Abstract
Based on its effects on both tumour cell intrinsic malignant properties as well as anti-tumour immune responses, tryptophan catabolism has emerged as an important metabolic regulator of cancer progression. Three enzymes, indoleamine-2,3-dioxygenase 1 and 2 (IDO1/2) and tryptophan-2,3-dioxygenase (TDO2), catalyse the first step of the degradation of the essential amino acid tryptophan (Trp) to kynurenine (Kyn). The notion of inhibiting IDO1 using small-molecule inhibitors elicited high hopes of a positive impact in the field of immuno-oncology, by restoring anti-tumour immune responses and synergising with other immunotherapies such as immune checkpoint inhibition. However, clinical trials with IDO1 inhibitors have yielded disappointing results, hence raising many questions. This review will discuss strategies to target Trp-degrading enzymes and possible down-stream consequences of their inhibition. We aim to provide comprehensive background information on Trp catabolic enzymes as targets in immuno-oncology and their current state of development. Details of the clinical trials with IDO1 inhibitors, including patient stratification, possible effects of the inhibitors themselves, effects of pre-treatments and the therapies the inhibitors were combined with, are discussed and mechanisms proposed that might have compensated for IDO1 inhibition. Finally, alternative approaches are suggested to circumvent these problems.
Subject terms: Immunosurveillance, Cancer metabolism
Background
Tryptophan (Trp) is the least abundant essential amino acid, and its supply in humans is exclusively covered by dietary intake. Trp is important not only for protein synthesis but also as a precursor for a variety of biologically active compounds. The majority of free Trp is metabolised via the kynurenine (Kyn) pathway, generating metabolites with crucial functions in neurotransmission and the regulation of immune responses.1 The first step of this Trp-degradation pathway can be catalysed by either indoleamine-2,3-dioxygenase 1 (IDO1), IDO2 or tryptophan-2,3-dioxygenase (TDO2). These enzymes catalyse the oxidative cleavage of the indole moiety of Trp that leads to the formation of N-formyl-l-kynurenine, which in a subsequent step is degraded to Kyn and further downstream metabolites.1
IDO1/2 and TDO2 modulate immune responses and promote cancer progression by mediating Trp deprivation and the production of metabolites along the Kyn pathway.1–4 The depletion of Trp has been reported to facilitate tumour immune escape through induction of regulatory T cells (Tregs),5,6 downregulation of the T-cell receptor ζ-chain (TCRζ) in CD8+ T cells,6 and the induction of the inhibitory receptors ILT3 and ILT4 on dendritic cells (DCs).5 Moreover, Trp catabolic products downstream of IDO1/2 and TDO2 regulate immune cell function and promote cancer progression through activation of the aryl hydrocarbon receptor (AHR).1,7–10
The importance of Trp catabolism in the induction of immune tolerance was initially established in the placenta, where IDO1 was shown to prevent rejection of the foetus.11 Such mechanisms responsible for self-tolerance are exploited by tumours to escape immunosurveillance. IDO1 mediates its immunosuppressive function by promoting the formation of Tregs and myeloid-derived suppressor cells (MDSCs), while suppressing the proliferation and function of effector T cells and natural killer (NK) cells.12–14 TDO2 has also been shown to decrease anti-tumour immune responses in a similar manner.7,15
Due to the role of Trp catabolism in promoting immune suppression, several small-molecule inhibitors targeting Trp catabolism have been developed and are currently tested in clinical trials.1,16,17 Moreover, clinical trials combining IDO1 inhibitors with other immunotherapies, such as anti-programmed cell death 1 (PD1) and anti-programmed cell death ligand 1 (PD-L1) immune checkpoint inhibitors, have elicited high hopes of a positive impact in the field of immuno-oncology by synergising to restore anti-tumour immune responses.18–22 The failure of the IDO1 inhibitor epacadostat in combination with pembrolizumab in a Phase 3 clinical trial in advanced melanoma, and other negative clinical results with IDO inhibitors,23,24 raises many questions.
This review will give a brief overview of Trp catabolism and the mechanisms involved in its immunosuppressive and tumour-promoting properties. In addition, it will detail specifics of inhibitors targeting Trp-degrading enzymes and will discuss downstream mechanisms that might have contributed to the failure of the IDO1 inhibitor epacadostat in clinical trials. Furthermore, we will suggest strategies that might circumvent mechanisms compensating for IDO1 inhibition.
Trp-catabolising enzymes (TCE)
IDO1 expression in normal tissues and in cancer
In line with studies implicating IDO1 in preventing the rejection of allogeneic foetuses,11 high IDO1 protein expression is found at the foeto–maternal junction during human pregnancy;25,26 it is also expressed in the epithelium of the cervical gland,26 in lung endothelial cells27 and in numerous lymphoid organs,28 particularly in lymph nodes, where it is enriched in activated DCs.28 In the majority of cases, IDO1 expression is increased in cancerous tissue compared with the untransformed tissue of origin;29,30 IDO1 protein has been detected in endothelial cells,31 immune cells,32 and in tumour cells themselves.28,31,33–35
High expression of IDO1 has been shown to correlate with poor patient prognosis in numerous cancer types.1,22 Cancer cells can either constitutively express IDO127,28 or can be induced to do so by tumour-infiltrating immune cells that secrete inflammatory cytokines, such as interferon γ (IFN-γ).36,37 In some tumour types, such as breast cancer or renal cell carcinoma (RCC), however, IDO1 expression has been linked to improved survival,31,38–40 most probably due to IDO1 acting as a surrogate marker for immune infiltration in these cancers,41 which often translates to improved survival rates.42 Notwithstanding the improved survival associated with IDO1 expression in these cancers, inhibition of IDO1 would be expected to further increase survival rates by enhancing the immune response against the tumour.
IDO1-mediated Trp catabolism and immunosuppression
Trp catabolism through increased IDO1 activity enables cancer progression by suppressing anti-tumour immune responses. High IDO1 expression has been shown to correlate with reduced levels of infiltrating CD3+ T cells, CD8+ T cells, CD57+ NK cells, B cells43–47 and increased levels of forkhead box P3 (Foxp3)+ Tregs48–52 in different cancer types. Accordingly, in breast cancer patients, the number of IDO1-expressing MDSCs is increased and their IDO1 expression is positively associated with Foxp3+ Treg cell infiltration in tumours and lymph node metastases.53 Moreover, IDO1 expression in melanoma cells was shown to promote immunosuppression by expanding, recruiting and activating MDSCs in a Treg-dependent manner.54
The concentration of Kyn in tumour tissue and in the plasma of cancer patients is also associated with immunosuppressive phenotypes. In patients with breast or colon cancer, the expression of the immune checkpoint component PD-1 on CD8+ T cells showed a strong positive correlation with the concentrations of Kyn in blood and tumour tissue.55 Collectively, these studies provide evidence of the role of Trp catabolism in promoting an immunosuppressive tumour microenvironment, thus confirming the importance of IDO1 as an immunotherapeutic target.
IDO2 and its role in cancer
IDO2 was discovered relatively recently,56,57 and was described to be present in human liver, small intestine, spleen, placenta, thymus, lung, brain, kidney and colon. Similar to IDO1, IDO2 is also expressed in antigen-presenting DCs,57 although IDO1 and IDO2 expression and their regulation differ among different populations of DCs.58 Under physiological conditions, IDO2 does not appear to contribute significantly to systemic Trp catabolism,59 and Kyn levels in the serum of Ido2–/– mice do not differ from those of wild-type mice.60 Ido2–/– mice showed no overt phenotypes in embryonic development, haematopoietic cell differentiation or their immune cell profile, but, upon immune stimulation, these mice displayed defective IDO1-dependent Treg cell formation.60 In support of this observation, human DCs promote the generation of Treg cells through a mechanism dependent on IDO2.58 Similar to IDO1, IDO2 has also been reported to inhibit the proliferation of CD4+ and CD8+ T cells.61
In addition to its functions in immune cells (reviewed by Prendergast and colleagues21,62), IDO2 has been implicated in promoting proliferation, survival and migration of cancer cells through a mechanism involving NAD+ production in a murine model.63 Although analysis of gene expression data from the cancer genome atlas (TCGA) reveals that IDO2 expression is not frequently upregulated in human cancer,64 IDO2 has been shown to be expressed in primary gastric, colon and RCCs,65 as well as in pancreatic ductal adenocarcinoma (PDAC).66–68 In mouse models, IDO2 expression promoted the development of PDAC.66,67 Moreover, the presence of biallelic IDO2-inactivating single-nucleotide polymorphisms (SNPs)57,68 was significantly associated with an improved disease-free survival in response to adjuvant radiotherapy in human patients with PDAC,66,67 suggesting that stratifying PDAC patients based on IDO2 genotype status could be beneficial for treatment decision-making.
TDO2 and its role in cancer
Under physiological conditions, TDO2 is expressed primarily in the liver, where its activity regulates systemic Trp levels.15,69,70 Furthermore, TDO2 expression has been detected in neurons and has been implicated in the regulation of neurogenesis and anxiety-related behaviour.71,72 TDO2 resembles IDO1 in that it is often expressed at higher levels in cancer tissue than in untransformed tissue.64,73 Specifically, TDO2 has been reported to be expressed in malignant glioma, hepatic carcinoma, non-small cell lung carcinoma (NSCLC), RCC, ovarian carcinoma, breast cancer, bladder cancer, basal cell carcinoma and melanoma.7,15,74–76 In co-cultures of glioblastoma cells with mixed leucocyte reactions (as an assay of T-cell competence), tumour cell TDO2 expression suppressed T-cell proliferation via the production of Kyn.7 This immunosuppressive effect was also observed in vivo, as TDO2 expression inhibited immune infiltration into gliomas7 and prevented tumour rejection in immunised mice.15 In addition, triple-negative breast cancer cells were reported to induce death of CD8+ T cells through TDO2-derived Kyn.77 Given the pro-tumour effects of TDO2 expression, recent research focus has been directed towards understanding factors regulating TDO2 expression in tumour cells. Tumour necrosis factor receptor-associated factor 7 (TRAF7) and AKT1 mutations in skull base meningiomas increase TDO2 expression in these tumours enabling suppression of immune responses.78 Apart from mutations, oncoviruses also appear to modulate TDO2 expression. Merkel cell polyomavirus (MCPyV)-positive Merkel cell carcinoma cells for instance show lower expression of TDO2 than MCPyV-negative cells.79 In malignant glioma, TDO2 expression is regulated via a steroid-responsive FK506-binding protein 4 (FKBP52)-dependent pathway,80 by prostaglandin E2 (PGE2) through activation of prostaglandin E receptor-4 (EP4)81 as well as by the homeobox transcription factor HOXC10, which directly binds to TDO2 promoter regions.82 In turn, expression of TDO2 has also been reported to regulate signalling in oesophageal squamous cell carcinoma cells, where knockdown of TDO2 expression inactivates the epidermal growth factor receptor (EGFR) signalling pathway.83
Mechanisms underlying the actions of Trp catabolism
Effects of Trp shortage
Trp depletion leads to the activation of the general control non-derepressible-2 (GCN2) kinase and consequent phosphorylation of the eukaryotic initiation factor-2 α (eIF2α), thus blocking protein synthesis (Fig. 1). In CD8+ T cells, activation of the GCN2 pathway has been reported to lead to an inhibition of proliferation and induction of anergy,84 as well as downregulation of the TCRζ chain, which impairs the cytotoxic effector function of these T cells.6 Decreased levels of Trp also modulate CD4+ T-cell proliferation by downregulating the expression of enzymes involved in fatty acid synthesis,85 as well as CD4+ T-cell function by promoting the conversion of CD4+CD25– cells into CD4+CD25+Foxp3+ Treg cells through a GCN2-pathway-dependent mechanism.6 Myeloid cells are also regulated by GCN2 kinase in response to low Trp concentrations. Under these circumstances, DCs become tolerogenic by increasing the expression of the inhibitory receptors immunoglobulin-like transcripts (ILTs) ILT3 and ILT4—hampering their ability to stimulate CD4+ T cells and promotes the induction of CD4+CD25+Foxp3+ Treg cells.5 Furthermore, induction of the IDO1–GCN2 pathway in macrophages can lead to an immunosuppressive phenotype, with increased production of the regulatory cytokines interleukin (IL)-10 and transforming growth factor-β (TGFβ), as well as suppression of the inflammatory cytokine, IL-12.86 In cancer cells, however, activation of GCN2 by Trp depletion induces the expression of tryptophanyl-tRNA-synthetase, which protects the cells against Trp shortage.87
Fig. 1.
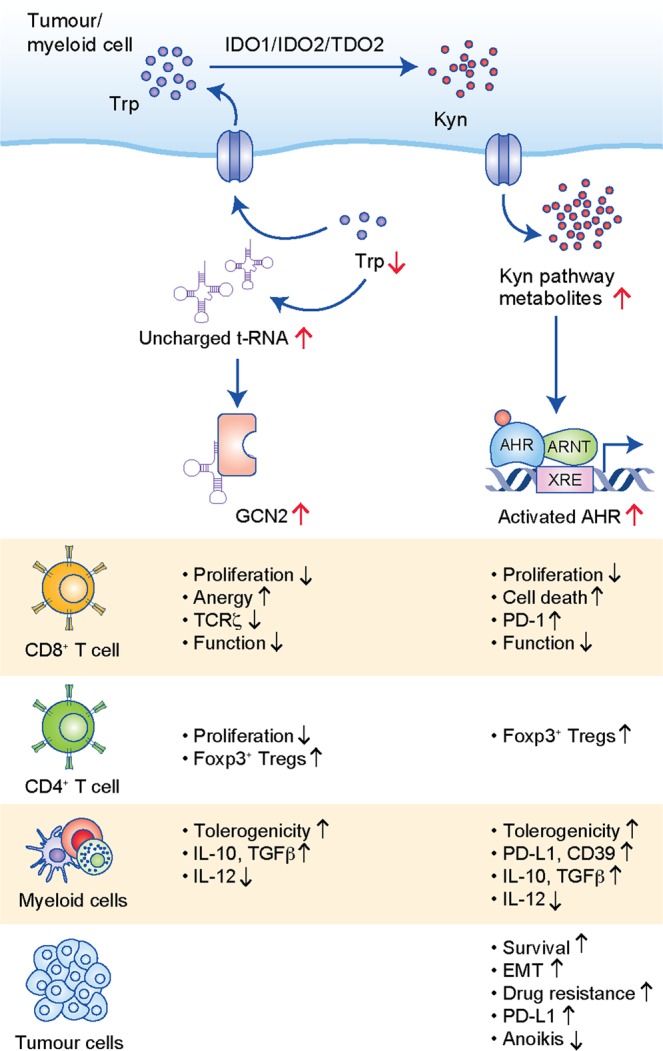
Mode of action and effects of Trp catabolism on cells in the tumour microenvironment. Effects of Trp depletion and Trp metabolites on CD8+ T cells, CD4+ T cells, myeloid cells and tumour cells are shown.
Despite this well-documented association between Trp catabolism and GCN2-pathway activation, little evidence from experimental models has supported this hypothesis. While GCN2 in T cells and DCs plays an important role in restricting autoimmunity in mouse models,88,89 GCN2 in T cells has recently been demonstrated to be irrelevant in suppressing anti-tumour immunity in B16 melanomas.90 Moreover, evidence also points to the existence of a GCN2-independent pathway in T cells that senses low amino acid concentration and promotes cell-cycle arrest.91 The mammalian target of rapamycin (mTOR) pathway appears to be a possible candidate as it has been described as an alternative pathway that senses Trp-deprived conditions.92,93 Insufficient Trp concentrations in the microenvironment have been reported to inhibit the mTOR complex 1 (mTORC1) pathway92 and to promote the induction of Foxp3+ Treg cells.93 Further investigation is required to understand the relevance of low Trp concentrations in vivo and the specific conditions under which reduced Trp levels promote immunoregulation through the GCN2 and/or the mTOR pathway.
Activation of the AHR by Trp catabolites
Trp catabolic products, such as Kyn7,94 and cinnabarinic acid (CA),95 regulate immune cells through activation of the AHR1,9,10,96,97 (Fig. 1). Additional Trp catabolites, such as kynurenic acid (KynA)98 and xanthurenic acid (XA),75 have also been shown to activate the AHR in cancer cells, implying a likely immunoregulatory role of these metabolites. Kyn-mediated AHR activation was shown to induce the death of CD8+ T cells77 and upregulate the expression of PD-1 in CD8+ T cells,55 as well as to stimulate the differentiation of CD4+CD25+Foxp3+ Treg cells.94,99 Differentiation of Foxp3+ Treg cells is also promoted indirectly following AHR activation through the generation of DCs with an immunosuppressive phenotype.99–101 These DCs increase the production of the anti-inflammatory cytokines TGF-β1 and IL-10, and lower the production of pro-inflammatory cytokines, such as IL-1β and IL-12.99 In macrophages, Kyn-mediated activation of AHR induces a tolerogenic phenotype by regulating the expression of immunosuppressive molecules such as PD-L1.97 Moreover, the Kyn–AHR pathway has been implicated in promoting the recruitment of tumour-associated macrophages (TAMs) into tumours. Mechanistically, AHR induces the expression of C–C motif chemokine receptor 2 (CCR2) in TAMs, which are then recruited in response to C–C motif chemokine ligand 2 (CCL2) generated by tumours.97 Moreover, in TAMs, AHR activation triggers an immunosuppressive phenotype by inducing the expression of the ectonucleoside CD39, which promotes dysfunctional tumour-infiltrating CD8+ T cells due to the production of adenosine in association with CD73.97 Interestingly, cancer cells exposed to carcinogens contained in tobacco smoke also evade immune surveillance by inducing the expression of the immune checkpoint molecule, PD-L1.102 Notably, knockdown of AHR in cancer cells decreased the percentage of immunosuppressive CD11b+PD-L1+ tumour-infiltrating cells, increased tumour-infiltrating CD4+ and CD8+ T cells and inhibited tumour growth in an immunocompetent orthotopic model of oral cancer.103 This suggests that AHR expression in cancer cells alone can promote immunosuppression.
Apart from modulating the functions of tumour-infiltrating immune cells, AHR activation in cancer cells also promotes the loss of cell–cell contact and adhesion, which enables epithelial–mesenchymal transition (EMT), by downregulating the expression of E-cadherin.104 In line, AHR activation in cancer cells also induces cell motility,7,74,75,105 promotes cells survival7 and resistance to anoikis.74 Furthermore, AHR activation was shown to promote resistance against several cancer drugs, including BRAF inhibitors (BRAFi),66 tyrosine kinase inhibitors targeting the EGFR106 and chemotherapeutic agents.107–109 Recently, Kyn-mediated AHR activation was reported to promote proliferation of MYC-dependent cancer cells.110 The AHR pathway therefore constitutes a key immunoregulatory and cancer-promoting mechanism downstream of Trp catabolism.
Other cancer-promoting effects of Trp degradation
Several lines of evidence point to a role for IDO1 in blood vessel formation. Compared with wild-type mice, mice with a genetic ablation of Ido1 had a significantly reduced density of pulmonary blood vessels.111 Furthermore, neovascularisation in mouse models of oxygen-induced retinopathy and lung metastasis was significantly reduced in mice lacking Ido1.112 In line with the cell motility-promoting effects of Trp catabolites mediated via AHR activation (see above), the expression of Trp-catabolic enzymes has been shown to affect metastasis formation. Downregulation of IDO1 decreased lung cancer cell motility, while overexpression of IDO1 enhanced it.113 Consistent with this result, IDO1 promoted metastasis formation in preclinical models of breast and lung cancer.111–114 IDO1 overexpression in human lung cancer cells increased metastasis formation in immune-deficient mice,113 while IDO1 knockdown or genetic ablation decreased metastasis formation in mouse models of breast carcinoma.111,114 Of clinical relevance, the degree of IDO1 expression in cancer tissue has been reported to be positively associated with the occurrence of distant metastases in hepatocellular cancer,115 liver metastases in colorectal cancer43 and nodal metastases in endometrial carcinoma.45 Recently, IDO1 has been reported to be involved in the maintenance of pluripotency of human embryonic stem cells by promoting glycolysis through increase of the NAD+/NADH ratio,116 and it remains to be investigated if this phenomenon also has implications for cancer stem cells.
Trp catabolism in preclinical models
Constitutive IDO1 expression is commonly observed in human tumour cells but is hardly present in mouse cancer cells27 (and own unpublished observations), which complicates preclinical studies investigating the effects of IDO1 in cancer, especially as syngeneic models are essential to investigate the immune effects of IDO1. Ido1 has therefore been overexpressed in murine cancer cells to investigate its in vivo functions, or ablated from mice to investigate the effect of host Ido1 on tumour development and progression. In mouse models of B16 melanoma54 and mastocytoma,117 Ido1 overexpression promoted tumour growth through mechanisms involving inhibition of tumour-specific T cells54,117 and expansion of MDSCs within the tumour microenvironment.54 Although Ido1 deficiency did not alter the size and number of colon tumours in Apc(Min/+) mice compared with wild-type mice, it increased the expression of pro-inflammatory cytokines in the tumour microenvironment while the number of Foxp3+ Treg cells was decreased.118 In a model of liver cancer, Ido1 deficiency decreased the incidence and multiplicity of hepatocellular carcinoma and reduced infiltration of Foxp3+ Treg cells into the livers.119 Ido1 ablation reduced tumour burden and infiltration of the tumours by MDSCs and PD-1-expressing CD8+ T cells in a mouse model of lung cancer.120 Furthermore, in a melanoma mouse model, knockout of Ido1 slightly enhanced the therapeutic efficacy of anti-PD-1 /PD-L1 and anti-CTLA-4.121
The regulation of tumoural Ido1 expression in mouse models can only be studied if endogenous Ido1 levels are present and regulated in a similar manner as they are in the corresponding human tissues and cells. As Ido1 is scarcely expressed in murine tumour cells, analysis of the effects of tumoural IDO1 expression is often only possible in mice implanted with human tumour cells, and reconstitution with human allogeneic lymphocytes has been employed for the analysis of the resulting immune effects.122 However, even in mouse cells expressing Ido1, its regulation can differ substantially from that of human IDO1 in the same cell types. Significant differences between IDO1 expression and regulation have, for example, been observed in human and murine mesenchymal stromal cells (MSCs).123–125 Similar to the case for IDO1, preclinical murine tumour models usually also lack TDO2 expression, distinguishing them from human cancers. Accordingly, strategies similar to the ones described above for Ido1 have also been employed to circumvent this issue. Injection of mice, immunised against a major antigen of tumour P815 cells, with murine TDO2-overexpressing P815 cells led to the development of tumours. In this model, TDO2 suppressed anti-tumour immune responses while the control P815 tumour cells were rejected.15 In summary, these issues render the preclinical evaluation of the function and regulation of Trp-degrading enzymes in murine in vivo models very challenging; hence, the analysis of human tissues and cells appears to be pivotal.
IDO1 inhibitors in clinical trials
Numerous studies have supported the notion that IDO1-mediated Trp catabolism promotes tumour progression,41,126–128 thus making IDO1 inhibitors attractive targets for clinical development. As IDO1 is expressed at low levels in normal tissues but is upregulated in tumours,64,73 inhibition of IDO1 would not be expected to cause major adverse effects. This theory is supported by the fact that Ido1-knockout mice do not show an overt phenotype across different mouse strains, unless challenged by the Toll-like receptor ligands.129 Moreover, preclinical data indicated that IDO1 inhibition could synergise with immune checkpoint blockade (ICB).19,121,130–132 In line, clinical data suggest that IDO1 might be induced by immune checkpoint inhibition, as the plasma Kyn:Trp ratio was enhanced upon therapy with an anti-PD-1 antibody in sarcoma patients.133 IDO1 inhibitors have thus sparked great interest and various compounds are currently being tested in clinical trials, mainly as an adjunct to other treatment modalities such as chemotherapy and immunotherapy.16,22,134 Patents on IDO1 inhibitors have been comprehensively reviewed by Cheong et al.16
Epacadostat
Epacadostat (INCB024360), developed by Incyte Corporation, is an IDO1 inhibitor that competes with Trp for IDO1 binding by forming a direct bond with the haem iron atom of IDO1.21,22 It was the most advanced IDO1 inhibitor in clinical studies, both as a single agent and in combination with anti-PD-1, anti-PD-L1 or anti-CTLA-4.18,24,135–137 Epacadostat boosts anti-tumour effects by enhancing T-cell and NK-cell proliferation and function.138 In a preclinical study, epacadostat synergistically enhanced the inhibitory action of anti-PD-L1, anti-PD-1 and anti-CTLA-4 on melanoma growth.139 Multiple clinical trials with epacadostat, ranging from Phase 1 to 3, have been performed in solid tumours and haematological malignancies.140 A Phase 1 clinical trial of epacadostat confirmed its safety and 80–90% IDO1 inhibitory activity measured in plasma at doses ≥100 mg twice daily as a single agent. In seven out of 52 patients, stable disease lasting ≥16 weeks was observed.141 The majority of clinical trials of epacadostat have investigated its effect as an adjunct to the anti-PD-1 antibody pembrolizumab, with or without additional treatment modalities.1,134 For the treatment of advanced melanoma, metastatic NSCLC, RCC, urothelial carcinoma and head and neck squamous cell carcinoma several of these trials reached Phase 3.140 However, in the Phase 3 ECHO-301 trial, the combination of epacadostat with pembrolizumab failed to meet the primary endpoint of progression-free survival in advanced melanoma.24 Consequently, the Phase 3 clinical trials of epacadostat combined with immune checkpoint inhibition were either suspended or turned into Phase 2 trials with randomisation.
BMS-986205
BMS-986205 is an orally administered, irreversible IDO1 inhibitor, which binds to the apo-IDO1 protein lacking haem.1,21,22 Bristol-Myers Squib evaluated the therapeutic efficacy of BMS-986205 in combination with nivolumab, another anti-PD-1 antibody, in several advanced cancers (NCT02658890), metastatic or non-resectable melanoma patients (NCT03329846), NSCLC (NCT03417037) and head and neck cancer (NCT03386838). Preliminary results from 42 patients had confirmed the potency of BMS-986205 in reducing plasma Kyn levels by more than 60% and tumoural Kyn by up to 90% at 100 or 200 mg orally once daily,142 but Bristol-Myers Squib halted its three Phase 3 trials of BMS-986205 in combination with nivolumab in response to the failure of epacadostat in combination with pembrolizumab. However, patients continued to be recruited into Phase 1/2 clinical trials with BMS-986205, including those in combination with immune checkpoint inhibitors. At this point no further results of these trials are available.
Indoximod
Also known as 1-methyl-d-tryptophan (D-1-MT), indoximod, in contrast to direct IDO1 enzymatic inhibitors, acts as an IDO1-pathway modulator.143 Similar to Bristol-Myers Squib’s BMS-986205, NewLink Genetics did not commence its Phase 3 clinical trial of indoximod combined with pembrolizumab or nivolumab for patients with advanced melanoma. Indoximod showed favourable bioavailability and tolerability in cancer patients at doses up to 2000 mg orally twice/day in combination with docetaxel for advanced solid tumours (NCT01191216)144 in a Phase 1 clinical trial and achieved a best response of stable disease for more than 6 months in five out of 48 patients.145 However, the combination of indoximod and taxane (NCT01792050), studied in a Phase 2 trial in patients with metastatic breast cancer, failed to meet its endpoints regarding progression-free survival, overall survival or objective response rate.
Navoximod
Another IDO1 inhibitor developed by NewLink Genetics, navoximod/NLG919/formerly GDC-0919—which also shows inhibitory activity against TDO2—showed favourable pharmacokinetic activity and a reduction of plasma and tissue Kyn concentrations of up to 50% in mice.146 Navoximod binds directly to the haem iron atom of the IDO1 enzyme.21,22 When tested as a single agent in multiple solid tumours in humans, navoximod was well tolerated at doses of up to 800 mg twice daily. Navoximod only transiently decreased plasma Kyn levels by 25–30% 2–4 h after drug intake, there were no objective responses, and the rate of stable disease was 12/22 (68%).147 Despite showing IDO1 inhibitory activity, the combination of navoximod with the anti-PD-L1 antibody atezolizumab showed no clinical benefit in a Phase 1 study of 157 patients with advanced solid tumours.23 In line, a Phase 2 trial of 61 patients with advanced solid tumours only yielded a 10% response rate, most of which were only partial responses (NCT02471846).
EOS-200271
EOS-200271, formerly also known as PF-06840003, is an IDO1 inhibitor developed by iTeos Therapeutics, which displays non-competitive kinetics with respect to Trp and does not bind to the haem iron atom.148 Preclinical studies of EOS-200271 showed an 80% reduction in intra-tumoural Kyn levels and inhibition of tumour growth in multiple syngeneic mouse models, both as a monotherapy and, with an enhanced efficacy, in combination with PD-L1 blockade (Avelumab).19,149 In GL261 murine glioma, EOS-200271 demonstrated excellent central nervous system (CNS) penetration and synergistic effects in combination with PD-1/PD-L1 blockade, CTLA-4 inhibition, radiation therapy and the chemotherapeutic drug temozolomide.130 Its penetration of the blood–brain barrier paved the way for the first study of EOS-200271 in patients with WHO Grade IV glioblastoma or WHO Grade III anaplastic gliomas (NCT02764151), and interim data demonstrated that EOS-200271 crossed the blood–brain barrier and was well tolerated at doses up to 500 mg twice daily. However, it lacked efficacy, resulting in Pfizer returning its EOS-200271 rights to iTeos Therapeutics.
KHK2455
KHK2455 is an IDO1 inhibitor developed by Kyowa Kirin Pharmaceutical Development, which acts by binding to the haem-free apo-IDO1 protein. KHK2455 has recently entered Phase 1 clinical trials for a host of cancer entities in combination therapy with the anti-PD-L1 monoclonal antibody (mAb) Avelumab in advanced bladder cancer (NCT03915405) and in combination with the anti-CCR4 mAb Mogamulizumab for locally advanced or metastatic solid tumours (NCT02867007).150 The clinical trials for KHK2455 have either not started or just started recruiting patients, therefore results are still awaited.
LY3381916
This Eli Lily-developed IDO1 inhibitor has entered Phase 1 clinical trials in late stage and metastatic solid tumours either alone or in combination with anti-PD-L1 therapies (NCT03343613). Recently pharmacodynamics data from a preclinical study in solid tumours were released, which revealed that LY3381916 does not bind the mature IDO1 enzyme but rather binds to the haem-binding pocket of the apo-IDO1 protein, thus preventing protein maturation.151
MK-7162
This IDO1 inhibitor with undefined mechanism of action has been developed by Merck. MK-7162 has entered into Phase 1b clinical trials in combination therapy with anti-PD-L1 treatments in adult participants with advanced metastatic solid tumours (NCT03364049). No results from this trial are in public domain at the moment.
NLG802
NLG802, another molecule from NewLink Genetics, is a prodrug of indoximod.152 Recently released Phase 1 clinical trial results suggest that NLG802 produced significantly higher pharmacokinetic exposure in advanced solid tumour patients as compared with the molar equivalent of indoximod, while maintaining promising safety parameters (NCT03164603).
Analysing IDO1 inhibitor clinical trial results: aspects of clinical trials
Given the mostly disappointing results from clinical trials, the question arises as to whether these failures can be attributed to aspects of the particular clinical trials. Crucial aspects of clinical trials include the choice of compound for IDO1 inhibition, dosing, the selection of treatment that IDO1 inhibition is combined with, the selection of cancer type and patient stratification.
IDO1 inhibitor choice
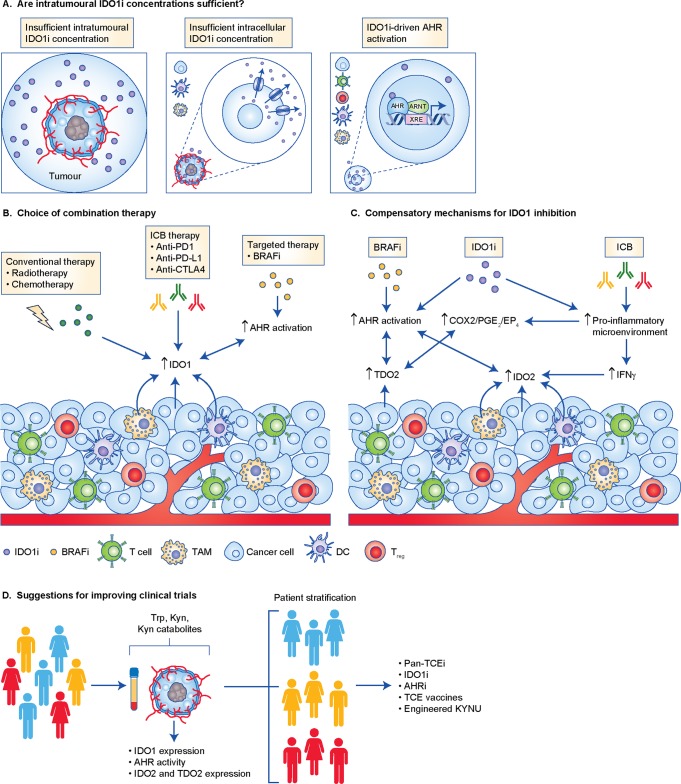
Intrinsic properties of the different IDO1 inhibitors, such as activity, intra-tumoural penetrance and off-target effects, can influence their efficacy. A reduction in systemic Kyn levels is only an indirect readout, given that the IDO1 inhibitors are targeting the tumour microenvironment. Tumoural Kyn levels in response to IDO1 inhibition were not routinely monitored in most of the clinical trials. For BMS-986025, the available data show a reduction of up to 90% in tumoural Kyn levels, which appears sufficient to exert biological effects. Such data are not available for epacadostat. Evaluation of the intra-tumoural concentrations of epacadostat would be important, as intra-tumoural penetrance of IDO1 inhibitors could be hindered due to diminished blood supply143,151 or elevated oncotic pressure in solid tumours8,143 (Fig. 2a, left). Moreover, epacadostat is a substrate of the ABC transporters P-glycoprotein (ABCB1) and the breast-cancer resistance protein (ABCG2),153 which are associated with drug resistance and expressed in malignant melanoma154 (Fig. 2a, middle). Consequently, intracellular epacadostat concentrations in melanoma could have been low, thus possibly not sufficiently inhibiting IDO1-mediated Trp degradation. Furthermore, IDO1 inhibitors can elicit unfavourable off-target effects, as some clinically tested IDO1 inhibitors have been implicated in activating the AHR,155,156 due to their structural resemblance to AHR agonists (Fig. 2a, right). As activation of AHR is one of the main mechanisms mediating immune regulation downstream of IDO1, IDO1-inhibitor-mediated activation of AHR could mitigate the effects of IDO1 inhibition, even if Kyn formation is sufficiently blocked. Furthermore, AHR activation could lead to induction of IDO1/2 and TDO2, as AHR has been demonstrated to be necessary for their upregulation.75,101,157–159
Fig. 2.
Analysing IDO1 inhibitors clinical trial results. a Intra-tumoural penetrance of IDO1 inhibitors (IDO1i) could be hindered due to diminished blood supply to tumours or elevated oncotic pressure in solid tumours (left). Even if IDO1i are able to access tumours, ABC transporters can interfere with their intracellular penetrance (middle). Cellular uptake of IDO1i can lead to AHR activation in various cell types and therefore contribute to cancer progression (right). b Combination of IDOi with other types of therapies can lead to the upregulation of IDO1 expression through various mechanisms (see text). In addition, clinical trials have included patients pre-treated with BRAF inhibitors (BRAFi), which were shown to promote an enrichment of a population of cells with constitutive AHR activity. This might further lead to the induction of IDO1 expression and contribute to cancer progression. c Even if IDO1 inhibition is effectively achieved, IDO2 and TDO2 might compensate for IDO1 blockade. BRAFi and IDO1i can promote AHR activation in various cells present in the tumour microenvironment, which unleashes the intrinsic cancer promoting effects driven by the AHR. Moreover, AHR activation leads to the upregulation of IDO2 and TDO2, which can further activate the AHR and deplete Trp. IDO1i and immune check point blockade (ICB) lead to an increase in cytotoxic TILs, which in turn secrete pro-inflammatory molecules, such as IFN-γ, which also induces IDO2. In addition, the pro-inflammatory microenvironment driven by IDO1i and ICB therapy can also lead to the upregulation of TDO2, via the COX2–PGE2–EP4 pathway. d In order to obtain a more robust view of the effects of IDO1 inhibition and improve the outcome of its use in clinical trials, we suggest stratifying patients based on the concentration of Trp, Kyn and Kyn-derived metabolites in plasma and tumours, as well as on the expression of the TCE and AHR activity present in tumours. Once stratified, patients can be treated more accurately with one or more therapies targeting Trp catabolism.
Dosing, possible effects of pre-treatments and combination therapy
Other reasons for the failure of IDO1 inhibitors could be inappropriate dosing and the selection of the therapy the IDO1 inhibitors were combined with. Diverse treatments such as radiotherapy,160 chemotherapy,161 demethylating agents162,163 and immune checkpoint inhibitors targeting PD-L1 and CTLA-419 have been reported to induce IDO1 expression and/or activity, so doses determined to be sufficient to inhibit IDO1 as a monotherapy might not be sufficient to inhibit the levels of IDO1 in combination therapies (Fig. 2b).
Moreover, IFN-γ, a known inducer of IDO1, can also induce IDO2 expression in cancer cell lines.65,68 Because PD-1 and PD-L1 blockade leads to an increase in cytotoxic TILs, which in turn secrete pro-inflammatory molecules, such as IFN-γ,164,165 IDO2 expression could be induced in the tumour and its activity might compensate for IDO1 inhibition (Fig. 2c). In a murine model of colon adenocarcinoma, dual inhibition of IDO1 and PD-L1 increased the percentage of intra-tumoural CD4+ IFN-γ+ and CD8+ IFN-γ+ T cells, compared with either therapy alone,19 suggesting that the increased levels of IFN-γ might mediate induction of IDO2. In agreement, PD-L1 blockade was recently shown to induce IDO1 and IDO2 in a preclinical syngeneic mouse model of sarcoma.165 Inhibition of IDO1 might channel Trp towards IDO2. Therefore, when IDO1 is pharmacologically inhibited, IDO2 might become active. Developing inhibitors against IDO2 is challenging as it is hard to express and purify.166 Therefore, currently, only few specific IDO2 inhibitors are known, none of which has entered the clinical phase.
Specific to the ECHO-301 trial,24 as patients previously having received adjuvant anti-CTLA-4 therapy were included in the study,24 it is possible that IDO1 induction by CTLA-4 inhibitors19 could have rendered the IDO1 inhibitor concentration insufficient (Fig. 2c). Moreover, this trial included patients pre-treated with BRAFi.24 The BRAFi vemurafenib was recently shown to promote an enrichment of dedifferentiated BRAFi persister cells with constitutively active AHR.66 As AHR activation is a key effector mechanism of IDO1, the constitutive AHR activity following vemurafenib treatment may counteract the effects of IDO1 inhibition. Moreover, as AHR activation promotes the expression of IDO1/2 and TDO2,101,157–159 the IDO1 inhibitor may not have been able to inhibit Trp catabolism due to induction of IDO2 and/or TDO2 (Fig. 2c).
In addition, PGE2, a molecule derived from cyclo-oxygenase-1/2 (COX1/2) activity, drives IDO1 and TDO2 expression and activity in human cancer cells through activation of EP4.35,167 COX2 is induced in response to inflammatory stimuli, which are present in the tumour microenvironment168,169 and enriched upon treatment with immune checkpoint blockade alone164,165 and also in combination with IDO1 inhibition.21 Therefore, activation of the COX2–PGE2–EP4 pathway may have increased IDO1 and TDO2 levels possibly rendering IDO1 inhibitors unable to inhibit Trp degradation (Fig. 2c).
Liu et al.55 demonstrated that IDO1 drives PD-1 expression via Kyn-mediated AHR activation. If IDO1 is indeed upstream of PD-1, then IDO1 inhibition might already inhibit PD-1 activity by downregulating its expression thus rendering a synergistic effect of PD-1 and IDO1 inhibition unlikely. Hence, the choice of combination partner is critical for the efficacy of IDO1 inhibition. Furthermore, inhibition of the immune checkpoint components PD-L1 and CTLA-4 when combined with simultaneous IDO1 blockade has recently been reported to result in hepatic injury mediated via immune infiltration in preclinical mouse models.4 It remains to be investigated if similar adverse effects also occur in humans.
Cancer type and patient stratification
Aside from the IDO1 inhibitors themselves, the type of cancer in which IDO1 inhibition is studied is critical, as IDO1 inhibitors can only be effective if IDO1 is expressed and active. Gliomas, for instance, scarcely express IDO17,28 and IDO1 does not account for the constitutive Trp degradation observed in these tumours.7 Therefore, efficacy of IDO1 inhibition by EOS-200271, for example, might not be expected in malignant gliomas. However, radiotherapy and/or PD-1 blockade may induce IDO1, thus resulting in IDO1 inhibition increasing overall survival as shown in a preclinical mouse model. Furthermore, IDO1 expression is very heterogeneous, even within a single cancer entity. Stratification of patients for IDO1 inhibitor treatment based on IDO1 expression and activity (based on Trp and Trp-derived metabolites, such as Kyn and Kyn catabolite levels) in the tumour tissues and body fluids therefore is critical (Fig. 2d). As Trp catabolism in the tumour does not necessarily lead to low Trp levels and Kyn can be further converted into downstream catabolites, measuring additional catabolites downstream of Kyn can provide important information about the efficacy of inhibition of Trp catabolism.7 Moreover, as several metabolites downstream of Kyn, such as KynA, XA and CA, are known AHR agonists,9,75,95,98 monitoring the concentration of these metabolites is relevant. In support of this approach, recent data showed that PD-L1 blockade in a syngeneic mouse model of sarcoma, induced the expression of several enzymes of the Kyn pathway, including IDO1, IDO2, kynurenine 3-mono-oxygenase (KMO) and kynureninase (KYNU).165 This leads to an increased intra-tumoural concentration of Kyn-derived catabolites and a decreased intra-tumoural Kyn/Trp ratio, suggesting that anti-PD-L1 therapy promotes Kyn catabolism.165 Unfortunately, stratification of patients to IDO1 inhibitors based on readouts of IDO1 expression and activity have been hardly performed in the clinical trials so far, despite a number of available approaches to assess these parameters (Fig. 3). Furthermore, assessing the activity of GCN2 and AHR pathways in tumour samples could provide additional information for stratifying patients. Most likely, the combined analysis of both enzyme expression and Trp-derived catabolite levels/Trp uptake will be most effective for stratification of patients to inhibitors of Trp catabolism.
Fig. 3.
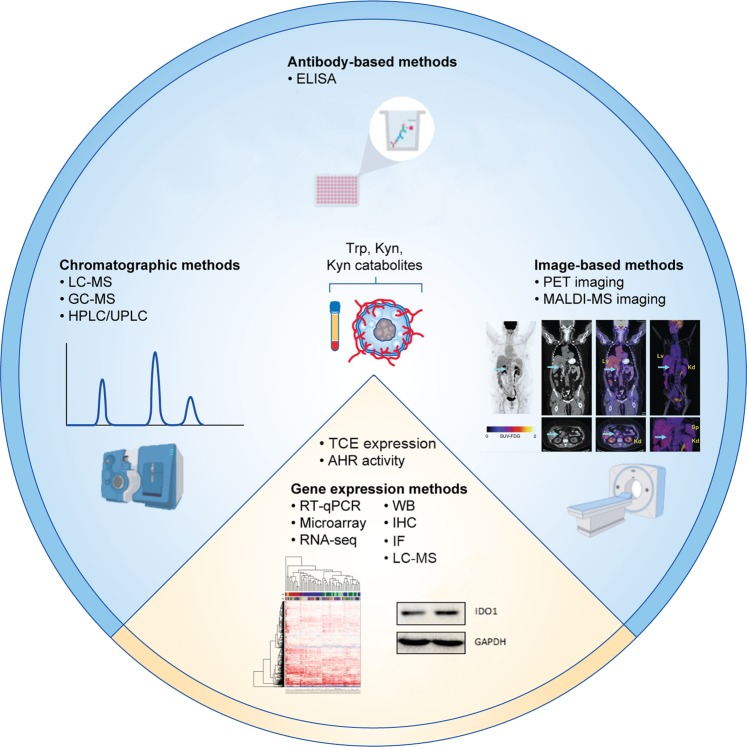
Analytical approaches for quantification expression and activity of tryptophan catabolising enzymes (TCEs) in cancer therapies. The levels of Trp and its metabolites can be measured by chromatographic methods, including high-performance liquid chromatography (HPLC), gas chromatography–mass spectrometry (GC-MS) or liquid chromatography–mass spectrometry (LC-MS).208 Antibodies detecting Trp, as well as specific metabolites of the Trp degradation pathway, enable enzyme-linked immunosorbent assay (ELISA) measurements.163,209–211 Furthermore, matrix-assisted laser desorption/ionisation (MALDI) mass spectrometry (MS) imaging allows visualisation of Trp and Kyn in tissue,90,212 while position-emission tomography (PET) imaging with Trp-derived tracers enables visualisation of Trp uptake in vivo.213–218 In addition, TCE expression can be detected on mRNA and protein levels, however care must be taken when selecting anti-IDO1 antibodies, as many lack selectivity, particularly for immunohistochemistry.27 The blue area of the circle illustrates methods used for measuring Trp and its metabolites; the yellow area illustrates methods used for detcting the expression of TCE and AHR activity. IF: immunofluorescence; WB: Western blot. PET-imaging example was reproduced under a Creative Commons CC-BY license from.219 RNA-seq example was reproduced under a Creative Commons CC-BY license from.220 Western Blot example was provided by the authors.
Alternative approaches to target Trp catabolism
As TDO2 catalyses the same reaction as IDO1, its activity might be able to substitute for IDO1 when it is inhibited. Therefore, TDO2 inhibitors and dual IDO1/TDO2 inhibitors have been developed.
TDO2 inhibitors
TDO2 inhibitors were initially developed as antidepressants to increase systemic Trp levels and thus enhance serotonin concentrations in the brain.170,171 Small-molecule inhibitors of TDO2 are being developed for cancer therapy but have not yet reached clinical trials. Patents on TDO2 inhibitors have recently been comprehensively reviewed.172
Single TDO2 inhibitors
The first TDO2 inhibitor developed, 680C91, also efficiently inhibited TDO2 in glioblastoma cells.7 Hsu et al. observed enhanced TDO2 expression in fibroblasts surrounding the implantation of murine lung cancer lines173 in a lung cancer model, and treatment with 680C91 resulted in an improved T-cell response, DC function and decreased tumour metastasis.173 However, 680C91 is poorly soluble in aqueous solutions and shows low oral bioavailability, limiting its in vivo use.170 In addition, 680C91 activates AHR target gene expression (Opitz, unpublished observation), suggesting that it might act as an AHR agonist, thus further limiting its usefulness in cancer therapy, as will be described below. LM10, a TDO2 inhibitor with improved solubility and bioavailability,174 was used to show that TDO2 inhibits anti-tumour immune responses and promotes tumour growth in a murine cancer model.15 Other single TDO2 inhibitors are also under development, several of these such as fused imidazo-indoles-based inhibitors developed by RedX Pharma (Patent WO 2016/051181, Patent WO 2016/059412) and indazole-based TDO2 inhibitors developed by IOmet Pharma (Patent WO 2016/071283, Patent WO 2015/150097) are currently in various stages of experimental or preclinical studies.
Dual IDO1–TDO2 inhibition
On the basis of the co-expression of IDO1 and TDO2 in several cancer types and the potential for TDO2 to undergo compensatory upregulation in response to IDO1 inhibition, combining IDO1 inhibitors with TDO2 inhibitors or using dual IDO1–TDO2 inhibitors might provide an effective means of targeting Trp catabolism. Accordingly, several dual inhibitors, such as HTI-1090 and DN1406131, are currently in various stages of development. Significantly reduced Kyn levels in response to the IDO1–TDO2 inhibitor RG70099 have been documented in preclinical cancer models,175 while reduced tumour volumes in response to the IDO1/TDO2 inhibitor EPL-1410 have been reported in preclinical trials.176 The IDO1/TDO2 dual inhibitors CB548 and CMG017 were recently described to elicit a robust anti-cancer immune effect and to synergistically inhibit cancer progression in combination with an immune checkpoint inhibitor.177 A further effort towards creating dual IDO1/TDO2 inhibitors as well as pan dioxygenase inhibitors that also target IDO2 has been reported.154,178 However, it is currently unknown how well such inhibitors would be tolerated, as even though mice seem to have more TDO2 expression in liver than humans,179 Tdo2–/– mice have extremely high systemic levels of Trp,70,71,180 which affect Trp metabolism along the Kyn pathway180 and might cause shunting of Trp into other pathways such as serotonin, or tryptamine formation. Moreover, Trp degradation down the Kyn pathway leads to the de novo synthesis of NAD+, particularly in the liver.181 Inhibition of TDO2 or dual inhibition of IDO1 and TDO2 might therefore reduce NAD+ levels. NAD+ plays a central role as a coenzyme in metabolism and redox reactions, in the removal of oxidative DNA damage by NAD+-dependent poly(ADP ribose) polymerases (PARPs) and in transcriptional regulation mediated through the action of NAD+-dependent sirtuins.182 Although no hepatic tumorigenicity has been described in Tdo2–/– mice so far, reduced expression of Tdo2 and consequent downstream Kyn-pathway enzymes has been reported to promote hepatic tumorigenesis through DNA damage by decreasing liver NAD+ levels.183 It therefore remains to be determined how well dual IDO1–TDO2 inhibition is tolerated.
Alternative approaches beyond small-molecule inhibition of dioxygenases
Vaccines against Trp catabolic enzymes
Although inhibition of IDO1/2 and TDO2 enzymatic activity has been the mainstay of efforts targeting these enzymes, alternative strategies have also been explored. One approach is based on T-cell reactivity against IDO1, IDO2 and TDO identified in cancer patients184–187 The detection of T-cell responses against IDO1 has sparked small Phase 1 and Phase 2 clinical trials testing vaccination with a synthetic IDO1 peptide either alone,188 in combination with the anti-CTLA-4 mAb ipilimumab189 or in combination with a vaccine against a survivin peptide and the chemotherapeutic agent temozolomide.190 A Phase 1 trial in stage III–IV NSCLC patients treated monthly with an IDO1 peptide vaccine alone, reported a 6-year overall survival of 20%.191 A recently developed technology termed T-win® aims to activate immune cells against both Tregs as well as malignant cells by vaccination against long peptide epitopes of IDO1 and/or TDO2.14 Furthermore, T-win® has also been proposed to target TAMs and revert them back to an M1 phenotype by converting the immunosuppressive tumour microenvironment into a pro-inflammatory one.17
Engineered KYNU
Another approach to limit the immunosuppressive and cancer-promoting effects of IDO1-mediated and/or TDO2-mediated Kyn formation is depletion of extracellular Kyn by engineered KYNU. Single doses of engineered KYNU potently reduced Kyn concentrations and increased the levels of CD8+ T cells in murine tumour models.192,193 KYNU converts Kyn into anthranilic acid (AA). Little information is available about the uptake of AA, although it is known to cross the blood–brain barrier and might contribute significantly to the cerebral pools of this metabolite.194 Although the effects of AA accumulation are unknown, it is taken up by cells and might be converted further into hydroxyanthranilic acid and the AHR agonist, CA.95 Given that many of the metabolites downstream of KYNU are also potent AHR ligands, depletion of the KYNU substrate, Kyn, might not be sufficient to exert the desired effects. Therefore, further studies are required to determine the tolerability and efficacy of engineered KYNU.
AHR inhibition to block the effects of enhanced Trp degradation in cancer
AHR expression is increased in aggressive malignancies and constitutively localises to the nucleus, suggesting that it is chronically activated to facilitate tumour progression.195,196 Moreover, AHR antagonism diminished cell viability in patient-derived glioma and meningioma cells following in vitro drug treatments,195 unlike inhibition of Trp-degrading enzymes. Therapeutic modulation of AHR is therefore currently being explored for cancer therapy.196 AHR inhibition represents a strategy to tackle immunosuppression and cancer cell intrinsic malignant properties mediated by Trp catabolites regardless of the enzymes involved in their formation. In support of this approach, a recent study showed that AHR inhibition elicited anti-tumour activity alone and further increased efficacy when combined with gemcitabine or PD-L1 blockade in diverse syngeneic mouse tumour models.197 Furthermore, by inhibiting the expression of IDO1, IDO2 and TDO2,33,34,75,157 AHR antagonism might even prevent effects of these enzymes that are independent of downstream AHR activation. Currently, the AHR inhibitors CH223191 and StemRegenin 1 (SR1) are commonly used to study AHR-activation mediated effects.1,55,66,97,198–200 CH223191 and SR1 exert their inhibitory effect through direct binding to AHR.201,202 AHR inhibitors are currently being developed by multiple pharmaceutical companies including Kyn Therapeutics,203 Hercules Pharmaceuticals,103 Phenex Pharmaceuticals,197 Ideaya Biosciences204 and Bayer AG.205–207 The AHR inhibitor HP163 (Hercules Pharmaceuticals), reduced the number of immunosuppressive CD11b+PD-L1+ or CD11b+CCR2+ cells in tumour-draining lymph nodes in a mouse model of oral cancer and reduced the tumour growth in immunocompetent orthotopic models of different types of cancer.103 The inhibitor from Ideaya Biosciences, IDE-AhRi, was recently shown to suppress the polarisation and immunosuppressive activity of M2 macrophages.204 Furthermore, the AHR inhibitor BAY-218 (Bayer AG) stimulated pro-inflammatory monocyte and T-cell responses in vitro and promoted anti-tumour immune responses and reduced tumour growth in the syngeneic mouse tumour models CT26 and B16-OVA. Notably, BAY-218 enhanced therapeutic efficacy of PD-L1 blockade in the CT26 model.205 This compound also recently entered a Phase 1 clinical trial in patients with advanced cancer.207 Although initial preclinical results look promising, only clinical trials will show how well AHR inhibitors are tolerated, whether they effectively inhibit tumour progression, and in which cancer entities they are effective.
Concluding remarks
Based on its immunosuppressive and cancer-promoting effects, Trp degradation remains an important target in immuno-oncology. In future clinical trials, it will be critical to stratify patients to inhibitors of Trp catabolism based on enzyme expression, Trp catabolite levels and the activation of downstream signalling pathways, as only those patients can benefit whose tumours indeed show expression and activity of Trp-catabolising enzymes. Collection of tumour tissue and biological fluids throughout treatment will be essential to monitor the efficacy of enzyme inhibition. Possible effects of pre-treatments and combination therapies on the inhibition of Trp degradation should be taken into account and closely monitored. IDO2 and TDO2 may compensate for IDO1 inhibition. Pan-Trp degradation inhibitors may circumvent this problem, but their safety and tolerability remain to be investigated. Vaccination against Trp-degrading enzymes and removal of Trp catabolites, e.g. by engineered KYNU, represent additional interesting approaches for cancer therapy. Furthermore, strategies targeting AHR activity might be beneficial in tackling immunosuppression and malignant properties of cancer cells mediated by Trp catabolites. By inhibiting the expression of IDO1/2 and TDO2, AHR inhibition might even prevent effects of these enzymes that are independent of downstream AHR activation. Subsequent to this audit of potential causes for the failure of IDO1 inhibition in recent clinical trials, we are hopeful that pharmaceutical companies will not abandon the entire concept of inhibition of Trp catabolism, but rather closely analyse the data that have been generated such that future clinical trials can overcome these obstacles.
Author contributions
C.A.O., L.F.S.P., S.R.M., D.L.D., A.S., M.P. and S.T. collected literature, wrote, read, reviewed and revised the manuscript. L.F.S.P. and S.R.M. conceptualised and prepared the figures in the manuscript.
Ethics approval and consent to participate
Not applicable.
Consent to publish
Not applicable.
Data availability
The data from The Cancer Genome Atlas (TCGA) and Genotype-Tissue Expression (GTEx) Project is publicly available.
Competing interests
C.A.O. and M.P. are listed as inventors on the patents “Means and methods for treating and/or preventing natural AHR ligand-dependent cancer” and “Isotopic method for measurement of tryptophan and metabolites thereof”. M.P. is listed on the patent “Treatment of Kynurenine-producing tumors with AHR antagonists”. M.P. has received research support and consulting honoraria from Bayer. The other authors have declared that no competing interests exist.
Funding information
This work was supported by grants from the BMBF e:Med initiative (GlioPATH, 01ZX1402) to S.T. and C.A.O. C.A.O. and A.S. were supported by funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement no. 754688. L.F.S.P. was supported by scholarships from the University of Costa Rica (UCR) and Costa Rica’s Ministry of Science, Technology and Telecommunications (MICITT).
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Luis F. Somarribas Patterson, Soumya R. Mohapatra, Dyah L. Dewi.
References
- 1.Platten M, Nollen EAA, Rohrig UF, Fallarino F, Opitz CA. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat. Rev. Drug Discov. 2019;18:379–401. doi: 10.1038/s41573-019-0016-5. [DOI] [PubMed] [Google Scholar]
- 2.Badawy AA, Namboodiri AM, Moffett JR. The end of the road for the tryptophan depletion concept in pregnancy and infection. Clin. Sci. (Lond). 2016;130:1327–1333. doi: 10.1042/CS20160153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sedlmayr P, Blaschitz A, Stocker R. The role of placental tryptophan catabolism. Front. Immunol. 2014;5:230. doi: 10.3389/fimmu.2014.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Affolter T, Llewellyn HP, Bartlett DW, Zong Q, Xia S, Torti V, et al. Inhibition of immune checkpoints PD-1, CTLA-4, and IDO1 coordinately induces immune-mediated liver injury in mice. PLoS ONE. 2019;14:e0217276. doi: 10.1371/journal.pone.0217276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brenk M, Scheler M, Koch S, Neumann J, Takikawa O, Hacker G, et al. Tryptophan deprivation induces inhibitory receptors ILT3 and ILT4 on dendritic cells favoring the induction of human CD4+CD25+ Foxp3+ T regulatory cells. J. Immunol. 2009;183:145–154. doi: 10.4049/jimmunol.0803277. [DOI] [PubMed] [Google Scholar]
- 6.Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, et al. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J. Immunol. 2006;176:6752–6761. doi: 10.4049/jimmunol.176.11.6752. [DOI] [PubMed] [Google Scholar]
- 7.Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478:197–203. doi: 10.1038/nature10491. [DOI] [PubMed] [Google Scholar]
- 8.Gabriely, G. & Quintana, F. J. Role of AHR in the control of GBM-associated myeloid cells. Semin Cancer Biol. (2019). https://www.sciencedirect.com/science/article/pii/S1044579X19300173?via%3Dihub. In press. [DOI] [PMC free article] [PubMed]
- 9.Gutierrez-Vazquez C, Quintana FJ. Regulation of the immune response by the aryl hydrocarbon receptor. Immunity. 2018;48:19–33. doi: 10.1016/j.immuni.2017.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rothhammer V, Quintana FJ. The aryl hydrocarbon receptor: an environmental sensor integrating immune responses in health and disease. Nat. Rev. Immunol. 2019;19:184–197. doi: 10.1038/s41577-019-0125-8. [DOI] [PubMed] [Google Scholar]
- 11.Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 12.Hwu P, Du MX, Lapointe R, Do M, Taylor MW, Young HA. Indoleamine 2,3-dioxygenase production by human dendritic cells results in the inhibition of T cell proliferation. J. Immunol. 2000;164:3596–3599. doi: 10.4049/jimmunol.164.7.3596. [DOI] [PubMed] [Google Scholar]
- 13.Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J. Exp. Med. 1999;189:1363–1372. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andersen MH. The T-win(R) technology: immune-modulating vaccines. Semin. Immunopathol. 2019;41:87–95. doi: 10.1007/s00281-018-0695-8. [DOI] [PubMed] [Google Scholar]
- 15.Pilotte L, Larrieu P, Stroobant V, Colau D, Dolusic E, Frederick R, et al. Reversal of tumoral immune resistance by inhibition of tryptophan 2,3-dioxygenase. Proc. Natl Acad. Sci. USA. 2012;109:2497–2502. doi: 10.1073/pnas.1113873109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheong JE, Ekkati A, Sun L. A patent review of IDO1 inhibitors for cancer. Expert Opin. Ther. Pat. 2018;28:317–330. doi: 10.1080/13543776.2018.1441290. [DOI] [PubMed] [Google Scholar]
- 17.Andersen MH. The targeting of tumor-associated macrophages by vaccination. Cell Stress. 2019;3:139–140. doi: 10.15698/cst2019.05.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gibney GT, Hamid O, Lutzky J, Olszanski AJ, Mitchell TC, Gajewski TF, et al. Phase 1/2 study of epacadostat in combination with ipilimumab in patients with unresectable or metastatic melanoma. J. Immunother. Cancer. 2019;7:80. doi: 10.1186/s40425-019-0562-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gomes B, Driessens G, Bartlett D, Cai D, Cauwenberghs S, Crosignani S, et al. Characterization of the selective indoleamine 2,3-dioxygenase-1 (IDO1) catalytic inhibitor EOS200271/PF-06840003 supports IDO1 as a critical resistance mechanism to PD-(L)1 blockade therapy. Mol. Cancer Ther. 2018;17:2530–2542. doi: 10.1158/1535-7163.MCT-17-1104. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell Tara C., Hamid Omid, Smith David C., Bauer Todd M., Wasser Jeffrey S., Olszanski Anthony J., Luke Jason J., Balmanoukian Ani S., Schmidt Emmett V., Zhao Yufan, Gong Xiaohua, Maleski Janet, Leopold Lance, Gajewski Thomas F. Epacadostat Plus Pembrolizumab in Patients With Advanced Solid Tumors: Phase I Results From a Multicenter, Open-Label Phase I/II Trial (ECHO-202/KEYNOTE-037) Journal of Clinical Oncology. 2018;36(32):3223–3230. doi: 10.1200/JCO.2018.78.9602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prendergast GC, Mondal A, Dey S, Laury-Kleintop LD, Muller AJ. Inflammatory reprogramming with IDO1 inhibitors: turning immunologically unresponsive ‘cold’ tumors ‘hot’. Trends Cancer. 2018;4:38–58. doi: 10.1016/j.trecan.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brochez L, Chevolet I, Kruse V. The rationale of indoleamine 2,3-dioxygenase inhibition for cancer therapy. Eur. J. Cancer. 2017;76:167–182. doi: 10.1016/j.ejca.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 23.Jung KH, LoRusso P, Burris H, Gordon M, Bang YJ, Hellmann MD, et al. Phase I study of the indoleamine 2,3-dioxygenase 1 (IDO1) inhibitor navoximod (GDC-0919) administered with PD-L1 inhibitor (atezolizumab) in advanced solid tumors. Clin. Cancer Res. 2019;25:3220–3228. doi: 10.1158/1078-0432.CCR-18-2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Long GV, Dummer R, Hamid O, Gajewski TF, Caglevic C, Dalle S, et al. Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in patients with unresectable or metastatic melanoma (ECHO-301/KEYNOTE-252): a phase 3, randomised, double-blind study. Lancet Oncol. 2019;20:1083–1097. doi: 10.1016/S1470-2045(19)30274-8. [DOI] [PubMed] [Google Scholar]
- 25.Honig A, Rieger L, Kapp M, Sutterlin M, Dietl J, Kammerer U. Indoleamine 2,3-dioxygenase (IDO) expression in invasive extravillous trophoblast supports role of the enzyme for materno-fetal tolerance. J. Reprod. Immunol. 2004;61:79–86. doi: 10.1016/j.jri.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Sedlmayr P, Blaschitz A. Placental expression of indoleamine 2,3-dioxygenase. Wien. Med. Wochenschr. 2012;162:214–219. doi: 10.1007/s10354-012-0082-3. [DOI] [PubMed] [Google Scholar]
- 27.Vigneron N, van Baren N, Van den Eynde BJ. Expression profile of the human IDO1 protein, a cancer drug target involved in tumoral immune resistance. Oncoimmunology. 2015;4:e1003012. doi: 10.1080/2162402X.2014.1003012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Theate I, van Baren N, Pilotte L, Moulin P, Larrieu P, Renauld JC, et al. Extensive profiling of the expression of the indoleamine 2,3-dioxygenase 1 protein in normal and tumoral human tissues. Cancer Immunol. Res. 2015;3:161–172. doi: 10.1158/2326-6066.CIR-14-0137. [DOI] [PubMed] [Google Scholar]
- 29.Sakurai K, Amano S, Enomoto K, Kashio M, Saito Y, Sakamoto A, et al. [Study of indoleamine 2,3-dioxygenase expression in patients with breast cancer] Gan to kagaku ryoho Cancer Chemother. 2005;32:1546–1549. [PubMed] [Google Scholar]
- 30.Hascitha J, Priya R, Jayavelu S, Dhandapani H, Selvaluxmy G, Sunder Singh S, et al. Analysis of kynurenine/tryptophan ratio and expression of IDO1 and 2 mRNA in tumour tissue of cervical cancer patients. Clin. Biochem. 2016;49:919–924. doi: 10.1016/j.clinbiochem.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 31.Riesenberg R, Weiler C, Spring O, Eder M, Buchner A, Popp T, et al. Expression of indoleamine 2,3-dioxygenase in tumor endothelial cells correlates with long-term survival of patients with renal cell carcinoma. Clin. Cancer Res. 2007;13:6993–7002. doi: 10.1158/1078-0432.CCR-07-0942. [DOI] [PubMed] [Google Scholar]
- 32.Munn DH, Sharma MD, Lee JR, Jhaver KG, Johnson TS, Keskin DB, et al. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science. 2002;297:1867–1870. doi: 10.1126/science.1073514. [DOI] [PubMed] [Google Scholar]
- 33.Calkins MA, Julien K, Marti P. The breakdown of the anelastic approximation in rotating compressible convection: implications for astrophysical systems. Proc. Math. Phys. Eng. Sci. 2015;471:20140689. doi: 10.1098/rspa.2014.0689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Litzenburger UM, Opitz CA, Sahm F, Katharina J, Trump S, Winter M, et al. Constitutive IDO expression in human cancer is sustained by an autocrine signaling loop involving IL-6, STAT3 and the AHR. Oncotarget. 2014;5:1038–1051. doi: 10.18632/oncotarget.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hennequart M, Pilotte L, Cane S, Hoffmann D, Stroobant V, Plaen E, et al. Constitutive IDO1 expression in human tumors is driven by cyclooxygenase-2 and mediates intrinsic immune resistance. Cancer Immunol. Res. 2017;5:695–709. doi: 10.1158/2326-6066.CIR-16-0400. [DOI] [PubMed] [Google Scholar]
- 36.Kim S, Park S, Cho MS, Lim W, Moon BI, Sung SH. Strong correlation of indoleamine 2,3-dioxygenase 1 expression with basal-like phenotype and increased lymphocytic infiltration in triple-negative breast cancer. J. Cancer. 2017;8:124–130. doi: 10.7150/jca.17437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yasui H, Takai K, Yoshida R, Hayaishi O. Interferon enhances tryptophan metabolism by inducing pulmonary indoleamine 2,3-dioxygenase: its possible occurrence in cancer patients. Proc. Natl Acad. Sci. USA. 1986;83:6622–6626. doi: 10.1073/pnas.83.17.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hendrickx W, Simeone I, Anjum S, Mokrab Y, Bertucci F, Finetti P, et al. Identification of genetic determinants of breast cancer immune phenotypes by integrative genome-scale analysis. OncoImmunology. 2017;6:e1253654. doi: 10.1080/2162402X.2016.1253654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jacquemier J, Bertucci F, Finetti P, Esterni B, Charafe-Jauffret E, Thibult ML, et al. High expression of indoleamine 2,3-dioxygenase in the tumour is associated with medullary features and favourable outcome in basal-like breast carcinoma. Int. J. Cancer. 2012;130:96–104. doi: 10.1002/ijc.25979. [DOI] [PubMed] [Google Scholar]
- 40.Denkert C, Von Minckwitz G, Brase JC, Sinn BV, Gade S, Kronenwett R, et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J. Clin. Oncol. 2015;33:983–991. doi: 10.1200/JCO.2014.58.1967. [DOI] [PubMed] [Google Scholar]
- 41.Munn DH, Mellor AL. IDO in the tumor microenvironment: inflammation, counter-regulation, and tolerance. Trends Immunol. 2016;37:193–207. doi: 10.1016/j.it.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Donskov F, Von Der Maase H. Impact of immune parameters on long-term survival in metastatic renal cell carcinoma. J. Clin. Oncol. 2006;24:1997–2005. doi: 10.1200/JCO.2005.03.9594. [DOI] [PubMed] [Google Scholar]
- 43.Brandacher G, Perathoner A, Ladurner R, Schneeberger S, Obrist P, Winkler C, et al. Prognostic value of indoleamine 2,3-dioxygenase expression in colorectal cancer: effect on tumor-infiltrating T cells. Clin. Cancer Res. 2006;12:1144–1151. doi: 10.1158/1078-0432.CCR-05-1966. [DOI] [PubMed] [Google Scholar]
- 44.Ben-Haj-Ayed A, Moussa A, Ghedira R, Gabbouj S, Miled S, Bouzid N, et al. Prognostic value of indoleamine 2,3-dioxygenase activity and expression in nasopharyngeal carcinoma. Immunol. Lett. 2016;169:23–32. doi: 10.1016/j.imlet.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 45.Ino K, Yamamoto E, Shibata K, Kajiyama H, Yoshida N, Terauchi M, et al. Inverse correlation between tumoral indoleamine 2,3-dioxygenase expression and tumor-infiltrating lymphocytes in endometrial cancer: its association with disease progression and survival. Clin. Cancer Res. 2008;14:2310–2317. doi: 10.1158/1078-0432.CCR-07-4144. [DOI] [PubMed] [Google Scholar]
- 46.Inaba T, Ino K, Kajiyama H, Yamamoto E, Shibata K, Nawa A, et al. Role of the immunosuppressive enzyme indoleamine 2,3-dioxygenase in the progression of ovarian carcinoma. Gynecol. Oncol. 2009;115:185–192. doi: 10.1016/j.ygyno.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 47.Carvajal-Hausdorf DE, Mani N, Velcheti V, Schalper KA, Rimm DL. Objective measurement and clinical significance of IDO1 protein in hormone receptor-positive breast cancer. J. Immunother. Cancer. 2017;5:81. doi: 10.1186/s40425-017-0285-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Curti A, Pandolfi S, Valzasina B, Aluigi M, Isidori A, Ferri E, et al. Modulation of tryptophan catabolism by human leukemic cells results in the conversion of CD25- into CD25+ T regulatory cells. Blood. 2007;109:2871–2877. doi: 10.1182/blood-2006-07-036863. [DOI] [PubMed] [Google Scholar]
- 49.Witkiewicz A, Williams TK, Cozzitorto J, Durkan B, Showalter SL, Yeo CJ, et al. Expression of indoleamine 2,3-dioxygenase in metastatic pancreatic ductal adenocarcinoma recruits regulatory T cells to avoid immune detection. J. Am. Coll. Surg. 2008;206:849–854. doi: 10.1016/j.jamcollsurg.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 50.Brody JR, Costantino CL, Berger AC, Sato T, Lisanti MP, Yeo CJ, et al. Expression of indoleamine 2,3-dioxygenase in metastatic malignant melanoma recruits regulatory T cells to avoid immune detection and affects survival. Cell Cycle. 2009;8:1930–1934. doi: 10.4161/cc.8.12.8745. [DOI] [PubMed] [Google Scholar]
- 51.Yu J, Sun J, Wang SE, Li H, Cao S, Cong Y, et al. Upregulated expression of indoleamine 2, 3-dioxygenase in primary breast cancer correlates with increase of infiltrated regulatory T cells in situ and lymph node metastasis. Clin. Dev. Immunol. 2011;2011:469135. doi: 10.1155/2011/469135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu XQ, Lu K, Feng LL, Ding M, Gao JM, Ge XL, et al. Up-regulated expression of indoleamine 2,3-dioxygenase 1 in non-Hodgkin lymphoma correlates with increased regulatory T-cell infiltration. Leuk. Lymphoma. 2014;55:405–414. doi: 10.3109/10428194.2013.804917. [DOI] [PubMed] [Google Scholar]
- 53.Yu J, Du W, Yan F, Wang Y, Li H, Cao S, et al. Myeloid-derived suppressor cells suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancer. J. Immunol. 2013;190:3783–3797. doi: 10.4049/jimmunol.1201449. [DOI] [PubMed] [Google Scholar]
- 54.Holmgaard RB, Zamarin D, Li Y, Gasmi B, Munn DH, Allison JP, et al. Tumor-expressed IDO recruits and activates MDSCs in a treg-dependent manner. Cell Rep. 2015;13:412–424. doi: 10.1016/j.celrep.2015.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu Y, Liang X, Dong W, Fang Y, Lv J, Zhang T, et al. Tumor-repopulating cells induce PD-1 expression in CD8(+) T cells by transferring kynurenine and AhR activation. Cancer Cell. 2018;33:480–94.e7. doi: 10.1016/j.ccell.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 56.Ball HJ, Sanchez-Perez A, Weiser S, Austin CJ, Astelbauer F, Miu J, et al. Characterization of an indoleamine 2,3-dioxygenase-like protein found in humans and mice. Gene. 2007;396:203–213. doi: 10.1016/j.gene.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 57.Metz R, Duhadaway JB, Kamasani U, Laury-Kleintop L, Muller AJ, Prendergast GC. Novel tryptophan catabolic enzyme IDO2 is the preferred biochemical target of the antitumor indoleamine 2,3-dioxygenase inhibitory compound D-1-methyl-tryptophan. Cancer Res. 2007;67:7082–7087. doi: 10.1158/0008-5472.CAN-07-1872. [DOI] [PubMed] [Google Scholar]
- 58.Trabanelli S, Ocadlikova D, Ciciarello M, Salvestrini V, Lecciso M, Jandus C, et al. The SOCS3-independent expression of IDO2 supports the homeostatic generation of T regulatory cells by human dendritic cells. J. Immunol. 2014;192:1231–1240. doi: 10.4049/jimmunol.1300720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jusof FFB, Supun M, Weiser Silvia, Too LayKhoon, Metz Richard, Prendergast GeorgeC, Fraser StuartT, Hunt NicholasH, Ball HelenJ. Investigation of the Tissue Distribution and Physiological Roles of Indoleamine 2,3-Dioxygenase-2. Int. J. Tryptophan Res. 2017;10:1–12. doi: 10.1177/1178646917735098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Metz R, Smith C, DuHadaway JB, Chandler P, Baban B, Merlo LM, et al. IDO2 is critical for IDO1-mediated T-cell regulation and exerts a non-redundant function in inflammation. Int. Immunol. 2014;26:357–367. doi: 10.1093/intimm/dxt073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qian F, Liao J, Villella J, Edwards R, Kalinski P, Lele S, et al. Effects of 1-methyltryptophan stereoisomers on IDO2 enzyme activity and IDO2-mediated arrest of human T cell proliferation. Cancer Immunol. Immunother. 2012;61:2013–2020. doi: 10.1007/s00262-012-1265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Prendergast GC, Metz R, Muller AJ, Merlo LM, Mandik-Nayak L. IDO2 in immunomodulation and autoimmune disease. Front. Immunol. 2014;5:585. doi: 10.3389/fimmu.2014.00585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu Y, Zhang Y, Zheng X, Zhang X, Wang H, Li Q, et al. Gene silencing of indoleamine 2,3-dioxygenase 2 in melanoma cells induces apoptosis through the suppression of NAD+ and inhibits in vivo tumor growth. Oncotarget. 2016;7:32329–32340. doi: 10.18632/oncotarget.8617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weinstein John N, Collisson Eric A, Mills Gordon B, Shaw Kenna R Mills, Ozenberger Brad A, Ellrott Kyle, Shmulevich Ilya, Sander Chris, Stuart Joshua M. The Cancer Genome Atlas Pan-Cancer analysis project. Nature Genetics. 2013;45(10):1113–1120. doi: 10.1038/ng.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lob S, Konigsrainer A, Zieker D, Brucher BL, Rammensee HG, Opelz G, et al. IDO1 and IDO2 are expressed in human tumors: levo- but not dextro-1-methyl tryptophan inhibits tryptophan catabolism. Cancer Immunol. Immunother. 2009;58:153–157. doi: 10.1007/s00262-008-0513-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Corre S, Tardif N, Mouchet N, Leclair HM, Boussemart L, Gautron A, et al. Sustained activation of the aryl hydrocarbon receptor transcription factor promotes resistance to BRAF-inhibitors in melanoma. Nat. Commun. 2018;9:4775. doi: 10.1038/s41467-018-06951-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nevler A, Muller AJ, Sutanto-Ward E, DuHadaway JB, Nagatomo K, Londin E, et al. Host IDO2 gene status influences tumor progression and radiotherapy response in KRAS-driven sporadic pancreatic cancers. Clin. Cancer Res. 2019;25:724–734. doi: 10.1158/1078-0432.CCR-18-0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Witkiewicz AK, Costantino CL, Metz R, Muller AJ, Prendergast GC, Yeo CJ, et al. Genotyping and expression analysis of IDO2 in human pancreatic cancer: a novel, active target. J. Am. Coll. Surg. 2009;208:781–787. doi: 10.1016/j.jamcollsurg.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu W, Nicolazzo JA, Wen L, Chung R, Stankovic R, Bao SS, et al. Expression of tryptophan 2,3-dioxygenase and production of kynurenine pathway metabolites in triple transgenic mice and human Alzheimer’s disease brain. PLoS ONE. 2013;8:e59749. doi: 10.1371/journal.pone.0059749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lanz TV, Williams SK, Stojic A, Iwantscheff S, Sonner JK, Grabitz C, et al. Tryptophan-2,3-Dioxygenase (TDO) deficiency is associated with subclinical neuroprotection in a mouse model of multiple sclerosis. Sci. Rep. 2017;7:41271. doi: 10.1038/srep41271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kanai M, Funakoshi H, Takahashi H, Hayakawa T, Mizuno S, Matsumoto K, et al. Tryptophan 2,3-dioxygenase is a key modulator of physiological neurogenesis and anxiety-related behavior in mice. Mol. Brain. 2009;2:8. doi: 10.1186/1756-6606-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miller CL, Llenos IC, Dulay JR, Barillo MM, Yolken RH, Weis S. Expression of the kynurenine pathway enzyme tryptophan 2,3-dioxygenase is increased in the frontal cortex of individuals with schizophrenia. Neurobiol. Dis. 2004;15:618–629. doi: 10.1016/j.nbd.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 73.GTEx Consortium. The Genotype-Tissue Expression (GTEx) project. Nat Genet.45, 580–585 (2013). [DOI] [PMC free article] [PubMed]
- 74.D’Amato NC, Rogers TJ, Gordon MA, Greene LI, Cochrane DR, Spoelstra NS, et al. A TDO2-AhR signaling axis facilitates anoikis resistance and metastasis in triple-negative breast cancer. Cancer Res. 2015;75:4651–4664. doi: 10.1158/0008-5472.CAN-15-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Novikov O, Wang Z, Stanford EA, Parks AJ, Ramirez-Cardenas A, Landesman E, et al. An aryl hydrocarbon receptor-mediated amplification loop that enforces cell migration in ER-/PR-/Her2- human breast cancer cells. Mol. Pharmacol. 2016;90:674–688. doi: 10.1124/mol.116.105361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tina E, Prosen S, Lennholm S, Gasparyan G, Lindberg M, Gothlin Eremo A. Expression profile of the amino acid transporters SLC7A5, SLC7A7, SLC7A8 and the enzyme TDO2 in basal cell carcinoma. Br. J. Dermatol. 2019;180:130–140. doi: 10.1111/bjd.16905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Greene LI, Bruno TC, Christenson JL, D’Alessandro A, Culp-Hill R, Torkko K, et al. A role for tryptophan-2,3-dioxygenase in CD8 T-cell suppression and evidence of tryptophan catabolism in breast cancer patient plasma. Mol. Cancer Res. 2019;17:131–139. doi: 10.1158/1541-7786.MCR-18-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hao S, Huang G, Feng J, Li D, Wang K, Wang L, et al. Non-NF2 mutations have a key effect on inhibitory immune checkpoints and tumor pathogenesis in skull base meningiomas. J. Neurooncol. 2019;144:11–20. doi: 10.1007/s11060-019-03198-9. [DOI] [PubMed] [Google Scholar]
- 79.Wardhani LO, Matsushita M, Iwasaki T, Kuwamoto S, Nonaka D, Nagata K, et al. Expression of the IDO1/TDO2-AhR pathway in tumor cells or the tumor microenvironment is associated with Merkel cell polyomavirus status and prognosis in Merkel cell carcinoma. Hum. Pathol. 2019;84:52–61. doi: 10.1016/j.humpath.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 80.Ott M, Litzenburger UM, Rauschenbach KJ, Bunse L, Ochs K, Sahm F, et al. Suppression of TDO-mediated tryptophan catabolism in glioblastoma cells by a steroid-responsive FKBP52-dependent pathway. Glia. 2015;63:78–90. doi: 10.1002/glia.22734. [DOI] [PubMed] [Google Scholar]
- 81.Ochs K, Ott M, Rauschenbach KJ, Deumelandt K, Sahm F, Opitz CA, et al. Tryptophan-2,3-dioxygenase is regulated by prostaglandin E2 in malignant glioma via a positive signaling loop involving prostaglandin e receptor-4. J. Neurochem. 2016;136:1142–1154. doi: 10.1111/jnc.13503. [DOI] [PubMed] [Google Scholar]
- 82.Li S, Zhang W, Wu C, Gao H, Yu J, Wang X, et al. HOXC10 promotes proliferation and invasion and induces immunosuppressive gene expression in glioma. FEBS J. 2018;285:2278–2291. doi: 10.1111/febs.14476. [DOI] [PubMed] [Google Scholar]
- 83.Pham QT, Oue N, Sekino Y, Yamamoto Y, Shigematsu Y, Sakamoto N, et al. TDO2 overexpression is associated with cancer stem cells and poor prognosis in esophageal squamous cell carcinoma. Oncology. 2018;95:297–308. doi: 10.1159/000490725. [DOI] [PubMed] [Google Scholar]
- 84.Munn DH, Sharma MD, Baban B, Harding HP, Zhang Y, Ron D, et al. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005;22:633–642. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 85.Dewhirst MW, Secomb TW. Transport of drugs from blood vessels to tumour tissue. Nat. Rev. Cancer. 2017;17:738–750. doi: 10.1038/nrc.2017.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ravishankar B, Liu H, Shinde R, Chaudhary K, Xiao W, Bradley J, et al. The amino acid sensor GCN2 inhibits inflammatory responses to apoptotic cells promoting tolerance and suppressing systemic autoimmunity. Proc. Natl Acad. Sci. USA. 2015;112:10774–10779. doi: 10.1073/pnas.1504276112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Adam Isabell, Dewi Dyah L., Mooiweer Joram, Sadik Ahmed, Mohapatra Soumya R., Berdel Bianca, Keil Melanie, Sonner Jana K., Thedieck Kathrin, Rose Adam J., Platten Michael, Heiland Ines, Trump Saskia, Opitz Christiane A. Upregulation of tryptophanyl-tRNA synthethase adapts human cancer cells to nutritional stress caused by tryptophan degradation. OncoImmunology. 2018;7(12):e1486353. doi: 10.1080/2162402X.2018.1486353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Keil M, Sonner JK, Lanz TV, Oezen I, Bunse T, Bittner S, et al. General control non-derepressible 2 (GCN2) in T cells controls disease progression of autoimmune neuroinflammation. J. Neuroimmunol. 2016;297:117–126. doi: 10.1016/j.jneuroim.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 89.Orsini H, Araujo LP, Maricato JT, Guereschi MG, Mariano M, Castilho BA, et al. GCN2 kinase plays an important role triggering the remission phase of experimental autoimmune encephalomyelitis (EAE) in mice. Brain Behav. Immun. 2014;37:177–186. doi: 10.1016/j.bbi.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 90.Sonner JK, Deumelandt K, Ott M, Thome CM, Rauschenbach KJ, Schulz S, et al. The stress kinase GCN2 does not mediate suppression of antitumor T cell responses by tryptophan catabolism in experimental melanomas. Oncoimmunology. 2016;5:e1240858. doi: 10.1080/2162402X.2016.1240858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Van de Velde LA, Guo XJ, Barbaric L, Smith AM, Oguin TH, 3rd, Thomas PG, et al. Stress kinase GCN2 controls the proliferative fitness and trafficking of cytotoxic T cells independent of environmental amino acid sensing. Cell Rep. 2016;17:2247–2258. doi: 10.1016/j.celrep.2016.10.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Metz R, Rust S, Duhadaway JB, Mautino MR, Munn DH, Vahanian NN, et al. IDO inhibits a tryptophan sufficiency signal that stimulates mTOR: A novel IDO effector pathway targeted by D-1-methyl-tryptophan. Oncoimmunology. 2012;1:1460–1468. doi: 10.4161/onci.21716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cobbold SP, Adams E, Farquhar CA, Nolan KF, Howie D, Lui KO, et al. Infectious tolerance via the consumption of essential amino acids and mTOR signaling. Proc. Natl Acad. Sci. USA. 2009;106:12055–12060. doi: 10.1073/pnas.0903919106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An Interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J. Immunol. 2010;185:3190–3198. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lowe MM, Mold JE, Kanwar B, Huang Y, Louie A, Pollastri MP, et al. Identification of cinnabarinic acid as a novel endogenous aryl hydrocarbon receptor ligand that drives IL-22 production. PLoS ONE. 2014;9:e87877. doi: 10.1371/journal.pone.0087877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stockinger B, Di Meglio P, Gialitakis M, Duarte JH. The aryl hydrocarbon receptor: multitasking in the immune system. Annu. Rev. Immunol. 2014;32:403–432. doi: 10.1146/annurev-immunol-032713-120245. [DOI] [PubMed] [Google Scholar]
- 97.Takenaka MC, Gabriely G, Rothhammer V, Mascanfroni ID, Wheeler MA, Chao CC, et al. Control of tumor-associated macrophages and T cells in glioblastoma via AHR and CD39. Nat. Neurosci. 2019;22:729–740. doi: 10.1038/s41593-019-0370-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.DiNatale BC, Murray IA, Schroeder JC, Flaveny CA, Lahoti TS, Laurenzana EM, et al. Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicol. Sci. 2010;115:89–97. doi: 10.1093/toxsci/kfq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Quintana FJ, Murugaiyan G, Farez MF, Mitsdoerffer M, Tukpah AM, Burns EJ, et al. An endogenous aryl hydrocarbon receptor ligand acts on dendritic cells and T cells to suppress experimental autoimmune encephalomyelitis. Proc. Natl Acad. Sci. USA. 2010;107:20768–20773. doi: 10.1073/pnas.1009201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li Q, Harden JL, Anderson CD, Egilmez NK. Tolerogenic phenotype of IFN-gamma-induced IDO+ dendritic cells is maintained via an autocrine IDO-kynurenine/AhR-IDO loop. J. Immunol. 2016;197:962–970. doi: 10.4049/jimmunol.1502615. [DOI] [PubMed] [Google Scholar]
- 101.Nguyen NT, Kimura A, Nakahama T, Chinen I, Masuda K, Nohara K, et al. Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc. Natl Acad. Sci. USA. 2010;107:19961–19966. doi: 10.1073/pnas.1014465107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang GZ, Zhang L, Zhao XC, Gao SH, Qu LW, Yu H, et al. The aryl hydrocarbon receptor mediates tobacco-induced PD-L1 expression and is associated with response to immunotherapy. Nat. Commun. 2019;10:1125. doi: 10.1038/s41467-019-08887-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sherr D, Kenison-Whte J, Wang Z. Abstract LB-128: the aryl hydrocarbon receptor as driver of cancer immunity. Cancer Res. 2018;78:LB–128. [Google Scholar]
- 104.Dietrich C, Kaina B. The aryl hydrocarbon receptor (AhR) in the regulation of cell-cell contact and tumor growth. Carcinogenesis. 2010;31:1319–1328. doi: 10.1093/carcin/bgq028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shadboorestan A, Tarfiei GA, Montazeri H, Sepand MR, Zangooei M, Khedri A, et al. Invasion and migration of MDA-MB-231 cells are inhibited by block of AhR and NFAT: role of AhR/NFAT1/beta4 integrin signaling. J. Appl. Toxicol. 2019;39:375–384. doi: 10.1002/jat.3728. [DOI] [PubMed] [Google Scholar]
- 106.Ye M, Zhang Y, Gao H, Xu Y, Jing P, Wu J, et al. Activation of the aryl hydrocarbon receptor leads to resistance to EGFR TKIs in non-small cell lung cancer by activating Src-mediated bypass signaling. Clin. Cancer Res. 2018;24:1227–1239. doi: 10.1158/1078-0432.CCR-17-0396. [DOI] [PubMed] [Google Scholar]
- 107.Chung Wei-Min, Ho Yen-Ping, Chang Wei-Chun, Dai Yuan-Chang, Chen Lumin, Hung Yao-Ching, Ma Wen-Lung. Increase Paclitaxel Sensitivity to Better Suppress Serous Epithelial Ovarian Cancer via Ablating Androgen Receptor/Aryl Hydrocarbon Receptor-ABCG2 Axis. Cancers. 2019;11(4):463. doi: 10.3390/cancers11040463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wangpaichitr M, Nguyen DJM, Li Y-Y, Wu C, Feun LG, Savaraj N. Abstract 4361: kynurenine - aryl hydrocarbon receptor axis: a crucial modulator of immunometabolism in cisplatin resistant lung cancer. Cancer Res. 2019;79:4361. [Google Scholar]
- 109.Yamashita N, Kanno Y, Saito N, Terai K, Sanada N, Kizu R, et al. Aryl hydrocarbon receptor counteracts pharmacological efficacy of doxorubicin via enhanced AKR1C3 expression in triple negative breast cancer cells. Biochem. Biophys. Res. Commun. 2019;516:693–698. doi: 10.1016/j.bbrc.2019.06.119. [DOI] [PubMed] [Google Scholar]
- 110.Venkateswaran N, Lafita-Navarro MC, Hao YH, Kilgore JA, Perez-Castro L, Braverman J, et al. MYC promotes tryptophan uptake and metabolism by the kynurenine pathway in colon cancer. Genes Dev. 2019;33:1236–1251. doi: 10.1101/gad.327056.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Smith C, Chang MY, Parker KH, Beury DW, DuHadaway JB, Flick HE, et al. IDO is a nodal pathogenic driver of lung cancer and metastasis development. Cancer Discov. 2012;2:722–735. doi: 10.1158/2159-8290.CD-12-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mondal A, Smith C, DuHadaway JB, Sutanto-Ward E, Prendergast GC, Bravo-Nuevo A, et al. IDO1 is an integral mediator of inflammatory neovascularization. EBioMedicine. 2016;14:74–82. doi: 10.1016/j.ebiom.2016.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tang D, Yue L, Yao R, Zhou L, Yang Y, Lu L, et al. P53 prevent tumor invasion and metastasis by down-regulating IDO in lung cancer. Oncotarget. 2017;8:54548–54557. doi: 10.18632/oncotarget.17408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Levina V, Su Y, Gorelik E. Immunological and nonimmunological effects of indoleamine 2,3-dioxygenase on breast tumor growth and spontaneous metastasis formation. Clin. Dev. Immunol. 2012;2012:173029. doi: 10.1155/2012/173029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pan K, Wang H, Chen MS, Zhang HK, Weng DS, Zhou J, et al. Expression and prognosis role of indoleamine 2,3-dioxygenase in hepatocellular carcinoma. J. Cancer. Res. Clin. Oncol. 2008;134:1247–1253. doi: 10.1007/s00432-008-0395-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu X, Wang M, Jiang T, He J, Fu X, Xu Y. IDO1 maintains pluripotency of primed human embryonic stem cells by promoting glycolysis. Stem Cells. 2019;37:1158–1165. doi: 10.1002/stem.3044. [DOI] [PubMed] [Google Scholar]
- 117.Uyttenhove C, Pilotte L, Théate I, Stroobant V, Colau D, Parmentier N, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat. Med. 2003;9:1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 118.Takamatsu M, Hirata A, Ohtaki H, Hoshi M, Ando T, Ito H, et al. Inhibition of indoleamine 2,3-dioxygenase 1 expression alters immune response in colon tumor microenvironment in mice. Cancer Sci. 2015;106:1008–1015. doi: 10.1111/cas.12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shibata Y, Hara T, Nagano J, Nakamura N, Ohno T, Ninomiya S, et al. The role of indoleamine 2,3-dioxygenase in diethylnitrosamine-induced liver carcinogenesis. PLoS ONE. 2016;11:e0146279. doi: 10.1371/journal.pone.0146279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schafer CC, Wang Y, Hough KP, Sawant A, Grant SC, Thannickal VJ, et al. Indoleamine 2,3-dioxygenase regulates anti-tumor immunity in lung cancer by metabolic reprogramming of immune cells in the tumor microenvironment. Oncotarget. 2016;7:75407–75424. doi: 10.18632/oncotarget.12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Holmgaard RB, Zamarin D, Munn DH, Wolchok JD, Allison JP. Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. J. Exp. Med. 2013;210:1389–1402. doi: 10.1084/jem.20130066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hennequart Marc, Pilotte Luc, Cane Stefania, Hoffmann Delia, Stroobant Vincent, Plaen Etienne De, Eynde Benoît J. Van den. Constitutive IDO1 Expression in Human Tumors Is Driven by Cyclooxygenase-2 and Mediates Intrinsic Immune Resistance. Cancer Immunology Research. 2017;5(8):695–709. doi: 10.1158/2326-6066.CIR-16-0400. [DOI] [PubMed] [Google Scholar]
- 123.Opitz CA, Litzenburger UM, Lutz C, Lanz TV, Tritschler I, Koppel A, et al. Toll-like receptor engagement enhances the immunosuppressive properties of human bone marrow-derived mesenchymal stem cells by inducing indoleamine-2,3-dioxygenase-1 via interferon-beta and protein kinase R. Stem Cells. 2009;27:909–919. doi: 10.1002/stem.7. [DOI] [PubMed] [Google Scholar]
- 124.Lanz TV, Opitz CA, Ho PP, Agrawal A, Lutz C, Weller M, et al. Mouse mesenchymal stem cells suppress antigen-specific TH cell immunity independent of indoleamine 2,3-dioxygenase 1 (IDO1) Stem Cells Dev. 2010;19:657–668. doi: 10.1089/scd.2009.0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Meisel R, Brockers S, Heseler K, Degistirici O, Bulle H, Woite C, et al. Human but not murine multipotent mesenchymal stromal cells exhibit broad-spectrum antimicrobial effector function mediated by indoleamine 2,3-dioxygenase. Leukemia. 2011;25:648–654. doi: 10.1038/leu.2010.310. [DOI] [PubMed] [Google Scholar]
- 126.Prendergast GC. Immune escape as a fundamental trait of cancer: focus on IDO. Oncogene. 2008;27:3889–3900. doi: 10.1038/onc.2008.35. [DOI] [PubMed] [Google Scholar]
- 127.van Baren N, Van den Eynde BJ. Tumoral immune resistance mediated by enzymes that degrade tryptophan. Cancer Immunol. Res. 2015;3:978–985. doi: 10.1158/2326-6066.CIR-15-0095. [DOI] [PubMed] [Google Scholar]
- 128.Mohinta S, Kannan AK, Gowda K, Amin SG, Perdew GH, August A. Differential regulation of Th17 and T regulatory cell differentiation by aryl hydrocarbon receptor dependent xenobiotic response element dependent and independent pathways. Toxicol. Sci. 2015;145:233–243. doi: 10.1093/toxsci/kfv046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chang MY, Smith C, DuHadaway JB, Pyle JR, Boulden J, Soler AP, et al. Cardiac and gastrointestinal liabilities caused by deficiency in the immune modulatory enzyme indoleamine 2,3-dioxygenase. Cancer Biol. Ther. 2011;12:1050–1058. doi: 10.4161/cbt.12.12.18142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Reardon D, Desjardins A, Rixe O, Cloughesy T, Alekar S, Gamelin E, et al. ATIM-29. A phase 1 study of PF-06840003, an oral indole 2,3-dioxygenase 1 (IDO1) inhibitor in patients with malignant gliomas. Neuro-Oncology. 2017;19:vi32. doi: 10.1007/s10637-020-00950-1. [DOI] [PubMed] [Google Scholar]
- 131.Spranger SK, H K, Horton B, Scherle PA, Newton R, Gajewski TF. Mechanism of tumor rejection with doublets of CTLA-4, PD-1:PD-L1, or IDO blockade involves restored IL-2 production and proliferation of CD8+ T cells directly within the tumor microenvironment. J. Immunother. Cancer. 2014;2:1–14. doi: 10.1186/2051-1426-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wainwright, D. A., Chang, A. L., Dey, M., Balyasnikova, I. V., Kim, C. K., Tobias, A., Cheng, Y., Kim, J. W., Qiao, J., Zhang, L., Han, Y. & Lesniak, M. S. Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4 and PD-L1 in mice with brain tumors. Clin. Cancer Res.20, 5290–5301 (2014). [DOI] [PMC free article] [PubMed]
- 133.Toulmonde M, Penel N, Adam J, Chevreau C, Blay JY, Le Cesne A, et al. Use of PD-1 targeting, macrophage infiltration, and IDO pathway activation in sarcomas: a phase 2 clinical trial. JAMA Oncol. 2017;4:93–97. doi: 10.1001/jamaoncol.2017.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhu MMT, Dancsok AR, Nielsen TO. Indoleamine dioxygenase inhibitors: clinical rationale and current development. Curr. Oncol. Rep. 2019;21:2. doi: 10.1007/s11912-019-0750-1. [DOI] [PubMed] [Google Scholar]
- 135.Hamid OG, T F, Frankel AE, Bauer TM, Olszanski AJ, Luke JJ, Balmanoukian AS, Schmidt EV, Sharkey B, Maleski J, Jones MJ, Gangadhar TC. Epacadostat plus pembrolizumab in patients with advanced melanoma: phase 1 and 2 efficacy and safety results from ECHO-202/KEYNOTE-037. Ann. Oncol. 2017;28:v428–v448. [Google Scholar]
- 136.Gibney, G. T., Hamid, O., Lutzky, J., Olszanski, A. J., Mitchell, T. C., Gajewski, T. F. et. al. Phase 1/2 study of epacadostat in combination with ipilimumab in patients with unresectable or metastatic melanoma. J. Immunother. Cancer7, 80 (2019). [DOI] [PMC free article] [PubMed]
- 137.Gangadhar TC, Hamid O, Smith DC, Bauer TM, Wasser JS, Luke JJ, et al. Preliminary results from a phase I / II study of epacadostat (incb024360) in combination with pembrolizumab in patients with selected advanced cancers. J. ImmunoTher. Cancer. 2015;3:O7. [Google Scholar]
- 138.Liu XS, N, Koblish HK, Yang G, Wang Q, Wang K, Leffet L, Hansbury MJ, Thomas B, Rupar M, Waeltz P, Bowman KJ, Polam P, Sparks RB, Yue EW, Li Y, Wynn R, Fridman JS, Burn TC, Combs AP, Newton RC, Scherle PA. Selective inhibition of IDO1 effectively regulates mediators of antitumor immunity. Blood. 2010;115:3520–3530. doi: 10.1182/blood-2009-09-246124. [DOI] [PubMed] [Google Scholar]
- 139.Spranger S, Koblish HK, Horton B, Scherle PA, Newton R, Gajewski TF. Mechanism of tumor rejection with doublets of CTLA-4, PD-1/PD-L1, or IDO blockade involves restored IL-2 production and proliferation of CD8+T cells directly within the tumor microenvironment. J. ImmunoTher. Cancer. 2014;2:1–14. doi: 10.1186/2051-1426-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.https://www.clinicaltrials.gov/.
- 141.Beatty Gregory L., O'Dwyer Peter J., Clark Jason, Shi Jack G., Bowman Kevin J., Scherle Peggy A., Newton Robert C., Schaub Richard, Maleski Janet, Leopold Lance, Gajewski Thomas F. First-in-Human Phase I Study of the Oral Inhibitor of Indoleamine 2,3-Dioxygenase-1 Epacadostat (INCB024360) in Patients with Advanced Solid Malignancies. Clinical Cancer Research. 2017;23(13):3269–3276. doi: 10.1158/1078-0432.CCR-16-2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Siu, L. L. Gelmon, K., Chu, Q., Pachynski, R., Alese, O., Basciano, P., Walker, J., Mitra, P., Zhu, L., Phillips, P., Hunt, J. & Desai, J. BMS-986205, an optimized indoleamine 2,3-dioxygenase 1 (IDO1) inhibitor, is well tolerated with potent pharmacodynamic (PD) activity, alone and in combination with nivolumab (nivo) in advanced cancers in a phase 1/2a trial. Cancer Res.77, CT116 LP–CT (2017).
- 143.Fox E, Oliver T, Rowe M, Thomas S, Zakharia Y, Gilman PB, et al. Indoximod: an immunometabolic adjuvant that empowers T cell activity in cancer. Front. Oncol. 2018;8:370. doi: 10.3389/fonc.2018.00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Soliman HH, Jackson E, Neuger T, Dees CE, Harvey DR, Han H, et al. A first in man phase I trial of the oral immunomodulator, indoximod, combined with docetaxel in patients with metastatic solid tumors. Oncotarget. 2014;5:8136–8146. doi: 10.18632/oncotarget.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Soliman HH, Minton SE, Han HS, Ismail-Khan R, Neuger A, Khambati F, et al. A phase I study of indoximod in patients with advanced malignancies. Oncotarget. 2016;7:22928–22938. doi: 10.18632/oncotarget.8216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mautino MR, Jaipuri FA, Waldo J, Kumar S, Adams J, Van Allen C, et al. Abstract 491: NLG919, a novel indoleamine-2,3-dioxygenase (IDO)-pathway inhibitor drug candidate for cancer therapy. Cancer Res. 2013;73:491LP. [Google Scholar]
- 147.Nayak-Kapoor A, Hao Z, Sadek R, Dobbins R, Marshall L, Vahanian NN, et al. Phase Ia study of the indoleamine 2,3-dioxygenase 1 (IDO1) inhibitor navoximod (GDC-0919) in patients with recurrent advanced solid tumors. J. Immunother. Cancer. 2018;6:61. doi: 10.1186/s40425-018-0351-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Crosignani S, Bingham P, Bottemanne P, Cannelle H, Cauwenberghs S, Cordonnier M, et al. Discovery of a novel and selective indoleamine 2,3-dioxygenase (IDO-1) inhibitor 3-(5-fluoro-1H-indol-3-yl)pyrrolidine-2,5-dione (EOS200271/PF-06840003) and its characterization as a potential clinical candidate. J. Med. Chem. 2017;60:9617–9629. doi: 10.1021/acs.jmedchem.7b00974. [DOI] [PubMed] [Google Scholar]
- 149.Tumang J, Gomes B, Wythes M, Crosignani S, Bingham P, Bottemanne P, et al. Abstract 4863: PF-06840003: a highly selective IDO-1 inhibitor that shows good in vivo efficacy in combination with immune checkpoint inhibitors. Cancer Res. 2016;76:4863LP. [Google Scholar]
- 150.Yap TA, Sahebjam S, Hong DS, Chiu VK, Yilmaz E, Efuni S, et al. First-in-human study of KHK2455, a long-acting, potent and selective indoleamine 2,3-dioxygenase 1 (IDO-1) inhibitor, in combination with mogamulizumab (Moga), an anti-CCR4 monoclonal antibody, in patients (pts) with advanced solid tumors. J. Clin. Oncol. 2018;36:3040. [Google Scholar]
- 151.Dorsey FC, Benhadji KA, Sams LL, Young DA, Schindler JF, Huss KL, et al. Abstract 5245: identification and characterization of the IDO1 inhibitor LY3381916. Cancer Res. 2018;78:5245. [Google Scholar]
- 152.Mautino MR, Kumar S, Zhuang H, Waldo J, Jaipuri F, Potturi H, et al. Abstract 4076: A novel prodrug of indoximod with enhanced pharmacokinetic properties. Cancer Res. 2017;77:4076. [Google Scholar]
- 153.Zhang Q, Zhang Y, Boer J, Shi JG, Hu P, Diamond S, et al. In vitro interactions of epacadostat and its major metabolites with human efflux and uptake transporters: implications for pharmacokinetics and drug interactions. Drug Metab. Dispos. 2017;45:612–623. doi: 10.1124/dmd.116.074609. [DOI] [PubMed] [Google Scholar]
- 154.Zhang W, Shannon WD, Duncan J, Scheffer GL, Scheper RJ, McLeod HL. Expression of drug pathway proteins is independent of tumour type. J. Pathol. 2006;209:213–219. doi: 10.1002/path.1955. [DOI] [PubMed] [Google Scholar]
- 155.Lewis HC, Chinnadurai R, Bosinger SE, Galipeau J. The IDO inhibitor 1-methyl tryptophan activates the aryl hydrocarbon receptor response in mesenchymal stromal cells. Oncotarget. 2017;8:91914–91927. doi: 10.18632/oncotarget.20166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Moyer BJ, Rojas IY, Murray IA, Lee S, Hazlett HF, Perdew GH, et al. Indoleamine 2,3-dioxygenase 1 (IDO1) inhibitors activate the aryl hydrocarbon receptor. Toxicol. Appl. Pharmacol. 2017;323:74–80. doi: 10.1016/j.taap.2017.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Vogel CF, Goth SR, Dong B, Pessah IN, Matsumura F. Aryl hydrocarbon receptor signaling mediates expression of indoleamine 2,3-dioxygenase. Biochem. Biophys. Res. Commun. 2008;375:331–335. doi: 10.1016/j.bbrc.2008.07.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Vogel CF, Wu D, Goth SR, Baek J, Lollies A, Domhardt R, et al. Aryl hydrocarbon receptor signaling regulates NF-kappaB RelB activation during dendritic-cell differentiation. Immunol. Cell Biol. 2013;91:568–575. doi: 10.1038/icb.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yamamoto T, Hatabayashi K, Arita M, Yajima N, Takenaka C, Suzuki T, et al. Kynurenine signaling through the aryl hydrocarbon receptor maintains the undifferentiated state of human embryonic stem cells. Sci. Signal. 2019;12:eaaw3306. doi: 10.1126/scisignal.aaw3306. [DOI] [PubMed] [Google Scholar]
- 160.Monjazeb AM, Kent MS, Grossenbacher SK, Mall C, Zamora AE, Mirsoian A, et al. Blocking indolamine-2,3-dioxygenase rebound immune suppression boosts antitumor effects of radio-immunotherapy in murine models and spontaneous canine malignancies. Clin. Cancer Res. 2016;22:4328–4340. doi: 10.1158/1078-0432.CCR-15-3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Creelan BC, Antonia S, Bepler G, Garrett TJ, Simon GR, Soliman HH. Indoleamine 2,3-dioxygenase activity and clinical outcome following induction chemotherapy and concurrent chemoradiation in Stage III non-small cell lung cancer. Oncoimmunology. 2013;2:e23428. doi: 10.4161/onci.23428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Barrero Maria. Epigenetic Strategies to Boost Cancer Immunotherapies. International Journal of Molecular Sciences. 2017;18(6):1108. doi: 10.3390/ijms18061108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dewi DL, Mohapatra SR, Blanco Cabanes S, Adam I, Somarribas Patterson LF, Berdel B, et al. Suppression of indoleamine-2,3-dioxygenase 1 expression by promoter hypermethylation in ER-positive breast cancer. Oncoimmunology. 2017;6:e1274477. doi: 10.1080/2162402X.2016.1274477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat. Rev. Cancer. 2016;16:275–287. doi: 10.1038/nrc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Shapira-Frommer R, Minei TR, Barshack I, Mendel I, Yakov N, Cohen YC, et al. Abstract 4979: Ofranergene Obadenovec (VB-111), an anti-cancer gene therapy, induces immunologic responses in solid tumors transforming cold tumors to hot tumors. Cancer Res. 2019;79:4979. [Google Scholar]
- 166.Rohrig UF, Majjigapu SR, Caldelari D, Dilek N, Reichenbach P, Ascencao K, et al. 1,2,3-Triazoles as inhibitors of indoleamine 2,3-dioxygenase 2 (IDO2) Bioorg. Med. Chem. Lett. 2016;26:4330–4333. doi: 10.1016/j.bmcl.2016.07.031. [DOI] [PubMed] [Google Scholar]
- 167.Ochs K, Ott M, Rauschenbach KJ, Deumelandt K, Sahm F, Opitz CA, et al. Tryptophan-2,3-dioxygenase is regulated by prostaglandin E2 in malignant glioma via a positive signaling loop involving prostaglandin E receptor-4. J. Neurochem. 2015;136:1142–1154. doi: 10.1111/jnc.13503. [DOI] [PubMed] [Google Scholar]
- 168.Hashemi Goradel N, Najafi M, Salehi E, Farhood B, Mortezaee K. Cyclooxygenase-2 in cancer: a review. J. Cell. Physiol. 2019;234:5683–5699. doi: 10.1002/jcp.27411. [DOI] [PubMed] [Google Scholar]
- 169.Tong D, Liu Q, Wang LA, Xie Q, Pang J, Huang Y, et al. The roles of the COX2/PGE2/EP axis in therapeutic resistance. Cancer Metastasis Rev. 2018;37:355–368. doi: 10.1007/s10555-018-9752-y. [DOI] [PubMed] [Google Scholar]
- 170.Salter M, Hazelwood R, Pogson CI, Iyer R, Madge DJ. The effects of a novel and selective inhibitor of tryptophan 2,3-dioxygenase on tryptophan and serotonin metabolism in the rat. Biochem. Pharmacol. 1995;49:1435–1442. doi: 10.1016/0006-2952(95)00006-l. [DOI] [PubMed] [Google Scholar]
- 171.Salter M, Hazelwood R, Pogson CI, Iyer R, Madge DJ, Jones HT, et al. The effects of an inhibitor of tryptophan 2,3-dioxygenase and a combined inhibitor of tryptophan 2,3-dioxygenase and 5-HT reuptake in the rat. Neuropharmacology. 1995;34:217–227. doi: 10.1016/0028-3908(94)00147-k. [DOI] [PubMed] [Google Scholar]
- 172.Kozlova A, Frederick R. Current state on tryptophan 2,3-dioxygenase inhibitors: a patent review. Expert Opin. Ther. Pat. 2019;29:11–23. doi: 10.1080/13543776.2019.1556638. [DOI] [PubMed] [Google Scholar]
- 173.Hsu Y-L, Hung J-Y, Chiang S-Y, Jian S-F, Wu C-Y, Lin Y-S, et al. Lung cancer-derived galectin-1 contributes to cancer associated fibroblast-mediated cancer progression and immune suppression through TDO2/kynurenine axis. Oncotarget. 2016;7:27584–27598. doi: 10.18632/oncotarget.8488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Dolusic E, Larrieu P, Moineaux L, Stroobant V, Pilotte L, Colau D, et al. Tryptophan 2,3-dioxygenase (TDO) inhibitors. 3-(2-(pyridyl)ethenyl)indoles as potential anticancer immunomodulators. J. Med. Chem. 2011;54:5320–5334. doi: 10.1021/jm2006782. [DOI] [PubMed] [Google Scholar]
- 175.Gyulveszi G, Fischer C, Mirolo M, Stern M, Green L, Ceppi M, et al. Abstract LB-085: RG70099: a novel, highly potent dual IDO1/TDO inhibitor to reverse metabolic suppression of immune cells in the tumor micro-environment. Cancer Res. 2016;76:LB-085. [Google Scholar]
- 176.Gullapalli S, Roychowdhury A, Khaladkar T, Sawargave S, Janrao R, Kalhapure V, et al. Abstract 1701: EPL-1410, a novel fused heterocycle based orally active dual inhibitor of IDO1/TDO2, as a potential immuno-oncology therapeutic. Cancer Res. 2018;78:1701. [Google Scholar]
- 177.Kim C, Kim JH, Kim JS, Chon HJ, Kim J-H. A novel dual inhibitor of IDO and TDO, CMG017, potently suppresses the kynurenine pathway and overcomes resistance to immune checkpoint inhibitors. J. Clin. Oncol. 2019;37:e14228. [Google Scholar]
- 178.Winters M, DuHadaway JB, Pham KN, Lewis-Ballester A, Badir S, Wai J, et al. Diaryl hydroxylamines as pan or dual inhibitors of indoleamine 2,3-dioxygenase-1, indoleamine 2,3-dioxygenase-2 and tryptophan dioxygenase. Eur. J. Med. Chem. 2019;162:455–464. doi: 10.1016/j.ejmech.2018.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wang J, Takahashi RH, DeMent K, Gustafson A, Kenny JR, Wong SG, et al. Development of a mass spectrometry-based tryptophan 2, 3-dioxygenase assay using liver cytosol from multiple species. Anal. Biochem. 2018;556:85–90. doi: 10.1016/j.ab.2018.06.025. [DOI] [PubMed] [Google Scholar]
- 180.Larkin PB, Sathyasaikumar KV, Notarangelo FM, Funakoshi H, Nakamura T, Schwarcz R, et al. Tryptophan 2,3-dioxygenase and indoleamine 2,3-dioxygenase 1 make separate, tissue-specific contributions to basal and inflammation-induced kynurenine pathway metabolism in mice. Biochim. Biophys. Acta. 2016;1860:2345–2354. doi: 10.1016/j.bbagen.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Liu Ling, Su Xiaoyang, Quinn William J., Hui Sheng, Krukenberg Kristin, Frederick David W., Redpath Philip, Zhan Le, Chellappa Karthikeyani, White Eileen, Migaud Marie, Mitchison Timothy J., Baur Joseph A., Rabinowitz Joshua D. Quantitative Analysis of NAD Synthesis-Breakdown Fluxes. Cell Metabolism. 2018;27(5):1067-1080.e5. doi: 10.1016/j.cmet.2018.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Verdin E. NAD(+) in aging, metabolism, and neurodegeneration. Science. 2015;350:1208–1213. doi: 10.1126/science.aac4854. [DOI] [PubMed] [Google Scholar]
- 183.Tummala KS, Gomes AL, Yilmaz M, Grana O, Bakiri L, Ruppen I, et al. Inhibition of de novo NAD(+) synthesis by oncogenic URI causes liver tumorigenesis through DNA damage. Cancer Cell. 2014;26:826–839. doi: 10.1016/j.ccell.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 184.Hjortso MD, Larsen SK, Kongsted P, Met O, Frosig TM, Andersen GH, et al. Tryptophan 2,3-dioxygenase (TDO)-reactive T cells differ in their functional characteristics in health and cancer. Oncoimmunology. 2015;4:e968480. doi: 10.4161/21624011.2014.968480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sorensen RB, Berge-Hansen L, Junker N, Hansen CA, Hadrup SR, Schumacher TN, et al. The immune system strikes back: cellular immune responses against indoleamine 2,3-dioxygenase. PLoS ONE. 2009;4:e6910. doi: 10.1371/journal.pone.0006910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sorensen RB, Hadrup SR, Svane IM, Hjortso MC, Thor Straten P, Andersen MH. Indoleamine 2,3-dioxygenase specific, cytotoxic T cells as immune regulators. Blood. 2011;117:2200–2210. doi: 10.1182/blood-2010-06-288498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sorensen RB, Kollgaard T, Andersen RS, van den Berg JH, Svane IM, Straten P, et al. Spontaneous cytotoxic T-cell reactivity against indoleamine 2,3-dioxygenase-2. Cancer Res. 2011;71:2038–2044. doi: 10.1158/0008-5472.CAN-10-3403. [DOI] [PubMed] [Google Scholar]
- 188.Iversen TZ, Engell-Noerregaard L, Ellebaek E, Andersen R, Larsen SK, Bjoern J, et al. Long-lasting disease stabilization in the absence of toxicity in metastatic lung cancer patients vaccinated with an epitope derived from indoleamine 2,3 dioxygenase. Clin. Cancer Res. 2014;20:221–232. doi: 10.1158/1078-0432.CCR-13-1560. [DOI] [PubMed] [Google Scholar]
- 189.Bjoern J, Iversen TZ, Nitschke NJ, Andersen MH, Svane IM. Safety, immune and clinical responses in metastatic melanoma patients vaccinated with a long peptide derived from indoleamine 2,3-dioxygenase in combination with ipilimumab. Cytotherapy. 2016;18:1043–1055. doi: 10.1016/j.jcyt.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 190.Nitschke NJ, Bjoern J, Iversen TZ, Andersen MH, Svane IM. Indoleamine 2,3-dioxygenase and survivin peptide vaccine combined with temozolomide in metastatic melanoma. Stem Cell Investig. 2017;4:77. doi: 10.21037/sci.2017.08.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kjeldsen JW, Iversen TZ, Engell-Noerregaard L, Mellemgaard A, Andersen MH, Svane IM. Durable clinical responses and long-term follow-up of stage III-IV non-small-cell lung cancer (NSCLC) patients treated with IDO peptide vaccine in a phase I study-a brief research report. Front. Immunol. 2018;9:2145. doi: 10.3389/fimmu.2018.02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Cheong, J. E. & Sun, L. Targeting the IDO1/TDO2–KYN–AhR pathway for cancer immunotherapy – challenges and opportunities. Trends Pharmacol. Sci.38, 307–325 (2018). [DOI] [PubMed]
- 193.Triplett TA, Garrison KC, Marshall N, Donkor M, Blazeck J, Lamb C, et al. Reversal of indoleamine 2,3-dioxygenase-mediated cancer immune suppression by systemic kynurenine depletion with a therapeutic enzyme. Nat. Biotechnol. 2018;36:758–764. doi: 10.1038/nbt.4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Fukui, S., Schwarcz, R., Rapoport, S. I., Takada, Y. & Smith, Q. R. Blood-brain barrier transport of kynurenines: implications for brain synthesis and metabolism. J. Neurochem.56, 2007–2017 (1991). [DOI] [PubMed]
- 195.Guastella Anthony R., Michelhaugh Sharon K., Klinger Neil V., Kupsky William J., Polin Lisa A., Muzik Otto, Juhász Csaba, Mittal Sandeep. Tryptophan PET Imaging of the Kynurenine Pathway in Patient-Derived Xenograft Models of Glioblastoma. Molecular Imaging. 2016;15:153601211664488. doi: 10.1177/1536012116644881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Murray IA, Patterson AD, Perdew GH. Aryl hydrocarbon receptor ligands in cancer: friend and foe. Nat. Rev. Cancer. 2014;14:801–814. doi: 10.1038/nrc3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Pinto, S., Steeneck, C., Albers, M., Anderhub, S., Birkel, M., Buselic-Wölfel, L. et al. Abstract 1210: targeting the IDO1-kynurenine-AhR pathway for cancer immunotherapy. Cancer Res.79, 1210 (2019).
- 198.Gentil M, Hugues P, Desterke C, Telliam G, Sloma I, Souza LEB, et al. Aryl hydrocarbon receptor (AHR) is a novel druggable pathway controlling malignant progenitor proliferation in chronic myeloid leukemia (CML) PLoS ONE. 2018;13:e0200923. doi: 10.1371/journal.pone.0200923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kim, D., Yoon, S.-S., Shin, D.-Y. Abstract 4675: aryl hydrocarbon receptor antagonist enhances cord blood-derived human hematopoietic stem cell expansion and platelet formation. Cancer Res.79, 4675 (2019).
- 200.Scoville SD, Nalin AP, Chen L, Chen L, Zhang MH, McConnell K, et al. Human AML activates the aryl hydrocarbon receptor pathway to impair NK cell development and function. Blood. 2018;132:1792–1804. doi: 10.1182/blood-2018-03-838474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Boitano AE, Wang J, Romeo R, Bouchez LC, Parker AE, Sutton SE, et al. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science. 2010;329:1345–1348. doi: 10.1126/science.1191536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zhao B, Degroot DE, Hayashi A, He G, Denison MS. CH223191 is a ligand-selective antagonist of the Ah (Dioxin) receptor. Toxicol. Sci. 2010;117:393–403. doi: 10.1093/toxsci/kfq217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Tchaicha J, Coma S, Walsh M, Cavanaugh J, Sanchez-Martin M, Zhang XM, et al. Abstract 4131: overcoming aryl hydrocarbon receptor mediated tumor immunosuppression. Cancer Res. 2019;79:4131. [Google Scholar]
- 204.Garcia C, Lemar H, Galang C, Joseph J, Gonzalez-Lopez M, Hager J, et al. Abstract 3255: a novel small molecule inhibitor of AhR suppresses the polarization and activity of M2 macrophages. Cancer Res. 2019;79:3255. [Google Scholar]
- 205.Gutcher, I., Kober, C., Roese, L., Roewe, J., Schmees, N., Prinz, F. et al. Abstract 1288: blocking tumor-associated immune suppression with BAY-218, a novel, selective aryl hydrocarbon receptor (AhR) inhibitor. Cancer Res.79, 1288 (2019).
- 206.Schmees, N., Gutcher, I., Roehn, U., Irlbacher, H., Stoeckigt, D., Bader, B. et al. Abstract 4454: identification of BAY-218, a potent and selective small-molecule AhR inhibitor, as a new modality to counteract tumor immunosuppression. Cancer Res.79, 4454 (2019).
- 207.US National Institutes of Health. ClinicalTrials.gov. NCT04069026. Accessed 28 Oct 2019. https://clinicaltrials.gov/ct2/show/NCT04069026 (2019).
- 208.Sadok I, Gamian A, Staniszewska MM. Chromatographic analysis of tryptophan metabolites. J. Sep. Sci. 2017;40:3020–3045. doi: 10.1002/jssc.201700184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Konishi M, Ebner N, Springer J, Schefold JC, Doehner W, Dschietzig TB, et al. Impact of plasma kynurenine level on functional capacity and outcome in heart failure― results from studies investigating co-morbidities aggravating heart failure (SICA-HF) Circ. J. 2017;81:52–61. doi: 10.1253/circj.CJ-16-0791. [DOI] [PubMed] [Google Scholar]
- 210.Maliniemi P, Laukkanen K, Väkevä L, Dettmer K, Lipsanen T, Jeskanen L, et al. Biological and clinical significance of tryptophan-catabolizing enzymes in cutaneous T-cell lymphomas. OncoImmunology. 2017;6:e1273310. doi: 10.1080/2162402X.2016.1273310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Reichetzeder C, Heunisch F, Einem GV, Tsuprykov O, Kellner KH, Dschietzig T, et al. Pre-interventional kynurenine predicts medium-term outcome after contrast media exposure due to coronary angiography. Kidney Blood Press. Res. 2017;42:244–256. doi: 10.1159/000477222. [DOI] [PubMed] [Google Scholar]
- 212.Ait-Belkacem R, Bol V, Hamm G, Schramme F, Van Den Eynde B, Poncelet L, et al. Microenvironment tumor metabolic interactions highlighted by qMSI: application to the tryptophan-kynurenine pathway in immuno-oncology. SLAS Discov. 2017;22:1182–1192. doi: 10.1177/2472555217712659. [DOI] [PubMed] [Google Scholar]
- 213.Giglio BC, Fei H, Wang M, Wang H, He L, Feng H, et al. Synthesis of 5-[(18)F]Fluoro-alpha-methyl tryptophan: new Trp based PET agents. Theranostics. 2017;7:1524–1530. doi: 10.7150/thno.19371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Henrottin J, Lemaire C, Egrise D, Zervosen A, Van den Eynde B, Plenevaux A, et al. Fully automated radiosynthesis of N(1)-[(18)F]fluoroethyl-tryptophan and study of its biological activity as a new potential substrate for indoleamine 2,3-dioxygenase PET imaging. Nucl. Med. Biol. 2016;43:379–389. doi: 10.1016/j.nucmedbio.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 215.Henrottin J, Zervosen A, Lemaire C, Sapunaric F, Laurent S, Van den Eynde B, et al. N (1)-Fluoroalkyltryptophan analogues: synthesis and in vitro study as potential substrates for indoleamine 2,3-dioxygenase. ACS Med. Chem. Lett. 2015;6:260–265. doi: 10.1021/ml500385d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Michelhaugh SK, Muzik O, Guastella AR, Klinger NV, Polin LA, Cai H, et al. Assessment of tryptophan uptake and kinetics using 1-(2-18F-fluoroethyl)-l-tryptophan and alpha-11C-methyl-l-tryptophan PET imaging in mice implanted with patient-derived brain tumor xenografts. J. Nucl. Med. 2017;58:208–213. doi: 10.2967/jnumed.116.179994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Tang T, Gill HS, Ogasawara A, Tinianow JN, Vanderbilt AN, Williams SP, et al. Preparation and evaluation of L- and D-5-[(18)F]fluorotryptophan as PET imaging probes for indoleamine and tryptophan 2,3-dioxygenases. Nucl. Med. Biol. 2017;51:10–17. doi: 10.1016/j.nucmedbio.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 218.Xin Y, Cai H. Improved radiosynthesis and biological evaluations of L- and D-1-[(18)F]fluoroethyl-tryptophan for PET imaging of IDO-mediated kynurenine pathway of tryptophan metabolism. Mol. Imaging Biol. 2017;19:589–598. doi: 10.1007/s11307-016-1024-z. [DOI] [PubMed] [Google Scholar]
- 219.Kimura, R. H., Wang, L., Shen, B., Huo, L., Tummers, W., Filipp, F. V. et al. Evaluation of integrin αvβ6 cystine knot PET tracers to detect cancer and idiopathic pulmonary fibrosis. Nat. Commun.10, 4673 (2019). [DOI] [PMC free article] [PubMed]
- 220.Schmidt Linnéa, Møller Mia, Haldrup Christa, Strand Siri H., Vang Søren, Hedegaard Jakob, Høyer Søren, Borre Michael, Ørntoft Torben, Sørensen Karina Dalsgaard. Exploring the transcriptome of hormone-naive multifocal prostate cancer and matched lymph node metastases. British Journal of Cancer. 2018;119(12):1527–1537. doi: 10.1038/s41416-018-0321-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data from The Cancer Genome Atlas (TCGA) and Genotype-Tissue Expression (GTEx) Project is publicly available.