Mcr is the key enzyme in methanogenesis and a promising candidate for bioengineering the conversion of methane to liquid fuel. Our knowledge of Mcr is still limited. In terms of complexity, uniqueness, and environmental importance, Mcr is more comparable to photosynthetic reaction centers than conventional enzymes. PTMs have long been hypothesized to play key roles in modulating Mcr activity. Here, we directly link the mmpX gene to the arginine PTM of Mcr, demonstrate its association with methanogenesis activity, and offer insights into its substrate specificity and putative cofactor binding sites. This is also the first time that a PTM of McrA has been shown to have a substantial impact on both methanogenesis and growth in the absence of additional stressors.
KEYWORDS: Methanococcus, methanogen marker protein, methanogenesis, methyl coenzyme M reductase, posttranslational modification
ABSTRACT
Catalyzing the key step for anaerobic production and/or oxidation of methane and likely other short-chain alkanes, methyl coenzyme M reductase (Mcr) and its homologs play a key role in the global carbon cycle. The McrA subunit possesses up to five conserved posttranslational modifications (PTMs) at its active site. It was previously suggested that methanogenesis marker protein 10 (Mmp10) could play an important role in methanogenesis. To systematically examine its physiological role, mmpX (locus tag MMP1554), the gene encoding Mmp10 in Methanococcus maripaludis, was deleted with a new genetic tool, resulting in the complete loss of the 5-C-(S)-methylarginine PTM of residue 275 in the McrA subunit. When the ΔmmpX mutant was complemented with the wild-type gene expressed by either a strong or a weak promoter, methylation was fully restored. Compared to the parental strain, maximal rates of methane formation by whole cells were reduced by 40 to 60% in the ΔmmpX mutant. The reduction in activity was fully reversed by the complement with the strong promoter. Site-directed mutagenesis of mmpX resulted in a differential loss of arginine methylation among the mutants in vivo, suggesting that activities of Mmp10 directly modulated methylation. R275 was present in a highly conserved PXRR275(A/S)R(G/A) signature sequence in McrAs. The only other protein in M. maripaludis containing a similar sequence was not methylated, suggesting that Mmp10 is specific for McrA. In conclusion, Mmp10 modulates the methyl-Arg PTM on McrA in a highly specific manner, which has a profound impact on Mcr activity.
IMPORTANCE Mcr is the key enzyme in methanogenesis and a promising candidate for bioengineering the conversion of methane to liquid fuel. Our knowledge of Mcr is still limited. In terms of complexity, uniqueness, and environmental importance, Mcr is more comparable to photosynthetic reaction centers than conventional enzymes. PTMs have long been hypothesized to play key roles in modulating Mcr activity. Here, we directly link the mmpX gene to the arginine PTM of Mcr, demonstrate its association with methanogenesis activity, and offer insights into its substrate specificity and putative cofactor binding sites. This is also the first time that a PTM of McrA has been shown to have a substantial impact on both methanogenesis and growth in the absence of additional stressors.
INTRODUCTION
Methyl coenzyme M reductase (Mcr) catalyzes the last step of methane formation in all methanogenic Archaea (methanogens) as well as the first step of anaerobic methane oxidation in the methanotrophic Archaea (ANME) (1–3). It catalyzes the reversible reaction shown below that results in the production of 500 to 600 Tg of CH4 and the oxidation of 70 to 300 Tg of CH4 per year (4), CH3-S-CoM + HS-CoB ↔ CH4 + CoM-S-S-CoB, where CH3-S-CoM is methyl coenzyme M, HS-CoB is coenzyme B, and CoM-S-S-CoB is the heterodisulfide of the two coenzymes. CH4 is an important biofuel as well as a potential feedstock for the chemical industry if it could be converted by Mcr to a liquid biofuel with a high energy density (5, 6). CH4 is also a potent greenhouse gas, increases of which are contributing to global warming (4). Therefore, understanding the biochemistry of Mcr is significant not only to advance a CH4-based bioeconomy but also to develop environmental CH4 mitigation strategies (7). Moreover, pathways for metabolizing methane have recently been found in novel archaeal lineages outside the well-established methanogens and ANME (7). Although the details of the pathways vary, they all possess homologs of Mcr (8). Some of these homologs are proposed to oxidize short-chain alkanes such as ethane, butane, and possibly propane (9, 10), essentially expanding our knowledge from ANME to NAOA (anaerobic alkane-oxidizing Archaea) (3, 11–15). These novel findings not only extend the roles of Mcr in the global carbon cycle but also present exciting new opportunities for biotechnology (11).
Although Mcr is notoriously difficult to study due to its extreme sensitivity to oxygen and the lability of the active reduced form, structural and biochemical studies have uncovered many unique aspects (1, 16, 17). Using Methanothermobacter marburgensis Mcr as a model, Mcr has been shown to be a hexameric, 300-kDa protein composed of three different subunits in an α2β2γ2 configuration (1). It contains two molecules of an unusual Ni tetrapyrrole, coenzyme F430 (or methylthio-F430 in ANME-1), which is tightly but not covalently bound (1, 16). During enzymatic catalysis, it was recently proposed that the Ni(I) of coenzyme F430 attacks the sulfur atom of methyl coenzyme M, producing a methyl radical intermediate (17). The enzyme possesses two identical active sites, each of which contains up to five posttranslationally modified amino acid residues (1). These posttranslational modifications (PTMs) are highly conserved but not universal. For instance, in Methanococcus maripaludis, PTMs include (M. maripaludis numbering) 1-N-methyl-His261, 5-C-(S)-methyl-Arg275, 2-(S)-methyl-Gln403, and thio-Gly448, which are all found within the McrA subunit (18). The S-methylation of cysteine, which is common in the Mcrs of many methanogens (19), is present at a low abundance or absent (18). Generally, the PTMs are highly conserved among the methanogens if not the ANME. Similarly, arginine methylation has been found in all methanogenic Mcrs examined but not ANME-1 Mcr (19). New PTMs continue to be discovered, such as didehydro-Asp and hydroxyl-Trp (20, 21). However, these PTMs do not appear to be widely distributed.
Given the wide occurrence but limited diversity of most of these PTMs across the Mcrs from distantly related species, they are believed to be important for catalysis. On the other hand, the absence of some of these PTMs in certain NAOA lineages may contribute to their diversification from methanogenesis to anaerobic alkane oxidation (12). However, little is known about the biosynthesis or function of these PTMs since the suggestion that S-adenosylmethionine (SAM) would be the methyl donor for the methylations (22). Recently, tfuA and ycaO homologs were found to be required for the thio-Gly PTM in Methanosarcina acetivorans (23). In vitro biochemical characterization of the TfuA and YcaO proteins suggests that they catalyze the thioamidation reaction in an ATP-dependent manner and require an external sulfide source (24). Notably, ycaO belongs to a list of conserved genes associated with methanogenesis previously identified by a bioinformatic study and named methanogen marker 1 (25).
The same list also identified methanogen marker 10, a gene that is divergently transcribed from the mcr operon in many methanogens (25). Sarmiento et al. subsequently showed that this gene is not essential for the growth of M. maripaludis, suggesting that its function could be studied by mutagenesis (26). In a preprint, we demonstrated that this gene (locus tag MMP1554, named here mmpX using the roman numeral X for 10 to conform with prokaryotic gene nomenclature), a gene that encodes a putative SAM-dependent methyltransferase, was required for the 5-C-(S)-methyl-Arg PTM in M. maripaludis (27). Similar findings were also reported for M. acetivorans (28), in which the mmpX gene was replaced by the puromycin antibiotic resistance cassette. Growth of the M. acetivorans mutant on methanol, trimethylamine, or acetate was largely unaffected. However, growth was more impaired in the presence of H2O2 or high temperature. The thermal stability of the unmethylated Mcr of the mutant was also reduced, suggesting that Arg methylation plays a role in Mcr stability and structural integrity. However, these results were ambiguous in two respects. First, complementation of the M. acetivorans mutation was not performed, so it was possible that the phenotype arose from an additional but unidentified mutation elsewhere in the genome. Second, the antibiotic resistance marker was not removed, and the possibilities of polar mutations and direct effects of the resistance marker were not addressed. More recently, the recombinant M. acetivorans protein MaMmp10 was shown to catalyze the methylation of a peptide mimicking the PTM site of Mcr (29). This radical SAM protein required cobalamin for activity (29). However, the roles of mmpX in PTM, methanogenesis, and growth are still incompletely understood. Here, we present our study addressing these roles through markerless gene deletion, gene complementation, and site-directed mutagenesis analyses.
RESULTS
A new tool for markerless deletion of mmpX.
To facilitate the construction of markerless deletions, a new plasmid vector, p5L-R, was constructed by assembling standardized genetic modules through BioBrick assembly (see Fig. S1 in the supplemental material). The standard modules included a pUC57 backbone, a methanococcal promoter, selectable markers, methanococcal ribosomal binding sites (RBSs), and repetitive elements (REs), which were all BioBrick compatible. The full protocol, standardized primers, and plasmid sequence for markerless deletions using p5L-R can be found in the supplemental material (Table S1 and Fig. S2). Briefly, both positive and negative markers were used to select for marker integration and removal from the methanococcal genome, respectively. The selectable markers were then flanked with two identical REs, forming an RMR module, i.e., RE-markers-RE, of which the REs can undergo homologous recombination to remove the markers and leave a short scar (Fig. S2). This marker removal strategy has been described for Pyrococcus furiosus, suggesting that RE size affects homologous-recombination efficiency (30). Of the various RE sizes of 20, 40, 60, 120, and 180 bp examined for Methanococcus, a minimal RE of 40 bp was necessary for detectable marker removal. With an RE of 60 bp, marker deletion was always observed when making several mutants, including the ΔmmpX mutant (Fig. S3).
Mmp10 is necessary for arginine PTM.
M. maripaludis mutants were constructed to investigate the role of Mmp10 in PTMs of Mcr. Since mmpX is located next to the mcr operon, in-frame deletion of mmpX was performed to avoid polar effects that might otherwise affect the expression of mcr (Fig. 1). This generated strain S0030 that possessed an mmpX deletion in the S0001 background (Table 1). Two additional strains, S0031 and S0034, were made in the S0030 background to complement the deletion. In strain S0031, the ΔmmpX mutation was complemented with the mmpX gene expressed under the control of Pnat, the predicted native promoter. This promoter included the entire 259-bp intergenic region and the predicted B recognition element (BRE) and TATA sequences 164 to 174 bp upstream of the mmpX start codon. In strain S0034, the deletion was complemented with mmpX expressed under the control of PhmvA, a constitutively strong promoter commonly used for M. maripaludis (31–33). The relative transcription rates of the Pnat and PhmvA promoters were examined using an mCherry reporter system in strains S0032 and S0033, respectively. The mCherry fluorescence for S0032 was around 30-fold lower than that for S0033; i.e., the arbitrary fluorescence values in optical density (OD) units per milliliter (averages and standard deviations from three measurements) were 19.3 ± 1.5 and 618 ± 40, respectively. Therefore, strains S0031 and S0034 were designated weak and strong mmpX complementations, respectively.
FIG 1.
Physical proximity of mmpX and the mcr operon in the genome of M. maripaludis. mmpX (locus tag MMP1554) is transcribed divergently from the adjacent mcrBCDGA operon that encodes the methyl coenzyme M reductase (59). The intergenic sequence between mmpX and mcr is only 259 bp long. Therefore, Pmcr, the ∼290-bp-long promoter that drives mcr expression (48), extends partially into the coding region of mmpX. The exact promoter sequence for mmpX is unknown, but the B recognition element (BRE) and TATA box sequences are predicted to be within the intergenic sequence. Thus, the entire 259-bp intergenic sequence was taken as the predicted mmpX native promoter, namely, Pnat, in this study.
TABLE 1.
Plasmids and microbial strains
| Plasmid or strain | Description | Reference |
|---|---|---|
| Plasmids | ||
| p5L-R | Template vector for PCR amplification of the RMR module | This study |
| Mm10 | Linear plasmid made by flanking RMR with 1554U and 1554D | This study |
| pMEV4 | Expression vector for the M. maripaludis S0001 chassis | 31 |
| p4MK10 | mmpX cloned into pMEV4 | This study |
| p4MK10t | Truncated mmpX cloned into pMEV4 | This study |
| pMEV4m | Synthetic mCherry reporter cloned into pMEV4 | This study |
| pM10m | pMEV4m modification by replacing the PhmvA promoter with the native promoter for mmpX | This study |
| pM10 | pM10m modification by replacing the mCherry gene with mmpX | This study |
| Strains | ||
| S0001 | Expression host containing ORF1 from pURB500 integrated into the M. maripaludis Mm900 genome | 60 |
| S0030 | ΔmmpX mutant in the S0001 background | This study |
| S0031 | Recombinant S0030 + pM10 vector (Pnat-mmpX) | This study |
| S0032 | Recombinant S0001 + pM10m vector (Pnat-mCherry) | This study |
| S0033 | Recombinant S0001 + pMEV4m vector (PhmvA-mCherry) | This study |
| S0034 | Recombinant S0030 + p4MK10 vector (PhmvA-mmpX) | This study |
| S0035 | Recombinant S0001 + pM10 vector (Pnat-mmpX) | This study |
| S0036 | Recombinant S0001 + p4MK10 vector (PhmvA-mmpX) | This study |
| S0037 | Recombinant S0001 + pMcrS1 vector (PhmvA-mcr-his) | This study |
| S0039 | Recombinant S0030 + p4MK10t vector (PhmvA-mmpXtrun) | This study |
| S0040 | Recombinant S0001 + pMEV0140 vector (PhmvA-0140-his) | This study |
Mcr proteins were purified from the parental and mutant strains to determine the PTMs by liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis. Through trypsin digestions, three of the PTMs, 1-N-methyl-His261, 2-(S)-methyl-Gln403, and thio-Gly448 of McrA, were found in the enzymes from the parental and ΔmmpX strains. However, trypsin digestion at the 5-C-(S)-methyl-Arg275 site caused the loss of most of the peptides containing this PTM (data not shown). To confirm the presence of the Arg275 PTM, a pepsin degradation was developed, which yielded the peptide PGRr275ARGPNEPGGIRF or MGNALPGRr275ARGPNEPGGIRF, where r is 5-C-(S)-methyl-Arg. This enabled the unambiguous quantification of the Arg PTM. Based on peak area integration, 5-C-(S)-methyl-Arg275 was absent in Mcr of the ΔmmpX strain (Table 2 and Fig. S4). In contrast, more than 98% of the Arg275-containing peptides were methylated in the complementation and wild-type strains (Table 2 and Fig. S5 to S7). In addition, complementation by a mutated form of mmpX containing an N212D213 → AA substitution plus a 220-amino-acid (aa) truncation at the C-terminal end of Mmp10 (Phmv-mmpXtrun) also failed to complement the deletion (Table 2 and Fig. S8). These results confirmed that mmpX and not some other component of the vector was required for Arg275 methylation. Moreover, when the recombinant Mcr protein was additionally expressed in trans in the wild-type background, the methylation of the recombinant Mcr protein was nearly complete (Table 2 and Fig. S9). Therefore, the Arg PTM did not depend on the mmpX and mcr genes being next to each other on the genome.
TABLE 2.
Deletion of mmpX affects methylation of R275
| mmpX genotype | % Ra | % rb | MS/MS spectrum location |
|---|---|---|---|
| ΔmmpX | 100 | ND | Fig. S4 |
| Parental (S0001) | 0.7 | 99.3 | Fig. S5 |
| ΔmmpX + Pnat-mmpX | 1.5 | 98.5 | Fig. S6 |
| ΔmmpX + PhmvA-mmpX | 1.5 | 98.5 | Fig. S7 |
| ΔmmpX + PhmvA-mmpXtrun | 100 | ND | Fig. S8 |
| Mcr overexpression in WTc | 3.7 | 96.3 | Fig. S9 |
Peptides were generated by pepsin degradation of McrA. R275 was not methylated.
Peptides where R275 was methylated. r indicates 5-C-(S)-methylarginine. ND, not detected.
Mcr was the recombinant His-tagged enzyme expressed in S0001 with the strong PhmvA promoter. WT, wild type.
Effect of methylation on methanogenesis and growth.
Mcr from M. maripaludis is very unstable even in cell extracts, and >90% of the activity of resting cells is lost within 1 h, even under the conditions that stabilize the enzyme from Methanothermobacter marburgensis (H. Shi, E. C. Duin, and W. B. Whitman, unpublished observations). Therefore, the rates of methanogenesis of the ΔmmpX mutant were compared to that of the parental strain S0001 in whole cells. Because the rates of methanogenesis by whole cells vary with the culture phase (34), the accumulation of methane in the culture headspace was monitored throughout growth, and the rate of methanogenesis was calculated from the increases in methane in the culture headspace (Fig. 2A). By this measure, the rates of methanogenesis by the ΔmmpX strain S0030 never exceeded 60% of the S0001 rate. To confirm that these results were not due to fluctuations in the medium composition during growth, the rates of methanogenesis were also measured in resting cells after washing and resuspension in fresh medium (Fig. 2B). Under these conditions, the maximal rates of methanogenesis of the ΔmmpX strain S0030 were about one-half that of S0001. In the complementation strains, the rate of methanogenesis throughout growth was restored to the S0001 levels in the strong complementation strain S0034 (Fig. 2A). Therefore, the impaired methanogenesis rate in the ΔmmpX mutant was due to the absence of Arg275 methylation. However, the rates of methanogenesis in cultures as well as in resting cells of the weak complementation strain S0031 were as low as those of the deletion mutant (Fig. 2B), suggesting that this complementation plasmid may have interfered with other cellular processes (see below).
FIG 2.
Cellular rate of methanogenesis in the ΔmmpX mutant and complementation strains. (A) Rates of CH4 production during growth of the cultures shown in panel C. The rates were calculated from the increases in CH4 in the headspace of the cultures. CH4 was measured for most of the time points during growth except the early lag phase, where the estimation of biomass based on the OD was inaccurate. The maximal rates were 0.91 ± 0.14 U · mg−1 (S0001), 0.49 ± 0.03 U · mg−1 (S0030), 0.60 ± 0.07 U · mg−1 (S0031), and 0.84 ± 0.06 U · mg−1 (S0034). (B) Specific CH4 production rates for resting cells sampled from cultures shown as open symbols in panel C. These sampling points represented the early, mid- (except for S0031), and late stages of growth, respectively. At each sampling point, methane from triplicate cell suspensions was measured over a period of 30 to 50 min when methane production was linear with time. The maximal rates were 1.44 ± 0.23 U · mg−1 (S0001), 0.61 ± 0.06 U · mg−1 (S0030), 0.70 ± 0.08 U · mg−1 (S0031), and 1.55 ± 0.20 U · mg−1 (S0034). Error bars indicate standard deviations for 3 independent measurements. U · mg−1 indicates micromoles of CH4 per minute per milligram (dry weight).
The growth of the ΔmmpX strain S0030 was also inhibited compared to the parental strain S0001. Not only did the lag phase increase, but the growth rate was also substantially reduced (P = 0.0006 by a t test), from 0.20 ± 0.03 h−1 (average ± standard deviation; n = 6) in S0001 to 0.13 ± 0.00 h−1 in S0030 (Fig. 2C and Fig. S10). This result would be expected if the rate of methanogenesis was growth limiting. In the complementation strains, substantial differences in growth were also evident. The strong complement strain S0034 grew significantly better than the deletion mutant (P = 0.005), albeit the lag was longer and the growth rate, 0.17 ± 0.02 h−1, was somewhat lower than but not statistically different (P = 0.07) from that of S0001 (Fig. 2C). In contrast, the weak complement strain S0031 had the longest lag and lowest growth rate, at 0.11 ± 0.00 h−1.
A possible explanation for the significantly lower rates of methanogenesis and slower growth of S0030 and S0031 is that the Mcr expression level might be lower in the mutants. To determine the levels of Mcr, whole-cell extracts were separated by SDS-PAGE (Fig. 3). Although the McrA and McrG subunits were not well separated, the McrB subunit was readily identifiable, and the Mcr abundance for each strain was calculated based on integration of the band intensity. The levels of Mcr (averages and standard deviations from three measurements) were 14.1% ± 0.7% of the total protein for S0001, the wild type; 15.7% ± 0.4% for S0030, the ΔmmpX mutant; 15.2% ± 0.5% for S0034, the strong complement; and 15.5% ± 0.3% for S0031, the weak complement. Statistically significant differences were found only between S0001 and S0030 (P = 0.02 by one-way analysis of variance [ANOVA]) as well as between S0001 and S0031 (P = 0.04). Therefore, the Mcr expression level actually went slightly higher in the ΔmmpX mutant and the weak complement than in the parental strain. These results reject the hypothesis that the observed slower methanogenesis and growth were caused by lower Mcr levels. The largely similar SDS profiles among the strains suggested similar expression levels for most other proteins (Fig. 3). However, an extra band near the position of 26 kDa appeared exclusively in the weak complement strain S0031. In a separate experiment where samples were overloaded (27), it was determined that the abundance of this band relative to the total protein in S0031 was 7.8% ± 0.7% (average and standard deviation from three measurements), much higher than the 2.0% ± 0.1% in S0001, 1.6% ± 0.5% in S0030, and 2.8% ± 0.2% in S0034.
FIG 3.
Mcr expression in the ΔmmpX mutant and complementation strains. Shown are SDS-PAGE profiles for strains S0001 (01) (the parental strain), S0030 (30) (the deletion mutant), S0034 (34) (the PhmvA-mmpX complement), and S0031 (31) (the Pnat-mmpX complement). M, purified wild-type His-tagged Mcr; L, protein ladder (catalog no. P7712; NEB). Although data for only one sample are shown here, calculations were based on triplicate samples run on a replicate SDS gel.
Matrix-assisted laser desorption ionization (MALDI) sequencing of the band from S0031 revealed that it was the puromycin N-acetyltransferase, which was encoded by the pac gene in the complementation plasmid and provided puromycin resistance. In the complementation plasmid, pac expression is under the control of the Mcr promoter from a closely related methanogen, Methanococcus voltae, which is similar in sequence to the reverse complement to the Pnat promoter. Presumably, an interaction between the Ppac and Pnat promoters led to the overexpression of the pac gene in S0031. Overexpression of the puromycin N-acetyltransferase could potentially interfere with growth by competing for resources for protein synthesis and by destabilizing the acetyl coenzyme A pool (31). The overexpression of this protein may at least partially explain the severely impaired growth of the weak complementation mutant S0031.
Collectively, these results suggested that the poor growth of the complementation strains was due to pleiotropic effects unrelated to the effect on Mcr PTM. To test this hypothesis, the complementation plasmids were transformed into the parental strain S0001, resulting in strains S0035 and S0036. Both strains grew poorly in comparison to the parental and ΔmmpX strains (Fig. S10A). Moreover, this inhibition was even more profound than in the corresponding complementation strains S0031 and S0034 in the ΔmmpX background (Fig. S10B). Thus, it appears that the growth of the complementation strains would have been fully rescued in the absence of the pleiotropic effects.
Specificity of Mmp10.
Because of the poor growth of the complementation strains, the possibility of additional targets for methylation by Mmp10 was examined. Since SAM-dependent protein methyltransferases specifically recognize the amino acid sequences flanking the target amino acid (22, 35, 36), this region was examined for a conserved signature sequence among the methanogen Mcrs. A conserved signature sequence, PXR274R275(A/S)R(G/A), was identified (Fig. 4A). Based upon the X-ray crystal structure of the closely related Methanothermococcus thermolithotrophicus Mcr, R274 could interact with coenzyme B at the active site (Fig. 4B and C) (21). While the methylated R275 would be too far away from coenzyme B to interact directly, the methylation could stabilize and/or enhance interactions between R274 and coenzyme B. To examine if proteins other than McrA have the same signature sequence, the M. maripaludis proteome was searched in silico. Although an identical match was not found, a similar peptide, RR388SRG, was identified in MMP0140. This gene encodes a putative hydrogenase maturation factor that possibly functions as a carbamoyltransferase. Hydrogenase is a key enzyme in methanogenesis, and MMP0140 is likely essential for growth (26, 37, 38). Thus, alteration of its PTM, if it occurred, could have a profound effect on growth. Therefore, a His-tagged MMP0140 protein was expressed in the parental background and subsequently purified to determine its methylation. Pepsin digestion resulted in three peptides that contained the RR388SRG signature sequence, including LRR388SRGFA, LRR388SRGFAPE, and MELLRR388SRGFAPEPVEVNYK (Fig. S11). However, methylation was not observed in any of these peptides. Therefore, arginine methylation appears to be specific to McrA in M. maripaludis.
FIG 4.
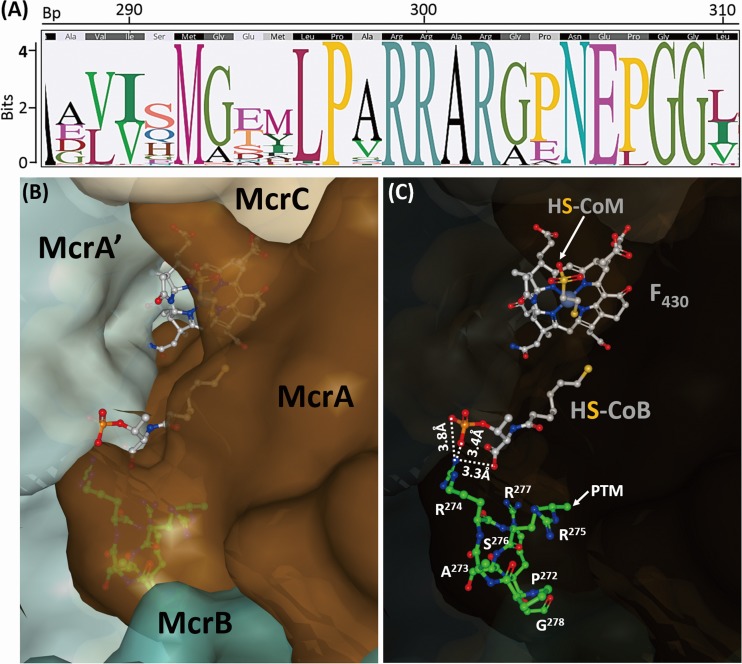
Potential Mmp10 recognition site in McrA and its three dimensional (3-D) location in the Mcr complex. (A) Sequence logo of the Met-Arg context from an alignment of 251 McrA amino acid sequences from all seven orders of methanogens available on the IMG platform at the time of analysis. On top of the logo, the sequence consensus and coordinates are shown, and Met-Arg is located at position 300 in this alignment. Because the McrA subunit is highly conserved near the active site, the region near R275 contained a large number of invariant residues. However, limiting a possible methylation consensus sequence to three residues on either side of the methylation site yielded a candidate motif, PXR274R275(A/S)R(G/A). (B and C) Methanococcal Mcr active site as illustrated by the crystal structure of Methanothermococcus thermolithotrophicus (PDB accession no. 5N1Q). (B) View from the surface of Mcr. Shown in a gray ball-and-stick model, coenzymes F430, M (HS-CoM), and B (HS-CoB) are embedded within the active-site pocket created primarily by the McrA and McrA′ subunits. (C) See-through view showing the PAR274R275SRG motif (shown in a green ball-and-stick model) of McrA contributing to the formation of the active-site pocket. The posttranslationally modified methyl group is indicated for R275, which is located far away from HS-CoB; e.g., the distance between the carbon atom of this PTM and the negatively charged oxygen atoms of the carboxyl group on HS-CoB is 6.7 Å. In contrast, the neighboring R274 is much closer to HS-CoB, suggesting potential interactions. The positively charged nitrogen atom of the amine group of R274 and the negatively charged oxygen atoms of the phosphate or carboxyl groups of HS-CoB were within 3.3 to 3.8 Å of each other, as indicated by the dashed lines.
Site-directed mutagenesis of mmpX.
Although Mmp10 is homologous to SAM-dependent methyltransferases, it does not possess high similarity to any of the five previously described classes, classes A to E (39). Each class contains one or more of the distinct functional domains that bind their cofactors, SAM, Fe-S clusters, cobalamin, CH3-THF (methyl-tetrahydrofolate), and/or heme, respectively (39). While SAM and Fe-S clusters are abundant in M. maripaludis, heme is absent (40). Moreover, in methanogens, CH3-THF is replaced by CH3-H4MPT (methyl-tetrahydromethanopterin), which is a major C-1 carrier in methanogenesis, and cobalamin is replaced by pseudo-vitamin B12 {Coα-[α-(7-adenyl)]-cobamide} (40, 41). In Mmp10, a SAM domain (pfam04055) of about 190 amino acids was readily recognizable in the N-terminal half of the protein. However, a functional domain for the C-terminal half could not be inferred by bioinformatic methods. Therefore, potential SAM, Fe-S cluster, cobamide, and CH3-H4MPT binding motifs were predicted in silico from the conserved amino acid residues of the methanogen Mmp10 homologs (Fig. 5).
FIG 5.
Sequence alignment of the Mmp10 homologs. Mmp10 homologs from six genomes representing each methanogen order and one ANME-2 genome were aligned to illustrate conserved motifs. At the N terminus, conserved motifs predicted to bind SAM are highlighted in purple, including CXXXCXXC, GGD, and GXTXGXGXXXXE/D. In fact, many of the x residues in the CXXXCXXC and GXTXGXGXXXXE/D motifs are highly conserved, but they are labeled in such a way to highlight just the residues conserved in other SAM binding proteins. Nevertheless, these conserved x residues may also play important roles. Near the CXXXCXXC motif, there are also two conserved CXXC motifs, highlighted in red, that may bind Fe-S clusters. In the middle region of Mmp10, residues highlighted in green form a conserved D-29X-N-D-35X-N-30X-R motif that may bind CH3-H4MPT, a potential methyl donor for SAM. At the C terminus, a conserved H residue is highlighted in brown (see the text).
First, conserved regions of Mmp10 were predicted by aligning the genes from diverse methanogens. Next, these regions were screened for cofactor binding sequences derived from known enzymes. Once identified, these putative motifs served as targets for site-directed mutagenesis of mmpX genes expressed in a methanococcal plasmid with the PhmvA promoter in the ΔmmpX background. The recombinant wild-type gene expressed in the same plasmid served as a control, and the effects of the mutations were evaluated by measuring changes in Arg275 methylation of McrA.
A typical SAM binding domain contains three conserved motifs, CXXXCXXC, GGE, and GXIXGXXE (42). While the first motif is highly conserved, the latter two motifs are often variable. For example, D/W/T can replace E in GGE, and V/T/M and N/Q/S can replace I and E in GXIXGXXE, respectively (42). An extra residue immediately prior to E is also often seen in the GXIXGXXE motif, making it GXIXGXXXE. CXXXCXXC binds a 4Fe-4S cluster that ligates the carboxy and amino groups of the methionine moiety in SAM, GGE interacts with the amino group of the methionine via a hydrogen bond, and GXIXGXXE interacts with the adenosine moiety (42). All three motifs could be identified in the N-terminal half of Mmp10 within the predicted SAM binding domain (Fig. 5), and the G140XTXG144XG146XXXXD/E motif was subjected to site-directed mutagenesis (G140,144,146 → A substitutions), leading to a loss of 16% of Arg275 methylation compared to the wild-type control (Table 3). However, Western blotting indicated that the mutated protein was expressed at levels 32-fold higher than those of the native protein (Fig. S12). Thus, the loss of Arg275 methylation in vivo appeared to be a poor surrogate for enzymatic activity, and it is likely that there was a great overcapacity of mutated Mmp10. In this case, even a large decrease in the specific activity of the mutated protein would not immediately translate into a similar loss of PTM. For this reason, the relatively small effect of the G140,144,146 → A substitutions on the PTM supported a role for this motif in SAM binding.
TABLE 3.
Loss of Arg PTM in vivo due to site-directed mutagenesis of Mmp10a
| Mutationb | Peak area (AU) for peptide 1c |
Loss of PTM for peptide 1 (%) | Peak area (AU) for peptide 2c |
Loss of PTM for peptide 2d (%) | Mean loss of PTM (%) | Relative protein expressione | ||
|---|---|---|---|---|---|---|---|---|
| r275 | R275 | r275 | R275 | |||||
| None | 37 | 0 | 0 | ND | ND | NA | 0 | (1) |
| G140,144,146 → A | 220 | 37 | 14.0 | 5.8 | 1.2 | 17.5 | 15.8 | 32 |
| N212D213 → AA | 96 | 52 | 35.0 | ND | ND | NA | 35.0 | 0.4 |
| H375 → G | 340 | 3.2 | 0.9 | 10 | 0.062 | 0.6 | 0.8 | 28 |
| H375 → F | 310 | 3.3 | 1.1 | 7.6 | ND | ND | 0.5 | ND |
| H375 → F, ΔN371 | 160 | 100 | 38.0 | 3.8 | 3.4 | 47 | 42.5 | 9 |
ND, not detected; NA, not applicable.
The mutated mmpX genes were expressed in a plasmid with the PhmvA promoter in the ΔmmpX background. The wild-type gene expressed in the same plasmid served as a positive control. All mutations were verified by Sanger sequencing of the plasmids. All the variants also had a His tag attached to the N terminus to allow confirmation of expression using MALDI.
Peptide 1 is MGNALPGRR275ARGPNEPGGIRF; peptide 2 is PGRR275ARGPNEPGGIRF. “R” indicates the presence of unmethylated arginine. “r” indicates the presence of 5-C-(S)-methylarginine. The peak area is multiplied by 107 to obtain the reported values, which are presented in arbitrary units (AU).
Loss of PTM = R275/(R275 + r275). The mean is calculated by averaging results from both peptides 1 and 2 if available. If a peptide was not detected, the value was taken as zero.
Protein expression was quantified by Western blotting (Fig. S12). The band intensity for the recombinant native Mmp10 was set at 1. Relative protein expression = band intensity of mutated Mmp10/band intensity of native Mmp10.
The sequence N-34X-D-53X-N-61X-D-34X-N-7X-R has been predicted to bind CH3-THF in some CH3-THF-dependent methyltransferases, and residues shared with the putative CH3-H4MPT binding motif are underlined (43). In Mmp10, a CH3-H4MPT binding motif was predicted to include D182-29X-N212-D213-35X-N249-30X-R280 (Fig. 5). Replacement of the N212D213 residues with AA resulted in a 35% loss in Arg275 methylation (Table 3), suggesting a significant role for these residues. However, in this mutant, the expression of the mutant protein was only 40% of that of the recombinant native protein (Fig. S12), so it is possible that the reduced PTM was due to reduced expression.
A typical DXHXXG motif for cobamide binding was not found in Mmp10. However, cobamide binding proteins do not always contain this motif, and the presence of a conserved His could still be indicative of cobamide binding (44). Indeed, an in vitro assay of Mmp10 from M. acetivorans has shown that its activity requires cobalamin, but its binding site remains elusive (29). His375 (M. maripaludis numbering) was the only conserved His residue in the C-terminal half of Mmp10 (Fig. 5). Replacement of this residue with either G or F resulted in a loss of less than 1% of Arg275 methylation (Table 3). However, for at least the H375 → G substitution, the mutated protein was expressed at levels 28 times those of the positive control, so the absence of an effect on PTM did not eliminate a role for this amino acid in activity. An additional mutant was constructed, which included an in-frame deletion of N371 in addition to the H375 → F substitution. This construct sustained a 43% loss in Arg275 methylation, even though it was expressed 9-fold more than the recombinant native protein. Thus, N371 and this highly conserved region appeared to be important for activity (Table 3). In most methanogens, N371 is replaced with R, another polar residue. Presumably, polarity at this position could be important for Mmp10 activity.
Although mutagenesis of suspected cofactor binding motifs failed to abolish Arg275 methylation, substitutions in or near the putative SAM, CH3-H4MPT, and cobamide binding motifs resulted in significant losses in activity, which was suggestive of important functional roles for these motifs. However, these in vivo experiments cannot eliminate the possibility that changes in activity were due to altered structural features unrelated to cofactor binding. Even though they did not provide definitive information on the cofactors of Mmp10 and their binding sites, these experiments strongly suggested a direct role of Mmp10 in modulating Arg275 methylation.
Distribution of mmpX in Archaea.
The important role of Mmp10 established here prompted us to survey its distribution across Archaea in a database of nearly 1,000 genomes. Homologs of mmpX were widely distributed in all well-established methanogen lineages except Methanoculleus bourgensis and Methanomassiliicoccales spp. (Table 4). Homologs were also found in all five ANME-2 methanotrophs and two unclassified euryarchaeotes, which appeared to be methanogens (Table 4). These results suggested that Mmp10 would play important roles in most methanogens and ANME-2 methanotrophs. Among methanogens and methanotrophs that possess mmpX homologs, they were adjacent to the mcr operon except in the orders Methanomicrobiales and Methanocellales (Table 4). This conserved location in the genome may be important for the regulation of the Arg PTM.
TABLE 4.
Distribution of the Mmp10 homologs within the genomes of Archaea
| Lineage | No. of genomes | No. of genomes encoding Mmp10 | No. of genomes with mmpX flanking mcra |
|---|---|---|---|
| Methanococcales | 19 | 19 | 19 |
| Methanopyrales | 1 | 1 | 1 |
| Methanobacteriales | 58 | 58 | 53b |
| Methanomicrobiales | 20 | 19c | 0 |
| Methanocellales | 3 | 3 | 0 |
| Methanosarcinales | 107 | 105d | 97e |
| Methanomassiliicoccales | 8 | 0 | 0 |
| NAOAf | 19 | 5g | 1h |
| Nonmethanogenic Euryarchaeota | 348i | 0 | 0 |
| “Candidatus Bathyarchaeota” | 24 | 0 | 0 |
| “Candidatus Verstraetearchaeota” | 7 | 0 | 0 |
| “Candidatus Nezhaarchaeota” | 2 | 0 | 0 |
| Other Archaea | 359 | 0 | 0 |
The mmpX position was examined by “Show neighborhood regions with the same top COG hit (via top homolog)” command at IMG/M ER.
Not linked to mcr in Methanobrevibacter smithii TS145B and ACE6, Methanobrevibacter curvatus DSM11111, and Methanobacterium sp. Maddingley. These are all draft genomes where mmpX and mcr are on different contigs. In Methanosphaera stadtmanae, mmpX flanks the mcrC-mtr operon, with mrtBDGA 16.8 kb downstream of the mcr operon.
Absent in Methanoculleus bourgensis BA1.
These homologs were present in the Euryarchaeota archaeon JGI 0000059-G05 and Euryarchaeota sp. strain AmaM. Both rRNA and mcrA, the conventional gene markers, were absent in the first archaeon. However, it encoded an McrC subunit which had 85% amino acid identity to that from Methanosaeta concilii. The second archaeon was recently named Methermicoccus shengliensis strain AmaM (61). Therefore, both archaeal species are in fact methanogens affiliated with Methanosarcinales. These homologs were absent in Methanosarcina horonobensis JCM 15518 (draft genome) and Methanosarcina mazei Tuc01.
Not linked in any of the four Methanosaeta genomes (including JGI 0000059-G05 described above), Methanosarcina mazei 1.H.A.2.8 (draft genome with mmpX and mcr on separate contigs), Methanosalsum zhilinae, Methanoflorens stordalenmirensis, and Methermicoccus shengliensis.
NAOA, anaerobic alkane-oxidizing Archaea, including anaerobic methane, ethane, propane, and butane oxidizers.
These homologs are absent in ANME-1 (GenBank accession no. FP565147), “Candidatus Polytropus marinifundus” (GenBank accession no. GCA_003935005) and other unclassified Archaeoglobi members (GenBank accession no. GCA_004347825, GCA_004347865, GCA_004347845, and GCA_002010305), “Candidatus Argoarchaeum ethanivorans” (GenBank accession no. GCA_004193545), “Candidatus Hadesarchaeota” (GenBank accession no. GCA_004347815, GCA_004347835, and GCA_004347925), “Candidatus Korarchaeota” (GenBank accession no. GCA_004347975), “Candidatus Syntrophoarchaeum butanivorans” (GenBank accession no. GCA_001766825), “Candidatus Syntrophoarchaeum caldarius” (GenBank accession no. GCA_001766815), and “Candidatus Syntrophoarchaeum” WYZ-LMO15 (GenBank accession no. GCA_003601555). These homologs are present in all five ANME-2 genomes.
Linked in one of the ANME-2 genomes.
A total of 350 genomes were originally classified as nonmethanogenic Euryarchaeota in IMG. However, two of them belonged to Methanosarcinales as described above. Therefore, they were added to their correct group.
Homologs of mmpX were not found in ANME-1 and any of the other archaeal species examined (Table 4). These included “Candidatus Bathyarchaeota,” “Candidatus Verstraetearchaeota,” and “Candidatus Nezhaarchaeota,” where methanogenesis and Mcr genes were recently found (14, 45, 46); “Candidatus Argoarchaeum ethanivorans,” which encoded Mcr homologs for anaerobic ethane oxidation (9); “Candidatus Syntrophoarchaeum,” which encoded Mcr homologs for anaerobic butane and possibly propane oxidation (10); and several Mcr-encoding members of the Archaeoglobi, including “Candidatus Polytropus marinifundus,” that potentially engage in anaerobic alkane oxidation (14, 15). Very recently, anaerobic alkane oxidation metabolism was also predicted in “Candidatus Helarchaeota” (13). However, its genome annotation was not publicly available at the time of our analyses, so we do not know if it encodes Mmp10.
In summary, Mmp10 is widely distributed in archaeal lineages that have Mcr, including most methanogens and ANME-2 anaerobic methanotrophs. However, it is not present in certain Mcr-bearing lineages that harbor either methanogens or most if not all of the remaining NAOA alkane oxidizers other than ANME-2.
DISCUSSION
Although the PTMs of McrA have been known for over 20 years (1, 19), little progress has been made in the identification of the source of these PTMs until recently (23, 27, 28). This study demonstrates that the 5-C-(S)-methyl-Arg275 PTM in M. maripaludis requires mmpX, the gene encoding the methanogenesis marker protein Mmp10. The PTM is lost in a ΔmmpX mutant and restored when the mutation is complemented with mmpX expressed from plasmids. Although Mmp10 contains structural features expected for a SAM-dependent methyltransferase, it is not easily assigned to any of the previously described classes (35). Nevertheless, site-directed mutagenesis of some of the motifs expected to play important roles in activity reduced the PTM in vivo and supported the hypothesis that Mmp10 catalyzes the methylation of Arg275. Because all other characterized arginine methylations take place at the guanidino nitrogen atoms instead of C-5 (47), more information about the mechanism and role of Mmp10 is of great interest. The various mutants generated here will facilitate future elucidation of this unique mode of methylation.
In resting cells, the rate of methanogenesis is reduced by 40 to 60% in the ΔmmpX mutant, suggesting a role of Arg methylation in modulating Mcr activity. A similar trend was observed in growth as well. These results are consistent with previous transposon insertion sequencing (Tn-seq) experiments which demonstrated that although mmpX was not an essential gene, transposon insertions led to a decrease in fitness (26). Thus, it is not surprising that unmethylated Mcr retains partial activity. This is the first time that a PTM is demonstrated to have a substantial impact on both methanogenesis and growth in the absence of additional stressors. In contrast, less-severe phenotypes were observed in two previous M. acetivorans studies where either the thio-Gly or methyl-Arg PTMs were removed (23, 28). However, the significance of such phenotypic differences between M. maripaludis and M. acetivorans is elusive. Compared to M. maripaludis, M. acetivorans grows much more slowly and is incapable of hydrogenotrophic growth, differences which confound direct comparisons between the two methanogens.
Two contrasting models can be envisioned for the profound loss in activity in the absence of the Arg PTM. First, Arg methylation could be an important structural feature of the active site and play an important role in catalysis. One possibility is that a loss of this methylation disorients the neighboring R274 and disrupt interactions between R274 and coenzyme B. Consequently, catalysis would be impaired. Alternatively, the PTM could also play a role in Mcr assembly or stability (28). Since the relative abundances of Mcr were comparable between the ΔmmpX mutant and the wild type, the in-frame deletion of mmpX did not appear to significantly change the expression or stability of Mcr. However, changes in folding or assembly might have occurred. Because the PTM is deeply buried in the mature protein, it is likely to occur before the insertion of coenzyme F430 and complete folding of the McrA subunit (1, 22). From this point of view, the low rates of methanogenesis of the ΔmmpX mutant could be a consequence of Mcr misfolding rather than a direct effect on catalysis. Currently, it is not possible to distinguish between these possibilities. Moreover, they are not necessarily mutually exclusive, and the Arg PTM may play multiple roles in Mcr activity.
The inability of complementation of the ΔmmpX mutation to fully restore the growth phenotype even when McrA was completely methylated raised the possibility of other physiological functions for Mmp10. For this reason, the possibility of PTMs of other proteins was examined. However, although the gene encoding a putative hydrogenase maturation factor, MMP0140, contains a potential methylation site similar to that in McrA, this site is not methylated. Therefore, Mmp10 appears to have high specificity. Instead, the complementing plasmids were inhibitory even in the parental strain background, and the plasmid with the lowest expression level of Mmp10 was the most inhibitory, suggesting that growth inhibition was unrelated to Mmp10 activity. Thus, the inhibition likely results from pleiotropic effects, such as the overexpression of the puromycin N-acetyltransferase in the complementation strain with the Pnat promoter.
In addition to establishing a direct link between mmpX and the Arg PTM, the site-directed mutagenesis analysis of mmpX presented here also helps elucidate the cofactor binding sites of Mmp10. The recently established in vitro assay of MaMmp10 from M. acetivorans confirmed the SAM and cobalamin binding nature of this enzyme (29), which is consistent with our mutagenesis results. Given that the in vitro activity of Mmp10 was still very low in the presence of both SAM and cobalamin, additional cofactors may be yet to be discovered.
In conclusion, this study identified a gene, mmpX, required for the Arg PTM of Mcr in Methanococcus maripaludis. Site-directed mutagenesis of mmpX further establishes that Mmp10 directly modulates Arg PTM activity. The reduction of methanogenesis of the mmpX deletion mutant suggests that this PTM influences Mcr activity, presumably through effects on catalytic activity, enzyme folding, and/or Mcr maturation. The wide distribution of mmpX in most but not all methanogenic lineages suggests that the Mmp10 function is ancient and has been highly conserved in the methanogens. The absence of mmpX in most but not all of the alkanotrophic NAOA lineages also suggests that Mmp10 might have played a transitional role during the diversification of the methanogenesis pathways.
MATERIALS AND METHODS
Strains and culture conditions.
Strains used in this study are listed in Table 1. The complex formate broth and solid medium for the cultivation and transformation of M. maripaludis have been described previously, except that cysteine hydrochloride was replaced with an equal amount of coenzyme M and the Fe(NH4)2(SO4)2 stock was replaced with a more diluted FeSO4 stock to reduce the final iron concentration from 35.1 μM to 10.9 μM (26, 34, 48, 49). This avoided small amounts of precipitates that formed occasionally, possibly due to the formation of iron sulfide. Puromycin (2.5 μg ml−1) and 8-azahypoxanthine (0.25 mg ml−1) were added from anaerobic, sterile stocks as needed. All M. maripaludis cultures were grown at 37°C with 15 lb/in2 of N2-CO2 in the headspace. Growth curves were monitored by measuring the optical densities at 600 nm. The cell dry weight was calculated from the optical densities based on previously reported calibration curves (34, 50).
Plasmid and strain construction.
Plasmids and PCR primers are listed in Table 1 and Table S2 in the supplemental material, respectively. Cloning was performed in Escherichia coli Top10, and selection was conducted with 100 μg ml−1 ampicillin. Genomic DNA extraction, DNA purification, and plasmid isolation were done with Zymo Research kits (catalog no. D6005, D4001/4003, and D4020, respectively) according to the manufacturer’s instructions. All PCRs were performed with the Phusion high-fidelity DNA polymerase (catalog no. M0530S; NEB). Site-directed mutagenesis was performed with the Q5 site-directed mutagenesis kit (catalog no. E0554S; NEB).
A linear DNA fragment, Mm10, was constructed for performing markerless deletion of mmpX from the parental strain S0001, producing strain S0030. This strategy is described in detail in the supplemental material. Upon transformation, genomic integration of the Mm10 construct through homologous recombination was positively selected with puromycin. Colonies of the selected transformants were subjected to PCR amplification using primers 1554-F and 1554-R (1554-F/R) to confirm complete integration. The removal of the markers was achieved by selection with azahypoxanthine for a second homologous recombination between the two repetitive elements (REs). The identities of colonies of the ΔmmpX mutant were confirmed by PCR amplification using primer pair 1554-F/R to confirm the complete removal of the markers (Fig. S3A). The PCR product was also sequenced to confirm that the promoter region of the mcr operon was intact. This conclusion was further confirmed by the sensitivity of the mutant to puromycin and the inability of primers 1554w-F and 1554w-R, which target an internal region of mmpX, to yield a product. As a positive control, PCR amplification under the same conditions detected 0.1% of parental S0001 DNA when mixed with the mutant DNA (Fig. S3B).
Following the transformation of expression vectors into M. maripaludis, individual colonies were isolated on plates with puromycin. The sequences of the mmpX gene were confirmed by sequencing of the PCR amplicons from the primer pairs pMEV4-F/R and 1554w-F/R. Expression plasmid vectors pM10 and p4MK10 were made to complement the ΔmmpX mutation in strain S0030, producing strains S0031 and S0034, respectively. The same two plasmids were also transformed into the parental strain S0001, resulting in strains S0035 and S0036, respectively. The expression of mmpX was under the control of the predicted native promoter Pnat in pM10. The expression of mmpX was under the control of the constitutive PhmvA promoter in p4MK10. The strengths of Pnat and PhmvA promoters were quantified in strains S0032 and S0033, which harbored vectors pM10m and pMEV4m, respectively. pMEV4m possessed the mCherry reporter under the control of PhmvA and enabled gene expression to be quantified by monitoring mCherry fluorescence (Z. Lyu and W. B. Whitman, unpublished observation). In pM10m, mCherry expression was controlled by Pnat. To make pM10m, two PCR products were made, digested with EcoRI and NdeI, and ligated by using T4 DNA ligase (catalog no. M0202S; NEB). The first PCR product was amplified from S0001 genomic DNA with primer pair 1554P-F/R, producing Pnat. The second PCR product was amplified from the pMEV4m plasmid with primers pair 4mp-F/R, producing the vector backbone with PhmvA removed. Similarly, another two PCR products were made, digested with NdeI and PstI, and ligated to make pM10. The first PCR product was amplified from S0001 genomic DNA with primer pair 1554c-Fa/R, producing the complete mmpX gene. The second PCR product was amplified from pM10m plasmid DNA with primers p4Brk-F and 1554P-R, producing the native promoter and vector backbone with the mCherry gene removed. p4MK10 was made by cloning mmpX into pMEV4 at the SpeI and PstI sites. Specifically, mmpX was amplified from S0001 genomic DNA with primer pair 1554c-Fb/R, and the product was digested with XbaI and PstI before ligation into the pMEV4 backbone. The pMEV4 backbone was amplified from pMEV4 plasmid DNA with primer pair p4Brk-F/R.
Another expression vector, pMcrS1, was constructed to express the wild-type mcr operon fused with a 6×His tag to the C terminus of the McrA subunit under the control of the PhmvA promoter in strain S0001, resulting in a new strain, S0037. Details regarding this construction will be reported elsewhere.
A His-tagged version of the MMP0140 gene was amplified by using primer pair 0140his-F/R, where a 6×His tag was introduced at the C terminus. The amplified product was cloned into a pMEV4 backbone via SpeI and NotI sites to form pMEV0140, which was transformed into strain S0001 to form strain S0040. In this vector, the MMP0140 gene was expressed under the control of the PhmvA promoter.
For site-directed mutagenesis of mmpX, the p4MK10 plasmid was amplified with 1554his-F/R and ligated at NdeI to introduce a 6×His tag at the N terminus of wild-type Mmp10. The resulting plasmid then served as a template to make all the site-directed mutations within mmpX by five sets of primers, G140–146-F/R, N212D213-F1/R1, H375G-F/R, H375F-F1/R1, and H375F-F2/R2, which resulted in the amino acid changes G140,144,146 → A, N212D213 → AA, H375 → G, H375 → F, and H375 → F and N371 deletion, respectively, as determined by Sanger sequencing (Table 3). In addition, a mutant with the N212D213 → AA substitution plus a 220-aa truncation at the C end of Mmp10 was formed with primer pair N212D213-F2/R2. This resulted in the plasmid p4MK10t (Table 1), also named PhmvA-mmpXtrun (Table 2). The strain carrying this mutation was named S0039. Both the wild-type and the mutant mmpX genes were expressed under the control of the PhmvA promoter in the ΔmmpX strain S0030. Following transformation into M. maripaludis, the sequences of the mutagenized mmpX gene were confirmed by sequencing of the PCR product.
Fluorescence quantification.
Triplicate cultures were grown to an absorbance of ∼0.4 to 0.6 in the presence of puromycin. Cultures of 2 ml were harvested by centrifugation at 17,000 × g for 1 min, resuspended in 200 μl of PIPES-K [piperazine-N,N'-bis(2-ethanesulfonic acid; pH adjusted with KOH] buffer (25 mM, pH 6.8), and frozen at −80°C overnight. For activating mCherry, cell extracts were thawed and incubated overnight in air with shaking at 30°C in the dark. The cell extracts were cleared by centrifugation at 17,000 × g for 1 min before measuring the fluorescence of the supernatant using the Qubit 2.0 fluorometer. For these measurements, excitation was determined at 600 to 645 nm, and emission was determined at 665 to 725 nm.
Purification of methyl coenzyme M reductase and His-tagged proteins.
Cultures were grown in 1.5 liters of complex formate broth to an absorbance of about 0.8. Cells were freshly harvested aerobically by centrifugation at 17,700 × g for 15 min at room temperature. Cells were resuspended in 2 ml of Mcr buffer g−1 (wet weight) and stored at −20°C. Mcr buffer contained 10 mM Ti(III) citrate, 10 mM coenzyme M, and 0.1 mM methyl viologen in 150 mM monosodium phosphate (pH 8.0) buffer. The cells in Mcr buffer were thawed and remained on ice during sonication, which was conducted with a W-380 sonicator (Heat Systems-Ultrasonics, Inc.) for 20 cycles of 5-s bursts with the output set at 5 and the duty cycle set at 90%. The lysate was then centrifuged at 17,000 × g for 5 min to remove cell debris. To achieve 50% (NH4)2SO4 saturation, an equal volume of a saturated (NH4)2SO4 solution was added to the supernatant. After 30 min, the precipitate was collected by centrifugation at 17,000 × g for 5 min and discarded. Additional (NH4)2SO4 powder was added to achieve 100% saturation. After 30 min, the precipitate was collected by centrifugation and resuspended in 4 ml of buffer A (1 mM coenzyme M in 10 mM Tris-HCl [pH 7.6]). The resulting proteins were desalted by concentration on an Amicon Ultra-4 centrifugal filter (10-kDa cutoff; Millipore) by centrifugation at 7,500 × g for 15 min at 4°C and resuspension in the same buffer. The desalted and concentrated proteins were resuspended with 2.5 ml buffer A and loaded onto a Q-Sepharose XK16 anion-exchange column equilibrated with the same buffer. The protein was eluted using the Äkta fast protein liquid chromatography (FPLC) system (GE Healthcare) with a linear gradient of 0% to 100% buffer B (buffer A plus 1 M NaCl). A similar protocol was used for the purification of His-tagged recombinant Mcr, except that coenzyme M was removed from the buffers and ammonium sulfate precipitation was replaced by a Ni-Sepharose column (GE Healthcare) step, where protein was eluted using a linear imidazole gradient from 0% to 100% before ion-exchange chromatography with Q-Sepharose. The Mcr subunits were separated by SDS-PAGE and stained with AcquaStain (Bulldog Bio) for 2 to 10 min until protein bands appeared. The gel was then washed with double-distilled water (ddH2O), and a slice of the gel containing the McrA subunit was excised and destained twice with 30% ethanol before being processed for mass spectrometry.
Other His-tagged proteins were purified as described previously (18).
Rates of growth and cellular methanogenesis.
Specific growth rates during exponential growth were analyzed by linear regression of the logarithm of the optical density with time. For each culture, the growth rate was calculated for each pair of neighboring points throughout the growth curve. The specific growth rate for the culture was then presumed to be the highest average from four adjacent time points. The specific growth rate of each strain was the average from six cultures.
To monitor CH4 production during growth, cultures were inoculated into 20 ml of formate medium without puromycin in 210-ml anaerobic bottles with fused-in side arms for convenient measurement of cell densities. The headspace was sampled for CH4 several times during the lag phase and throughout the growth phase. CH4 production rates in the cultures were corrected for the removal of cells during sampling for assays of resting cells. In this experiment, 4 ml of the culture was harvested at times representative of the early, mid-, and late stages of growth by centrifugation at 2,800 × g for 10 min inside an anaerobic chamber. Triplicate cell pellets were washed and resuspended in 1.6 ml of formate medium in a 4.6-ml anaerobic vial sealed with a butyl rubber stopper. Vials were flushed immediately with an atmosphere of N2-CO2 for 30 s, and the assay was initiated by incubating the mixture at 37°C. Headspace gas was withdrawn from the vials at intervals of 3 to 6 min for 30 to 50 min, and methane was detected with an SRI 8610-C gas chromatograph as described previously (34). One unit was defined as 1 μmol of CH4 produced per min.
Mcr abundance in cell extracts.
Cell extracts were separated in an SDS gel to examine protein expression profiles (51). Each lane was scanned, and the intensity of individual bands was compared to the total staining with ImageJ (52). The band representing the McrB subunit was readily identifiable and used for calculation of the Mcr abundance. In separate experiments, McrB accounted for 34% of the total staining by the purified Mcr protein. Therefore, the percentage of Mcr in extracts was calculated as (percentage of the total staining by McrB)/0.34. All samples were prepared in triplicate, and mean values for the triplicates ± standard deviations are reported.
Western blotting of recombinant proteins.
Cells expressing His-tagged recombinant Mmp10 proteins were grown in complex formate medium containing puromycin to early stationary phase or an absorbance of about 0.80. Cell lysates were prepared as described above. Equal amounts of proteins were separated on precast 12% SDS-PAGE gels (Bio-Rad) and then transferred onto methanol-activated polyvinylidene difluoride (PVDF) membranes. Nonspecific binding was blocked with 5% milk in phosphate-buffered saline (PBS) and 0.1% Tween 20 (PBST) for 1.5 h at room temperature. The PVDF membranes were then incubated with primary antibodies against the His tag (1:2,000 dilution) (catalog no. MA1-21315; Invitrogen) for 1.5 h at room temperature, washed three times for 15 min with PBST, and then incubated with horseradish peroxidase (HRP)-conjugated goat anti-mouse secondary antibodies (1:20,000 dilution) (catalog no. 31430; Thermo Scientific) for 1 h. After additional washing with PBST, PVDF membranes were developed using the Western HRP substrate (ECL, catalog no. 32132; Thermo Scientific). The relative intensity of each immunoreactive band was estimated by using ImageJ.
Mass spectrometry analysis.
For trypsin digestion, the gel bands were sliced into small pieces and rinsed twice with 50% acetonitrile–20 mM ammonium bicarbonate (pH ∼7.5 to 8.0). Proteins in the gel pieces were then alkylated with 50 mM iodoacetamide in 20 mM ammonium bicarbonate for 1 h in the dark. The gel pieces were rinsed twice with 50% acetonitrile in 20 mM ammonium bicarbonate, dehydrated by adding 100% acetonitrile, and dried by using a SpeedVac. Next, small amounts of a trypsin solution (0.01 μg μl−1 in 20 mM ammonium bicarbonate; Promega) were added until the gel pieces totally absorbed the solution. The tubes were placed into an incubator at 37°C overnight. The tryptic peptides were extracted twice from gel pieces with 50% acetonitrile in 0.1% formic acid. The extracts were then combined and evaporated to dryness on a SpeedVac. A similar protocol was used for pepsin digestion, except that the pH was adjusted to ∼2 with 0.04 M HCl and the digestion was performed for 48 h.
The mass spectrometry analyses were performed on a Thermo Fisher (Waltham, MA) LTQ Orbitrap Elite mass spectrometer coupled with a Proxeon Easy NanoLC system located at the Proteomics and Mass Spectrometry Facility, University of Georgia. The peptides were loaded onto a reversed-phase column (Dionex PepMap 100 C8 or a 100-μm-internal-diameter column/emitter self-packed with 200-Å 5 μM Bruker MagicAQ C18 resin) and then eluted into the mass spectrometer. Briefly, the two-buffer gradient elution at a flow rate of 500 nl min−1 (0.1% formic acid as buffer A and 99.9% acetonitrile with 0.1% formic acid as buffer B) started with 5% buffer B for 2 min, which was then increased to 25% buffer B in 60 min, 40% buffer B in 10 min, and finally 95% buffer B in 10 min.
The data-dependent acquisition (DDA) method was used to acquire MS data. A survey MS scan was acquired first, and the top 5 ions in the MS scan were then selected for collision-induced dissociation (CID) and high-energy collisional dissociation (HCD) MS/MS experiments. Whenever necessary, electron transfer dissociation (ETD) was used instead of CID for better identification of posttranslational modifications (53). MS and MS/MS scans were acquired by using an Orbitrap instrument at resolutions of 120,000 and 30,000, respectively. Data were acquired using Xcalibur software (version 2.2; Thermo Fisher Scientific). Protein identification and modification characterizations were performed using Thermo Proteome Discoverer (version 1.3/1.4) with the Mascot (Matrix Science) or SEQUEST (Thermo) program.
Bioinformatic analyses.
The distribution of the methanogenesis marker 10 gene family TIGR03278 across Archaea was assessed using the Integrated Microbial Genomes and Microbiomes Expert Review (IMG/M ER) platform (54, 55). Genome assemblies from the novel methanogen and NAOA lineages were often not hosted by IMG/M ER at the time of analysis (Table 4). Instead, their annotation files were downloaded from GenBank and searched using BLASTp (default parameters) for the marker 10 homologs from ANME-2 and M. maripaludis. The search was conducted at either the NCBI online server or a local server if the online server had not yet hosted the genome assemblies. Protein structural analysis was conducted with either EMBOSS 6.5.7 (56) or Protein Workshop (57). Unless otherwise mentioned, all other analyses were done with Geneious versions 8 and 10 (58).
Nucleotide sequence accession number.
The p5L-R plasmid sequence and annotation were deposited in GenBank under accession number MN251049.
Supplementary Material
ACKNOWLEDGMENTS
We thank Aynom Tsegay Misghina and Bhavana Nagareddy for their assistance in developing the p5L-R tool, the University of Georgia iGEM team for their contribution in developing the methanococcal mCherry reporter system, Hannah Bullock for assistance using the FPLC system, and the Whitman lab members, especially Suet Yee Chong, for assistance with crystal structure analysis.
Indirect support of laboratory facilities and services for research was provided by the Office of the Vice-President for Research at the University of Georgia. The Korea Institute of Ocean Science and Technology supported development of the genetic deletion tool. National Science Foundation grant MCB-1410102 enabled construction of the initial deletion of mmpX, and contract DE-SC0018028 from the U.S. Department of Energy supported the construction and characterization of additional mutants and publication costs.
The experiments were conceived and designed by Z.L., C.-W.C., E.C.D., and W.B.W. They were executed by Z.L., N.S., C.-W.C., H.S., and R.P. The manuscript was written by Z.L., C.-W.C., and W.B.W.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Ermler U, Grabarse W, Shima S, Goubeaud M, Thauer RK. 1997. Crystal structure of methyl-coenzyme M reductase: the key enzyme of biological methane formation. Science 278:1457–1462. doi: 10.1126/science.278.5342.1457. [DOI] [PubMed] [Google Scholar]
- 2.Scheller S, Goenrich M, Boecher R, Thauer RK, Jaun B. 2010. The key nickel enzyme of methanogenesis catalyses the anaerobic oxidation of methane. Nature 465:606–608. doi: 10.1038/nature09015. [DOI] [PubMed] [Google Scholar]
- 3.Thauer RK. 5 April 2019. Methyl (alkyl)-coenzyme M reductases: nickel F-430-containing enzymes involved in anaerobic methane formation and in anaerobic oxidation of methane or of short chain alkanes. Biochemistry doi: 10.1021/acs.biochem.9b00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conrad R. 2009. The global methane cycle: recent advances in understanding the microbial processes involved. Environ Microbiol Rep 1:285–292. doi: 10.1111/j.1758-2229.2009.00038.x. [DOI] [PubMed] [Google Scholar]
- 5.Rittmann S, Seifert A, Herwig C. 2015. Essential prerequisites for successful bioprocess development of biological CH4 production from CO2 and H2. Crit Rev Biotechnol 35:141–151. doi: 10.3109/07388551.2013.820685. [DOI] [PubMed] [Google Scholar]
- 6.Soo VW, McAnulty MJ, Tripathi A, Zhu F, Zhang L, Hatzakis E, Smith PB, Agrawal S, Nazem-Bokaee H, Gopalakrishnan S, Salis HM, Ferry JG, Maranas CD, Patterson AD, Wood TK. 2016. Reversing methanogenesis to capture methane for liquid biofuel precursors. Microb Cell Fact 15:11. doi: 10.1186/s12934-015-0397-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lyu Z, Shao N, Akinyemi T, Whitman WB. 2018. Methanogenesis. Curr Biol 28:R727–R732. doi: 10.1016/j.cub.2018.05.021. [DOI] [PubMed] [Google Scholar]
- 8.Evans PN, Boyd JA, Leu AO, Woodcroft BJ, Parks DH, Hugenholtz P, Tyson GW. 2019. An evolving view of methane metabolism in the Archaea. Nat Rev Microbiol 17:219–232. doi: 10.1038/s41579-018-0136-7. [DOI] [PubMed] [Google Scholar]
- 9.Chen S-C, Musat N, Lechtenfeld OJ, Paschke H, Schmidt M, Said N, Popp D, Calabrese F, Stryhanyuk H, Jaekel U, Zhu Y-G, Joye SB, Richnow H-H, Widdel F, Musat F. 2019. Anaerobic oxidation of ethane by archaea from a marine hydrocarbon seep. Nature 568:108–111. doi: 10.1038/s41586-019-1063-0. [DOI] [PubMed] [Google Scholar]
- 10.Laso-Perez R, Wegener G, Knittel K, Widdel F, Harding KJ, Krukenberg V, Meier DV, Richter M, Tegetmeyer HE, Riedel D, Richnow HH, Adrian L, Reemtsma T, Lechtenfeld OJ, Musat F. 2016. Thermophilic archaea activate butane via alkyl-coenzyme M formation. Nature 539:396–401. doi: 10.1038/nature20152. [DOI] [PubMed] [Google Scholar]
- 11.Lyu Z, Whitman WB. 2019. Transplanting the pathway engineering toolbox to methanogens. Curr Opin Biotechnol 59:46–54. doi: 10.1016/j.copbio.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 12.Borrel G, Adam PS, McKay LJ, Chen L-X, Sierra-García IN, Sieber CMK, Letourneur Q, Ghozlane A, Andersen GL, Li W-J, Hallam SJ, Muyzer G, de Oliveira VM, Inskeep WP, Banfield JF, Gribaldo S. 2019. Wide diversity of methane and short-chain alkane metabolisms in uncultured archaea. Nat Microbiol 4:603–613. doi: 10.1038/s41564-019-0363-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seitz KW, Dombrowski N, Eme L, Spang A, Lombard J, Sieber JR, Teske AP, Ettema TJG, Baker BJ. 2019. Asgard archaea capable of anaerobic hydrocarbon cycling. Nat Commun 10:1822. doi: 10.1038/s41467-019-09364-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Wegener G, Hou J, Wang F, Xiao X. 2019. Expanding anaerobic alkane metabolism in the domain of Archaea. Nat Microbiol 4:595–602. doi: 10.1038/s41564-019-0364-2. [DOI] [PubMed] [Google Scholar]
- 15.Boyd JA, Jungbluth SP, Leu AO, Evans PN, Woodcroft BJ, Chadwick GL, Orphan VJ, Amend JP, Rappé MS, Tyson GW. 2019. Divergent methyl-coenzyme M reductase genes in a deep-subseafloor Archaeoglobi. ISME J 13:1269–1279. doi: 10.1038/s41396-018-0343-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shima S, Krueger M, Weinert T, Demmer U, Kahnt J, Thauer RK, Ermler U. 2011. Structure of a methyl-coenzyme M reductase from Black Sea mats that oxidize methane anaerobically. Nature 481:98–101. doi: 10.1038/nature10663. [DOI] [PubMed] [Google Scholar]
- 17.Wongnate T, Sliwa D, Ginovska B, Smith D, Wolf MW, Lehnert N, Raugei S, Ragsdale SW. 2016. The radical mechanism of biological methane synthesis by methyl-coenzyme M reductase. Science 352:953–958. doi: 10.1126/science.aaf0616. [DOI] [PubMed] [Google Scholar]
- 18.Lyu Z, Chou CW, Shi H, Wang L, Ghebreab R, Phillips D, Yan Y, Duin EC, Whitman WB. 2018. Assembly of methyl coenzyme M reductase in the methanogenic archaeon Methanococcus maripaludis. J Bacteriol 200:e00746-17. doi: 10.1128/JB.00746-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kahnt J, Buchenau B, Mahlert F, Krüger M, Shima S, Thauer RK. 2007. Post-translational modifications in the active site region of methyl-coenzyme M reductase from methanogenic and methanotrophic archaea. FEBS J 274:4913–4921. doi: 10.1111/j.1742-4658.2007.06016.x. [DOI] [PubMed] [Google Scholar]
- 20.Wagner T, Kahnt J, Ermler U, Shima S. 2016. Didehydroaspartate modification in methyl-coenzyme M reductase catalyzing methane formation. Angew Chem Int Ed Engl 55:10630–10633. doi: 10.1002/anie.201603882. [DOI] [PubMed] [Google Scholar]
- 21.Wagner T, Wegner CE, Kahnt J, Ermler U, Shima S. 2017. Phylogenetic and structural comparisons of the three types of methyl coenzyme M reductase from Methanococcales and Methanobacteriales. J Bacteriol 199:e00197-17. doi: 10.1128/JB.00197-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Selmer T, Kahnt J, Goubeaud M, Shima S, Grabarse W, Ermler U, Thauer RK. 2000. The biosynthesis of methylated amino acids in the active site region of methyl-coenzyme M reductase. J Biol Chem 275:3755–3760. doi: 10.1074/jbc.275.6.3755. [DOI] [PubMed] [Google Scholar]
- 23.Nayak DD, Mahanta N, Mitchell DA, Metcalf WW. 2017. Post-translational thioamidation of methyl-coenzyme M reductase, a key enzyme in methanogenic and methanotrophic Archaea. Elife 6:e29218. doi: 10.7554/eLife.29218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahanta N, Liu A, Dong S, Nair SK, Mitchell DA. 2018. Enzymatic reconstitution of ribosomal peptide backbone thioamidation. Proc Natl Acad Sci U S A 115:3030–3035. doi: 10.1073/pnas.1722324115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Basu MK, Selengut JD, Haft DH. 2011. ProPhylo: partial phylogenetic profiling to guide protein family construction and assignment of biological process. BMC Bioinformatics 12:434. doi: 10.1186/1471-2105-12-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarmiento F, Mrazek J, Whitman WB. 2013. Genome-scale analysis of gene function in the hydrogenotrophic methanogenic archaeon Methanococcus maripaludis. Proc Natl Acad Sci U S A 110:4726–4731. doi: 10.1073/pnas.1220225110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lyu Z, Chou C, Hao S, Patel R, Duin EC, Whitman WB. 2017. Mmp10 is required for post-translational methylation of arginine at the active site of methyl-coenzyme M reductase. bioRxiv doi: 10.1101/211441. [DOI]
- 28.Deobald D, Adrian L, Schone C, Rother M, Layer G. 2018. Identification of a unique radical SAM methyltransferase required for the sp(3)-C-methylation of an arginine residue of methyl-coenzyme M reductase. Sci Rep 8:7404. doi: 10.1038/s41598-018-25716-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Radle MI, Miller DV, Laremore TN, Booker SJ. 2019. Methanogenesis marker protein 10 (Mmp10) from Methanosarcina acetivorans is a radical S-adenosylmethionine methylase that unexpectedly requires cobalamin. J Biol Chem 294:11712–11725. doi: 10.1074/jbc.RA119.007609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farkas J, Stirrett K, Lipscomb GL, Nixon W, Scott RA, Adams MW, Westpheling J. 2012. Recombinogenic properties of Pyrococcus furiosus strain COM1 enable rapid selection of targeted mutants. Appl Environ Microbiol 78:4669–4676. doi: 10.1128/AEM.00936-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lyu Z, Jain R, Smith P, Fetchko T, Yan Y, Whitman WB. 2016. Engineering the autotroph Methanococcus maripaludis for geraniol production. ACS Synth Biol 5:577–581. doi: 10.1021/acssynbio.5b00267. [DOI] [PubMed] [Google Scholar]
- 32.Kim W, Whitman WB. 1999. Isolation of acetate auxotrophs of the methane-producing archaeon Methanococcus maripaludis by random insertional mutagenesis. Genetics 152:1429–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gardner WL, Whitman WB. 1999. Expression vectors for Methanococcus maripaludis: overexpression of acetohydroxyacid synthase and beta-galactosidase. Genetics 152:1439–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lupa B, Hendrickson EL, Leigh JA, Whitman WB. 2008. Formate-dependent H2 production by the mesophilic methanogen Methanococcus maripaludis. Appl Environ Microbiol 74:6584–6590. doi: 10.1128/AEM.01455-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kusevic D, Kudithipudi S, Jeltsch A. 2016. Substrate specificity of the HEMK2 protein glutamine methyltransferase and identification of novel substrates. J Biol Chem 291:6124–6133. doi: 10.1074/jbc.M115.711952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paik WK, Paik DC, Kim S. 2007. Historical review: the field of protein methylation. Trends Biochem Sci 32:146–152. doi: 10.1016/j.tibs.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 37.Major TA, Liu Y, Whitman WB. 2010. Characterization of energy-conserving hydrogenase B in Methanococcus maripaludis. J Bacteriol 192:4022–4030. doi: 10.1128/JB.01446-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lie TJ, Costa KC, Lupa B, Korpole S, Whitman WB, Leigh JA. 2012. Essential anaplerotic role for the energy-converting hydrogenase Eha in hydrogenotrophic methanogenesis. Proc Natl Acad Sci U S A 109:15473–15478. doi: 10.1073/pnas.1208779109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fay AW, Wiig JA, Lee CC, Hu Y. 2015. Identification and characterization of functional homologs of nitrogenase cofactor biosynthesis protein NifB from methanogens. Proc Natl Acad Sci U S A 112:14829–14833. doi: 10.1073/pnas.1510409112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hendrickson EL, Kaul R, Zhou Y, Bovee D, Chapman P, Chung J, Conway de Macario E, Dodsworth JA, Gillett W, Graham DE, Hackett M, Haydock AK, Kang A, Land ML, Levy R, Lie TJ, Major TA, Moore BC, Porat I, Palmeiri A, Rouse G, Saenphimmachak C, Soll D, Van Dien S, Wang T, Whitman WB, Xia Q, Zhang Y, Larimer FW, Olson MV, Leigh JA. 2004. Complete genome sequence of the genetically tractable hydrogenotrophic methanogen Methanococcus maripaludis. J Bacteriol 186:6956–6969. doi: 10.1128/JB.186.20.6956-6969.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stupperich E, Kräutler B. 1988. Pseudo vitamin B12 or 5-hydroxybenzimidazolyl-cobamide are the corrinoids found in methanogenic bacteria. Arch Microbiol 149:268–271. doi: 10.1007/BF00422016. [DOI] [Google Scholar]
- 42.Vey JL, Drennan CL. 2011. Structural insights into radical generation by the radical SAM superfamily. Chem Rev 111:2487–2506. doi: 10.1021/cr9002616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Svetlitchnaia T, Svetlitchnyi V, Meyer O, Dobbek H. 2006. Structural insights into methyltransfer reactions of a corrinoid iron-sulfur protein involved in acetyl-CoA synthesis. Proc Natl Acad Sci U S A 103:14331–14336. doi: 10.1073/pnas.0601420103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Banerjee R, Ragsdale SW. 2003. The many faces of vitamin B12: catalysis by cobalamin-dependent enzymes. Annu Rev Biochem 72:209–247. doi: 10.1146/annurev.biochem.72.121801.161828. [DOI] [PubMed] [Google Scholar]
- 45.Vanwonterghem I, Evans PN, Parks DH, Jensen PD, Woodcroft BJ, Hugenholtz P, Tyson GW. 2016. Methylotrophic methanogenesis discovered in the archaeal phylum Verstraetearchaeota. Nat Microbiol 1:16170. doi: 10.1038/nmicrobiol.2016.170. [DOI] [PubMed] [Google Scholar]
- 46.Evans PN, Parks DH, Chadwick GL, Robbins SJ, Orphan VJ, Golding SD, Tyson GW. 2015. Methane metabolism in the archaeal phylum Bathyarchaeota revealed by genome-centric metagenomics. Science 350:434–438. doi: 10.1126/science.aac7745. [DOI] [PubMed] [Google Scholar]
- 47.Bedford MT, Clarke SG. 2009. Protein arginine methylation in mammals: who, what, and why. Mol Cell 33:1–13. doi: 10.1016/j.molcel.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sarmiento F, Leigh JA, Whitman WB. 2011. Genetic systems for hydrogenotrophic methanogens. Methods Enzymol 494:43–73. doi: 10.1016/B978-0-12-385112-3.00003-2. [DOI] [PubMed] [Google Scholar]
- 49.Long F, Wang L, Lupa B, Whitman WB. 2017. A flexible system for cultivation of Methanococcus and other formate-utilizing methanogens. Archaea 2017:7046026. doi: 10.1155/2017/7046026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jones WJ, Paynter MJB, Gupta R. 1983. Characterization of Methanococcus maripaludis sp. nov., a new methanogen isolated from salt marsh sediment. Arch Microbiol 135:91–97. doi: 10.1007/BF00408015. [DOI] [Google Scholar]
- 51.Franklin MJ, Wiebe WJ, Whitman WB. 1988. Populations of methanogenic bacteria in a Georgia salt marsh. Appl Environ Microbiol 54:1151–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schindelin J, Rueden CT, Hiner MC, Eliceiri KW. 2015. The ImageJ ecosystem: an open platform for biomedical image analysis. Mol Reprod Dev 82:518–529. doi: 10.1002/mrd.22489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Syka JE, Coon JJ, Schroeder MJ, Shabanowitz J, Hunt DF. 2004. Peptide and protein sequence analysis by electron transfer dissociation mass spectrometry. Proc Natl Acad Sci U S A 101:9528–9533. doi: 10.1073/pnas.0402700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Markowitz VM, Chen I-MA, Chu K, Szeto E, Palaniappan K, Pillay M, Ratner A, Huang J, Pagani I, Tringe S, Huntemann M, Billis K, Varghese N, Tennessen K, Mavromatis K, Pati A, Ivanova NN, Kyrpides NC. 2014. IMG/M 4 version of the integrated metagenome comparative analysis system. Nucleic Acids Res 42:D568–D573. doi: 10.1093/nar/gkt919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Markowitz VM, Chen I-MA, Palaniappan K, Chu K, Szeto E, Pillay M, Ratner A, Huang J, Woyke T, Huntemann M, Anderson I, Billis K, Varghese N, Mavromatis K, Pati A, Ivanova NN, Kyrpides NC. 2014. IMG 4 version of the integrated microbial genomes comparative analysis system. Nucleic Acids Res 42:D560–D567. doi: 10.1093/nar/gkt963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rice P, Longden I, Bleasby A. 2000. EMBOSS: the European Molecular Biology Open Software Suite. Trends Genet 16:276–277. doi: 10.1016/s0168-9525(00)02024-2. [DOI] [PubMed] [Google Scholar]
- 57.Moreland JL, Gramada A, Buzko OV, Zhang Q, Bourne PE. 2005. The Molecular Biology Toolkit (MBT): a modular platform for developing molecular visualization applications. BMC Bioinformatics 6:21. doi: 10.1186/1471-2105-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A. 2012. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yoon SH, Turkarslan S, Reiss DJ, Pan M, Burn JA, Costa KC, Lie TJ, Slagel J, Moritz RL, Hackett M, Leigh JA, Baliga NS. 2013. A systems level predictive model for global gene regulation of methanogenesis in a hydrogenotrophic methanogen. Genome Res 23:1839–1851. doi: 10.1101/gr.153916.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Walters AD, Smith SE, Chong JP. 2011. Shuttle vector system for Methanococcus maripaludis with improved transformation efficiency. Appl Environ Microbiol 77:2549–2551. doi: 10.1128/AEM.02919-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mayumi D, Mochimaru H, Tamaki H, Yamamoto K, Yoshioka H, Suzuki Y, Kamagata Y, Sakata S. 2016. Methane production from coal by a single methanogen. Science 354:222–225. doi: 10.1126/science.aaf8821. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.