncology. 2013;22:167–171. doi: 10.1016/j.suronc.2013.05.002. [DOI] [PubMed] [Google Scholar]
- Mahar AL, Coburn NG, Viola R, et al. Predictors of hospital stay and home care services use: a population-based, retrospective cohort study in stage IV gastric cancer. Palliative Medicine. 2015;29:147–156. doi: 10.1177/0269216314554325. [DOI] [PubMed] [Google Scholar]
- Marrelli D, Morgagni P, de Manzoni G, et al. Prognostic value of the 7th AJCC/UICC TNM classification of noncardia gastric cancer: analysis of a large series from specialized Western centers. Annals of Surgery. 2012;255:486–491. doi: 10.1097/SLA.0b013e3182389b1a. [DOI] [PubMed] [Google Scholar]
- McGhan LJ, Pockaj BA, Gray RJ, et al. Validation of the updated 7th edition AJCC TNM staging criteria for gastric adenocarcinoma. Journal of Gastrointestinal Surgery. 2012;16:53–61. doi: 10.1007/s11605-011-1707-3. [DOI] [PubMed] [Google Scholar]
- National Cancer Registration and Analysis Service . Stage breakdown by CCG 2014. London: NCRAS; 2016. [Google Scholar]
- Northern Ireland Cancer Registry . Incidence by stage 2010–2014. Belfast: Queens University Belfast; 2016. [Google Scholar]
- Novotny AR, Schuhmacher C, Busch R, et al. Predicting individual survival after gastric cancer resection: validation of a U.S.-derived nomogram at a single high-volume center in Europe. Annals of Surgery. 2006;243:74–81. doi: 10.1097/01.sla.0000194088.81126.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel MI, Rhoads KF, Ma Y, et al. Seventh edition (2010) of the AJCC/UICC staging system for gastric adenocarcinoma: is there room for improvement? Annals of Surgical Oncology. 2013;20:1631–1638. doi: 10.1245/s10434-012-2724-5. [DOI] [PubMed] [Google Scholar]
- Pernot S, Voron T, Perkins G, et al. Signet-ring cell carcinoma of the stomach: impact on prognosis and specific therapeutic challenge. World Journal of Gastroenterology. 2015;21:11428–11438. doi: 10.3748/wjg.v21.i40.11428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postlewait LM, Squires MH, 3rd, Kooby DA, et al. The prognostic value of signet-ring cell histology in resected gastric adenocarcinoma. Annals of Surgical Oncology. 2015;22(Suppl 3):S832–S839. doi: 10.1245/s10434-015-4724-8. [DOI] [PubMed] [Google Scholar]
- Rausei S, Ruspi L, Galli F, et al. Seventh tumor-node-metastasis staging of gastric cancer: five-year follow-up. World Journal of Gastroenterology. 2016;22:7748–7753. doi: 10.3748/wjg.v22.i34.7748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reim D, Loos M, Vogl F, et al. Prognostic implications of the seventh edition of the international union against cancer classification for patients with gastric cancer: the Western experience of patients treated in a single-center European institution. Journal of Clinical Oncology. 2013;31:263–271. doi: 10.1200/JCO.2012.44.4315. [DOI] [PubMed] [Google Scholar]
- Sano T, Coit DG, Kim HH, et al. Proposal of a new stage grouping of gastric cancer for TNM classification: International Gastric Cancer Association staging project. Gastric Cancer. 2017;20:217–225. doi: 10.1007/s10120-016-0601-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu P, Qin J, Shen K, et al. The IGCA staging system is more accurate than AJCC7 system in stratifying survival of patients with gastric cancer in stage III. BMC Cancer. 2017;17:238. doi: 10.1186/s12885-017-3235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: A Cancer Journal for Clinicians. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- Steyerberg EW. Clinical prediction models: a practical approach to development, validation, and updating. New York: Springer New York; 2009. [Google Scholar]
- Zhang J, Zhou Y, Jiang K, et al. Evaluation of the seventh AJCC TNM staging system for gastric cancer: a meta-analysis of cohort studies. Tumour Biology. 2014;35:8525–8532. doi: 10.1007/s13277-014-1848-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 17 kb)