Abstract
Background:
Leukocyte telomere has been shown to be related to insulin resistance-related diseases, such as type 2 diabetes mellitus (T2DM) and non-alcoholic fatty liver disease (NAFLD). This cross-sectional study investigated the association of leukocyte telomere length (LTL) with NAFLD in T2DM patients.
Methods:
Clinical features were collected and LTL was measured by Southern blot-based terminal restriction fragment length analysis in 120 T2DM patients without NAFLD and 120 age-matched T2DM patients with NAFLD. NAFLD was clinically defined by manifestations of ultrasonography. The correlation between LTL and clinical and biochemical parameters were analyzed by Pearson correlation or Spearman correlation analysis. Factors for NAFLD in T2DM patients were identified using multiple logistic regressions.
Results:
LTL in T2DM patients with NAFLD were significantly longer than those without NAFLD (6400.2 ± 71.8 base pairs [bp] vs. 6023.7 ± 49.5 bp, P < 0.001), especially when diabetes duration was less than 2 years. Meanwhile, the trend of shorter LTL was associated with the increased diabetes duration in T2DM patient with NAFLD, but not in T2DM patients without NAFLD. Finally, LTL (odds ratio [OR]: 1.001, 95% confidence interval [CI]: 1.000–1.002, P = 0.001), as well as body mass index (OR: 1.314, 95% CI: 1.169–1.477, P < 0.001) and triglycerides (OR: 1.984, 95% CI: 1.432–2.747, P < 0.001), had a significant association with NAFLD status in T2DM patients.
Conclusions:
T2DM patients with NAFLD had a significantly longer LTL than those without NAFLD. The longer LTL was especially evident in the early stage of T2DM, indicating that longer LTL may be used as a biomarker for NAFLD in T2DM patients.
Keywords: Non-alcoholic fatty liver disease, Telomere, Type 2 diabetes mellitus
Introduction
Non-alcoholic fatty liver disease (NAFLD) is the most frequent cause of liver disease,[1,2] and has a sharp rise in the prevalence in many parts of the world.[3,4] NAFLD and type 2 diabetes mellitus (T2DM) coexist frequently, since they share the common pathogenic characteristics of insulin resistance and excess adiposity.[5–7] Several studies suggest that the prevalence of NAFLD in T2DM may range from 29.6% to 87.1%.[8–13] T2DM may increase the risk of cirrhosis, hepatocellular carcinoma, and liver-related death.[14,15] Meanwhile, NAFLD may increase the risk of major adverse cardiac events and the overall mortality in T2DM.[16,17] These findings indicate that screening for NAFLD should be considered in T2DM patients, and biomarkers for screening of NAFLD to facilitate diagnosis are critical.
Telomeres are regions of repetitive G-rich DNA at the ends of eukaryotic chromosomes that protect the chromosomes tips from end-to-end fusion and degradation.[18] Leukocyte telomere length (LTL) is assumed to be a marker of chronic inflammation and systemic oxidative stress resulting in higher leukocyte turnover.[19] Previous cross-sectional clinical studies indicate that telomere length could be a useful indicator of risk for metabolic disease.[20] And it is suggested that shorter telomere length is related to components of metabolic dysfunction, such as insulin resistance, abdominal obesity, and hypertension.[19] Shorter telomere length also predicts development of insulin resistance and T2DM [20–22] and progression of the metabolic syndrome.[23] Telomere length is also linked to the NAFLD,[24] but there are conflicting findings related to telomere length and NAFLD. Aravinthan et al[25] demonstrated hepatic telomere length was shorter in NAFLD. Additionally, shorter telomere length in peripheral blood leukocytes was also reported in NAFLD.[26] However, one clinical study with a large population in USA indicated that telomere length was not associated with NAFLD after adjustment for confounders in the National Health and Nutrition Examination Survey. And shorter telomere length was only found in certain ethnical groups, such as the Mexican-origin population.[27] Furthermore, another study suggested that only the subjects aged 20 to 39 years with NAFLD had shorter telomere length, whereas elderly subjects with NAFLD had even longer telomere length.[28] Thus, the relationship between NAFLD and LTL is still ambiguous in particularly whether such relationship, could be affected by ethnicity and age. In the present study, we assess the association between LTL and NAFLD in Chinese T2DM patients.
Methods
Ethical approval
This study was approved by the ethics committee of Tongji Hospital. The procedures complied with the provisions of the Declaration of Helsinki. Informed consent has been obtained from patients.
Patients
Participants were T2DM patients from the Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology, between August 2012 and April 2017. Patients were excluded under the following conditions: (1) current drinkers and ex-drinkers, (2) virus hepatitis, (3) malignant neoplasm, (4) exposure history of radioactive substances, (5) auto-immune hepatitis, (6) treatment with systemic corticosteroids, (7) pregnancy, (8) drug-induced liver disease, (9) secondary diabetes, (10) Wilson disease, and (11) biliary obstructive diseases. A total of 240 Chinese Han ethnicity patients were included in this study. The age of patients ranged from 24 to 84 years. All 240 T2DM patients were divided into two groups, and one group consisted of 120 T2DM patients with NAFLD. The other consisted of 120 age-matched T2DM patients without NAFLD.
Terminal restriction fragment (TRF) length
AxyPrep Blood Genomic DNA Miniprep kit (Axygen, Corning, Inc., NY, USA) was used for blood genomic DNA extraction. The concentrations of DNA samples were measured by Nanodrop spectrophotometer (NanoDrop2000; Thermo Scientific, Carlsbad, CA, USA). Genomic DNA was digested by Hinf I (R0155L; New England Bio Labs, Beverly, MA, USA) and RsaI (R0167L; New England Bio Labs). Then, agarose gel electrophoresis was applied to separate the digested DNA. After that, the gel was denatured. The gel was neutralized after drying. Then, the gel was pre-hybridized with pre-hybridization solution. A 32P-labeled telomeric probe was used to detect telomeres. After the gel was washed, it was then exposed to a phosphor imager (Storm 860 Molecular Dynamics, Sunnyvale, CA, USA) and scanned with a Typhoon scanners (FLA 9500; GE Healthcare, Pittsburgh, PA, USA). The weighted mean telomere length was calculated.[29]
Clinical diagnosis for NAFLD
Guideline for diagnosis of NAFLD was in accordance with the Asia-Pacific Working Party.[30] NAFLD was determined by ultrasonography. Ultrasonography was carried out by senior radiologists (each having more than 10 years’ experience). Hepatic steatosis was estimated by the increase of hepatic-renal echo-intensity ratio.
Statistical analysis
All data analysis was performed using SPSS (version 22.0; SPSS Inc., Chicago, IL, USA). Distribution of the continuous variables was carried out by Kolmogorov-Smirnov test. Normally distributed data were expressed as the mean ± standard deviation, and data with non-normal distributions were expressed as median (interquartile range). Student's t test was used to test the difference between means of normally distributed data, and the Mann-Whitney U test was applied for data that were not normally distributed. Categorical variables were presented as n and were compared by the Chi-square test. Pearson correlation analysis was performed to examine the relationship between LTL and normally distributed parameters, and Spearman correlation was used to test the association between LTL and non-normally distributed parameters. Multiple logistic regressions were performed with relevant variables that were significantly different between T2DM patients with and without NAFLD. The analysis of variance trend analysis with polynomial contrast was used to estimate the relationship between the LTL and diabetes duration. The accuracy of LTL as biomarker for NAFLD in T2DM patients was calculated by area under the receiver operating characteristic (ROC) curve (AUC). A value of P < 0.05 was considered statistically significant.
Results
Characteristics of patients
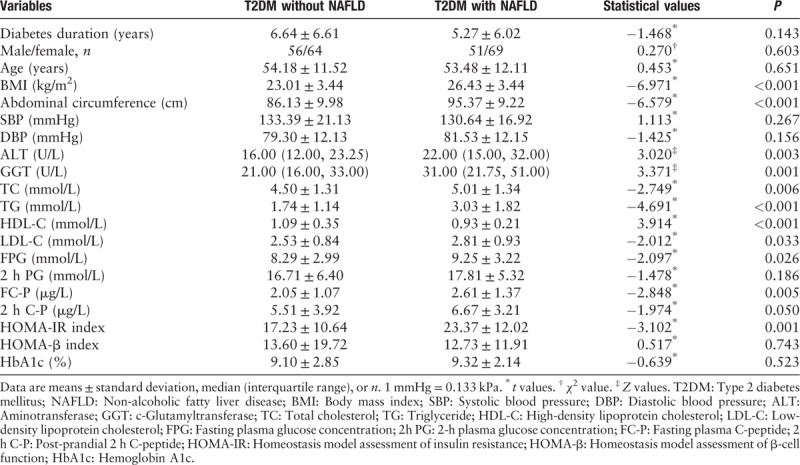
General characteristics of the patients in the study are summarized in Table 1. There was no statistical difference in age between T2DM patients with or without NAFLD. T2DM patients with NAFLD had higher body mass index (BMI) and abdominal circumference than those without (all P < 0.001). Aminotransferase (ALT), c-glutamyltransferase (GGT), total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), fasting plasma glucose (FPG), fasting plasma C-peptide (FC-P), and homeostasis model assessment of insulin resistance (HOMA-IR) index in T2DM patients with NAFLD were significantly higher compared to those in T2DM subjects without NAFLD (all P < 0.05). After adjustment for age, BMI, abdominal circumference, serum levels of ALT, GGT, TC, TG, FPG, and FC-P, and HOMA-IR index in T2DM patients with NAFLD were still significantly higher than those in T2DM patients without NAFLD (all P < 0.05) (data not shown). By contrast, high-density lipoprotein cholesterol (HDL-C) in T2DM patients with NAFLD was significantly lower than that in T2DM patients without NAFLD (P < 0.01).
Table 1.
Anthropometric and biochemical variables of participants.
Leukocyte telomere length in T2DM patients with and without NAFLD
For patients in this study, LTL of patients ranged from 4502 to 8993 base pairs (bp). After adjustment for age, LTL in T2DM patients with NAFLD (6400.2 ± 71.8 bp) was significantly longer than that in T2DM patients without NAFLD (6023.7 ± 49.5 bp) (P < 0.001). In addition, T2DM patients with NAFLD had longer LTL than those without NAFLD within all age groups (20–39 years, 40–59 years, and ≥60 years) [Figure 1].
Figure 1.
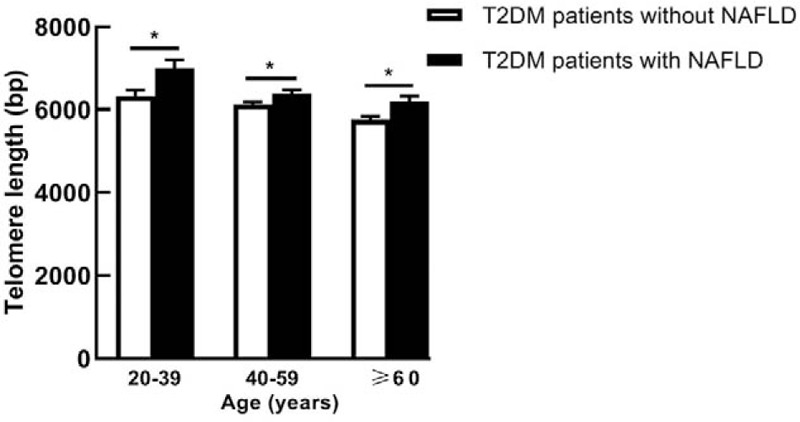
Leukocyte telomere length in T2DM patients with and without NAFLD of different age subgroups. Leukocyte telomere length is shown as mean ± standard error. ∗P < 0.05. bp: Base pairs; NAFLD: Non-alcoholic fatty liver disease; T2DM: Type 2 diabetes mellitus.
Association of LTL with age and diabetes duration
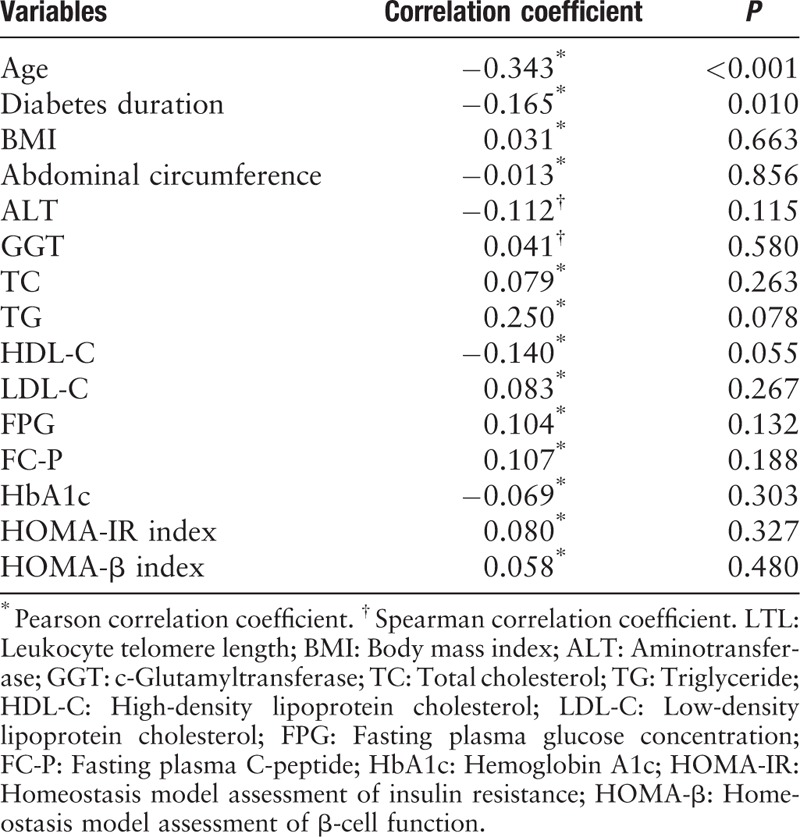
We next investigated the correlation between LTL and a cluster of anthropometric and biochemical parameters by Pearson correlation or Spearman correlation analysis [Table 2]. The analysis indicated a statistically significant negative association of LTL with age (r = −0.343, P < 0.001) and diabetes duration (r = −0.165, P = 0.010).
Table 2.
Correlations of LTL with anthropometric and biochemical variables in all patients.
Relationship between diabetes duration and LTL
To further explore the effect of diabetes duration on LTL, both T2DM patients with and without NAFLD were stratified into four subgroups by diabetes duration (<2 years, 2–4.9 years, 5–8 years, and >8 years). T2DM patient with NAFLD had significantly longer LTL than those without NAFLD when diabetes duration was less than two years (P < 0.005) [Figure 2A]. Notably, we observed that the trend of shorter LTL was associated with the increased diabetes duration in T2DM patient with NAFLD, but not in T2DM patients without NAFLD [Figure 2B and 2C].
Figure 2.
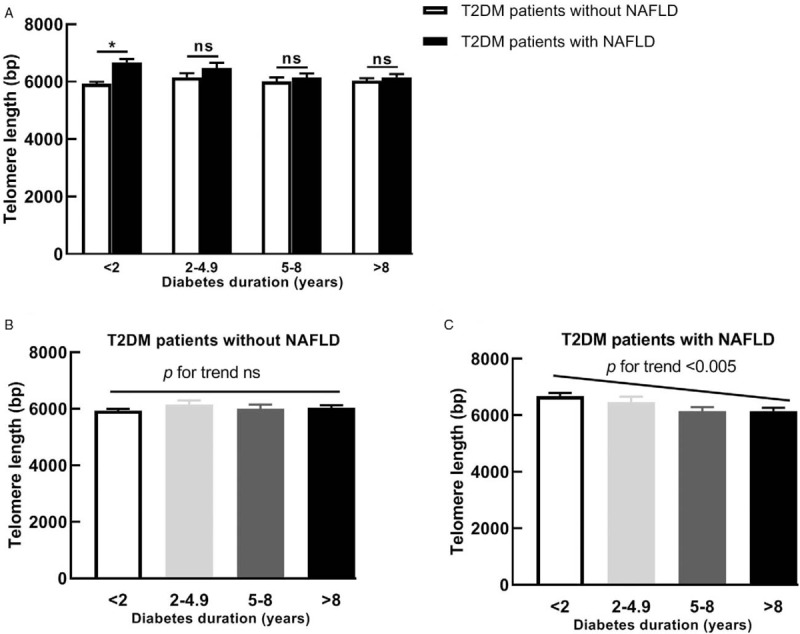
Leukocyte telomere length in T2DM patients with and without NAFLD of different diabetes duration subgroups. Comparison of Leukocyte telomere length between the two groups (A); Leukocyte telomere length in T2DM patients without NAFLD group (B) and T2DM patients with NAFLD group (C). Leukocyte telomere length is presented as the mean ± standard error. ∗P < 0.005. bp: Base pairs; NAFLD: Non-alcoholic fatty liver disease; ns: Non-significant; T2DM: Type 2 diabetes mellitus.
Leukocyte telomere length is an independent factor for NAFLD in T2DM patients
Factors for NAFLD in T2DM patients were identified using multiple logistic regressions that included BMI, abdominal circumference, ALT, GGT, TC, TG, LDL-C, HDL, FPG, FC-P, and LTL, since these variables were significantly different between T2DM patients with and without NAFLD. LTL significantly associated with NAFLD in T2DM patients (odds ratio [OR]: 1.001, 95% confidence interval [CI]: 1.000–1.002, P = 0.001), together with BMI (OR: 1.314, 95% CI: 1.169–1.477, P < 0.001), and TG (OR: 1.984, 95% CI: 1.432–2.747, P < 0.001). After adjusting for HOMA-IR values which represent the extent of insulin resistance, LTL, BMI, and TG were still significantly associated with NAFLD.
ROC curves for NAFLD and comparison of AUCs
The ROC curves shown in Figure 3 represented the diagnostic accuracy of the LTL and telomere model (LTL + BMI + TG) for NAFLD. The area under the ROC curve AUC was 0.860 (95% CI: 0.804–0.917) for the telomere model and 0.654 (95% CI: 0.573–0.734) for LTL alone. The AUC of the telomere model was larger than that of LTL alone (P < 0.001). Setting cut-off value at 6346.22 bp as calculated by the Youden index, sensitivity of LTL as biomarker for NAFLD in T2DM patients was 51.7% and specificity was 75.0%.
Figure 3.
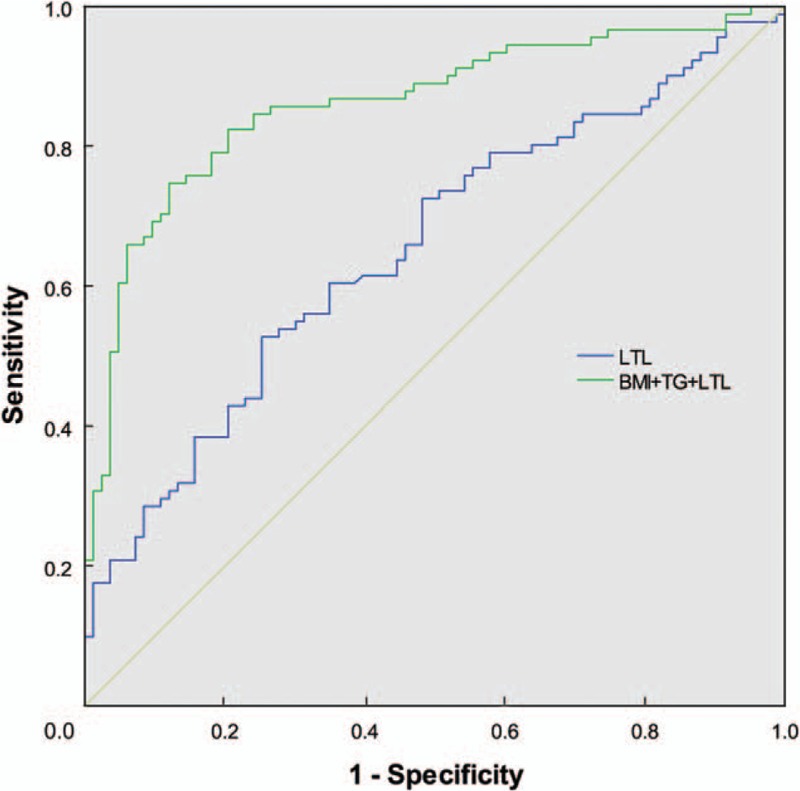
ROC curves for diagnosis of NAFLD and comparison of AUCs. BMI + TG + LTL, AUC = 0.860 (95% CI: 0.804–0.917); LTL, AUC = 0.654 (95% CI: 0.573–0.734); BMI + TG + LTL vs. LTL, P < 0.001. AUC: Area under the receiver operating characteristic curve; BMI: Body mass index; CI: Confidence interval; LTL: Leukocyte telomere length; ROC: Receiver operating characteristic; TG: Triglyceride.
Discussion
The present cross-sectional study indicated that T2DM patients with NAFLD had significantly longer LTL at the early stage of T2DM, and LTL was negatively associated with age and diabetes duration. The trend of shorter LTL was associated with the increased diabetes duration in T2DM patient with NAFLD, but not in T2DM patients without NAFLD. In addition, LTL was significantly associated with the NAFLD status in T2DM patients.
Our results identified that T2DM patients with NAFLD had significantly longer LTL. Agreement with recent report by Kim et al,[28] they found that NAFLD had a longer LTL in 2419 elderly participants (aged over 60 years). Indeed, NAFLD had longer LTL within all age groups in our study when the T2DM patients were stratified by age (20–39 years, 40–59 years, and ≥ 60 years). The common feature of T2DM patients and elderly subjects were insulin resistance. Therefore, these two studies may suggest that NALFD presented with longer LTL under severe insulin resistance condition. Other studies had found that NAFLD had shorter LTL, which were only observed in the USA adults (aged 20–39 years)[28] or Mexican-origin population.[27] This suggests that the LTL in NAFLD might partially depend on ethnicity and age.
In terms of NAFLD, the mechanism linking LTL with NAFLD is still unclear. Although there is evidence that short LTL is closely related to systemic oxidative stress and chronic inflammation, which were pathogenic characteristics of NAFLD, longer LTL was also observed in some other oxidative stress and inflammation-related diseases, such as chronic pancreatitis,[31] hepatitis B virus-related hepatocellular carcinoma,[32] and telomeres in visceral adipose tissue were longer even in T2DM patients.[33] In addition, LTL had been shown to increase with time in obese patients with impaired glucose.[34] Therefore, longer LTL seemed to be often associated with metabolic diseases. Our findings that longer LTL was negatively associated with age and diabetes duration were in line with previous studies,[35,36] although the association of LTL and diabetes duration lost statistical significance after adjustment for age. Notably, in this study we could not find any evidence that LTL was correlated with insulin secretion and sensitivity. Although our results may be due to the fact that subjects in our study had severe insulin resistance, our results were consistent with a previous study which also identified that LTL was not associated with insulin resistance in cross-sectional study, and LTL is more likely to predict insulin resistance later in life.[22]
We also found that NAFLD presented with significantly longer LTL mainly in the early stage of diabetes. Interestingly, another study by Fan et al[37] showed that T2DM patients who developed NAFLD had shorter LTL at the last follow-up, but their baseline LTL was longer in T2DM patients who tended to develop NAFLD later (the differences were not statistically significant possibly due to a small sample size in their study). In addition, T2DM patients tended to develop NAFLD in that study also had shorter diabetes duration.[37] Based on these observations, we hypothesized the following scenarios: (1) T2DM patients with NAFLD had a longer LTL in the early the stage of T2DM; (2) the shortening rate of LTL in T2DM patients with NAFLD was higher than those without with the extension of diabetes, thus T2DM patients with NAFLD may finally have shorter LTL. If our hypotheses are true, longer LTL might be a useful biomarker for early diagnosis and intervention of NAFLD in T2DM patients, which might be more important than shorter LTL just as the indicator in later stage of NAFLD.
Our results also suggested that telomere length was associated with the NAFLD status in addition to BMI and TG in T2DM patients. A recent study revealed that BMI was related to the long-term prognosis in NAFLD patients.[38] Hypertriglyceridemia was also associated with NAFLD.[39,40] Subjects with metabolic syndrome tended to develop NAFLD.[41] HOMA-IR index was also considered as risk factor for NAFLD.[42] In this study, HOMA-IR index was included into the logistic regression analyses, which was in agreement with our previous report.[43] Telomere length was shown as a biomarker of NAFLD, which was independent of higher BMI, TG, and insulin resistance.
We would like to underline the strengths and limitations of the study. First, our study uncovered that the positive correlation between longer LTL and NAFLD at the early stage of T2DM. It may provide a clinically feasible, safe, and effective method to detect NAFLD. Second, TRF length analysis was applied in our study to estimate LTL, which is a Southern blot-based method and is considered as a gold standard. This method is more accurate and repeatable compare to quantitative polymerase chain reaction in other studies. More importantly, TRF analysis provided an exact value of LTL. Third, ultrasonography was used to diagnose NAFLD in our study. The previous studies [27,28] that aimed to examine the relationship between LTL and NAFLD defined NAFLD according to high serum ALT level, which may result in misdiagnosis of NAFLD.
The limitations of our study include the follows. First, our study lacked a population-based healthy control group and patients only with NAFLD. Second, our study did not apply more accurate NAFLD diagnosis methods such as computed tomography scan, magnetic resonance imaging (MRI), or biopsy-proven steatosis. However, liver biopsy, considered the gold standard for NAFLD, had been recommended only in NAFLD patients tended to develop non-alcoholic steatohepatitis because of invasive nature.[44] MRI gave the highest precision to detect hepatic steatosis, but was time-consuming and expensive.[45–47] Therefore, ultrasonography was still the most widely available test due to safety, low cost, and accessibility, and with acceptable sensitivity and specificity.[48,49] Third, our study is cross-sectional and longitudinal studies are essential to evaluate the role of telomere length as a potential predictor to assess pathogenesis of NAFLD in T2DM patients.
In summary, our findings may indicate a critical role of longer LTL as an early response to the metabolic stress induced by NAFLD in T2DM patients. In addition, our results suggest that LTL may play a role in the detection of NAFLD in T2DM patients. Further studies are necessary to explore the mechanism linking longer LTL and NAFLD in T2DM patients.
Funding
This work was supported by grants from the National Natural Science Foundation of China (Nos. 81670754 and 81600661).
Conflicts of interest
None.
Footnotes
How to cite this article: Zhang M, Hu ML, Huang JJ, Xia SS, Yang Y, Dong K. Association of leukocyte telomere length with non-alcoholic fatty liver disease in patients with type 2 diabetes. Chin Med J 2019;132:2927–2933. doi: 10.1097/CM9.0000000000000559
References
- 1.Younossi ZM, Stepanova M, Afendy M, Fang Y, Younossi Y, Mir H, et al. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol 2011; 9:9524–9530.. doi: 10.1016/j.cgh.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 2.Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2018; 15:11–20.. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 3.Ruhl CE, Everhart JE. Fatty liver indices in the multiethnic United States National Health and Nutrition Examination Survey. Aliment Pharmacol Ther 2015; 41:65–76.. doi: 10.1111/apt.13012. [DOI] [PubMed] [Google Scholar]
- 4.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016; 64:73–84.. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 5.Forlani G, Giorda C, Manti R, Mazzella N, De Cosmo S, Rossi MC, et al. The burden of NAFLD and its characteristics in a nationwide population with type 2 diabetes. J Diabetes Res 2016; 2016:2931985.doi: 10.1155/2016/2931985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes 2001; 50:1844–1850.. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- 7.Tilg H, Moschen AR. Insulin resistance, inflammation, and non-alcoholic fatty liver disease. Trends Endocrinol Metab 2008; 19:371–379.. doi: 10.1016/j.tem.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Dai W, Ye L, Liu A, Wen SW, Deng J, Wu X, et al. Prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus: a meta-analysis. Medicine 2017; 96:e8179.doi: 10.1097/MD.0000000000008179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ortiz-Lopez C, Lomonaco R, Orsak B, Finch J, Chang Z, Kochunov VG, et al. Prevalence of prediabetes and diabetes and metabolic profile of patients with nonalcoholic fatty liver disease (NAFLD). Diabetes Care 2012; 35:873–878.. doi: 10.2337/dc11-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lazo M, Hernaez R, Eberhardt MS, Bonekamp S, Kamel I, Guallar E, et al. Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988–1994. Am J Epidemiol 2013; 178:38–45.. doi: 10.1093/aje/kws448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williamson RM, Price JF, Glancy S, Perry E, Nee LD, Hayes PC, et al. Prevalence of and risk factors for hepatic steatosis and nonalcoholic fatty liver disease in people with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Diabetes Care 2011; 34:1139–1144.. doi: 10.2337/dc10-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schindhelm RK, Heine RJ, Diamant M. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care 2007; 30:e94.doi: 10.2337/dc07-0982. [DOI] [PubMed] [Google Scholar]
- 13.Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology 2011; 140:124–131.. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 14.Kim D, Kim WR, Kim HJ, Therneau TM. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology 2013; 57:1357–1365.. doi: 10.1002/hep.26156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Portillo-Sanchez P, Bril F, Maximos M, Lomonaco R, Biernacki D, Orsak B, et al. High prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus and normal plasma aminotransferase levels. J Clin Endocrinol Metab 2015; 100:2231–2238.. doi: 10.1210/jc.2015-1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Targher G, Bertolini L, Rodella S, Tessari R, Zenari L, Lippi G, et al. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular events in type 2 diabetic patients. Diabetes Care 2007; 30:2119–2121.. doi: 10.2337/dc07-0349. [DOI] [PubMed] [Google Scholar]
- 17.Targher G, Bertolini L, Rodella S, Zoppini G, Lippi G, Day C, et al. Non-alcoholic fatty liver disease is independently associated with an increased prevalence of chronic kidney disease and proliferative/laser-treated retinopathy in type 2 diabetic patients. Diabetologia 2008; 51:444–450.. doi: 10.1007/s00125-007-0897-4. [DOI] [PubMed] [Google Scholar]
- 18.Blackburn EH. Structure and function of telomeres. Nature 1991; 350:569–573.. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- 19.Demissie S, Levy D, Benjamin EJ, Cupples LA, Gardner JP, Herbert A, et al. Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham Heart Study. Aging cell 2006; 5:325–330.. doi: 10.1111/j.1474-9726.2006.00224.x. [DOI] [PubMed] [Google Scholar]
- 20.Tamura Y, Takubo K, Aida J, Araki A, Ito H. Telomere attrition and diabetes mellitus. Geriatr Gerontol Int 2016; 16: Suppl 1: 66–74.. doi: 10.1111/ggi.12738. [DOI] [PubMed] [Google Scholar]
- 21.Gardner JP, Li S, Srinivasan SR, Chen W, Kimura M, Lu X, et al. Rise in insulin resistance is associated with escalated telomere attrition. Circulation 2005; 111:2171–2177.. doi: 10.1161/01.CIR.0000163550.70487.0B. [DOI] [PubMed] [Google Scholar]
- 22.Verhulst S, Dalgard C, Labat C, Kark JD, Kimura M, Christensen K, et al. A short leucocyte telomere length is associated with development of insulin resistance. Diabetologia 2016; 59:1258–1265.. doi: 10.1007/s00125-016-3915-6. [DOI] [PubMed] [Google Scholar]
- 23.Revesz D, Verhoeven JE, Picard M, Lin J, Sidney S, Epel ES, et al. Associations between cellular aging markers and metabolic syndrome: findings from the CARDIA study. J Clin Endocrinol Metab 2018; 103:148–157.. doi: 10.1210/jc.2017-01625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donati B, Valenti L. Telomeres, NAFLD and chronic liver disease. Int J Mol Sci 2016; 17:383.doi: 10.3390/ijms17030383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aravinthan A, Scarpini C, Tachtatzis P, Verma S, Penrhyn-Lowe S, Harvey R, et al. Hepatocyte senescence predicts progression in non-alcohol-related fatty liver disease. J Hepatol 2013; 58:549–556.. doi: 10.1016/j.jhep.2012.10.031. [DOI] [PubMed] [Google Scholar]
- 26.Laish I, Mannasse-Green B, Hadary R, Biron-Shental T, Konikoff FM, Amiel A, et al. Telomere dysfunction in nonalcoholic fatty liver disease and cryptogenic cirrhosis. Cytogenet Genome Res 2016; 150:93–99.. doi: 10.1159/000454654. [DOI] [PubMed] [Google Scholar]
- 27.Wojcicki JM, Rehkopf D, Epel E, Rosenthal P. Shorter leukocyte telomere length in relation to presumed nonalcoholic fatty liver disease in Mexican-American Men in NHANES 1999–2002. Int J Hepatol 2017; 2017:8435178.doi: 10.1155/2017/8435178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim D, Li AA, Ahmed A. Leucocyte telomere shortening is associated with nonalcoholic fatty liver disease-related advanced fibrosis. Liver Int 2018; 38:1839–1848.. doi: 10.1111/liv.13886. [DOI] [PubMed] [Google Scholar]
- 29.Liu J, Ge Y, Wu S, Ma D, Xu W, Zhang Y, et al. Association between antidiabetic agents use and leukocyte telomere shortening rates in patients with type 2 diabetes. Aging (Albany NY) 2019; 11:741–755.. doi: 10.18632/aging.101781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chitturi S, Farrell GC, Hashimoto E, Saibara T, Lau GK, Sollano JD. Non-alcoholic fatty liver disease in the Asia-Pacific region: definitions and overview of proposed guidelines. J Gastroenterol Hepatol 2007; 22:778–787.. doi: 10.1111/j.1440-1746.2007.05001.x. [DOI] [PubMed] [Google Scholar]
- 31.Wu H, Zou WB, Zhou DZ, Gu YT, Liao Z, Li ZS. Longer leukocyte telomere length is associated with an increased risk of chronic pancreatitis. Pancreas 2017; 46:e65–e66.. doi: 10.1097/MPA.0000000000000887. [DOI] [PubMed] [Google Scholar]
- 32.Liu J, Yang Y, Zhang H, Zhao S, Liu H, Ge N, et al. Longer leukocyte telomere length predicts increased risk of hepatitis B virus-related hepatocellular carcinoma: a case-control analysis. Cancer 2011; 117:4247–4256.. doi: 10.1002/cncr.26015. [DOI] [PubMed] [Google Scholar]
- 33.Jones DA, Prior SL, Barry JD, Caplin S, Baxter JN, Stephens JW. Changes in markers of oxidative stress and DNA damage in human visceral adipose tissue from subjects with obesity and type 2 diabetes. Diabetes Res Clin Pract 2014; 106:627–633.. doi: 10.1016/j.diabres.2014.09.054. [DOI] [PubMed] [Google Scholar]
- 34.Hovatta I, de Mello VD, Kananen L, Lindstrom J, Eriksson JG, Ilanne-Parikka P, et al. Leukocyte telomere length in the Finnish Diabetes Prevention Study. PloS One 2012; 7:e34948.doi: 10.1371/journal.pone.0034948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murillo-Ortiz B, Albarran-Tamayo F, Arenas-Aranda D, Benitez-Bribiesca L, Malacara-Hernandez JM, Martinez-Garza S, et al. Telomere length and type 2 diabetes in males, a premature aging syndrome. Aging Male 2012; 15:54–58.. doi: 10.3109/13685538.2011.593658. [DOI] [PubMed] [Google Scholar]
- 36.Gurung RL, Yiamunaa M, Liu S, Liu JJ, Chan SM, Moh MC, et al. Ethnic disparities in relationships of obesity indices with telomere length in Asians with type 2 diabetes. J Diabetes 2019; 11:386–393.. doi: 10.1111/1753-0407.12864. [DOI] [PubMed] [Google Scholar]
- 37.Ping F, Li ZY, Lv K, Zhou MC, Dong YX, Sun Q, et al. Deoxyribonucleic acid telomere length shortening can predict the incidence of non-alcoholic fatty liver disease in patients with type 2 diabetes mellitus. J Diabetes Investig 2017; 8:174–180.. doi: 10.1111/jdi.12555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu FB, Hu ED, Xu LM, Chen L, Wu JL, Li H, et al. The relationship between obesity and the severity of non-alcoholic fatty liver disease: systematic review and meta-analysis 2018; 12:491–502.. doi: 10.1080/17474124.2018.1460202. [DOI] [PubMed] [Google Scholar]
- 39.Liao XH, Cao X, Liu J, Xie XH, Sun YH, Zhong BH. Prevalence and features of fatty liver detected by physical examination in Guangzhou. World J Gastroenterol 2013; 19:5334–5339.. doi: 10.3748/wjg.v19.i32.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang H, He SM, Sun J, Wang C, Jiang YF, Gu Q, et al. Prevalence and etiology of abnormal liver tests in an adult population in Jilin, China. Int J Med Sci 2011; 8:254–262.. doi: 10.7150/ijms.8.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hamaguchi M, Kojima T, Takeda N, Nakagawa T, Taniguchi H, Fujii K, et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med 2005; 143:722–728.. doi: 10.7326/0003-4819-143-10-200511150-00009. [DOI] [PubMed] [Google Scholar]
- 42.Zelber-Sagi S, Lotan R, Shlomai A, Webb M, Harrari G, Buch A, et al. Predictors for incidence and remission of NAFLD in the general population during a seven-year prospective follow-up. J Hepatol 2012; 56:1145–1151.. doi: 10.1016/j.jhep.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 43.Li H, Dong K, Fang Q, Hou X, Zhou M, Bao Y, et al. High serum level of fibroblast growth factor 21 is an independent predictor of non-alcoholic fatty liver disease: a 3-year prospective study in China. J Hepatol 2013; 58:557–563.. doi: 10.1016/j.jhep.2012.10.029. [DOI] [PubMed] [Google Scholar]
- 44.Kamal S, Khan MA, Seth A, Cholankeril G, Gupta D, Singh U, et al. Beneficial effects of statins on the rates of hepatic fibrosis, hepatic decompensation, and mortality in chronic liver disease: a systematic review and meta-analysis. Am J Gastroenterol 2017; 112:1495–1505.. doi: 10.1038/ajg.2017.170. [DOI] [PubMed] [Google Scholar]
- 45.Noureddin M, Lam J, Peterson MR, Middleton M, Hamilton G, Le TA, et al. Utility of magnetic resonance imaging versus histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials. Hepatology 2013; 58:1930–1940.. doi: 10.1002/hep.26455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Le Y, Kroeker R, Kipfer HD, Lin C. Development and evaluation of TWIST Dixon for dynamic contrast-enhanced (DCE) MRI with improved acquisition efficiency and fat suppression. J Magn Reson Imaging 2012; 36:483–491.. doi: 10.1002/jmri.23663. [DOI] [PubMed] [Google Scholar]
- 47.Schwenzer NF, Springer F, Schraml C, Stefan N, Machann J, Schick F. Non-invasive assessment and quantification of liver steatosis by ultrasound, computed tomography and magnetic resonance. J Hepatol 2009; 51:433–445.. doi: 10.1016/j.jhep.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 48.Hernaez R, Lazo M, Bonekamp S, Kamel I, Brancati FL, Guallar E, et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology 2011; 54:1082–1090.. doi: 10.1002/hep.24452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goulart AC, Oliveira IR, Alencar AP, Santos MS, Santos IS, Martines BM, et al. Diagnostic accuracy of a noninvasive hepatic ultrasound score for non-alcoholic fatty liver disease (NAFLD) in the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Sao Paulo Med J 2015; 133:115–124.. doi: 10.1590/1516-3180.2014.9150812. [DOI] [PMC free article] [PubMed] [Google Scholar]


