Abstract
Renal injury almost always accompanies the multisystem organ failure that precedes cardiac transplantation and renal function is further compromised by the nephrotoxicity of calcineurin inhibitors posttransplant. Renal dysfunction in turn causes significant morbidity and mortality. The development of belatacept was motivated by need for an alternative to calcineurin-based immunosuppression, particularly in renal transplantation where the nephrotoxicity of calcineurin inhibitors reduce graft longevity and adverse cardiovascular effects of calcineurin inhibitors increase overall mortality. In 2011, the FDA approved belatacept for use in renal transplantation. Seven-year data from the multicenter randomized phase III BENEFIT trial, which compared belatacept with cyclosporine in renal transplant recipients, show belatacept therapy offers both improved renal function and 43% risk reduction for the combined endpoint of graft loss and death. At present, belatacept use is predominantly confined to renal transplant recipients; however, reports of belatacept use in other transplant settings are emerging. Here, we describe successful long-term use of belatacept in a kidney-after-heart transplant recipient and review use of belatacept in cardiothoracic and other nonrenal transplant settings.
Solid organ transplantation saw enormous gains in short-term graft survival with the advent of calcineurin inhibitors (CNIs) in the 1980s. Paradoxically, CNI-related morbidity, including significant nephrotoxicity, now stands as a leading barrier to long-term graft survival. CNIs are universally used for immunosuppression in cardiac transplantation, and renal injury is common through the induction of chronic allograft nephropathy and interstitial fibrosis and tubular atrophy. Indeed over 10% of heart transplant recipients have a GFR <30 mL/min by 5 years posttransplant, and the risk of ESRD is estimated at 1%–1.5% per year of follow-up.1 CNIs also potentiate diabetes, hypertension, and hyperlipidemia leading to more rampant cardiovascular disease and increased overall mortality in transplant recipients when compared with the general population.2 Despite these known adverse effects, CNIs remain a mainstay of contemporary immunosuppression because few proven alternatives exist.
Belatacept was FDA approved in 2011 for use in renal transplantation as a nonnephrotoxic CNI-alternative for maintenance immunosuppression. Belatacept is a fusion protein composed of the Fc fragment of human IgG1 linked to the extracellular domain of cytotoxic T-lymphocyte-associated antigen 4 that selectively inhibits T-cell activation through co-stimulation blockade. Despite higher rates of early acute rejection, 7-year outcomes demonstrated improved renal function and 43% reduction in the risk of patient death and graft loss in comparison to the CNI cyclosporine.3 CNI-avoidance with belatacept has the additional benefits of equivalent safety and improved cardiovascular and metabolic risk profiles along with lower rates of de novo donor-specific antibody formation in renal transplant recipients.4,5 This favorable toxicity profile and improved long-term outcomes in renal transplantation render belatacept as an attractive alternative immunosuppressant for CNI-avoidance in cardiac transplantation.
To date, belatacept has been predominantly administered in kidney transplant recipients and scant data describe use in heart transplant recipients. Enderby et al6 reported on use of belatacept in a noncompliant 26-year-old female heart transplant recipient with postpartum cardiomyopathy who experienced 6 episodes of grade 3R rejection associated with de novo DSA within the first 20 months posttransplant. After the initiation of belatacept to mitigate her noncompliance, 2 allograft biopsies during an 8-month period showed no histologic evidence of cellular or humoral rejection. However, the long-term impact of belatacept utilization in this heart transplant recipient could not be ascertained due to her premature death from an unexplained cardiac arrest 2 years and 3 months posttransplant. Recently, Kumar et al7 reported on a 61-year-old female simultaneous heart-liver transplant recipient who required kidney transplant 3 years after SHLT that was converted to belatacept for oliguric DGF, resulting in good function of all 3 allografts 1 year post kidney transplant. Here, we provide the first report of de novo belatacept-based immunosuppression in a kidney-after-heart transplant recipient with excellent long-term renal function.
PRESENTATION OF CASE
The patient is a 27-year-old Asian-born female who developed congestive heart failure of unclear etiology at the age of 14. She was born at 38 weeks without perinatal complications, moved to the United States at the age of 6, and had no significant past medical history. Thirty-one days after presentation, she received a 6-antigen disparate heart transplant. Her early posttransplant course was complicated by tamponade, need for mediastinal reexploration, cardiac arrest, transient need for ECMO, and delayed sternal closure. Despite these initial setbacks, she was discharged to home 26 days after heart transplant and continues to have normal cardiac function 11 years later. Pathology from the native explant was suggestive of a “burned out” hypertrophic cardiomyopathy. Daclizumab and methylprednisolone were used for induction therapy. Initial maintenance immunosuppression was with cyclosporine, mycophenolate mofetil, and a steroid taper. After 60 days, cyclosporine was discontinued and tacrolimus therapy was started with an initial trough goal of 8–12 mg/dL.
Before transplantation, the patient had normal renal function with a serum creatinine of 0.9 mg/dL (Figure 1). She experienced perioperative acute kidney injury at the time of heart transplantation with a maximum serum Cr of 2.0 mg/dL but recovered fully and was discharged with a Cr of 0.9. After discontinuing cyclosporine, tacrolimus trough goals were slowly reduced during posttransplant years 1–5. Renal function remained normal until an acute kidney injury associated with a UTI 2 years posttransplant at which time the serum creatinine reached a maximum value of 3.2 mg/dL. Thereafter, there was residual chronic renal insufficiency, proteinuria, and the serum Cr never fell below 1.3 mg/dL. A native renal biopsy showed glomerular and vascular changes suggestive of acute and chronic thrombotic microangiopathy as well as acute and chronic tubulointerstitial nephritis associated with diffuse mild to moderate interstitial fibrosis and 30% tubular atrophy. At 5 years posttransplant, mild distal pruning of the coronary arteries was observed on a left heart catheterization. This prompted discontinuation of mycophenelate and an attempt to begin sirolimus and wean tacrolimus. Renal function abruptly declined and the treating clinicians feared sirolimus was potentiating the nephrotoxic effects of tacrolimus. Although the original regimen of tacrolimus, mycophenolate, and steroid was restarted renal function never recovered.
FIGURE 1.
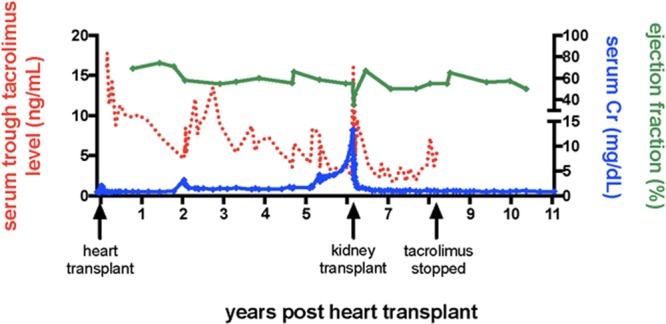
Serum tacrolimus level, serum creatinine, and cardiac ejection fraction as a function of time following heart and kidney transplantation.
Dialysis was initiated 6 years and 2 months post heart transplant and 1 day after her first hemodialysis treatment the patient received a haploidentical living related renal transplant from her father. There were no A, B, or DR alleles shared between the heart and kidney donors. Induction immunosuppression consisted of methylprednisolone and basiliximab. Belatacept, tacrolimus, mycophenolate, and prednisone were initiated for maintenance therapy according to institutional protocol.8 A brief period of delayed graft function was managed with 2 posttransplant hemodialysis sessions. An echocardiogram on POD#5 showed a mildly reduced ejection fraction of 35%–40% and a renal allograft protocol biopsy showed mild acute tubular necrosis. By POD#12 the ejection fraction had recovered to 45%–50% and on POD#13 the patient was discharged to home, off dialysis, on mycophenolate, prednisone, tacrolimus, and once monthly belatacept. The initial tacrolimus trough goal was 4–6 mg/dL.
After kidney transplantation, the patient has maintained excellent renal function without any episodes of rejection. The most recent serum Cr 5 years post renal transplant was 0.9 mg/dL. Tacrolimus was slowly weaned and then discontinued in the first 2 years following renal transplant. The current maintenance regimen consists of 5 mg prednisone daily, 750 mg mycophenolic acid twice daily, and 5 mg/kg belatacept monthly. Three protocol myocardial biopsies after kidney transplant have twice shown ISHLT grade 0 pathology and once shown grade 1A pathology. Allomap analyses were also followed in liu of additional cardiac biopsies and scores have yet to exceed 29. The ejection fraction was 50% on the most recent ECHO now >11 years after heart transplant. Aside from chronic sinusitis, the patient has had few complications. With respect to protective immunity the patient has never developed CMV viremia and had a single low-positive EBV titer that spontaneously resolved. At her most recent office visit, a low-positive BK titer was noted. Importantly, she has not developed any detectable donor-specific anti-HLA antibodies.
DISCUSSION
The 5-year risk of stage IV or V chronic kidney disease in nonrenal organ transplant recipients is estimated between 7% and 21% with an associated 4-fold increased risk of death.1 It is estimated that 4.6%–8%,9 7.7%,10 and 22%11 of heart, lung, and liver transplant recipients progress to require renal replacement therapy. The need to transplant for CNI-induced renal failure adds to an already overwhelming shortage of donor organs. The renal-sparing properties of belatacept are appealing for heart transplant recipients that suffer from CNI-associated nephrotoxicity, hypertension, hyperlipidemia, and new onset diabetes mellitus after transplant. Additionally, given belatacept’s IV formulation and infusion requirement, transplant centers have the opportunity to better track compliance. Importantly, posttransplant de novo donor-specific antibodies are an increasingly recognized source of graft dysfunction and death following heart transplantation, suggesting the need to reassess immunosuppressant strategies in this population.12 Costimulation blockade remains the most promising agent in this regard, as the risk of de novo donor-specific antibodies is considerably lower with belatacept compared with a CNI-based regimen in the renal transplant population.3 It remains to be determined if this holds true in a nonrenal transplant population.
Few reports exist documenting the use of belatacept following nonrenal solid organ transplants. At the University of Maryland 8 lung transplant recipients were transitioned to belatacept because of acute or chronic renal insufficiency (median GFR 24).13 GFR stabilized in 2 patients and increased in 5 while 1 patient died from multisystem organ failure after only 1 dose of belatacept. During 6 months of follow-up, there was a single instance of mild acute cellular rejection. Hui et al14 describe 24 months of successful use of belatacept in a lung transplant recipient taken off of CNIs because of thrombotic microangiopathy, and Haidar et al15 issue a cautionary note describing fatal invasive tracheobronchiolar aspergillosis in a lung transplant recipient treated with belatacept after developing HUS/TTP on tacrolimus and posterior reversible encephalopathy on cyclosporine.
One hundred fifty-three liver transplant recipients were treated with belatacept in a phase II randomized trial designed to evaluate the safety and efficacy of belatacept relative to tacrolimus.16 Belatacept use was associated with higher rates of graft loss and death leading to termination of the study after 1 year; however, some speculate that liver disease itself creates a high degree of endogenous immunosuppression that cannot safely be combined with potent costimulatory blockade.17 In a much smaller series, LaMattina et al18 describe safe 1- to 3-month use of belatacept “bridge” therapy in hepatitis C positive liver transplant recipients with perioperative renal dysfunction. Isolated reports of belatacept use in islet19 and pancreas20 transplantation also exist.
Belatacept use in renal transplant recipients has been associated with higher rates of early acute rejection, yet long-term outcomes and renal function remain superior to CNI-based regimens.3 Identifying strategies to overcome this observed belatacept-resistant rejection is an active area of investigation. We have adopted transient CNI therapy as one method to prevent higher rates of acute rejection with belatacept and facilitate improved long-term renal allograft function.8 This alternative belatacept-based strategy reduces rates of early acute rejection to levels equivalent with standard-of-care therapy while still allowing for long-term CNI-free maintenance immunosuppression. While this strategy proved effective in the case reported here, much remains to be determined regarding the use of costimulation blockade in heart transplantation. Clinicians unfamiliar with belatacept should remember that use is constrained to transplant recipients with known EBV-positive serostatus as there is an increased risk of posttransplant lymphoproliferative disease in recipients with negative or unknown EBV serostatus. In addition, clinicians should be aware that progressive multifocal leukoencephalopathy, polyoma-virus associated nephropathy, and other serious infections, though rare, have been associated with belatacept use.
In summary, there is indisputable need for nonnephrotoxic immunosuppression in all organ transplant recipients. Safety and efficacy of belatacept are proven in renal transplant recipients and selective use in nonrenal transplant recipients has been reported. Here, we describe successful long-term use of de novo belatacept in a young kidney-after-heart transplant recipient who developed CNI-induced renal failure. Clinicians should take a cautious and thoughtful approach when extrapolating results of the BENEFIT trials and considering belatacept use in nonrenal transplant recipients; however, belatacept will likely have utility in many extra-renal transplant settings. Larger comparative studies are needed. This case report highlights belatacept as an option for long-term maintenance immunosuppression in heart transplant recipients and should stimulate interest in clinical trials to prospectively evaluate efficacy.
Footnotes
Published online 24 December, 2019.
A.D.S., D.J.A., R.T.C., I.R.B. performed article writing/editing. C.P.L. performed article writing/editing and patient care.
C.P.L. is an investigator on BMS sponsored clinical trials. The other authors declare no conflicts of interest.
REFERENCES
- 1.Ojo AO, Held PJ, Port FK, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:931–940. doi: 10.1056/NEJMoa021744. [DOI] [PubMed] [Google Scholar]
- 2.Wheeler DC, Steiger J. Evolution and etiology of cardiovascular diseases in renal transplant recipients. Transplantation. 2000;70(11 Suppl):SS41–SS45. [PubMed] [Google Scholar]
- 3.Vincenti F, Rostaing L, Grinyo J, et al. Belatacept and long-term outcomes in kidney transplantation. N Engl J Med. 2016;374:333–343. doi: 10.1056/NEJMoa1506027. [DOI] [PubMed] [Google Scholar]
- 4.Rostaing L, Vincenti F, Grinyó J, et al. Long-term belatacept exposure maintains efficacy and safety at 5 years: results from the long-term extension of the BENEFIT study. Am J Transplant. 2013;13:2875–2883. doi: 10.1111/ajt.12460. [DOI] [PubMed] [Google Scholar]
- 5.Vanrenterghem Y, Bresnahan B, Campistol J, et al. Belatacept-based regimens are associated with improved cardiovascular and metabolic risk factors compared with cyclosporine in kidney transplant recipients (BENEFIT and BENEFIT-EXT studies). Transplantation. 2011;91:976–983. doi: 10.1097/TP.0b013e31820c10eb. [DOI] [PubMed] [Google Scholar]
- 6.Enderby CY, Habib P, Patel PC, et al. Belatacept maintenance in a heart transplant recipient. Transplantation. 2014;98:e74–e75. doi: 10.1097/TP.0000000000000404. [DOI] [PubMed] [Google Scholar]
- 7.Kumar D, Yakubu I, Cooke RH, et al. Belatacept rescue for delayed kidney allograft function in a patient with previous combined heart-liver transplant. Am J Transplant. 2018;18:2613–2614. doi: 10.1111/ajt.15003. [DOI] [PubMed] [Google Scholar]
- 8.Adams AB, Goldstein J, Garrett C, et al. Belatacept combined with transient calcineurin inhibitor therapy prevents rejection and promotes improved long-term renal allograft function. Am J Transplant. 2017;17:2922–2936. doi: 10.1111/ajt.14353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wyatt CM, Arons RR. The burden of acute renal failure in nonrenal solid organ transplantation. Transplantation. 2004;78:1351–1355. doi: 10.1097/01.tp.0000140848.05002.b8. [DOI] [PubMed] [Google Scholar]
- 10.Rocha PN, Rocha AT, Palmer SM, et al. Acute renal failure after lung transplantation: incidence, predictors and impact on perioperative morbidity and mortality. Am J Transplant. 2005;5:1469–1476. doi: 10.1111/j.1600-6143.2005.00867.x. [DOI] [PubMed] [Google Scholar]
- 11.Paramesh AS, Grosskreutz C, Florman SS, et al. Thrombotic microangiopathy associated with combined sirolimus and tacrolimus immunosuppression after intestinal transplantation. Transplantation. 2004;77:129–131. doi: 10.1097/01.TP.0000092522.36410.D0. [DOI] [PubMed] [Google Scholar]
- 12.Smith JD, Banner NR, Hamour IM, et al. De novo donor HLA-specific antibodies after heart transplantation are an independent predictor of poor patient survival. Am J Transplant. 2011;11:312–319. doi: 10.1111/j.1600-6143.2010.03383.x. [DOI] [PubMed] [Google Scholar]
- 13.Timofte I, Terrin M, Barr E, et al. Belatacept for renal rescue in lung transplant patients. Transpl Int. 2016;29:453–463. doi: 10.1111/tri.12731. [DOI] [PubMed] [Google Scholar]
- 14.Hui C, Kern R, Wojciechowski D, et al. Belatacept for maintenance immunosuppression in lung transplantation. J Investig Med High Impact Case Rep. 2014;2:2324709614546866. doi: 10.1177/2324709614546866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haidar G, Crespo M, Maximous S, et al. Invasive tracheobronchial aspergillosis in a lung transplant recipient receiving belatacept as salvage maintenance immunosuppression: a case report. Transplant Proc. 2016;48:275–278. doi: 10.1016/j.transproceed.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Klintmalm GB, Feng S, Lake JR, et al. Belatacept-based immunosuppression in de novo liver transplant recipients: 1-year experience from a phase II randomized study. Am J Transplant. 2014;14:1817–1827. doi: 10.1111/ajt.12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knechtle SJ, Adams AB. Belatacept: is there BENEFIT for liver transplantation too? Am J Transplant. 2014;14:1717–1718. doi: 10.1111/ajt.12806. [DOI] [PubMed] [Google Scholar]
- 18.LaMattina JC, Jason MP, Hanish SI, et al. Safety of belatacept bridging immunosuppression in hepatitis C-positive liver transplant recipients with renal dysfunction. Transplantation. 2014;97:133–137. doi: 10.1097/01.TP.0000438635.44461.2e. [DOI] [PubMed] [Google Scholar]
- 19.Posselt AM, Szot GL, Frassetto LA, et al. Islet transplantation in type 1 diabetic patients using calcineurin inhibitor-free immunosuppressive protocols based on T-cell adhesion or costimulation blockade. Transplantation. 2010;90:1595–1601. doi: 10.1097/TP.0b013e3181fe1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mujtaba MA, Sharfuddin AA, Taber T, et al. Conversion from tacrolimus to belatacept to prevent the progression of chronic kidney disease in pancreas transplantation: case report of two patients. Am J Transplant. 2014;14:2657–2661. doi: 10.1111/ajt.12863. [DOI] [PubMed] [Google Scholar]


