Abstract
Background.
Calcineurin inhibitors (CNIs) and steroids are strongly associated with new-onset diabetes after transplantation, worsening of pre-existing diabetes, and cardiovascular events. We assessed the benefit of conversion from CNI-based to belatacept-based immunosuppression in diabetic kidney-transplant (KT) recipients on glucose control and cardiovascular risk factors.
Methods.
In this retrospective, noncontrolled single-study conducted between May 2016 and October 26, 2018, we recruited KT recipients converted from CNIs to belatacept at least 6 months after KT. The primary endpoint was the evolution of hemoglobin A1c (HbA1c) between baseline and after 6 months of treatment. Secondary endpoints included modifications to antidiabetic drugs, other cardiovascular risk factors, and renal function.
Results.
One hundred and three KT recipients were included. Of these, 26 (25%) had type 2 diabetes. The patients were either receiving oral antidiabetic drugs (n = 21; 75%) or insulin therapy (n = 14; 54%). Overall HbA1c decreased significantly from 6.2 ± 1 to 5.8 ± 1%, P < 0.001. In diabetic patients, HbA1c decreased from 7.2 ± 1 to 6.5 ± 1%, P = 0.001. HbA1c significantly decreased in the subgroup of patients with new-onset diabetes after transplantation and whether diabetes was controlled at inclusion or not (ie, HA1c ≤7% or >7%). Moreover, no diabetic patient increased the number of oral antidiabetic drugs and the dose of basal insulin was not statistically different from baseline to 6 months (16 international unit at baseline and 16 international unit at 6 mo, P = 1). One patient had to start treatment by insulin pump. During follow-up, the renal function, body mass index, and hemoglobin level of all 103 patients remained stable, 2 patients presented acute cellular rejection, and no patient suffered from graft loss.
Conclusions.
A late switch from CNI to belatacept was a valuable therapeutic option for diabetic kidney recipients and substantially improved glycemic parameters.
Cardiovascular events are a major cause of morbidity and mortality following solid-organ transplantation. In kidney transplantation, the cornerstone of the immunosuppressive regimen still relies on anticalcineurin inhibitors (CNIs; ie, tacrolimus or cyclosporine A). CNIs and steroids are strongly associated with new-onset diabetes after transplantation (NODAT) and cardiovascular events.1–4 Diabetes is a serious complication that negatively impacts on graft- and patient-survival rates.5,6 Moreover, NODAT is a risk factor of cardiovascular events.7 A study from the Brooklyn University compared 40 kidney-transplant (KT) recipients with NODAT to 38 non–diabetic-matched patients. After an average follow-up of 9 years, patient survival was similar in the 2 groups, but graft survival was lower in the diabetic group (relative risk of graft loss at 3.72).8 Other risk factors for NODAT have been reported in the literature such as recipient age, obesity, β-blocker use, albuminuria, high blood pressure (BP), and high renal-resistive index.9,10
An ideal immunosuppressive drug should maintain good immunosuppression but also reduce nephrotoxicity and cardiovascular side-effects, such as diabetes. In this context, belatacept has emerged as a preferred treatment.11,12 Belatacept is a fusion protein that blocks the cluster of differentiation (CD)80/86-CD28 costimulation pathway between antigen-presenting cells and effector T cells.13 The use of belatacept after kidney transplantation was approved by the US Food and Drug Administration after the Belatacept Evaluation of Nephroprotection and Efficacy as a First-Line Immunosuppression Trial (BENEFIT) and the BENEFIT-Extended Criteria Donors (BENEFIT-EXT) trials.14–16
Although belatacept has been associated with a higher glomerular filtration rate (GFR) compared with CNI-based therapy, its real benefit to reduce diabetes remains to be clarified. In a previous smaller retrospective cohort study, we reported that conversion to belatacept was associated with reduced hemoglobin A1c (HbA1c) levels in diabetic patients.17 In the present study, we assessed the benefit of belatacept on glycemic parameters and other cardiovascular risk factors in our cohort of KT recipients.
MATERIALS AND METHODS
Study Population
All patients were followed-up during post–kidney transplantation in our university hospital. This retrospective noncontrolled study included KT recipients aged >18 years and undergoing conversion from CNI- to belatacept-based immunosuppression between May 2016 and October 26, 2018. Conversion was conducted to avoid sustained CNI nephrotoxicity in the setting of allograft dysfunction, whatever the initial cause of it. Among these patients, we focused on all type 2 diabetes (NODAT or pre-existing diabetes) recipients that had received insulin therapy and/or oral antidiabetics (OAD) at the time of conversion. We excluded patients converted during the first 6 months post-KT. All patients gave their informed and written consent.
Immunosuppressive Protocol
Initiation of belatacept consisted of 1 intravenous injection on day 1, a second injection on day 14, a third on day 28, and then 1 injection every 4 weeks. Each belatacept injection was dosed at 5 mg/kg. During the first month of belatacept treatment, patients still received CNIs at the same dose preceding belatacept initiation. After the third belatacept injection, the dose of CNIs was halved for 4 weeks and then finally interrupted after 8 weeks. For patients with a history of acute antibody-mediated rejection before the switch or a high level of sensitization, the dose of CNIs was halved after 4 weeks and was then continued. Apart from belatacept, standard immunosuppressive therapy consisted of either on mycophenolate mofetil (1 g/d) or mammalian target of rapamycin inhibitor (everolimus aiming at trough levels between 6 and 8 ng/mL) or tacrolimus (trough concentration target 3–5 ng/mL after 1 mo post-transplantation) or cyclosporine A (peak concentration target 300–500 ng/mL after 1 mo post-transplantation). Blood and urine samples were monitored monthly. HbA1c dosage was assessed at month 6 post–belatacept conversion.
Study Design
The hospital’s electronic medical records were used to collect demographic data on recipients and donors. We collected clinical and biological data at baseline and at 6 months. We collected data on comorbidities when available. All medical data were collected from our database (CNIL [French national committee for data protection] approval number 1987785v0).
NODAT was defined in our study as patients with random glucose level ≥200 mg/dL (11.1 mmol/L) and/or HbA1c levels ≥6.5% and/or the need of diabetes medication post-transplantation. A diagnosis of cardiac disease was based on a history of rhythmic, ischemic, or hypertensive cardiopathy. Peripheral vascular disease consisted of obliterative arteriopathy of the lower limbs or carotid-artery stenosis based on a Doppler ultrasound. Dyslipidemia was defined as total cholesterol >2 g/L. Sleep apnea was diagnosed based on polysomnography. A body mass index (BMI) of 25–29.9 kg/m2 defined overweight patients; obesity was defined as a BMI of >30 kg/m2. Diabetic retinopathy was assessed by an ophthalmologic annual evaluation. Diabetic neuropathy was assessed in a clinical examination. We used the Chronic Kidney Disease Epidemiology Collaboration equation to estimate GFR.18
Data from diabetic treatments were collected at baseline and at 6 months after switching to belatacept: that is, the use and quantity of basal insulin, the use of fast insulin, the use of an insulin pump, and/or the intake of OADs.
Late conversion to belatacept was considered when the time between transplantation and the switch was performed at least 6 months after transplantation. Controlled diabetes was defined as a HbA1c strictly <7% during the 6 months before conversion.
Endpoints
The primary endpoint was the evolution of HbA1c rate between the first day of belatacept initiation (baseline) and month 6 postinitiation. Secondary endpoints assessed modifications to antidiabetic drugs during the follow-up for diabetic patients and the evolution of cardiovascular risk factors (BP, antihypertensive treatments, BMI, hypercholesterolemia, renal function: GFR and albuminuria) for all the switched patients.
Statistical Analyses
Symmetrically distributed variables are shown as their means ± standard deviations. For a heterogeneous distribution, variables are shown as the median [interquartile range]. Categorical variables are expressed as a percentage. Student’s t test or the Mann–Whitney U test was used to compare continuous variables. The χ2 test was used to compare categorical variables. A pairwise comparison was used between pairs of proportions with correction for multiple testing for categorical variables between baseline and month 6. A P value of <0.05 was considered to be statistically significant. Analyses were performed using R software.
RESULTS
Recipients’ Characteristics
Between May 2016 and October 26, 2018, 112 CNI-treated KT recipients were converted after transplantation to receive belatacept for nephroprotection. Of those patients, we included 103 patients in our analyses. Nine patients were excluded because of belatacept conversion occurred during the first 6 months post-KT. Two of them were diabetic (Figure 1). Baseline characteristics at inclusion are summarized in Table 1. Of these recipients, 26 (25%) had type 2 diabetics, of which 21 (75%) were treated by OAD and 14 (56%) were treated by insulin therapy. Eleven (11% and 42% of the diabetic patients) had pre-existing diabetes before transplantation and 15 (15% and 58% of the diabetic patients) had NODAT. NODAT occurred at a median time of 3 [0.2–73] months post-KT. Twenty-six (25%) patients received a kidney from a living donor, 69 patients (67%) from a brain-dead donor, and 6 patients (6%) from a donor after cardiovascular death. The median follow-up period was 54 [11–242] months after kidney transplantation. Conversion to belatacept occurred at a median of 40 [6–398] months post-transplantation. The median follow-up period after switching to belatacept was 13 [7–37] months.
FIGURE 1.
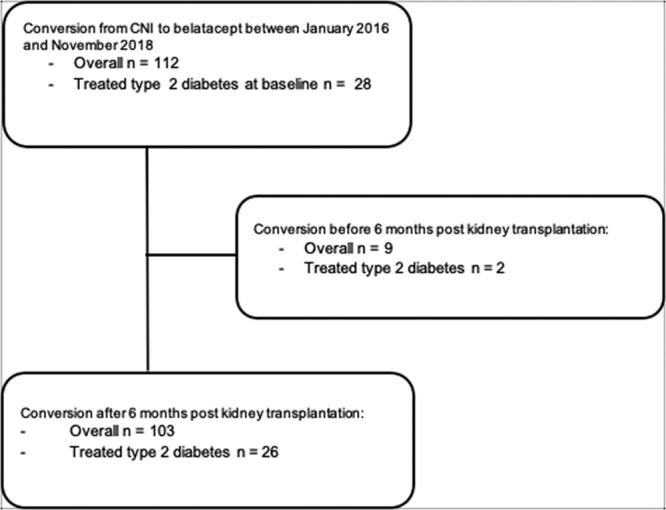
Study flow chart. CNI, calcineurin inhibitors.
TABLE 1.
Baseline characteristics of kidney recipients (n = 103)
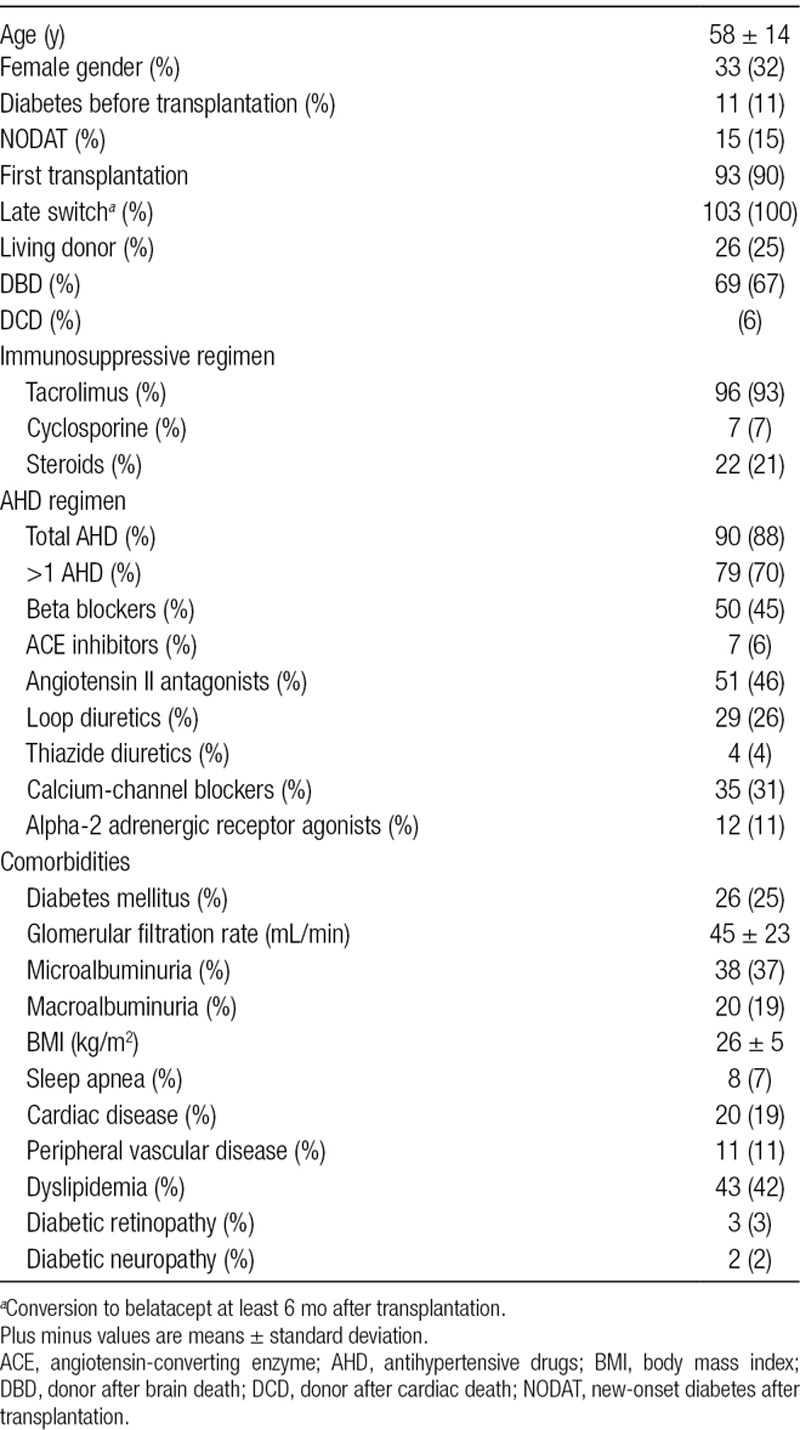
Diabetic nephropathy was the cause of end-stage renal disease in 7 patients (7%). Other main nephropathies were polycystic kidney disease for 17 patients (17%) and hypertensive nephropathy for 6 patients (5.8%). Regarding BMI, 36 patients were overweight (34.9%) and 20 were obese (19.4%). At the time of initiating belatacept, 96 patients were receiving tacrolimus (93.3%), 7 received cyclosporine (6.7%), and 22 were still receiving corticosteroids (21.3%) at a dose of 5 mg of prednisolone per day. Indications of steroid maintenance were acute rejection and high degree of sensitization. Tacrolimus blood trough level was monitored to be between 3 and 5 ng/mL. The induction therapy consisted of thymoglobulin for all patients. The dose of cyclosporine and everolimus did not change during the follow-up.
At the time of inclusion, mean estimated GFR was 45 ± 23 mL/min/1.73 m2, 38 (37%) patients had microalbuminuria (albuminuria between 30 and 300 mg/L on urine sample), and 20 (19%) had macroalbuminuria (albuminuria over 300 mg/L on urine sample). Forty-four patients (43%) were receiving statins for hyperlipidemia.
Hemoglobin level was stable between inclusion and month 6: 127 ± 17 g/dL and 128 ± 15 mg/dL, respectively.
Improvement to Diabetes at 6 Months Post-belatacept
Only 2 patients remained on low-dose tacrolimus after initiating belatacept because of a history of antibody-mediated rejection (1 was diabetic). Baseline treatments of diabetic recipients are summarized in Table 2. Decrease of HbA1c was assessed at 6 months. In the entire cohort, HbA1c decreased significantly from 6.2 ± 1 to 5.8 ± 1%, P < 0.001. In diabetic patients, HbA1c decreased from 7.2 ± 1 to 6.5 ± 1%, P = 0.001 (Figure 2). Seventy-seven patients were nondiabetic and HbA1c data were available for 74 of them. In these patients, HbA1c also decreased from 5.5 ± 0.5 to 5.4 ± 0.4, P = 0.008.
TABLE 2.
Diabetes treatment at baseline and 6 mo after the introduction of belatacept (n = 26)
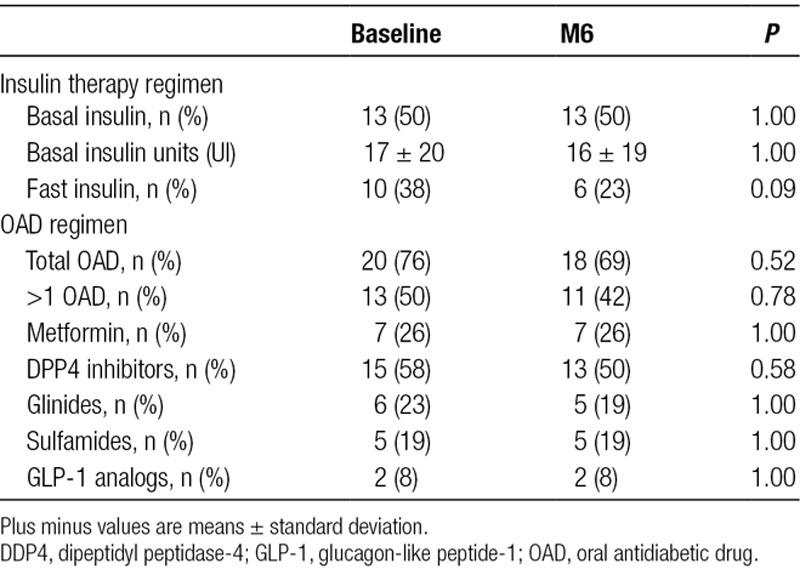
FIGURE 2.
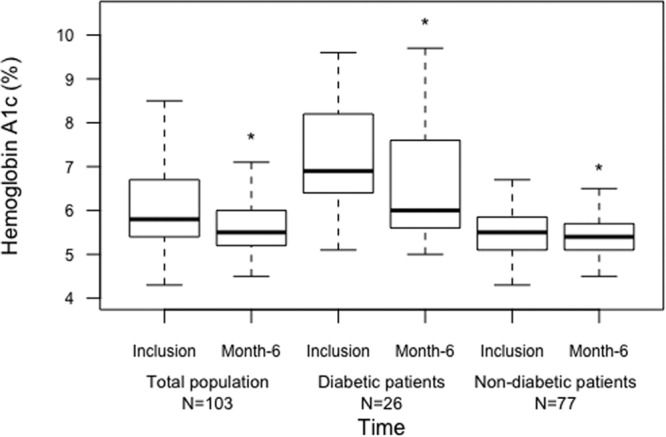
Improvement in HbA1c at 6 mo post–belatacept conversion. Box plots represent HbA1c percentage at inclusion for each patient and at 6 mo. Black lines represent the average. *P < 0.05 as compared with inclusion. HbA1c, hemoglobin A1c.
Results for diabetes and HbA1c were assessed in the different subgroups (Figure 3). We considered that the duration of diabetes may have impacted on improvements to HbA1c after CNI withdrawal and the initiation of belatacept: thus, we focused the analyses on pre-existing diabetes and NODAT. The HbA1c in the group of patients with pre-existing diabetes before kidney transplantation (n = 11) was 7.5 ± 1.2 at inclusion and significantly decreased to 7.1 ± 1.4 at 6 months (P = 0.05). In the group of patients with NODAT (n = 15), HbA1c decreased also significantly from 6.9 ± 1.4 at inclusion to 6.0 ± 1.0 at 6 months (P = 0.003).
FIGURE 3.
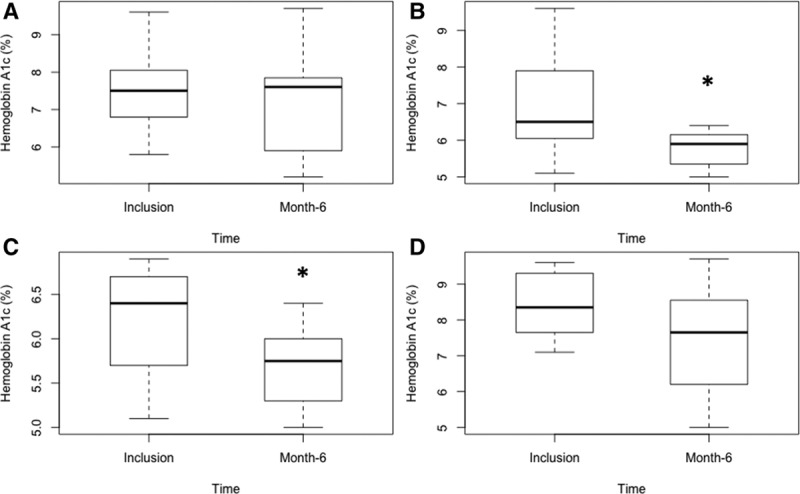
Evolution of HbA1c at postswitch from CNI to belatacept at 6 mo in the subgroup analyses. Box plots represent the HbA1c percentage at inclusion for each patient and at 6 mo. A, Patients with pre-existing diabetes before kidney transplantation. B, Patients with new-onset diabetes after transplantation. C, Patients with controlled diabetes at inclusion (HbA1c ≤7%). D, Patients with uncontrolled diabetes at inclusion (HbA1c >7%). CNI, calcineurin inhibitors; HbA1c, hemoglobin A1c. *P < 0.05.
The initial control of diabetes at inclusion may also be a confounding factor. We separated patients’ data according to an initial HbA1c of <7% or strictly >7% (Figure 3). In the group of patients with HbA1c <7 % at inclusion (n = 13), HbA1c decreased significantly from 6.2 ± 0.5 at inclusion to 5.7 ± 0.4 after 6 months (P = 0.005). In the group with uncontrolled diabetes at inclusion, that is, HbA1c >7% (n = 13), Hba1C decreased from 8.4 ± 0.9 at inclusion to 7.4 ± 1.5 at 6 months (P = 0.029).
Finally, we hypothesized that a reduction in HbA1c may be indirectly due to a more frequent antidiabetic drug use or a difference in hemoglobin levels between baseline and 6 months. Ten patients (39%) diminished or discontinued antidiabetic drug usage at 6 months, and no diabetic patient had to increase the number of OAD drugs. The dose of basal insulin was not statistically different from baseline to 6 months (16.36 international unit at baseline and 16.36 international unit at 6 mo, P = 1). One patient had to start treatment by insulin pump. OAD consisted mainly of metformin for 7 patients and sitagliptin for 15 patients. The mean dose of metformin was 1535 mg/d at baseline and 1485 mg/d at 6 months. The mean dose of sitagliptin was 63 mg/d at baseline and at 6 months.
During follow-up, the renal function, BMI, and hemoglobin level of the 103 patients remained stable (see below).
Cardiovascular Comorbidities
In our analyses, we assessed the evolution of other cardiovascular risk factors and the potential impact of a switch to belatacept and/or CNI discontinuation. When assessed in our entire cohort, BMI did not differ at inclusion and 6 months (26 ± 6 versus 26 ± 6 kg/m2, respectively). In subgroup analyses, the BMI remained similar in the diabetic group and nondiabetic group. Arterial BP was also similar at inclusion and at month 6, that is, mean systolic BP 137 ± 25 versus 151 ± 134 mm Hg, and mean diastolic BP was 80 ± 16 versus 79 ± 12 mm Hg, respectively, P = 0.8 and P = 0.16. Yet, the number of antihypertensive drugs was similar between inclusion and month 6: 2 [0–6] different drugs and 88% of patients treated. Thirty-two patients (31%) were receiving statins at inclusion and 33 (32) at 6 months. At inclusion, 38 patients (37%) had microalbuminuria and 20 (19.4%) had macroalbuminuria. At month 6, 42 patients (40.7%) had microalbuminuria and 18 (17.4%) had macroalbuminuria. These differences were not significant (P = 0.67 and P = 0.86, respectively).
Safety
All patients and grafts survived at 6 months. Two patients experienced acute cellular rejection (class IV of the 2013 Banff classification) after the switch to belatacept. One was grade IA and 1 was grade II. Both were treated with high doses of intravenous corticosteroids and antithymocyte globulin treatment was needed for 1 patient. Both were maintained on oral steroids. These 2 patients recovered their basal renal function after rejection treatment. None of them were diabetic. No patient suffered from antibody-mediated rejection. Renal function did not improve at 6 months (mean estimated GFR was 45 ± 23 before and 45 ± 19 mL/min/1.73 m2 at 6 mo after conversion).
DISCUSSION
In this single-center study, we found that conversion from CNI- to belatacept-based immunosuppression was associated with a significant improvement in HbA1C for treated type 2 diabetic patients. The HbA1c improvement was also observed in nondiabetic patients but this result was not clinically relevant. We may argue a specific effect of the belatacept on top of CNI withdrawal benefit regarding this differential effect. Medicoeconomic studies showed a benefit of antidiabetic drugs of HbA1c decrease above 0.5%. Previous findings from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications studies suggest that a 10% decrease in HbA1c levels could lead to a 39% reduction of retinopathy progression and 25% reduction in microalbuminuria onset in type 1 diabetes.19 In the strategy of belatacept conversion plus CNI withdrawal we studied, we showed a relative decrease of 9.7 % of HbA1c in KT diabetic patients (from 7.2% to 6.5%).
CNI agents (and mainly tacrolimus) remain the cornerstone of solid-organ transplantation immunosuppression regimens worldwide.20 Tacrolimus has been associated with a decreased risk of acute rejection and improved long-term graft survival.14 However, tacrolimus is also known to be associated with 2 major dose-dependent side-effects: nephrotoxicity and NODAT.21–23 Belatacept has emerged as an interesting alternative to CNIs to avoid these complications, but only a few randomized studies have compared tacrolimus to belatacept in the setting of kidney transplantation.24,25
Several mechanisms have been described to explain the effect of CNIs on diabetes. First, in vitro, it has been shown that beta cells have diminished insulin secretion when they are exposed to CNIs.26 Moreover, tacrolimus decreases the number of basal insulin secretion cells and the glucose-induced release of insulin by pancreatic beta cells by inhibiting glucokinase activity.27–30 Another assumed mechanism is that CNIs directly induce beta-cell apoptosis and reduce cellular proliferation of beta cells.31 In addition, CNIs induce insulin resistance by stimulating endocytic removal of glucose transporter type-4 from the cell membranes of adipocytes and muscle cells.32,33
Even if the effects of CNIs (and specifically tacrolimus) to impair insulin secretion and insulin resistance are well described in the literature, few data confirm their involvement in the development of NODAT or the worsening of pre-existing type 2 diabetes. In the SYMPHONY study, no statistical difference in the antidiabetic treatments between different groups was found, that is, a standard dose of cyclosporine, a low dose of cyclosporine, a low dose of tacrolimus, or a low dose of sirolimus.34
A recent Cochrane meta-analysis reported that patients receiving a belatacept immunosuppressive regimen at post-transplantation had a lower risk of developing NODAT compared with those receiving CNI-based immunosuppression.35 Data were collected from 4 randomized studies that included 1049 patients. The analysis showed there were 50 per 1000 cases of NODAT in the belatacept group compared with 67.2 per 1000 in the CNI group (relative risk = 0.61 [95% confidence interval] 0.40–0.93). In these studies, belatacept was given immediately at post-transplantation. No study (to date) has specifically evaluated the fate of diabetes after a late switch from CNI to belatacept in diabetic KT recipients.
The effect of these strategies on glycemic parameters may be explained by the withdrawal of CNIs rather than a specific benefit of belatacept, although some data suggest both implications. Yet, we may hypothesize that the improvement in glucose metabolism may be due to the direct effect of belatacept. Many studies on animals have assessed the effects of glucose metabolism by discontinuing tacrolimus. Triñanes et al28 reported a reversible effect on gluco-lipotoxicity when tacrolimus was stopped in Zucker rats. Recently, Jin et al36 published a study on tacrolimus-induced diabetes in rats. They administered tacrolimus for 3 weeks and assessed de novo diabetes. Tacrolimus was then continued, withdrawn, or replaced by CTLA4-Ig (1 or 2 mg/kg) for an additional 3 weeks. Tacrolimus withdrawal improved pancreatic islet dysfunction compared with the group that remained on tacrolimus. The administration of belatacept was not diabetogenic compared with the control group that had no immunosuppressive therapy. These authors showed that oxidative stress, apoptotic cell death, and infiltration of macrophages decreased with tacrolimus withdrawal. Interestingly, they showed that CTLA4-Ig conversion further reduced those improvements. This may be explained by the CD-86 playing a role in insulin resistance via its interaction with the adiponectin axis.37
In our subgroup analysis, we found improved HbA1c mainly occurred in patients with uncontrolled type 2 diabetes.
One strength of our study was to assess the decrease of HbA1c with no difference in treatment, BMI, renal function, and hemoglobin level between baseline and 6 months after conversion, considering those factors as potential confusion factors.
KT recipients converted to belatacept come to a medical structure every month and have an increase medical surveillance which may impact diabetes management. However, KT recipients included in our study were converted to belatacept to avoid CNI nephrotoxicity, but not because of metabolism or glucose abnormalities.
The secondary outcomes of this study were to assess the evolution of metabolic and cardiovascular risk factors after a switch to belatacept in the general transplant population. In our cohort, at 6 months, we did not find any improvement in BMI, renal function, proteinuria, arterial BP, or a decrease in antihypertensive or cholesterol-lowering drugs. In 2011, Vanrenterghem et al38 reported that the BENEFIT and BENEFIT-EXT cohorts at 1 year had improved systolic BP (from 6 to 9 mm Hg), mean diastolic BP (from 3 to 4 mm Hg), and serum lipids in patients receiving a belatacept regimen. In contrast, we did not include our 1-year results because the median follow-up was only 10.9 months after the belatacept switch and not enough patients could be included in this analysis. We did not assess serum lipid at 6 months in our cohort due to missing data. Yet, many studies suggest a potential reduction in cardiovascular risk factors and arterial stiffness.39–41 Based on the BENEFIT cohort, some authors have estimated a 20% long-term reduction in major adverse cardiac events in belatacept-treated patients compared with those receiving CNIs.42
To summarize, our results suggest that a late switch from CNI to belatacept in type 2 diabetic KT recipients is a safe and effective strategy to improve diabetic parameters and/or decrease antidiabetic drug use at 6 months. However, in this small cohort of diabetic patients, belatacept-based immunosuppression failed to show advantages regarding other metabolic parameters.
Footnotes
Published online 24 December, 2019.
The authors declare no funding or conflicts of interest.
F.T. collected the data and participated in data analysis; F.T. and J.N. designed the study; T.J., P.M., B.J., J.N., H.N.-B., and L.R. recruited the patients; D.G. reviewed the kidney allograft biopsies; F.T., P.-Y.B., J.N., and L.R. participated in the writing of the article.
REFERENCES
- 1.Starzl TE, Fung J, Jordan M, et al. Kidney transplantation under FK 506. JAMA. 1990;264:63–67. [PMC free article] [PubMed] [Google Scholar]
- 2.Myers BD, Ross J, Newton L, et al. Cyclosporine-associated chronic nephropathy. N Engl J Med. 1984;311:699–705. doi: 10.1056/NEJM198409133111103. [DOI] [PubMed] [Google Scholar]
- 3.Hošková L, Málek I, Kopkan L, et al. Pathophysiological mechanisms of calcineurin inhibitor-induced nephrotoxicity and arterial hypertension. Physiol Res. 2017;66:167–180. doi: 10.33549/physiolres.933332. [DOI] [PubMed] [Google Scholar]
- 4.Rodrigo E, Fernández-Fresnedo G, Valero R, et al. New-onset diabetes after kidney transplantation: risk factors. J Am Soc Nephrol. 2006;17(12 Suppl 3):S291–S295. doi: 10.1681/ASN.2006080929. [DOI] [PubMed] [Google Scholar]
- 5.Kasiske BL, Snyder JJ, Gilbertson D, et al. Diabetes mellitus after kidney transplantation in the United States. Am J Transplant. 2003;3:178–185. doi: 10.1034/j.1600-6143.2003.00010.x. [DOI] [PubMed] [Google Scholar]
- 6.Cosio FG, Pesavento TE, Kim S, et al. Patient survival after renal transplantation: IV. Impact of post-transplant diabetes. Kidney Int. 2002;62:1440–1446. doi: 10.1111/j.1523-1755.2002.kid582.x. [DOI] [PubMed] [Google Scholar]
- 7.Ducloux D, Kazory A, Chalopin JM. Posttransplant diabetes mellitus and atherosclerotic events in renal transplant recipients: a prospective study. Transplantation. 2005;79:438–443. doi: 10.1097/01.tp.0000151799.98612.eb. [DOI] [PubMed] [Google Scholar]
- 8.Miles AM, Sumrani N, Horowitz R, et al. Diabetes mellitus after renal transplantation: as deleterious as non-transplant-associated diabetes? Transplantation. 1998;65:380–384. doi: 10.1097/00007890-199802150-00014. [DOI] [PubMed] [Google Scholar]
- 9.Mutinelli-Szymanski P, Caille A, Tranquart F, et al. Renal resistive index as a new independent risk factor for new-onset diabetes mellitus after kidney transplantation. Transpl Int. 2012;25:464–470. doi: 10.1111/j.1432-2277.2012.01445.x. [DOI] [PubMed] [Google Scholar]
- 10.Roland M, Gatault P, Al-Najjar A, et al. Early pulse pressure and low-grade proteinuria as independent long-term risk factors for new-onset diabetes mellitus after kidney transplantation. Am J Transplant. 2008;8:1719–1728. doi: 10.1111/j.1600-6143.2008.02308.x. [DOI] [PubMed] [Google Scholar]
- 11.Noble J, Jouve T, Janbon B, et al. Belatacept in kidney transplantation and its limitations. Expert Rev Clin Immunol. 2019;15:359–367. doi: 10.1080/1744666X.2019.1574570. [DOI] [PubMed] [Google Scholar]
- 12.Perez CP, Patel N, Mardis CR, et al. Belatacept in solid organ transplant: review of current literature across transplant types. Transplantation. 2018;102:1440–1452. doi: 10.1097/TP.0000000000002291. [DOI] [PubMed] [Google Scholar]
- 13.Malvezzi P, Jouve T, Rostaing L. Costimulation blockade in kidney transplantation: an update. Transplantation. 2016;100:2315–2323. doi: 10.1097/TP.0000000000001344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vincenti F, Charpentier B, Vanrenterghem Y, et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study). Am J Transplant. 2010;10:535–546. doi: 10.1111/j.1600-6143.2009.03005.x. [DOI] [PubMed] [Google Scholar]
- 15.Durrbach A, Pestana JM, Pearson T, et al. A phase III study of belatacept versus cyclosporine in kidney transplants from extended criteria donors (BENEFIT-EXT study). Am J Transplant. 2010;10:547–557. doi: 10.1111/j.1600-6143.2010.03016.x. [DOI] [PubMed] [Google Scholar]
- 16.Durrbach A, Pestana JM, Florman S, et al. Long-term outcomes in belatacept- versus cyclosporine-treated recipients of extended criteria donor kidneys: final results from BENEFIT-EXT, a phase III randomized study. Am J Transplant. 2016;16:3192–3201. doi: 10.1111/ajt.13830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malvezzi P, Fischman C, Rigault G, et al. Switching renal transplant recipients to belatacept therapy: results of a real-life gradual conversion protocol. Transpl Immunol. 2019;56:101207. doi: 10.1016/j.trim.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Andrassy KM. Comments on ‘KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease’. Kidney Int. 2013;84:622–623. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 19.Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA. 2002;287:2563–2569. doi: 10.1001/jama.287.19.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Webster AC, Woodroffe RC, Taylor RS, et al. Tacrolimus versus ciclosporin as primary immunosuppression for kidney transplant recipients: meta-analysis and meta-regression of randomised trial data. BMJ. 2005;331:810. doi: 10.1136/bmj.38569.471007.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mourad G, Glyda M, Albano L, et al. Advagraf-based immunosuppression regimen examining new onset diabetes mellitus in kidney transplant recipients (ADVANCE) study investigators. Incidence of posttransplantation diabetes mellitus in de novo kidney transplant recipients receiving prolonged-release tacrolimus-based immunosuppression with 2 different corticosteroid minimization strategies: ADVANCE, A randomized controlled trial. Transplantation. 2017;101:1924–1934. doi: 10.1097/TP.0000000000001453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montori VM, Basu A, Erwin PJ, et al. Posttransplantation diabetes: a systematic review of the literature. Diabetes Care. 2002;25:583–592. doi: 10.2337/diacare.25.3.583. [DOI] [PubMed] [Google Scholar]
- 23.Naesens M, Kuypers DR, Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol. 2009;4:481–508. doi: 10.2215/CJN.04800908. [DOI] [PubMed] [Google Scholar]
- 24.Vincenti F. Belatacept and long-term outcomes in kidney transplantation. N Engl J Med. 2016;374:2600–2601. doi: 10.1056/NEJMc1602859. [DOI] [PubMed] [Google Scholar]
- 25.Abdelwahab Elhamahmi D, Heilman RL, Smith B, et al. Early conversion to belatacept in kidney transplant recipients with low glomerular filtration rate. Transplantation. 2018;102:478–483. doi: 10.1097/TP.0000000000001985. [DOI] [PubMed] [Google Scholar]
- 26.Redmon JB, Olson LK, Armstrong MB, et al. Effects of tacrolimus (FK506) on human insulin gene expression, insulin mRNA levels, and insulin secretion in HIT-T15 cells. J Clin Invest. 1996;98:2786–2793. doi: 10.1172/JCI119105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Øzbay LA, Smidt K, Mortensen DM, et al. Cyclosporin and tacrolimus impair insulin secretion and transcriptional regulation in INS-1E beta-cells. Br J Pharmacol. 2011;162:136–146. doi: 10.1111/j.1476-5381.2010.01018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Triñanes J, Rodriguez-Rodriguez AE, Brito-Casillas Y, et al. Deciphering tacrolimus-induced toxicity in pancreatic β cells. Am J Transplant. 2017;17:2829–2840. doi: 10.1111/ajt.14323. [DOI] [PubMed] [Google Scholar]
- 29.Heit JJ, Apelqvist AA, Gu X, et al. Calcineurin/NFAT signalling regulates pancreatic beta-cell growth and function. Nature. 2006;443:345–349. doi: 10.1038/nature05097. [DOI] [PubMed] [Google Scholar]
- 30.Radu RG, Fujimoto S, Mukai E, et al. Tacrolimus suppresses glucose-induced insulin release from pancreatic islets by reducing glucokinase activity. Am J Physiol Endocrinol Metab. 2005;288:E365–E371. doi: 10.1152/ajpendo.00390.2004. [DOI] [PubMed] [Google Scholar]
- 31.Drachenberg CB, Klassen DK, Weir MR, et al. Islet cell damage associated with tacrolimus and cyclosporine: morphological features in pancreas allograft biopsies and clinical correlation. Transplantation. 1999;68:396–402. doi: 10.1097/00007890-199908150-00012. [DOI] [PubMed] [Google Scholar]
- 32.Pereira MJ, Palming J, Rizell M, et al. Cyclosporine A and tacrolimus reduce the amount of GLUT4 at the cell surface in human adipocytes: increased endocytosis as a potential mechanism for the diabetogenic effects of immunosuppressive agents. J Clin Endocrinol Metab. 2014;99:E1885–E1894. doi: 10.1210/jc.2014-1266. [DOI] [PubMed] [Google Scholar]
- 33.Fonseca ACRG, Carvalho E, Eriksson JW, et al. Calcineurin is an important factor involved in glucose uptake in human adipocytes. Mol Cell Biochem. 2018;445:157–168. doi: 10.1007/s11010-017-3261-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ekberg H, Tedesco-Silva H, Demirbas A, et al. ELITE-Symphony Study. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med. 2007;357:2562–2575. doi: 10.1056/NEJMoa067411. [DOI] [PubMed] [Google Scholar]
- 35.Masson P, Henderson L, Chapman JR, et al. Belatacept for kidney transplant recipients. Cochrane Database Syst Rev. 2014:CD010699. doi: 10.1002/14651858.CD010699.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jin L, Lim SW, Jin J, et al. Effect of conversion to CTLA4IG on tacrolimus-induced diabetic rats. Transplantation. 2018;102:e137–e146. doi: 10.1097/TP.0000000000002048. [DOI] [PubMed] [Google Scholar]
- 37.Pang TT, Chimen M, Goble E, et al. Inhibition of islet immunoreactivity by adiponectin is attenuated in human type 1 diabetes. J Clin Endocrinol Metab. 2013;98:E418–E428. doi: 10.1210/jc.2012-3516. [DOI] [PubMed] [Google Scholar]
- 38.Vanrenterghem Y, Bresnahan B, Campistol J, et al. Belatacept-based regimens are associated with improved cardiovascular and metabolic risk factors compared with cyclosporine in kidney transplant recipients (BENEFIT and BENEFIT-EXT studies). Transplantation. 2011;91:976–983. doi: 10.1097/TP.0b013e31820c10eb. [DOI] [PubMed] [Google Scholar]
- 39.Melilli E, Manonelles A, Montero N, et al. Impact of immunosuppressive therapy on arterial stiffness in kidney transplantation: are all treatments the same? Clin Kidney J. 2018;11:413–421. doi: 10.1093/ckj/sfx120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Melilli E, Bestard-Matamoros O, Manonelles-Montero A, et al. Arterial stiffness in kidney transplantation: a single center case-control study comparing belatacept versus calcineurin inhibitor immunosuppressive based regimen. Nefrologia. 2015;35:58–65. doi: 10.3265/Nefrologia.pre2014.Sep.12615. [DOI] [PubMed] [Google Scholar]
- 41.Seibert FS, Steltzer J, Melilli E, et al. Differential impact of belatacept and cyclosporine A on central aortic blood pressure and arterial stiffness after renal transplantation. Clin Transplant. 2014;28:1004–1009. doi: 10.1111/ctr.12413. [DOI] [PubMed] [Google Scholar]
- 42.Soveri I, Snyder J, Holdaas H, et al. The external validation of the cardiovascular risk equation for renal transplant recipients: applications to BENEFIT and BENEFIT-EXT trials. Transplantation. 2013;95:142–147. doi: 10.1097/TP.0b013e31827722c9. [DOI] [PubMed] [Google Scholar]


