Abstract:
Interleukin-15 is a pleotropic factor, capable of modulating metabolism, survival, proliferation, and differentiation in many different cell types. The rationale behind this study relates to previous work demonstrating that IL-15 is a major factor present in stem cell extracts, which protects cardiomyocytes subjected to hypoxic stress in vitro. The objective of this current study was to assess whether administration of IL-15 peptide will also show protective effects in vivo. The data indicate that administration of IL-15 reduces cell death, increases vascularity, decreases scar size, and significantly improves left ventricular ejection fraction in a mouse model of myocardial infarction.
Key Words: interleukin-15, myocardial infarction, cardiomyocyte, ejection fraction, vascular
INTRODUCTION
Several laboratories have demonstrated that stem cells exert their beneficial therapeutic effects in the heart through paracrine mechanisms.1–5 For example, mesenchymal stem cells or bone marrow mononuclear cells can mediate cardiomyocyte (CM) survival/cardioprotective effects through paracrine factors.3,6,7 Of the many paracrine factors present in stem cell extracts, individual ones, on their own, have shown to account for some of the beneficial effects observed.8,9 Our original work demonstrated that administration of bone marrow derived stem cell extract, in a mouse model of infarction induced by permanent ligation of the left anterior descending coronary artery (LAD), reduced CM death, decreased infarct scar size, and improved vascularity and cardiac function.5 One of the factors highly present in the extract was the cytokine interleukin-15 (IL-15), which protected CMs subjected to hypoxic stress in vitro.10 In this article, we have assessed whether IL-15 would also have protective effects in vivo by using a LAD-infarction mouse model. The data demonstrate that administration of IL-15 peptide in vivo improves left ventricular ejection fraction (LVEF), which correlates with diminished CM cell death and scar size as well as increased vascularity.
METHODS
Animals and Study Groups
Ten- to 12-week-old male C57BL/6 mice were used for all experiments and handled in accordance with the guidelines of the Institutional Animal Care and Use Committee.
MI and Echocardiography
Details of the experimental techniques have been reported previously.11 All analyses were performed blinded.
Animal Treatment and Control
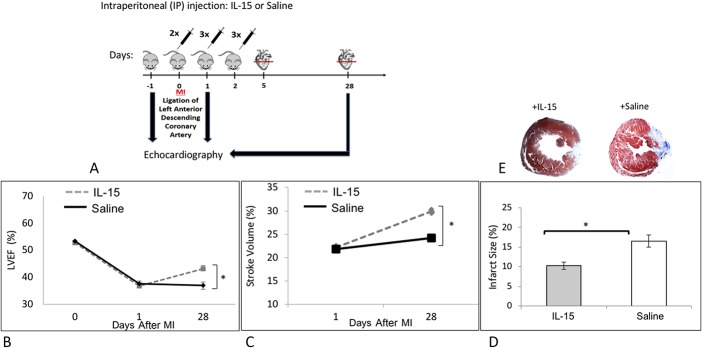
Animals were randomized into 2 groups: IL-15 therapy versus control. One-hundred microliter IL-15/saline solution or 100-μL 0.9% saline solution was injected every 6 hours intraperitoneally (IP) into experimental and control animals, respectively, twice at the day of Myocardial infarction (MI) and 3 times at days 1 and 2 after MI. IL-15 level was evaluated from blood serum 1 hour after IP injection. IP administration of 10-µg IL-15 resulted in maximum levels in blood serum, and higher doses of IL-15 (up to 40 µg) did not increase blood serum IL-15 level any more than the 10-µg dose. IL-15 was sustained up to 4 hours after each single injection. Animals were sacrificed at days 5 and 28 after MI heart tissues were embedded in paraffin. Apical half of each heart was further analyzed. The treatment procedure is presented in Figure 1A.
FIGURE 1.
Effect of IL-15 on left ventricular function and infarct size. A, MI was induced in C57BL/6 mice at day 0. IL-15 (10 μg) or saline as control was administered through IP injection every 6 hours into the animals twice at the day of MI and 3 times on the following days as indicated. B–C, Left ventricular function was evaluated by echocardiography on the day before MI (−1) and at days 1 and 28 after MI. B, IL-15 significantly improved LVEF. C, Stroke volume was significantly higher after IL-15 treatment. D, On average, IL-15 treatment significantly reduced the infarct size by 1.7-fold. E, As indicated by Masson's trichrome staining in the representative figures, IL-15 treatment resulted significantly reduced infarct size compared with the saline-treated control.  IL-15, n = 28.
IL-15, n = 28.  saline, n = 26. *P < 0.05. Error bars are SEM.
saline, n = 26. *P < 0.05. Error bars are SEM.
Tissue Analysis
Details of the tissue analysis techniques have been reported previously.5 Briefly, tissue sections from the midventricular level were analyzed by Masson's trichrome kit (American MasterTech, KTMTR) and Image-Pro Plus 6.0 (MediaCybernetics) to detect infarct size. Cardiomyocytes undergoing apoptosis were detected by 2 methods: TUNEL assay using ApopTag Plus Peroxidase In Situ Apoptosis Detection Kit (Milipore Sigma, S7101, Burlington, MA) and colocalization of cleaved caspase-3 (Cell Signaling, 9661S, 1:200) and cardiac troponin-I (Abcam 19615, 1:50, Cambridge, United Kingdom). Secondary antibodies used were Alexa Flour 546 (Invitrogen A-11035, Carlsbad, CA) and 488 (Invitrogen, A-11001), respectively. Apoptotic CMs were counted in 5 random high-power fields in each of 3 zones: infarct zone, peri-infarct zone (PZ), and remote zone. Tissues were imaged using Olympus fluorescent microscope at 20X. A maximum of 5 random high-power fields per each zone was taken. All analyses were performed blinded.
Arteriole and Capillary Staining
For vessel staining, Rat Detection Kit for Anti-Mouse CD31 from Biocare (CM303) was used.
In Vitro Angiogenesis Assay
Angiogenesis Starter Kit (Gibco A1460901) was used to examine the effect of IL-15 on tube formation of human umbilical vein endothelial cells (HUVECs). Briefly, passage-2 HUVECs were cultured in large vessel endothelial supplement (LVES)-supplemented Medium 200 (M200), and media were changed every other day until the HUVECs reached 80% confluency. On the day of assay, 96-well plates (Corning Inc, Corning, NY) were coated with Geltrex matrix. The HUVECs were added to Geltrex matrix-coated plates at density of 9000 cells/well and incubated for 7 hours. The experimental conditions contained LVES-supplemented M200 with IL-15 while the control contained only LVES-supplemented M200. Tube formation was visualized with 2-mM Calcein AM (Invitrogen C3099), and each well was imaged using an inverted fluorescent Olympus microscope with 4X objective and quantified using Angiogenesis Analyzer tool of Image J. The experiment was repeated 3 times. All analyses were performed blinded.
Statistics
Statistical results were calculated based on Student's t-test. A P value below 0.05 was deemed as statistically significant. Data error bars are presented as SEM.
RESULTS
IL-15 Treatment Improves Cardiac Function In Vivo
To establish baseline heart function, C57BL/6 mice were evaluated through echocardiography 1 day before induction of MI (−1 in Fig. 1A). After establishing the baseline heart function, MI was induced in mice (day 0 in Fig. 1A) and IL-15 (10 μg) or saline as control was administered through IP injection into the animals twice (every 6 hours) on the day of the MI and then 3x/d on days 1 and 2 after MI (Fig. 1A). At days 1 and 28 after MI, mice were examined through echocardiography (Fig. 1A). Echocardiography showed no differences in ejection fraction (LVEF) and stroke volume between treated and control groups 1 day after infarction (Figs. 1B, C). However, these parameters were significantly improved 28 days after infarct with the IL-15–treated group. These data indicate that IL-15 can improve cardiac function after an infarct in a mouse of infarction. We then questioned how the improved heart function would correlate with infarct size. As indicated graphically in Figure 1D, and as a representative figure in 1E, IL-15–treated group had significantly smaller infarct size as compared to control mice 28 days after infarct.
IL-15 Treatment Reduces Cardiomyocyte Cell Death
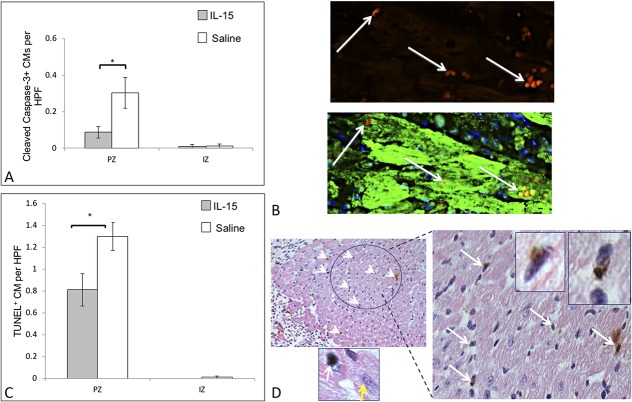
We then questioned whether decreased infarct size after 28 days is resultant of diminished cell death at an earlier time point. Immunohistochemical analysis performed on heart sections 5 days after MI indicated diminished cell death in the PZ. We used immunohistochemistry and immunofluorescence techniques to specifically identify changes occurring in CMs. At day 5, IL-15 treatment resulted in significantly lower cleaved caspase-3 positive and TUNEL-positive CMs in the PZ, indicating diminished apoptotic CMs (Figs. 2A–D). As indicated graphically in Figure 2A, cleaved caspase 3-positive CM count was diminished in IL-15–treated group. Figure 2B indicates an example of colocalized cleaved caspase 3 with troponin I that is indicative of CMs. As indicated graphically in Figure 2C, less TUNEL-positive apoptotic CMs are evident in the IL-15–treated group. Figure 2D shows examples of TUNEL-positive CMs as observed microscopically. IL-15 has been previously shown to protect hypoxic CMs in vitro attributed to the IL-15 receptor complex activating the STAT3 and ERK1/2 pathway.10 Therefore, the effects of IL-15 in reducing caspase-3 cleavage and TUNEL-positive CMs, may be due to STAT3 and ERK1/2 signaling.
FIGURE 2.
Effect of IL-15 on cell death. A, IL-15 treatment significantly reduced cardiomyocyte (CM) death, as evaluated by cleaved caspase-3 in PZ at day 5 after MI. B, Representative figure showing analysis of cleaved caspase as bright red stain colocalizing with cardiomyocytes that were stained with troponin I. White arrows indicate cleaved caspase 3-positive CMs. C, TUNEL assay at day 5 after MI also indicated diminished apoptotic CMs in PZ. D, Representative figure showing how apoptotic cells were evaluated: MM-AP (pink tone) and DAB (brown tone) against cTnI and terminal deoxynucleotidyl transferase (TdT), respectively. Nuclei were stained with hematoxylin. White arrows indicate apoptotic CMs. Zoomed in pictures show TUNEL-positive cardiomyocytes. Yellow arrow shows an example of negative stain for TUNEL.  IL-15, n = 28.
IL-15, n = 28.  saline, n = 26. IZ, infarct zone; PZ, Peri-infarct zone. *P < 0.05. Error bars are SEM.
saline, n = 26. IZ, infarct zone; PZ, Peri-infarct zone. *P < 0.05. Error bars are SEM.
IL-15 Treatment Effects Vascularity
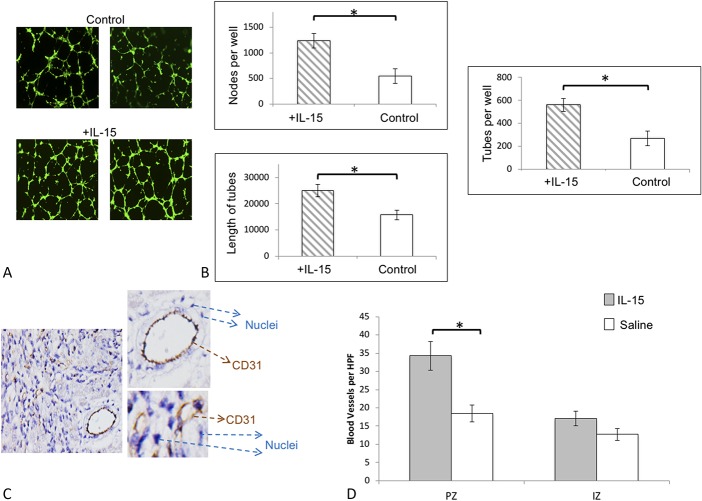
IL-15 has previously been reported to modulate angiogenesis.12 Thus, we first examined the effect of IL-15 on in vitro HUVEC angiogenesis assay (Figs. 3A, B). We incorporated IL-15 into HUVEC angiogenesis assays by adding 50-ng/mL IL-15 to the assay media and assessed the number of nodes as well as the extend of tube formation. Figure 3A shows examples of the HUVEC assay as observed microscopically with and without IL-15. There was an increase in the number of nodes, number of tubes, and tube length when IL-15 was added (Fig. 3B). These results indicated that IL-15 could modulate angiogenesis in vitro. We next examined whether IL-15 treated mice had increased vascularity (Figs. 3C, D). Indeed, IL-15 treatment significantly increased vessel area density at the PZ 5 days after MI, as evaluated by CD31 staining. Figure 3C indicates vessels as observed microscopically and graphically in Figure 3D.
FIGURE 3.
Effect of IL-15 on angiogenesis and cardiac vascularity. A, Representative images of HUVEC angiogenesis assay with (+IL-15) and without IL-15 (control). B, Addition of IL-15 to the culture media of HUVECs resulted in significantly greater number of nodes, tubes, and tube length compared with the control. C, Representative figure showing CD31 staining and visualization for evaluating vessel density. D, Vessel area density was significantly increased in PZ with the IL-15–treated group.  IL-15 n = 28;
IL-15 n = 28;  saline, n = 26. *P < 0.05. Error bars are SEM. IZ, infarct zone; PZ, peri-infarct Zone.
saline, n = 26. *P < 0.05. Error bars are SEM. IZ, infarct zone; PZ, peri-infarct Zone.
DISCUSSION
This brief report provides evidence that IL-15 can have cardioprotective effects in vivo. Several other stem cell factors have been shown to regulate vascularization3,7,13 or prevent the metabolic changes associated with infarction and have survival effects on CMs.3,6,8,9,14 Similarly, IL-15 can modulate cell death,15 metabolism,16 stem cell differentiation,17 endoplasmic reticulum stress/unfolded protein response,18 oxidative stress,19 and angiogenesis,12 which are parameters affected by MI. Therefore, it is highly likely that the effects of IL-15 on heart function reported in this article are due to pleiotropic effects such as modulating endoplasmic reticulum stress20,21 and glucose uptake.22 IL-15 has been previously shown to protect hypoxic CMs in vitro attributed to the STAT3 and ERK1/2 pathway.10 In addition, a characteristic of the ischemic microenvironment is decreased levels of oxygen and glucose deprivation, which can result in increased ROS and DNA damage. Indeed, IL-15 has been shown to diminish H2O2-mediated oxidative stress in skeletal muscle cells.19 Similarly, we observed a reduction in H2O2-mediated oxidative stress with cardiac fibroblast in vitro when treated with IL-15 (data not shown). Interestingly, deletion of STAT3 can sensitize cells to oxidative stress, and hence, the antioxidant effect of IL-15 may be due to STAT3 activation.23 Another target of IL-15, namely ERK1/2, may additionally modify STAT3 phosphorylation (S727), boosting the transcriptional activity of STAT3.24 Whether activation of ERK1/2 through IL-15 occurs in cardiac cells on treatment leading to STAT3 phosphorylation (S727) needs to be investigated.
It should be also noted that in the setting of myocardial infarction, an initial proinflammatory response plays a major role in modulating repair in the infarcted region.
We do not rule out immune-modularity effects of IL-15 that could be beneficial for the infarcted heart in an acute setting, such as suppressing B-cell activation for beneficial purposes,25 modulating macrophages for reparative purposes,26 or Gr-1+ neutrophil subsets that may play important repair roles.27 Indeed, IL-15 administration to mice did modulate markers associated with the aforementioned immune cells (data not shown). However, prolonged IL-15 administration may result in undesired inflammatory response, and hence, we did not perform prolonged administration of IL-15.
It is encouraging that IL-15 shows some beneficial effects in an in vivo infarction model; however, further detailed in vivo work, including a wider ischemia model spectrum, such as reperfusion models, is needed to assess for any potential clinical benefits of IL-15 after an infarct. Our laboratory is currently delineating the pleiotropic effects of IL-15 for improving heart function after an infarct and investigating IL-15 in additional mouse models to see whether it has any therapeutic potential.
CONCLUSIONS
IL-15 peptide administration improves heart function after an infarct in a mouse model of infarction.
ACKNOWLEDGMENTS
Perkins-Leone Gift; Khachaturian Foundation; Barbara and Gerson Bakar Foundation; Michael and Catherine Podell Fund; Mr C. Preston Butcher and Mrs Carolyn Butcher Gift; Vadasz Family Foundation; Xandex, Inc; Rosenberg Family Foundation; Madden Charities, Inc; Mr David Chamberlain and Mrs Karin Chamberlain Gift; National Institutes of Health Bridges to the Future (2R25GMO48972); and California Institute for Regenerative Medicine Bridges (TB1-01194).
Footnotes
The authors report no conflicts of interest.
K. Ameri and D. Bayardorj are primary authors.
REFERENCES
- 1.Crisostomo PR, Abarbanell AM, Wang M, et al. Embryonic stem cells attenuate myocardial dysfunction and inflammation after surgical global ischemia via paracrine actions. Am J Physiol Heart Circ Physiol. 2008;295:H1726–H1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gnecchi M, He H, Liang OD, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–368. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi M, Li TS, Suzuki R, et al. Cytokines produced by bone marrow cells can contribute to functional improvement of the infarcted heart by protecting cardiomyocytes from ischemic injury. Am J Physiol Heart Circ Physiol. 2006;291:H886–H893. [DOI] [PubMed] [Google Scholar]
- 4.Uemura R, Xu M, Ahmad N, et al. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ Res. 2006;98:1414–1421. [DOI] [PubMed] [Google Scholar]
- 5.Yeghiazarians Y, Zhang Y, Prasad M, et al. Injection of bone marrow cell extract into infarcted hearts results in functional improvement comparable to intact cell therapy. Mol Ther. 2009;17:1250–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gnecchi M, He H, Noiseux N, et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006;20:661–669. [DOI] [PubMed] [Google Scholar]
- 7.Tse HF, Siu CW, Zhu SG, et al. Paracrine effects of direct intramyocardial implantation of bone marrow derived cells to enhance neovascularization in chronic ischaemic myocardium. Eur J Heart Fail. 2007;9:747–753. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Z, Deb A, Zhang Z, et al. Secreted frizzled related protein 2 protects cells from apoptosis by blocking the effect of canonical Wnt3a. J Mol Cell Cardiol. 2009;46:370–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang J, Guo J, Beigi F, et al. HASF is a stem cell paracrine factor that activates PKC epsilon mediated cytoprotection. J Mol Cell Cardiol. 2014;66:157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yeghiazarians Y, Honbo N, Imhof I, et al. IL-15: a novel prosurvival signaling pathway in cardiomyocytes. J Cardiovasc Pharmacol. 2014;63:406–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Springer ML, Sievers RE, Viswanathan MN, et al. Closed-chest cell injections into mouse myocardium guided by high-resolution echocardiography. Am J Physiol Heart Circ Physiol. 2005;289:H1307–H1314. [DOI] [PubMed] [Google Scholar]
- 12.Angiolillo AL, Kanegane H, Sgadari C, et al. Interleukin-15 promotes angiogenesis in vivo. Biochem Biophys Res Commun. 1997;233:231–237. [DOI] [PubMed] [Google Scholar]
- 13.Kinnaird T, Stabile E, Burnett MS, et al. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94:678–685. [DOI] [PubMed] [Google Scholar]
- 14.Feygin J, Mansoor A, Eckman P, et al. Functional and bioenergetic modulations in the infarct border zone following autologous mesenchymal stem cell transplantation. Am J Physiol Heart Circ Physiol. 2007;293:H1772–H1780. [DOI] [PubMed] [Google Scholar]
- 15.Shinozaki M, Hirahashi J, Lebedeva T, et al. IL-15, a survival factor for kidney epithelial cells, counteracts apoptosis and inflammation during nephritis. J Clin Invest. 2002;109:951–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crane JD, MacNeil LG, Lally JS, et al. Exercise-stimulated interleukin-15 is controlled by AMPK and regulates skin metabolism and aging. Aging Cell. 2015;14:625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gomez-Nicola D, Valle-Argos B, Pallas-Bazarra N, et al. Interleukin-15 regulates proliferation and self-renewal of adult neural stem cells. Mol Biol Cell. 2011;22:1960–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang HT, Luo LJ, Chen WJ, et al. IL-15 expression increased in response to treadmill running and inhibited endoplasmic reticulum stress in skeletal muscle in rats. Endocrine. 2015;48:152–163. [DOI] [PubMed] [Google Scholar]
- 19.Li F, Li Y, Tang Y, et al. Protective effect of myokine IL-15 against H2O2-mediated oxidative stress in skeletal muscle cells. Mol Biol Rep. 2014;41:7715–7722. [DOI] [PubMed] [Google Scholar]
- 20.Hong J, Kim K, Kim JH, et al. The role of endoplasmic reticulum stress in cardiovascular disease and exercise. Int J Vasc Med. 2017;2017:2049217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deldicque L, Hespel P, Francaux M. Endoplasmic reticulum stress in skeletal muscle: origin and metabolic consequences. Exerc Sport Sci Rev. 2012;40:43–49. [DOI] [PubMed] [Google Scholar]
- 22.Krolopp JE, Thornton SM, Abbott MJ. IL-15 activates the jak3/STAT3 signaling pathway to mediate glucose uptake in skeletal muscle cells. Front Physiol. 2016;7:626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barry SP, Townsend PA, McCormick J, et al. STAT3 deletion sensitizes cells to oxidative stress. Biochem Biophys Res Commun. 2009;385:324–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zouein FA, Altara R, Chen Q, et al. Pivotal importance of STAT3 in protecting the heart from acute and chronic stress: new advancement and unresolved issues. Front Cardiovasc Med. 2015;2:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zouggari Y, Ait-Oufella H, Bonnin P, et al. B lymphocytes trigger monocyte mobilization and impair heart function after acute myocardial infarction. Nat Med. 2013;19:1273–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shintani Y, Ito T, Fields L, et al. IL-4 as a repurposed biological drug for myocardial infarction through augmentation of reparative cardiac macrophages: proof-of-concept data in mice. Sci Rep. 2017;7:6877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Puhl SL, Steffens S. Neutrophils in post-myocardial infarction inflammation: damage vs. resolution? Front Cardiovasc Med. 2019;6:25. [DOI] [PMC free article] [PubMed] [Google Scholar]