Abstract
Background.
Renal arteriovenous fistula (rAVF) is a rare complication after a total nephrectomy, with only 72 cases reported in the last literature review published in 1997. AVF has never been described in a renal transplant recipient, and the possible consequences of hemodetournement on the graft function are unknown.
Methods.
We hereby reported the first case of rAVF occurring in a renal transplant recipient and analyzed all cases of postnephrectomy rAVF reported between 1997 and 2017.
Results.
A 75-year-old woman who underwent a right nephrectomy and kidney transplant 16 years earlier, and complaining of mild exercise dyspnea, was discovered with a lumbar continuous murmur. Echocardiography showed a moderate to severe dilatation of the left ventricle, with a decreased ejection fraction. Serum creatinine was slightly raised but returned to normal value with hydration. An injected computed tomography scan demonstrated a communication between the stump of the right renal artery and inferior vena cava. Total occlusion of the rAVF was obtained with Amplatzer plug and coils placed in the distal renal stump, just upstream of rAVF. Exercise dyspnea disappeared immediately, and regression of left ventricular dilatation was objectified at 6-month echocardiography follow-up.
Conclusions.
Postnephrectomy rAVF is rare, frequently diagnosed late, and may be responsible for high-output heart failure by left-to-right shunt, with abdominal/lumbar bruit being the only manifestation. Renal complications concern 15% of the patients. Endovascular procedure is nowadays the treatment of choice. Occluding rAVF permits cardiac hemodynamic features and heart failure symptoms resolution.
Renal arteriovenous fistulas (rAVFs) are rare and may be classified as congenital or acquired. Acquired forms represent up to 70% of all rAVFs and occur following percutaneous transrenal procedure, surgery (including partial or total nephrectomy), trauma, renal malignancy, inflammation, or infection.1 Postnephrectomy AVF was first described by Hollingsworth2 in 1934, followed by 61 cases between 1934 and 1985,3 then 10 additional cases until 1997.4 Frequently diagnosed late (up to 50 y postsurgery), they can be responsible for high-output heart failure. As cardiac consequences are reversible after arteriovenous fistula (AVF) occlusion, their detection and treatment are recommended.
To our knowledge, we hereby report the first case of postnephrectomy rAVF occurring in a renal transplant recipient, which is particularly interesting regarding the possible consequences of hemodetournement on the graft function. We also analyzed all cases of postnephrectomy renal AVF described between 1997 and 2017 and compared them with the cases reported by Lacombe3 in 1985 and El Rassi et al4 in 1997.
CASE REPORT
A 75-year-old woman underwent right nephrectomy (900 g) and deceased donor kidney transplant in right iliac fossa 16 years earlier for autosomal hepatorenal polycystic disease. While she presented to the emergency room for viral gastroenteritis, the clinical exam revealed a loud continuous murmur in the right flank and in the back. When asking, she noticed exercise dyspnea and an easy fatigability progressively appearing for a year. Her serum creatinine was slightly raised but returned to normal value with hydration; urinalysis was normal. Chest X ray revealed cardiomegaly (cardiothoracic ratio 57%), and echocardiography showed a moderate to severe dilatation of the left ventricle, with a decreased ejection fraction of 50% (normal echocardiography in 2014). A contrast-enhanced thoraco-abdominal computed tomography (CT) scanner demonstrated a communication between the stump of the right renal artery (measuring 5 mm in diameter) and inferior vena cava (Figure 1A and B).
FIGURE 1.
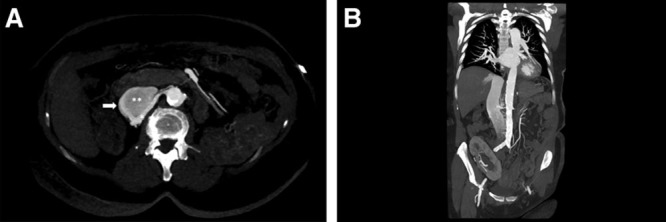
Computed tomography (CT) angiography in arterial phase. Axial (A) and coronal (B) maximum intensity projection reconstruction images demonstrating the communication (arrow) between the right renal artery stump (*) and inferior vena cava (**) with dilatation and early opacification of the inferior vena cava. The coronal plane also shows the dilated left atrium. Note the presence of the renal graft in the right iliac fossa.
Following a multidisciplinary discussion with specialists in interventional radiology, angiology, cardiovascular surgery, and nephrology, an endovascular approach to occlude the AVF was decided. The procedure was performed under local anesthesia and light sedation, with retrograde puncture of the left common femoral artery and catheterization of the right renal artery. Antegrade right renal arteriography showed a high-flow AVF between the right renal artery stump and inferior vena cava (Figure 2A). A successful embolization was performed with a distal Amplatzer Vascular Plug II (10 mm), 2 detachable coils (Azur framing coil 10 mm/26 cm and 20 mm/50 cm, Terumo), 2 pushable coils (Azur helical hydro coil 8 mm/14 cm, Terumo), and a second Amplatzer Vascular Plug II (12 mm), sequentially placed in the distal part of the main feeding artery, the right renal artery stump. Total occlusion of the rAVF (Figure 2B) was obtained. The Doppler ultrasonography performed on the same day showed the absence of diastolic flow in the renal artery and aorta and absence of arterialized flow in the inferior vena cava. The patient recovered uneventfully and was discharged the day after. Symptoms of exercise dyspnea and easy fatigability disappeared. No AVF recurrence was shown on Doppler ultrasonography at 1-, 3-, and 6-month follow-up. Regression of left ventricular dilatation has been found at 6-month echocardiography follow-up, but no improvement in left ventricular ejection fraction
FIGURE 2.
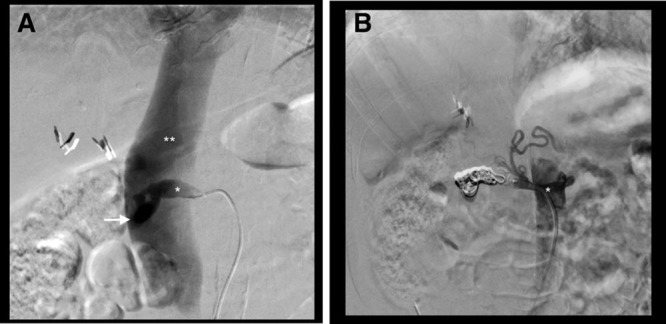
Antegrade right renal arteriography. Selective right renal artery stump (*) angiogram showing early opacification of the inferior vena cava (**), confirming the presence of a high-flow arteriovenous fistula (arrow). A, Complete embolization of the arteriovenous fistula (AVF; no residual opacification of the inferior vena cava) after deployment of detachable and pushable coils in-between 2 Amplatzer Vascular Plugs. B, Note the opacification of the right inferior phrenic artery and superior suprarenal branches proximal to the occluded arterial segment.
DISCUSSION
To our knowledge, we described here the first case of postnephrectomy AVF in a renal transplant recipient. Graft function was not altered, and AVF was successfully occluded. Heart failure of unknown origin in a postnephrectomy renal transplant recipient must raise suspicion for rAVF.
Renal AVF is a rare complication after total nephrectomy. The last literature review published in 1997 reported a total of 72 cases,4 and since 1997, 18 series analyzed 20 new patients, which makes a total of 21 including our patient. In 14 of 21 cases (67%), the right side was involved, as already observed in other studies.3,4 Anatomic features could contribute to this right dominance, with a right renal artery typically longer than the left5 one. Indeed, a long residual section could promote the formation of an aneurysm at the blind end by lack of shear stress, and then the growing aneurysm could erode the vein’s wall and lead to the formation of the AVF.6-8 En bloc ligation of the renal pedicle (instead of dissection and suture of the artery and the vein separately) and local inflammation have been reported by most studies as main risk factors for the development of AVF,3,4 with subsequent necrosis of the vessel’s wall and perforation.9,10 In only 2 of the 20 cases reported since 1997, the surgical technique was described as en bloc ligation of renal pedicle.11 In our case, renal pedicle was dissected and the artery and the vein separately sutured. rAVF mainly follows posttraumatic nephrectomies (7 cases), because these nephrectomies are performed in an emergency setting and careful dissection and suture of each vessel are difficult to achieve. rAVF also frequently complicates nephrectomies performed in case of lithiasis (3 cases), infection (tuberculosis 5 cases, pyelonephritis 1 case), and cancer (3 cases), because local inflammation is a great factor for vessel’s wall necrosis8 and secondary AVF formation. In 1 case, nephrectomy was performed because of angiomyolipoma bleeding. To notice, AVF has never been described after elective nephrectomies in living donors.
Latency between nephrectomy and the diagnosis of AVF varies a lot, from a couple of weeks (the fact of only 4 patients),12-15 to several years, with a mean follow-up of 19 years. In the 4 patients with a very short delay of diagnosis, nephrectomies were performed in an emergency setting following trauma, and AVFs were immediately suspected because of persistent postoperative anemia.12-15 Clinical manifestations are summarized in Table 1 and are most of the time insidious, delaying the diagnosis up to 50 years.4 AVFs could induce left-to-right shunt, leading to an increase of the cardiac output to compensate this arterial steal. Both the increased workload and venous return can finally lead to heart failure.16 Most of the patients have already established heart failure at time of diagnosis. Echocardiographies have been described in 6 cases5,7,10,17,18: all but one10 with normal ejection fraction, and all showing left ventricle dilatation. Hemodynamic studies performed in 5 patients have revealed increased cardiac index.5,17-20
TABLE 1.
Main manifestations of a renal arteriovenous fistula
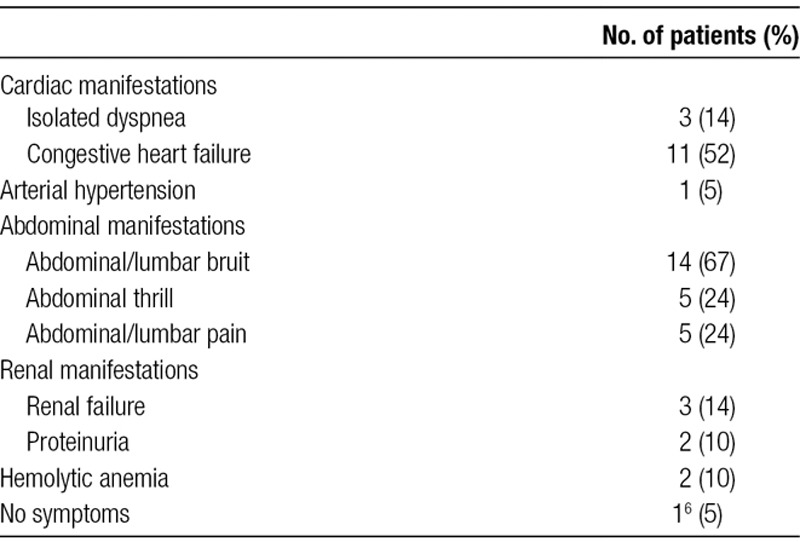
In a literature review, El Rassi et al4 found that all patients have an abdominal bruit at the time of diagnosis. In our review from 1997 until 2007, this clinical manifestation was found in 67% of patients and was the only rAVF manifestation in 2 patients.19,21 A good clinical examination including abdominal and lumbar auscultation is therefore mandatory in postnephrectomy follow-up for early detection of rAVF.
Renal failure is rare and found in only 3 cases.5,7,16 Two mechanisms could be involved: for Marcaggi et al,22 a process of hemostealing could cause renal ischemia, followed by renal failure and hypertension; for Kajbafzadeh and Broumand,16 the increased cardiac output increases renal flow with secondary hyperfiltration, proteinuria, and renal failure.
Once clinical suspicion is raised, the diagnosis must be confirmed by radiological tests. Doppler sonography is an excellent screening exam, which also allows controlling the AVF occlusion after surgery or endovascular intervention.14 Eight of 21 patients (38%) had a Doppler sonography as the initial exam, which confirmed the clinical suspicion. For more precise anatomy depiction, either CT scan (7/8 patients) or contrast-enhanced magnetic resonance angiography (1/8 patient)19 completed Doppler sonography. CT scan can also be the initial screening test, as it was the case of our patient. The gold standard radiological exam is the arteriography, for diagnostic confirmation and management. Ninety percent of patients (19/21) underwent an arteriography, and 24% (5/21) as first exam.
Renal AVFs being most of the time symptomatic when discovered, a treatment is mandatory. Surgery was the treatment of choice for many years. Of the 62 cases described by Lacombe3 in 1985 and the 72 cases described by El Rassi et al4 in 1997, 90% and 89%, respectively, have been treated surgically, by simple ligature and total excision of the AVF. Although total excision can be hazardous due to inflammation surrounding the AVF that limits dissection, it remains the treatment of choice. Indeed, simple ligation puts the patient at risk of recurrence, due to possible missed collateral vessels that may still feed the AVF after the main vessels ligation.22 In our review from 1997, only 7 of 21 patients (33%) have been treated by surgery.5,9,16,17,19,20 All interventions were successful, without any complication, and all patients have been discharged a few days later.
When implemented, endovascular treatments (mainly coiling) were associated with great risk of embolic materials migration17,23 and therefore initially proposed to high operative and anesthetic risk patients. In our review, 2 rAVFs have been successfully occluded by coils,12,13 but in 2 cases, the first attempt of coiling resulted in migration into pulmonary artery, which could be removed during arteriography.6,23 To prevent further migration, AVF flow was first reduced by inflation of an occlusion balloon in the renal vein in one case,6 and in another case, the diameter of the AVF was reduced by the deployment of a Wallstent preliminarily sutured at midportion.23 To circumvent migration, larger devices have been recently developed, such as Amplatzer Vascular Plugs (AVPs), which are self-expanding occluding devices, available in a large array of size and which provided full cross-sectional coverage of large vessels. This device can be easily repositioned and redeployed during procedures for greater precision, although some authors used through-and-through access for more stability, achieving by simultaneous transarterial and transvenous access.8 AVPs have been successfully used in 5 cases.6-8,14,15 In our case, we used a mix of AVPs and coils, with a single transarterial approach. Other devices for occlusion are available such as Patent Ductus Arteriosus Occluder, cheaper and more accessible in China.11 Recently, Manresa-Manresa et al21 successfully designed a custom-made stent graft with suprarenal fixation and a scallop for the contralateral renal artery. With the improvement of endovascular technique and development of new devices such as AVPs, endovascular treatment has superseded surgery, having the advantage of being minimally invasive, with lower blood loss, decreased morbidity, and a shorter hospital stay.6,8,23
When rAVF is considered too important for the endovascular procedure alone, a mixed technique of endovascular procedure and surgery can be performed.10,18 Initially, inflation of a balloon in renal artery stump allows precise localization of the AVF, improvement of cardiac hemodynamic, decompression of inferior vena cava, and prevention of serious bleeding.10 Then, a safe surgical ligature or excision of AVF can be performed.
Since 1997, all but one rAVF occlusion—either performed by surgery, endovascular or mixed procedure—were successful. The unique recanalization 2 months after coiling was in a second time treated successfully with Amplatzer embolization.6
Following therapeutic management, symptoms and chest congestion improved in all cases with all hemodynamic studies showing a decreased cardiac index.5,18-20 The longest follow-up reported is 7 years,9 with most of cases performed between 1 month and 1 year.
CONCLUSION
To our knowledge, we hereby report the first case of rAVF in a renal transplant recipient, 16 years posttransplantation and ipsilateral right nephrectomy. The fistula was successfully treated by endovascular procedure, with coils and AVPs sequentially placed in the renal artery stump. Patient recovered uneventfully, and graft function was not altered.
Although rare, rAVF should be suspected in autosomal hepatorenal polycystic disease recipients with dyspnea of unknown origin who underwent native nephrectomy.
Footnotes
Published online 12 December, 2019.
J.S. involved in investigation, research design, data acquisition, data analysis, manuscript writing, revision, and editing the paper. A.P. performed the procedure, revision, and editing the paper. T.E. involved in investigation, revision, and editing the paper. C.H.-P. performed the procedure, revision, and editing the paper. C.T. involved in revision and editing the paper. K.H. involved in conceptualization, manuscript writing, revision, and editing the paper.
The authors declare no funding or conflicts of interest.
REFERENCES
- 1.Tarkington MA, Matsumoto AH, Dejter SW, et al. Spectrum of renal vascular malformation. Urology. 1991; 38297–300 [DOI] [PubMed] [Google Scholar]
- 2.Hollingsworth EW. Arteriovenous fistula of the renal vessels. Am J Med Sci. 1934; 188399–403 [Google Scholar]
- 3.Lacombe M. Renal arteriovenous fistula following nephrectomy. Urology. 1985; 2513–16 [DOI] [PubMed] [Google Scholar]
- 4.El-Rassi I, Jebara I, Khoury A, et al. [Cardiac failure caused by renal arteriovenous fistula fifty years after nephrectomy. A new case and review of the literature]. Arch Mal Coeur Vaiss. 1997; 901427–1430 [PubMed] [Google Scholar]
- 5.Ozaki K, Kubo T, Hanayama N, et al. High-output heart failure caused by arteriovenous fistula long after nephrectomy. Heart Vessels. 2005; 20236–238 [DOI] [PubMed] [Google Scholar]
- 6.Abdel-Aal AK, Elsabbagh A, Soliman H, et al. Percutaneous embolization of a postnephrectomy arteriovenous fistula with intervening pseudoaneurysm using the Amplatzer vascular plug 2. Vasc Endovascular Surg. 2014; 48516–521 [DOI] [PubMed] [Google Scholar]
- 7.Stefańczyk L, Religa W, Kasprzak J, et al. Giant postnephrectomy arteriovenous fistula in a patient with tuberous sclerosis and anomalous inferior vena cava: treatment with amplatzer vascular plug embolization. Ann Vasc Surg. 2014; 281318.e7–1318.e10 [DOI] [PubMed] [Google Scholar]
- 8.Kayser O, Schäfer P. Transcatheter Amplatzer vascular plug-embolization of a giant postnephrectomy arteriovenous fistula combined with an aneurysm of the renal pedicle by through-and-through, arteriovenous access. Ger Med Sci. 2013; 11Doc01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baptista-Silva JC, de Figueiredo LF, Castro MJ, et al. Postnephrectomy arteriovenous fistula. Sao Paulo Med J. 1997; 1151444–1447 [DOI] [PubMed] [Google Scholar]
- 10.Ferrari M, Bonanomi G, Catalano G, et al. Combined percutaneous and surgical approach to a postnephrectomy arteriovenous fistula. J Cardiovasc Surg (Torino). 2001; 42393–395 [PubMed] [Google Scholar]
- 11.Yin H, Zhao Y, Wang M, et al. Endovascular management of early-onset post nephrectomy renal arteriovenous fistula: a report of two cases. SAGE Open Med Case Rep. 2015; 32050313X15621856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kocakoc E, Poyraz AK, Cetinkaya Z, et al. Postnephrectomy renal arteriovenous fistula. J Ultrasound Med. 2004; 23965–968 [DOI] [PubMed] [Google Scholar]
- 13.Bozgeyik Z, Ozdemir H, Orhan I, et al. Pseudoaneurysm and renal arteriovenous fistula after nephrectomy: two cases treated by transcatheter coil embolization. Emerg Radiol. 2008; 15119–122 [DOI] [PubMed] [Google Scholar]
- 14.Taneja M, Metupalle V, Hiong TK, et al. Postnephrectomy fistula between the renal artery stump and inferior vena cava treated with Amplatzer vascular plug: gray-scale and Doppler sonographic findings. J Clin Ultrasound. 2008; 36497–499 [DOI] [PubMed] [Google Scholar]
- 15.Taneja M, Lath N, Soo TB, et al. Renal artery stump to inferior vena cava fistula: unusual clinical presentation and transcatheter embolization with the Amplatzer vascular plug. Cardiovasc Intervent Radiol. 2008; 31Suppl 2S92–S95 [DOI] [PubMed] [Google Scholar]
- 16.Kajbafzadeh AM, Broumand B. Arteriovenous fistula following nephrectomy. Eur Urol. 1997; 31112–114 [DOI] [PubMed] [Google Scholar]
- 17.Okamoto M, Hashimoto M, Akita T, et al. Congestive heart failure caused by aortocaval fistula after nephrectomy. Intern Med. 2001; 401113–1116 [DOI] [PubMed] [Google Scholar]
- 18.Chen J, Liu M, Ke J, et al. An unusual cause of high-output heart failure: renal arteriovenous fistula after nephrectomy. Circ Heart Fail. 2014; 7378–379 [DOI] [PubMed] [Google Scholar]
- 19.Develing L, Leiner T, Kitslaar PJ. Magnetic resonance angiography for postnephrectomy arteriovenous fistula. Eur J Vasc Endovasc Surg. 2002; 23178–179 [DOI] [PubMed] [Google Scholar]
- 20.Hirai S, Hamanaka Y, Mitsui N, et al. High-output heart failure caused by a huge renal arteriovenous fistula after nephrectomy: report of a case. Surg Today. 2001; 31468–470 [DOI] [PubMed] [Google Scholar]
- 21.Manresa-Manresa F, Sánchez-Rodríguez JM, Nacarino-Mejías V, et al. Aortic customized stent-graft treatment of postnephrectomy renal pedicle arteriovenous fistula. Vasc Endovascular Surg. 2017; 51191–194 [DOI] [PubMed] [Google Scholar]
- 22.Marcaggi X, Boyer L, Lusson JR, et al. [Arteriovenous fistula of the renal pedicle following nephrectomy responsible for heart failure. Report of 2 cases]. Arch Mal Coeur Vaiss. 1990; 831721–1724 [PubMed] [Google Scholar]
- 23.Resnick S, Chiang A. Transcatheter embolization of a high-flow renal arteriovenous fistula with use of a constrained wallstent to prevent coil migration. J Vasc Interv Radiol. 2006; 172 Pt 1363–367 [DOI] [PubMed] [Google Scholar]


