To the Editor: A 37-year-old Chinese woman, a non-smoker, presented with adenocarcinoma of the left lung (stage IV) [Figure 1A], malignant pleural effusion, and multiple bone metastases in December 2014. Deoxyribonucleic acid sequencing of the tumor biopsy revealed an epidermal growth factor receptor (EGFR) exon 19 deletion, and treatment with gefitinib was consequently initiated in December 2014. There were no treatment-related side effects except for a grade 1 rash, and stable disease was achieved. Unfortunately, the patient did not experience long-term benefits and developed disease progression after only two months. A follow-up examination showed that extracranial disease and a small intracranial lesion had developed gradually. The patient then received whole-brain radiotherapy (30 Gy in ten fractions) and four cycles of single-agent pemetrexed in April 2015. Stable disease was achieved, which coincided with an improvement in the patient's cough, shortness of breath, and general condition. The chemotherapy course was changed to two cycles of pemetrexed combined with cisplatin. A partial response (PR) was achieved, and the patient was treated with 14 cycles of pemetrexed alone as maintenance chemotherapy. The follow-up evaluations showed the patient's condition to be stable; however, the patient's disease progressed after 23 months. A computed tomography (CT) scan in March 2017 revealed that both the lung lesion and the malignant pleural effusion had increased in size [Figure 1B]. The subsequent biopsy specimen was subjected to next-generation sequencing (NGS) and a Syndecan 4-c-ros oncogene 1 (SDC4-ROS1) rearrangement was detected [Figure 1C]. The patient then received crizotinib in April 2017, and a PR was achieved [Figure 1D]. A CT scan done in August 2018 indicated the progression of the primary lesion in the left lung and malignant pleural effusion. However, the growth of the remaining lesions remained stable.
Figure 1.
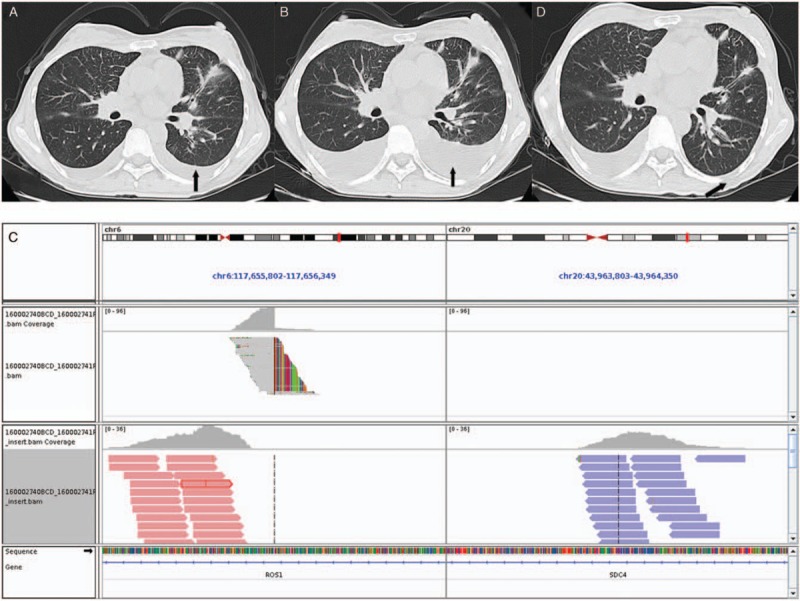
Representative image of the patient. (A) CT scans of adenocarcinoma of the left lung, malignant pleural effusion. (B) CT revealed that the lung lesion and the malignant pleural effusion had grown. SDC4-ROS1 fusion is clinically actionable. (C) An Integrative Genomics Viewer snapshot of SDC4-ROS1, respectively. Soft-clipped bases reverse complement each other. (D) Follow-up CT scan of the chest showing a partial response and disappearance of the malignant pleural effusion after 1 month of crizotinib. CT: Computed tomography; SDC4-ROS1: Syndecan 4-c-ros oncogene 1.
EGFR genomic aberrations in lung cancer mostly occur in the intracellular-coding domain (exon 18–21), including exon 19 deletions and the Leu858Arg (L858R) point mutation in exon 21, which accounts for up to 90% of all EGFR mutations in the clinic.[1] Compared with traditional chemotherapy, EGFR-tyrosine kinase inhibitor (TKI) targeted therapy has several advantages and has become an effective treatment for advanced non-small-cell lung cancer (NSCLC) patients with specific EGFR mutations. However, primary and acquired drug resistance inevitably make targeted therapy treatment for lung cancer difficult. In the study, we did not detect additional resistance mechanisms to first- or second-generation EGFR-TKIs, such as an EGFR (Thr790Met) T790M mutation, human epidermal growth factor receptor-2 amplification, phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha mutation, mesenchymal-epithelial transition amplification, and small cell transformation. Therefore, this report indicates that the SDC4-ROS1 rearrangement may function as a possible mechanism of acquired resistance to EGFR-TKIs in EGFR-mutant lung adenocarcinomas (LADCs).
The ROS1 gene was first identified as an oncogenic sequence in the avian sarcoma virus (UR2) in 1982. ROS1 is a proto-oncogene highly expressed in multiple tumor cell lines. Genomic aberrations of the ROS1 gene lead to the dissonance of ROS1 proteins and can activate multiple downstream oncogenic signaling pathways including phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin, Signal transducer and activator of transcription 3, rat sarcoma viral oncogene/mitogen-activated protein kinase/extracellular regulated protein kinases, VAV gene family 3, and tyrosine phosphatase-1/2. The first ROS1 rearrangement detected in NSCLC was reported by Rikova et al[2] in 2007. Since then, 52 different ROS1 rearrangements have been identified in NSCLC, including cluster of differentiation74-syndecan 4-ROS1 (CD74-ROS1), solute carrier family 34 member 2-syndecan 4-ROS1, ezrin-syndecan 4-ROS1, SDC4-ROS1, tropomyosin 3-ROS1, golgi associated PDZ and coiled-coil motif containing-ROS1 (GOPC-ROS1), leucine rich repeats and immunoglobulin like domains 3-syndecan 4-ROS1, Homo sapiens fidgetin like 1-syndecan 4-ROS1, trafficking from ER to golgi regulator-syndecan 4-ROS1, LIM domain and actin binding 1-syndecan 4-ROS1, Moesin-ROS1, clathrin heavy chain-syndecan 4-ROS1, TPD52 like 1-syndecan 4-ROS1, centrosomal protein 72-syndecan 4-ROS1, KDEL endoplasmic reticulum protein retention receptor 2-syndecan 4-ROS1, RNA binding region containing 3-syndecan 4-ROS1, zinc finger CCHC-type containing 8-syndecan 4-ROS1, myosin heavy chain 9-syndecan 4-ROS1, G protein-coupled receptor class C group 6 member A-syndecan 4-ROS1, regulatory factor X6-syndecan 4-ROS1, aquaporin 4-syndecan 4-ROS1, adhesion G protein-coupled receptor G6-syndecan 4-ROS1, tripartite motif containing 33-syndecan 4-ROS1, coiled-coil domain containing 6-ROS1, syndecan 4-ROS1-family with sequence similarity 135 member B, solute carrier family 6 member 17-syndecan 4-ROS1, ROS1-adhesion G protein-coupled receptor G6, myosin VA-syndecan 4-ROS1, myosin VC-syndecan 4-ROS1, catenin delta 2syndecan 4-ROS1, opioid receptor Mu 1-syndecan 4-ROS1, serine and arginine rich splicing factor 6-syndecan 4-ROS1, UncharacterizedLOC101927919-syndecan4-ROS1, breast cancer-associated transcript 107-syndecan 4-ROS1, osteoblast specific factor-ROS1, muscle RAS oncogene homolog-ROS1, adhesion G protein-coupled receptor V1-syndecan 4-ROS1, pumilio RNA binding family member 1-syndecan 4-ROS1, BTB domain containing 9-syndecan 4-ROS1, X-prolyl aminopeptidase 1-syndecan 4-ROS1, WNK lysine deficient protein kinase 1-syndecan 4-ROS1, PPFIA binding protein 1-syndecan 4-ROS1, PWWP domain containing 2A-syndecan 4-ROS1, ELKS/RAB6-interacting/CAST family member 1-ROS1, HLA-A-syndecan 4-ROS1, CAP-Gly domain containing linker protein 1-syndecan 4-ROS1, shootin 1-syndecan 4-ROS1, sperm flagellar 2-syndecan 4-ROS1, syndecan 4-ROS1-occludin, phosphatase and actin regulator 3syndecan 4-ROS1, HMG-box containing 3-syndecan 4-ROS1, and RAD18 E3 ubiquitin protein ligase-syndecan 4-ROS1. The CD74 gene has been identified as the most common fusion partner with ROS1, with 42% of ROS1 rearrangements involving CD74. NSCLC tumors with ROS1 rearrangements have similar features to tumors with an anaplastic lymphoma kinase (ALK) rearrangement, and ROS1 fusions are more frequent in female non-smokers. NSCLC tumors harboring ROS1 rearrangements can be sensitive to TKIs and pemetrexed-based chemotherapies.[3]
We present a rare report on the coexistence of an SDC4-ROS1 rearrangement and an activating EGFR mutation in NSCLC. Although the coexistence of two driver gene mutations in NSCLC is infrequent, reports have recently shown the coexistence of activating alterations of EGFR, ROS1, ALK, and kirsten rat sarcoma viral oncogene. One study reported a case of a LADC harboring a coexisting GOPC-ROS1 rearrangement and EGFR mutation, as detected by NGS. Zeng et al[4] reported a case of a LADC patient harboring an EGFR exon 19 deletion in the primary lesion and who received icotinib treatment. The patient acquired drug resistance after 14 months and the treatment was changed to osimertinib. Acquired drug resistance developed after 10 months. A rebiopsy showed the coexistence of a GOPC-ROS1 rearrangement and an EGFR exon 19 deletion. The patient then received osimertinib combined with crizotinib and a PR was achieved. The GOPC-ROS1 rearrangement might be a novel acquired resistance mechanism to EGFR-TKIs, and crizotinib proved to be effective in this case. In addition, Zhu et al[5] reported a LADC patient with concurrent EGFR exon 21 with an L858R point mutation, as well as a CD74-ROS1 rearrangement. However, because EGFR-TKIs were not prescribed to this patient, the patient's response to EGFR-TKIs is unknown.
In conclusion, this report provides the basis for the premise that an SDC4-ROS1 rearrangement might function as a potential mechanism of acquired resistance to EGFR-TKIs, and crizotinib will likely be an effective treatment strategy for patients with acquired resistance to EGFR-TKIs. For patients with this molecular subtype, more research is needed to explore optimal treatment regimens and to further understand the biologic characteristics of these tumors.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given her consent for her images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Funding
This study was supported by a grant from the Science and Technology Planning Project of Zhejiang Province (No. LGF19H160002).
Conflicts of interest
None.
Footnotes
How to cite this article: Zhu YC, Xu CW, Zhang QX, Wang WX, Lei L, Zhuang W. Syndecan 4-c-ros oncogene 1 fusion as a mechanism of acquired resistance in epidermal growth factor receptor mutant lung adenocarcinoma. Chin Med J 2019;132:3015–3017. doi: 10.1097/CM9.0000000000000555
You-Cai Zhu and Chun-Wei Xu contributed equally to this work.
References
- 1.Westover D, Zugazagoitia J, Cho BC, Lovly CM, Paz-Ares L. Mechanisms of acquired resistance to first- and second-generation EGFR tyrosine kinase inhibitors. Ann Oncol 2018; 29:i10–i19.. doi: 10.1093/annonc/mdx703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rikova K, Guo A, Zeng Q, Possemato A, Yu J, Haack H, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell 2007; 131:1190–1203.. doi: 1016/j.cell.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 3.Gainor JF, Shaw AT. Novel targets in non-small cell lung cancer: ROS1 and RET fusions. Oncologist 2013; 18:865–875.. doi: 10.1634/theoncologist.2013-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeng L, Yang N, Zhang Y. GOPC-ROS1 rearrangement as an acquired resistance mechanism to osimertinib and responding to crizotinib combined treatments in lung adenocarcinoma. J Thorac Oncol 2018; 13:e114–e116.. doi: 10.1016/j.jtho.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Zhu Y, Xu C, Ye X, Yin M, Zhang J, Du K, et al. Lung cancer with concurrent EGFR mutation and ROS1 rearrangement: a case report and review of the literature. Onco Targets Ther 2016; 9:4301–4305.. doi: 10.2147/OTT.S109415. [DOI] [PMC free article] [PubMed] [Google Scholar]


