Abstract
To identify long-term effects of traumatic brain injury (TBI) on levels of plasma neuron-derived exosome (NDE) protein biomarkers of cognitive impairment (CI), plasmas were obtained from four groups of older veterans, who were matched for age and sex: no TBI or CI (n = 42), no TBI with CI (n = 19), TBI without CI (n = 21), and TBI with CI (n = 26). The TBI was sustained 12 to 74 years before the study in 75%. The NDEs were enriched by sequential precipitation and anti-L1CAM antibody immunoabsorption, and extracted protein biomarkers were quantified by enzyme-linked immunosorbent assays. Chronic NDE biomarkers known to increase for three to 12 months after TBI, including cellular prion protein (PrPc), synaptogyrin-3, P-T181-tau, P-S396-tau, Aβ42, and interleukin (IL)-6, were elevated significantly in subjects who had TBI and CI compared with controls with TBI but no CI. Chronic NDE biomarker levels in subjects without TBI showed significantly higher levels of PrPc, synaptogyrin-3, P-T181-tau, and Aβ42, but not P-S396-tau and IL-6, in those with CI compared with controls without CI. The acute NDE biomarkers claudin-5, annexin VII, and aquaporin-4 were not increased in either group with CI. The NDE biomarkers P-S396-tau and IL-6, which are increased distinctively with CI after TBI, may prove useful in evaluating CI in older patients. Aβ42 and P-tau species, as well as their respective putative receptors, PrPc and synaptogyrin-3, remain elevated for decades after TBI and may mediate TBI-associated CI and be useful targets for development of drugs.
Keywords: amyloid, biomarkers, phospho-tau, prion cellular protein, proteinopathic neurodegeneration, synaptogyrin-3
Introduction
Traumatic brain injury (TBI) of different degrees of severity is a common medical problem worldwide that is especially frequent in young adults exposed to shockwaves of explosions in military combat or violent contact in various sports.1,2 Some effects of TBI develop and subside rapidly, but many others, including mild cognitive impairment (CI) and dementia, appear and worsen over extended periods of neuronal degeneration. Although it was thought initially that late-onset dementia was evoked only by moderate to severe TBI, military veterans showed very significant increases in risk of dementia even in those with mild TBI (mTBI).3
Acute TBI evokes a complex of neural responses that includes changes in neural fluid and vesicle movements, multi-factorial inflammation with increased oxidation and proteolysis, and initiation of regional synaptic and neuronal loss that may slowly progress to a state of proteinopathic neurodegeneration with CI.4–6 Some aspects of the neurodegenerative pathology observed in TBI-associated CI resemble those of Alzheimer disease (AD), but there also are distinguishing features.7,8 Hyperphosphorylated tau (P-tau) neurofibrillary tangles in neurons, astrocytes, and clusters of neurites deep in cortical sulci around blood vessels are more prominent in TBI-associated CI than AD. Neuronal deposition of amyloid β 1-42 (Aβ42) peptides is greater in AD than in TBI-associated CI. Investigations of a wide range of neurally derived proteins in cerebrospinal fluid (CSF) and plasma so far have not yielded biomarkers that reliably predict progression of TBI to chronic dementia or offer useful targets for therapy.7,9–14
Neuron-derived exosomes (NDEs) collect a wide range of neuronal proteins of cellular membranes and cytosol as they move from the plasma membrane through endosomal pathways prior to being secreted into extracellular fluids of the central nervous system (CNS) and entry into other neural cells or passage through the blood–brain barrier (BBB) into blood.15 We thus had conducted an initial investigation of changes in NDE cargo protein levels that occur acutely within the first two weeks after sports-induced mild TBI (mTBI) and those seen chronically at up to 3 to 12 months after sports-induced mTBI.16
In the first week after mTBI, levels of NDEs in plasma were decreased significantly, possibly as a result of diminished vesicular traffic associated with concurrently depressed neuronal levels of the small guanosine triphosphate (GTP)ases—including ras-related small GTPase 10 (RAB10), RAB35, and others.16 In the same acute period, NDE levels of cargo proteins with known neural functions were elevated, including annexin VII, ubiquitin C-terminal hydrolase L1 (UCH-L1), fragments of AII spectrin, claudin-5, sodium-potassium-chloride co-transporter-1 (NKCC-1), aquaporins 1 and 4, and synaptogyrin-3. Only NDE levels of aquaporin 4 and synaptogyrin-3 were still elevated significantly but less at 3 to 12 months after the most recent mTBI.16
Of the neural proteins with putative pathogenic activities that were detected in plasma NDEs, levels of Aβ42, P-T181-tau, P-S396-tau, interleukin (IL)-6, and prion cellular protein (PrPc) were significantly elevated at 3 to 12 months after the most recent mTBI, and that of total tau was elevated marginally. Compared with elevated NDE levels in the first week after mTBI, those at 3 to 12 months were higher for Aβ42, P-T181-tau, and P-S396-tau, the same for PrPc, and lower for IL-6. We report now the persistent effects of TBI of different degrees of severity up to decades later on plasma NDE levels of proteins with neural pathogenic activities and their putative neuronal receptors relevant to TBI-associated CI.
Methods
Research subject recruitment, evaluation, and blood collection
All participants were military veterans residing at the Veterans Home of California in Yountville, CA (VHCY). Residents of VHCY were recruited for the study through flyers, social events, and word of mouth. The total cohort of 108 had an age range of 50 to 95 years, and all were able to provide informed consent for research. Individuals were excluded because of a cognition level too low to allow informed consent as judged by a Mini-Mental State Examination (MMSE) score17 < 20, past penetrating head injury, inability or unwillingness to provide a blood sample, or a comorbid serious medical condition or severe hearing or vision impairment. The study was approved by the Human Research Institutional Review Committee of the University of California, San Francisco, and all participants gave written informed consent.
TBI was defined for the affected groups three and four as a head injury resulting in any form of medical care. The two groups classified as never having sustained a TBI had no history of any head injury that produced neurological symptoms or required medical care by self-report. A history of TBI was found in VHCY medical records of 52% of the TBI participants and was absent from the medical records of all non-TBI participants. The Ohio State University TBI Identification Method,18 a structured clinical interview recommended by the National Institute of Neurological Disorders and Stroke as a Common Data Element for the retrospective assessment of lifetime TBI in clinical research, was used to assess TBI history and determine the severity of TBI.
General cognitive abilities were assessed by the MMSE (range of scores, 0–30), a frequently used test for general cognition. The Auditory Verbal Learning Task (AVLT)19,20 is a verbal memory test with five Learning Trials and a 30-min Delayed Recall test. The Learning Trials (total trials 1–5) and Delayed Recall were used to determine learning and memory performance, respectively. Mental processing speed was quantified by the Wechsler Adult Intelligence Scale-Revised (WAIS-R) Digit Symbol Task,21 a timed task involving number and symbol matching.
Given that norms are less established for the very old, we defined CI operationally using a composite Z-score from the AVLT learning score, AVLT delay score, MMSE, and WAIS Digit Symbol. The composite score was based on the Alzheimer's Disease Cooperative Study Preclinical Alzheimer Cognitive Composite score,22 a measure of early cognitive impairment that has been used in multiple studies.
First, each participant's raw cognitive test scores were compared with demographically corrected normative data for each measure—Mayo's Older Americans Normative Studies age-corrected norms for AVLT Learning Trials23 and Delayed Recall and Alzheimer's Disease Centers' Uniform Data Set age-, gender-, and education-corrected norms24 for MMSE and WAIS-R Digit Symbol. Then, each individual's raw test scores were converted into demographically corrected Z-scores using these normative data, indicating how much each individual's performance differs from that of healthy, demographically similar peers. Finally, the individual Z-scores for each participant were averaged to create a composite cognitive Z-score.
CI was defined as a cognitive composite score below normative values by more than one standard deviation, diagnosis of dementia in medical records, or history of taking a dementia prescription medication (donepezil, memantine, or rivastigmine) that was recorded in their medical records.
The first and second study groups were composed of subjects who had not experienced any TBI. Subjects of the first group (n = 42) had normal cognition, whereas CI had developed in those of the second group (n = 19). Subjects of groups three (n = 21) and four (n = 26) had experienced one to four episodes of TBI in the one to 74 years before blood sampling that was not followed by CI in those of group three, but was followed by CI in those of group four. Seventy-five percent of subjects of groups three and four had experienced TBI decades ago, and 25% had TBI one to 10 years ago.
Venous blood was obtained for plasma by the standard phlebotomy technique using EDTA for anticoagulation. Plasma was stored in 0.25 mL portions at -80°C.
Enrichment of plasma NDEs for extraction and enzyme-linked immunosorbent assay (ELISA) quantification of proteins
Aliquots of 0.25 mL plasma were incubated with 0.1 mL thromboplastin D (Thermo Fisher Scientific, Waltham, MA), followed by addition of 0.15 mL of calcium- and magnesium-free Dulbecco balanced salt solution with protease inhibitor cocktail (Roche, Indianapolis, IN) and phosphatase inhibitor cocktail (Thermo Fisher Scientific), as described.25 After centrifugation at 3000g for 30 min at 4°C, NDEs were harvested from resultant supernatants by sequential ExoQuick (System Biosciences, Mountain View, CA) precipitation and immunoabsorption enrichment with mouse anti-human CD171 (L1CAM neural adhesion protein) biotinylated antibody (clone 5G3; eBiosciences, San Diego, CA) as described.25
The NDEs were counted and their size ranges were determined, as described previously15 Then NDEs were lysed in mammalian protein extraction reagent (M-PER; ThermoFisher Scientific) that contained protease and phosphatase inhibitor cocktails prior to storage at -80°C before ELISAs. These procedures all have been described in greater detail elsewhere along with an estimate that 8–10% of NDEs may be attributable to other cellular sources.15
The NDE proteins were quantified by ELISA kits for human claudin-5 and tetraspanning exosome marker CD81 (Cusabio-American Research Products, Waltham, MA), PrPc, aquaporin-1 and aquaporin-4 (Cloud-Clone Corp.-American Research Products), synaptogyrin-3 (Abbkine, Wuhan, China-American Research Products), IL-6 (R&D Systems, Minneapolis, MN), annexin VII (Biomatik, Wilmington, DE), Aβ42 (ultrasensitive ELISA) and P-S396-tau (Invitrogen, ThermoFisher Scientific, Vienna, Austria), and P-T181-tau (FUJIREBIO, US, Inc., Malvern, PA).
The mean value for all determinations of CD81 in the total population was set at 1.00, and relative values of CD81 for each sample were used to normalize their recovery. There were no differences in recovery of NDEs between the groups based on mean (± standard error of the mean) relative levels of CD81: no TBI or CI (0.97 ± 0.07), no TBI with CI (0.98 ± 0.08), TBI without CI (1.11 ± 0.12), and TBI with CI (0.91 ± 0.07). For each group, there was a significant correlation between NDE counts and CD81 levels with Pearson coefficient r values >0.85 for all. One investigator (EJG) conducted all ELISAs without knowledge of the clinical data for any subject.
Statistical analyses
The Shapiro-Wilks test showed whether data in each set were distributed normally. Data for only two analytes (IL-6 and Aβ42) were not distributed normally, and the significance of the differences between their group values was determined by the Mann-Whitney rank-sum test. For all other analytes, groups were compared using an unpaired Student t test, including a Bonferroni correction, and analysis of variance (ANOVA) analyses (Prism 7; GraphPad Software, La Jolla, CA). Correlative evaluations used a Pearson correlation method (GraphPad).
Results
There were no significant differences in age among subjects of the four study groups, nor were there significant differences between the two groups without TBI and those with TBI with respect to the percentages of male or minority race participants or total years of education (Table 1). Levels of cognition, quantified by the two distinct methods of MMSE and Composite Z score, were significantly higher for group 1 than 2 without TBI and for group 3 than 4 with TBI (Table 1).
Table 1.
Demographics and Cognitive Levels for the Four Study Groups
| |
Control wo TBI |
CI wo TBI |
TBI wo CI |
TBI w CI |
|
|---|---|---|---|---|---|
| n = 42 | n = 19 | n = 21 | n = 26 | p | |
| Demographics | |||||
| Age in years (SD) | 78.6 (8.8) | 79.5 (9.7) | 79.4 (9.6) | 74.7 (10.6) | NS |
| Number of males (%) | 35 (83.3) | 18 (94.7) | 18 (85.7) | 26 (100.0) | NS |
| Number of minority race (%) | 4 (9.5) | 3 (15.8) | 2 (9.5) | 1 (3.9) | NS |
| Education in years (SD) | 15.3 (1.8) | 14.9(2.2) | 15.3 (2.5) | 14.7 (2.1) | NS |
| Cognition | |||||
| MMSE score (SD) | 29.0 (1.3) | 26.4 (2.8) | 29.0 (1.0) | 27.6 (1.8) | <0.001* |
| Composite Z score (SD) | 0.25 (0.46) | -0.40 (0.49) | 0.24 (0.59) | -0.22 (0.58) | <0.001* |
TBI, traumatic brain injury; CI, cognitive impairment; wo, without; w, with; SD, standard deviation; MMSE, Mini Mental State Examination.
p values for comparisons of two without TBI and two TBI groups.
In comparing groups 3 and 4 with TBI, the numbers of subjects in each group who sustained one TBI and two or more TBIs were no different. Six subjects in each of the TBI groups sustained their most recent TBI one to 10 years before donating blood for this study, and neither the mean times or the ranges of times since the most recent TBI differed between groups 3 and 4 (Table 2). Similarly, there was no difference between the percentages of subjects in groups 3 and 4 who had moderate or severe TBIs (Table 2).
Table 2.
TBI Characteristics
| |
TBI wo CI |
TBI w CI |
|
|---|---|---|---|
| n = 21 | n = 26 | p | |
| Years since most recent TBI, mean (SD) | 33.9 (26.2) | 25.4 (23.9) | NS |
| Number of TBIs (SD) | |||
| 1 TBI | 9 (42.9) | 14 (53.9) | NS |
| >2 TBIs | 12 (57.1) | 12 (46.2) | NS |
| Moderate/severe TBI, n (% of total) | 7 (33.3) | 10 (38.5) | NS |
TBI, traumatic brain injury; wo, without; CI, cognitive impairment; w, with; SD, standard deviation; n, number; NS, not significant.
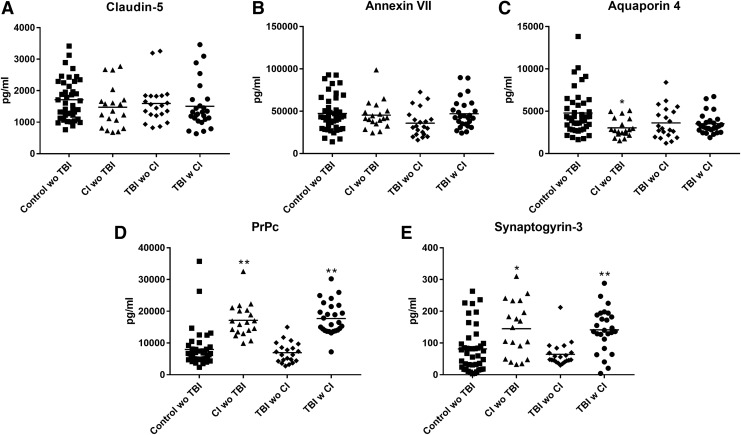
The CD81-normalized levels of acutely responsive NDE cargo proteins, such as claudin-5 and annexin VII, that are representative of those elevated only transiently within weeks after an impact sport-induced mTBI16 were the same for cognitively normal controls, subjects with CI without TBI, and those who had TBI years before testing without or with subsequent CI (Fig. 1 A,B). The CD81-normalized levels of NDE aquaporin 4, which were elevated significantly within one week and less for at least three to 12 months after an impact sport-induced mTBI,16 also were the same for controls, subjects with CI without TBI, and those who had TBI years before testing without or with subsequent CI (Fig.1 C).
FIG. 1.
Neuron-derived exosome (NDE) levels of normal functional cargo proteins in cross-sectional control, cognitive impairment (CI) with no history of traumatic brain injury (TBI), TBI without (w/o) CI, and TBI with (w) CI groups. Each point represents the value for one participant, and the horizontal line in point clusters is the mean level for that group. Mean ± standard error of the mean for control w/o TBI, CI w/o TBI, TBI w/o CI, and TBI w CI, respectively, are 1711 ± 101 pg/mL, 1474 ± 154 pg/mL, 1595 ± 139 pg/mL, and 1505 ± 145 pg/mL for claudin-5 (A), 47256 ± 3249 pg/mL, 45274 ± 3882 pg/mL, 35831 ± 3503 pg/mL, and 47087 ± 3486 pg/mL for annexin VII (B), 4787 ± 397 pg/mL, 3029 ± 251 pg/mL, 3623 ± 408 pg/mL, and 3522 ± 241 pg/mL for aquaporin 4 (C), 7986 ± 922 pg/mL, 17149 ± 1207 pg/mL, 6949 ± 702 pg/mL, and 17730 ± 995 pg/mL for PrPc (D), and 81.7 ± 11.0 pg/mL, 145 ± 19.7 pg/mL, 64.3 ± 9.14 pg/mL, and 142 ± 13.3 pg/mL for synaptogyrin-3 (E). The significance of differences shown between values for controls and CI w/o TBI subjects and between values for TBI w/o CI and TBI with CI subjects, respectively, were calculated by an unpaired Student t test; *p < 0.01, **p < 0.001.
The CD81-normalized NDE levels of PrPc and synaptogyrin-3, which were elevated within one week and for at least three to 12 months after an impact sport-induced mTBI,16 were significantly higher in those with CI than without CI years after TBI and also were significantly higher in subjects with CI without TBI than cognitively normal controls (Fig.1 D,E).
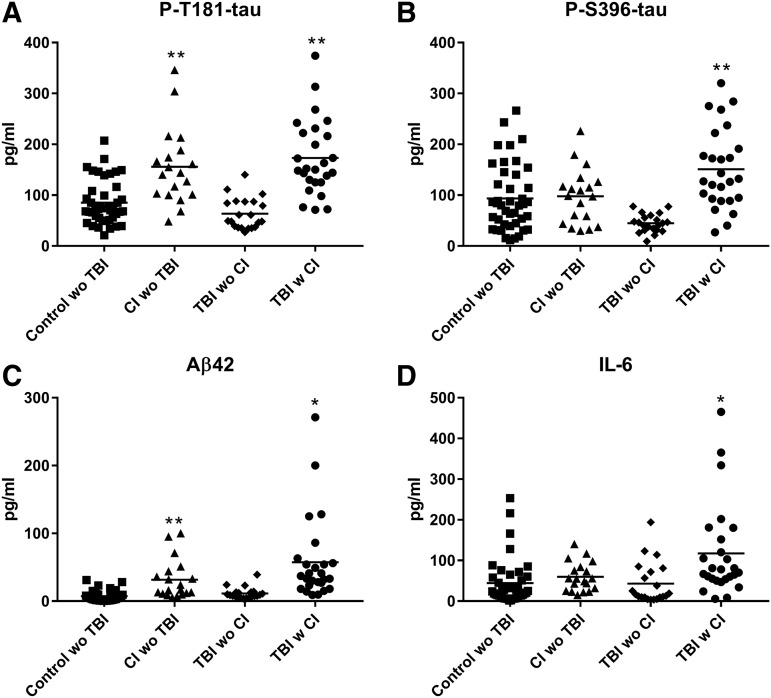
The CD81-normalized NDE levels of the neuropathogenic proteins P-T181-tau and Aβ42 were shown previously to be significantly elevated within one week with persistence for at least three to 12 months after an impact sport-induced mTBI and also are elevated up to 10 years before clinical presentation of AD.16,26,27 The present results show that these proteins also were significantly higher in those with CI than without CI many years after TBI and in subjects with CI without TBI compared with their cognitively normal controls (Fig. 2 A,C).
FIG. 2.
Neuron-derived exosome (NDE) levels of putatively proteinopathic cargo proteins in cross-sectional control, cognitive impairment (CI) with no history of traumatic brain injury (TBI), TBI without (w/o) CI, and TBI with (w) CI groups. Each point represents the value for one participant, and the horizontal line in point clusters is the mean level for that group. Mean ± standard error of the mean for control w/o TBI, CI w/o TBI, TBI w/o CI, and TBI w CI, respectively, are 85.2 ± 6.67 pg/mL, 156 ± 17.3 pg/mL, 63.5 ± 6.70 pg/mL, and 173 ± 14.6 pg/mL for P-T181-tau (A), 93.5 ± 10.0 pg/mL, 97.7 ± 12.4 pg/mL, 44.7 ± 3.92 pg/mL, and 151 ± 15.4 pg/mL for P-S396-tau (B), 7.49 ± 1.16 pg/mL, 31.5 ± 6.64 pg/mL, 11.3 ± 1.83 pg/mL, and 57.5 ± 12.1 pg/mL for Aβ42 (C), and 44.2 ± 8.54 pg/mL, 59.7 ± 8.21 pg/mL, 43.2 ± 11.2 pg/mL and 117 ± 22.2 pg/mL for IL-6 (D). The significance of differences shown between values for controls and CI w/o TBI subjects and between values for TBI w/o CI and TBI with CI subjects, respectively, were calculated by an unpaired Student t test; *p < 0.01, **p < 0.001.
The CD81-normalized NDE levels of the neuropathogenic proteins P-S396-tau and IL-6 are elevated variably in AD depending on the stage and severity of neurodegeneration. The same two NDE proteins are consistently elevated after sport-induced mTBI with significant increases in P-S396-tau only after three months and for at least 12 months and in IL-6 within one week with persistence for at least three to 12 months.16
For this set of plasmas from elderly military veterans, both P-S396-tau and IL-6 were significantly higher in those with CI than without CI many years after TBI, but were not higher in subjects with CI without TBI than cognitively normal controls (Fig. 2 B,D). The ANOVA analyses were conducted to evaluate possible interactions between TBI and CI in increasing the levels of biomarkers. TBI with CI resulted in significantly higher levels than CI alone (p < 0.05) for P-S396-tau, IL-6, and Aβ42, but not for P-T181-tau, PrPc, or synaptogyrin-3. For most analytes, there is considerable overlap of the values for mTBI subjects without and with CI. For PrPc, P-T181-tau and P-S396-tau, however, only 20% or fewer of the values for subjects with CI overlap into the range for those without CI.
In summary, neuronal putative binding proteins PrPc and synaptogyrin-3 along with their respective neuropathogenic protein ligands Aβ42 and the P-tau species P-T181-tau and P-S396-tau all were significantly higher in military veteran TBI subjects with CI than without CI years after the injury. Further statistical analyses of values for the total set of plasma NDE analytes showed no significant correlations between those of any NDE cargo protein and the time interval between TBI and obtaining plasma for study. For the total set of plasma NDE analytes, however, there are very significant inverse correlations between cognitive composite Z scores and the CD81-normalized NDE levels of PrPc, P-T181-tau, and Aβ42 (p < 0.0001 from Pearson correlation coefficients).
Discussion
Our previous analyses of the time courses of abnormal elevations in levels of plasma NDE proteins after TBI had so far been limited to one year after the most recent mTBI in college athletes.16 Plasma NDE proteins in the present study were harvested from veterans whose most recent TBI was at least one decade before the study for 75% of group 3 and 4 participants and a mean of several decades before for the total group (Table 2). The current similar results substantially extend our previous findings (Fig. 1,2).
No increases were found in levels of any of the three acutely responsive NDE proteins—claudin-5, annexin VII, or aquaporin 4—that were elevated only for weeks to months after a sports-related TBI (Fig. 1). In contrast, levels of the plasma NDE proteins that were elevated for at least one year after a sports-related TBI also were significantly increased in the subjects with CI after very remote TBI relative to levels in those without CI at the same time after TBI. This set of NDE proteins that are increased relative to controls both for at least one year after sports-related TBI and now for elderly in a veterans home up to seven decades after TBI included PrPc, synaptogyrin-3, P-T181-tau, P-S396-tau, Aβ42, and IL-6 (Fig. 1,2).16 For military veterans resident in the same facility but with no history of TBI, elevations of plasma NDE proteins of this cluster in those with CI relative to those without CI included PrPc, synaptogyrin-3, P-T181-tau, and Aβ42, but not P-S396-tau or IL-6 (Fig. 1,2).
It is difficult to compare our current results with previous findings of elevated levels of total tau and P-T181-tau in total plasma exosomes from military subjects who had their last TBI more recently.28 In that earlier study, there was no enrichment of NDEs from total plasma exosomes, protease and phosphatase inhibitors were added late in the harvesting process, and analytes levels were one-third to 1/100 of those we and others now report.
Finding significant elevations of NDE levels of Aβ42-binding PrPc and P-tau-binding synaptogyrin-3, as well as Aβ42, two species of P-tau, and IL-6, decades after the last known TBI suggests that the putative progression factors PrPc and synaptogyrin-3 have been persistently trapping and concentrating their respective neurotoxic ligands on neurons over a very long interval. The importance of PrPc as a neuronal receptor for Aβ peptides has been supported by results of several recent studies.
Analyses of binding of synthetic Aβ to COS-7 cell transfectants expressing equal levels of a wide range of putative Aβ receptors demonstrated binding only by PrPc, Nogo receptor 1, and leukocyte immunoglobulin-like receptor subfamily B member 2.29 Further, the decrease in Aβ binding to mouse hippocampal neurons lacking PrPc was more than twice as great as those lacking both Nogo receptor 1 and leukocyte immunoglobulin-like receptor subfamily B member 2. Both PrPc and Nogo receptor 1 bound neurotoxic oligomeric Aβ (Aβo) preferentially over monomeric Aβ. Aβo eluted from human Alzheimer disease brain tissue was bound with high affinity only by PrPc.
In human neuroblastoma cells, activation of the endogenous metalloproteinase ADAM10 evoked shedding of PrPc and concurrent reduction in binding of Aβo, that were both affected oppositely in parallel by inhibition of ADAM10 activity.30 Knockdown of ADAM10 reduced shedding of PrPc and concomitantly increased neuroblastoma cell binding and toxicity of Aβo, that also were both blocked by a PrPc-blocking antibody. Thus, overall increases in neuronal expression of PrPc after a TBI would be expected to increase neuronal binding and toxicity of Aβo species. Similar studies have not yet been conducted with synaptogyrin-3 and P-tau species.
Current data add to the originally proposed progression factor hypothesis—the possibility that levels of Aβ42-PrPc and P-tau-synaptogyrin-3 complexes on the surface of neurons may be increased for decades after sustaining a TBI as a result of elevated neuronal surface expression of PrPc and synaptogyrin-3 evoked by the TBI.16,31 We hypothesize that the persistently elevated levels of these complexes in NDEs and on neurons deliver into neurons for many years a toxic level of their ligands Aβo and P-tau species, and that interference with this toxic mechanism could prevent the development of TBI-associated CI and possibly AD.
Much additional research will be needed, however, to clarify the potential neural pathogenic roles in TBI of PrPc, synaptogyrin-3, and perhaps other progression factors, such as Aβ42-binding and aggregating coagulation factor XIII and the inflammatory cytokine IL-6. Such progression factors are defined by their persistent elevation in NDEs and neurons affected by TBI, and their capacity to bind and thus concentrate neurotoxic Aβ peptides, especially Aβo, and various P-tau species.
Many other characteristics of such progression factor-Aβo or progression factor-P-tau complexes remain to be established. For example, are the complexes Aβ42-PrPc and P-tau-synaptogyrin-3 similarly or differently neurotoxic than the unconjugated proteins? Are cellular trafficking and intercellular movement of the neurotoxic proteins altered by binding to their membrane progression factor partners? Do PrPc and synaptogyrin-3 function as true cellular receptors in the sense that binding of their respective ligands sends biochemical or functional signals to host neurons and/or modifies neuronal distribution of PrPc or synaptogyrin-3?
There also may be therapeutic implications of the pathogenic roles of the progression factor-conjugated neurotoxic proteins. If drugs can be identified that dissociate these complexes or alter their distribution and activities in neurons, will the pathogenic effects of Aβo or P-tau species be diminished in chronic traumatic encephalopathy or perhaps in AD?
As accumulating evidence implicates several progression factors in neuronal delivery of neurotoxic Aβ peptides and P-tau species in mTBI and possibly also in early AD, a rationale is developing for expanding drug discovery efforts to include agents that affect neuronal expression of such proteins and their binding interactions with neurotoxic Aβo and P-tau.
Acknowledgments
This research was supported in part by the Department of Defense (W81XHW-14-2-0137) (KY) and the Intramural Research Program of the National Institute on Aging of the National Institutes of Health (MM, DK). The authors thank the research participants, the Veterans Home of California in Yountville, CA, and the dedicated study staff, including Kim Kelley and Dan Freimer. The authors are grateful to J.H. Goetzl for expert preparation of the illustrations.
Author Disclosure Statement
Dr. Goetzl has pending patent applications covering the basic exosome methods used in this publication. For the remaining authors, no competing financial interests exist.
References
- 1. Hawryluk G.W. and Manley G.T. (2015). Classification of traumatic brain injury: past, present, and future. Handb. Clin. Neurol. 127, 15–21 [DOI] [PubMed] [Google Scholar]
- 2. Nguyen R., Fiest K.M., McChesney J., Kwon C.S., Jette N., Frolkis A.D., Atta C., Mah S., Dhaliwal H., Reid A., Pringsheim T., Dykeman J., and Gallagher C. (2016). The International incidence of traumatic brain injury: a systematic review and meta-analysis. Can. J. Neurol. Sci. 43, 774–785 [DOI] [PubMed] [Google Scholar]
- 3. Barnes D.E., Byers A.L., Gardner R.C., Seal K.H., Boscardin W.J., and Yaffe K. (2018). Association of mild traumatic brain injury with and without loss of consciousness with dementia in US military veterans. JAMA Neurol. 75, 1055–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Roozenbeek B., Maas A.I., and Menon D.K. (2013). Changing patterns in the epidemiology of traumatic brain injury. Nat. Rev. Neurol. 9, 231–236 [DOI] [PubMed] [Google Scholar]
- 5. McKee A.C., Alosco M.L., and Huber B.R. (2016). Repetitive head impacts and chronic traumatic encephalopathy. Neurosurg. Clin. N. Am. 27, 529–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Quillinan N., Herson P.S., and Traystman R.J. (2016). Neuropathophysiology of brain injury. Anesthesiol. Clin. 34, 453–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. DeKosky S.T., Blennow K., Ikonomovic M.D., and Gandy S. (2013). Acute and chronic traumatic encephalopathies: pathogenesis and biomarkers. Nat. Rev. Neurol. 9, 192–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McKee A.C., Stein T.D., Kiernan P.T., and Alvarez V.E. (2015). The neuropathology of chronic traumatic encephalopathy. Brain Pathol. 25, 350–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Woodcock T. and Morganti-Kossmann M.C. (2013). The role of markers of inflammation in traumatic brain injury. Front. Neurol. 4, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Agoston D.V., Shutes-David A., and Peskind E.R. (2017). Biofluid biomarkers of traumatic brain injury. Brain Inj. 31, 1195–1203 [DOI] [PubMed] [Google Scholar]
- 11. Werhane M.L., Evangelista N.D., Clark A.L., Sorg S.F., Bangen K.J., Tran M., Schiehser D.M., and Delano-Wood L. (2017). Pathological vascular and inflammatory biomarkers of acute- and chronic-phase traumatic brain injury. Concussion 2, CNC30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim H.J., Tsao J.W., and Stanfill A.G. (2018). The current state of biomarkers of mild traumatic brain injury. JCI Insight 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roselli F., Chandrasekar A., and Morganti-Kossmann M.C. (2018). Interferons in traumatic brain and spinal cord injury: current evidence for translational application. Front. Neurol. 9, 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Di Battista A.P., Rhind S.G., and Baker A.J. (2013). Application of blood-based biomarkers in human mild traumatic brain injury. Front. Neurol. 4, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mustapic M., Eitan E., Werner J.K. Jr., Berkowitz S.T., Lazaropoulos M.P., Tran J., Goetzl E.J., and Kapogiannis D. (2017). Plasma extracellular vesicles enriched for neuronal origin: a potential window into brain pathologic processes. Front. Neurosci. 11, 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goetzl E.J., Elahi F.M., Mustapic M., Kapogiannis D., Pryhoda M., Gilmore A., Gorgens K.A., Davidson B., Granholm A.C., and Ledreux A. (2019). Altered levels of plasma neuron-derived exosomes and their cargo proteins characterize acute and chronic mild traumatic brain injury. FASEB J. 33, 5082–5088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Folstein M.F., Folstein S.E., and McHugh P.R. (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12, 189–198 [DOI] [PubMed] [Google Scholar]
- 18. Corrigan J.D. and Bogner J. (2007). Initial reliability and validity of the Ohio State University TBI Identification Method. J. Head Trauma Rehabil. 22, 318–329 [DOI] [PubMed] [Google Scholar]
- 19. Rey A. (1964). L'examenCclinique en Psychologie. Presses, Universitaires de France: Paris, France [Google Scholar]
- 20. Taylor E.M. (1959). Psychological Appraisal of Children with Cerebral Deficits. Harvard University Press: Cambridge, MA [Google Scholar]
- 21. Ivnik R.J., Malec J.F., Tangalos E.G., Petersen R.C., Kokmen E. and Kurland L.T. (1990). The Auditory-Verbal Learning Test (AVLT): norms for ages 55 years and older. Psychological Assessment 2, 304 [Google Scholar]
- 22. Donohue M.C., Sperling R.A., Salmon D.P., Rentz D.M., Raman R., Thomas R.G., Weiner M., Aisen P.S., Australian Imaging, Biomarkersand , Lifestyle Flagship Study of, Ageing; Alzheimer's Disease Neuroimaging Initiative and Alzheimer's Disease Cooperative Study. (2014). The preclinical Alzheimer cognitive composite: measuring amyloid-related decline. JAMA Neurol. 71, 961–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ivnik R.J., Malec J., Smith G.E., Tangalos E.G., Petersen R.C., Kokmen E., and Kurland L.T. (1992). Mayo's older americans normative studies: updated AVLT norms for ages 56 to 97. Clinical Neuropsychologist 6, 83–104 [Google Scholar]
- 24. Shirk S.D., Mitchell M.B., Shaughnessy L.W., Sherman J.C., Locascio J.J., Weintraub S., and Atri A. (2011). A web-based normative calculator for the uniform data set (UDS) neuropsychological test battery. Alzheimers Res. Ther. 3, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goetzl E.J., Kapogiannis D., Schwartz J.B., Lobach I.V., Goetzl L., Abner E.L., Jicha G.A., Karydas A.M., Boxer A., and Miller B.L. (2016). Decreased synaptic proteins in neuronal exosomes of frontotemporal dementia and Alzheimer's disease. FASEB J. 30, 4141–4148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fiandaca M.S., Kapogiannis D., Mapstone M., Boxer A., Eitan E., Schwartz J.B., Abner E.L., Petersen R.C., Federoff H.J., Miller B.L., and Goetzl E.J. (2015). Identification of preclinical Alzheimer's disease by a profile of pathogenic proteins in neurally derived blood exosomes: a case-control study. Alzheimers Dement. 11, 600–607.e601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goetzl E.J., Abner E.L., Jicha G.A., Kapogiannis D., and Schwartz J.B. (2018). Declining levels of functionally specialized synaptic proteins in plasma neuronal exosomes with progression of Alzheimer's disease. FASEB J. 32, 888–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kenney K., Qu B.X., Lai C., Devoto C., Motamedi V., Walker W.C., Levin H.S., Nolen T., Wilde E.A., Diaz-Arrastia R., Gill J., and CENC Multisite Observational Investigators. (2018). Higher exosomal phosphorylated tau and total tau among veterans with combat-related repetitive chronic mild traumatic brain injury. Brain Inj. 32, 1276-1284 [DOI] [PubMed] [Google Scholar]
- 29. Smith L.M., Kostylev M.A., Lee S., and Strittmatter S.M. (2019). Systematic and standardized comparison of reported amyloid-beta receptors for sufficiency, affinity, and Alzheimer's disease relevance. J. Biol. Chem. 294, 6042–6053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jarosz-Griffiths H.H., Corbett N.J., Rowland H.A., Fisher K., Jones A.C., Baron J., Howell G.J., Cowley S.A., Chintawar S., Cader M.Z., Kellett K.A., and Hooper N.M. (2019). Proteolytic shedding of the prion protein via activation of metallopeptidase ADAM10 reduces cellular binding and toxicity of amyloid-beta oligomers. J. Biol. Chem. 294, 7085–7097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Goetzl E.J., Ledreux A., Granholm A.C., Elahi F.M., Goetzl L., Hiramoto J., and Kapogiannis D. (2019). Neuron-derived exosome proteins may contribute to progression from repetitive mild traumatic brain injuries to chronic traumatic encephalopathy. Front. Neurosci. 13, 452. [DOI] [PMC free article] [PubMed] [Google Scholar]