Supplemental Digital Content is available in the text
Keywords: Pulmonary tuberculosis, Vitamin D, Adults, Meta-analysis
Abstract
Background:
Tuberculosis (TB) is one of the most debilitating diseases worldwide. Current studies have shown that vitamin D plays a significant role in host immune defense against Mycobacterium tuberculosis, but clinical trials reported inconsistent results. Therefore, we systematically reviewed the literature to investigate whether vitamin D supplementation could improve the effect of anti-TB therapy.
Methods:
We systematically searched PubMed, Embase, and the Cochrane Central Register of Controlled Trials from their inception to February 8th, 2019 for randomized controlled trials on vitamin D supplementation in patients with pulmonary TB receiving anti-TB therapy. The primary outcomes were time to sputum culture and smear conversion and proportion of participants with negative sputum culture. The secondary outcomes were clinical response to treatment and adverse events. A random-effects model was used to pool studies. Data were analyzed using RevMan 5.3 software.
Results:
Five studies with a total of 1126 participants were included in our meta-analysis. Vitamin D supplementation did not shorten the time to sputum culture and smear conversion (hazard ratio [HR] 1.04, 95% confidence interval [CI] 0.89–1.23, P = 0.60; HR 1.15, 95% CI 0.93–1.41, P = 0.20, respectively) and did not lead to an increase in the proportion of participants with negative sputum culture (relative risk [RR] 1.04, 95% CI 0.97–1.11, P = 0.32). However, it reduced the time to sputum culture conversion in the sub-group of participants with TaqI tt genotype (HR 8.09, 95% CI 1.39–47.09, P = 0.02) and improved the multidrug-resistant (MDR) TB sputum culture conversion rate (RR 2.40, 95% CI 1.11–5.18, P = 0.03). There was no influence on secondary outcomes.
Conclusions:
Vitamin D supplementation had no beneficial effect on anti-TB treatment, but it reduced the time to sputum culture conversion in participants with tt genotype of the TaqI vitamin D receptor gene polymorphism and improved the MDR TB sputum culture conversion rate.
Introduction
Tuberculosis (TB) is an ancient human disease, which has existed for thousands of years since the origin and evolution of human beings.[1] The World Health Organization reported that TB remains one of the world's deadliest communicable diseases with 10.4 million incident cases and 1.674 million deaths in 2016, despite the advancement in disease control.[2] It was estimated that 490,000 cases developed into multidrug-resistant (MDR) TB in 2016.[2] Therefore, it is urgent to find a new anti-TB medication to control the global TB epidemic.
It is suggested that vitamin D deficiency (VDD) is associated with the development of active TB and could increase TB risk.[3–7] Patients with latent TB with VDD were more likely to exhibit disease progression.[8] Current evidence shows that vitamin D plays a significant role in host immune defense against Mycobacterium tuberculosis,[6,9–15] which has the potential to shorten the time of anti-microbial therapy in drug-sensitive disease or improve results in drug-resistant diseases.[16] Calcitriol, the active metabolite of vitamin D, can modulate immune responses by binding to vitamin D receptor (VDR) to regulate transcription of vitamin D-responsive genes.[17] Patients with pulmonary TB (PTB) with the TaqI Tt genotype, compared with those with the TT genotype, have more rapid sputum culture conversion.[18]
Therefore, it is crucial to determine whether vitamin D supplementation can benefit patients with PTB. There are several published clinical trials[19–27] and meta-analyses[28–30] on the effects of vitamin D as adjunctive therapy in patients with TB. However, the results are still inconsistent. Moreover, no meta-analysis conducted to date has primarily investigated the influence of TaqI VDR genotypes on responses to vitamin D supplementation in PTB and the effects of adjunctive vitamin D on adult TB treatment. Hence, we conducted an updated meta-analysis to investigate the effects of vitamin D supplementation on adult TB treatment.
Methods
Literature search
We systematically searched PubMed, Embase, and the Cochrane Central Register of Controlled Trials from their inception to February 8th, 2019 for randomized controlled trials (RCTs) using a combination of Medical Subject Headings or Emtree and related keywords in all fields. The keywords used were “tuberculosis” or “tuberculosis” or “Mycobacterium tuberculosis infection” and “vitamin D” or “cholecalciferol” or “calcifediol” or “calcitriol” or “ergocalciferol” or “25-hydroxyvitamin D” or “vitamin D2” or “vitamin D3.” We also scanned the reference lists of pertinent trials and key review articles to identify relevant studies.
Inclusion criteria
Studies meeting the following criteria were included: (1) participants: patients were aged ≥18 years with newly diagnosed PTB with sputum smear-positive result; (2) intervention: vitamin D3 or vitamin D supplementation was conducted within 7 days of anti-TB therapy initiation; (3) comparison: placebo was administered at the time of vitamin D supplementation; (4) outcomes: the primary outcomes were time to sputum culture conversion, time to sputum smear conversion, and proportion of participants with negative sputum culture. The secondary outcomes were body mass index (BMI), radiological examinations, and adverse events (hypercalcemia and mortality); and (5) design: studies were RCTs. If data were duplicated or shared in more than one study, the first published study was included in the meta-analysis. The language was restricted to English. Discrepancies regarding study inclusion between authors were resolved through discussion. We also conducted sub-group analyses of time to sputum culture conversion in participants with TaqI VDR polymorphism and MDR TB sputum culture conversion rate.
Data extraction and risk of bias assessment
Two of the authors (JZ and CC) independently evaluated the eligibility of all studies obtained from the databases according to the above selection criteria. We extracted data in the placebo and vitamin D arms. Disagreements between authors were resolved by discussion. The following data were extracted from the studies: study name (name of the first author with the publication year), country and design, participants (sample size, sex, and age), intervention arms and controls (intervention drug and dose, follow-up duration, and anti-TB therapy protocol), and outcomes (primary and secondary outcomes). The Cochrane Collaboration's tool for assessing risk of bias was used for each RCT, which includes the following criteria: adequacy of sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, incomplete outcome data, selective reporting, and other biases.[31] We also used the grading of recommendations assessment, development and evaluation (GRADE) system to rate the quality of evidence from our meta-analysis by using GRADEpro software which was supported by the Italian Ministry of Health and developed by the GRADE Working Group.
Statistical analysis
To evaluate the effect of vitamin D on PTB, we calculated relative risks (RRs) with 95% confidence intervals (95% CIs) for dichotomous outcomes. For continuous outcomes, mean differences (MDs) or standard mean differences between the experimental and control groups were combined. Time-to-event outcomes were analyzed using hazard ratios (HRs). The proportion of participants with negative sputum culture after treatment was evaluated separately using intention-to-treat (ITT) and per-protocol (PP) analyses, while other outcomes were analyzed by ITT analysis only because of the availability of data. Heterogeneity in results across studies was examined using the Cochran Q and I2 statistics.[32] The null hypothesis that the studies are homogeneous was rejected if the P value for heterogeneity was <0.10 or I2 was >50%. Studies with an I2 statistic >50% were considered to have significant heterogeneity. A random-effects model was used to pool studies.
A sensitivity analysis was conducted to assess the influence of individual studies on the pooled result when the P value was <0.10 or I2 was >50% by excluding each study one by one and recalculating the combined results of the remaining studies. To test for publication bias, we used a test for asymmetry of the funnel plot proposed by Egger et al[33] All data analyses were performed with Review Manager 5.3 (Cochrane Informatics and Knowledge Management Department, available from http://tech. cochrane.org/; UK).
Results
Study flow diagram
Figure 1 shows a flow diagram of the selection process. A total of 713 records were initially identified from the database search. Of these, 80 records were excluded for duplicates, and 621 records were excluded after screening the titles and abstracts. The remaining 12 full-text articles were assessed for eligibility, of which seven were further excluded because of the inclusive age of <18 years in the lower side,[19,20,22,27,34] presence of respiratory disease,[35] and inclusion of children[36] in the analysis. The remaining five RCTs[21,23–26] were included in the final meta-analysis.
Figure 1.
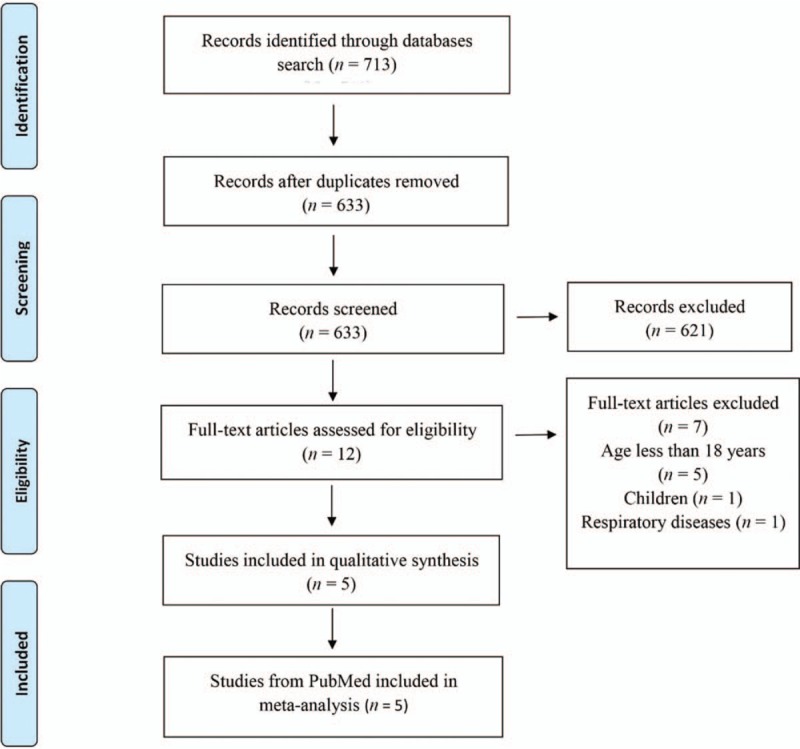
Study flow diagram. All studies were randomized controlled trials.
Characteristics of included studies
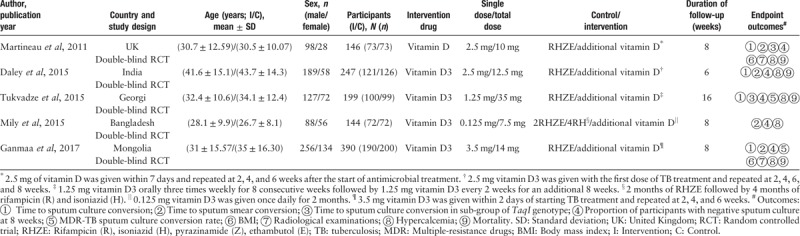
The characteristics of studies included in our meta-analysis are summarized in Table 1. Overall, the five trials enrolled 1126 patients with newly diagnosed TB who were predominantly male (758, 67.32%), and a total of 556 patients were administered vitamin D supplements, while 570 patients were administered placebo. The sample size varied from 144 to 390 participants. Two studies reported MDR TB.[24,25] Three RCTs[21,24,25] reported sputum culture conversion in VDR polymorphism, two[21,24] of which reported the TaqI genotype. The study by Mily et al[26] investigated the effect of phenylbutyrate (PBA) and vitamin D on PTB and reported the proportion of patients with TB who had negative culture at week 8 and time to sputum smear conversion, with three arms separately investigating the benefit of adding PBA or PBA + vitamin D or vitamin D in PTB treatment compared with placebo. Only the two arms exploring adjunctive vitamin D on PTB were included in the systematic review. The other four included trials had two arms: standard anti-TB therapy (including 2RHZE/4RH,[26] RHZE,[21,23–25] and appropriate treatment regimen for MDR TB[24,25]) plus vitamin D administration versus standard anti-TB therapy with placebo. All studies focused on adults with a mean age of 28.1 to 41.6 years and 26.7 to 43.7 years in intervention and control arms, respectively. The total doses of vitamin D3 varied from 7.5 to 35 mg and were all administered orally. The vitamin D doses and supplementation duration were different across studies: 2.5 mg within 7 days of anti-TB therapy initiation and repeated at 2, 4, and 6 weeks in Martineau et al's[21] study; 2.5 mg with the first dose of TB treatment and repeated at 2, 4, 6, and 8 weeks in Daley et al's[23] study; 1.25 mg three times weekly for 8 consecutive weeks followed by 1.25 mg every 2 weeks for additional 8 weeks in Tukvadze et al's[24] study; 0.125 mg/day for 2 months in Mily et al's[26] study; and 3.5 mg within 2 days of TB treatment initiation and repeated at 2, 4, and 6 weeks in Ganmaa et al's[25] study. Because almost all endpoints of the included trials were 8 weeks, all trials reported the outcomes at 8 weeks. To ensure consistency of the results, we extracted the data at 8 weeks.
Table 1.
Characteristics of included studies in meta-analysis.
Assessment of risk of bias and publication bias
Risk-of-bias assessment of the included studies is presented in Figure 2. The included trials had some methodological strengths and limitations. One trial[25] was adjudicated to be of unclear risk of bias in blinding of outcome assessment, two studies[23,24] were adjudicated to be of unclear risk of bias in incomplete outcome data, and two trials[21,26] were adjudicated to be of low risk of bias.
Figure 2.
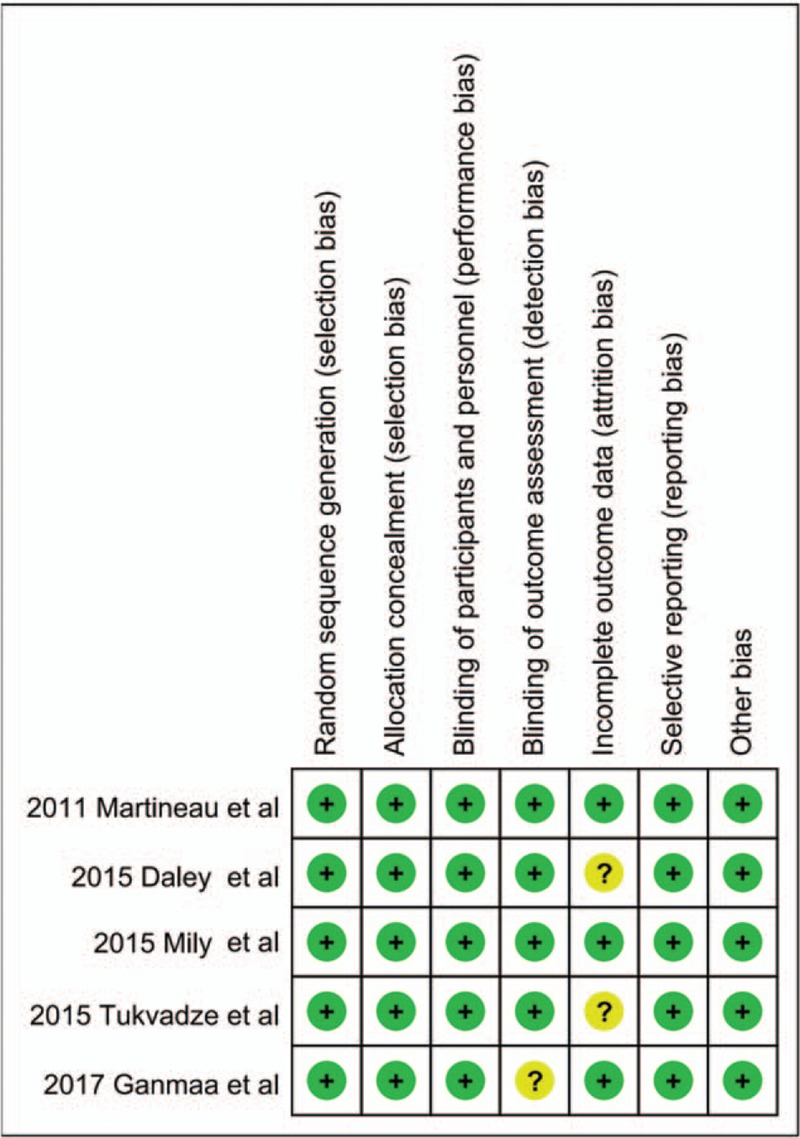
Risk of bias summary of the included studies.
We were unable to assess the publication bias using a funnel plot due to the small number of studies (<10) included in this analysis. Therefore, publication bias cannot be excluded.
Heterogeneity and sensitivity analysis
No heterogeneity was observed in time to sputum culture conversion, proportion of participants with negative sputum culture, MDR TB sputum culture conversion rate, BMI, chest radiography finding, and mortality. We found a moderate statistical heterogeneity in the incidence of hypercalcemia (I2 = 54%, RR 1.28, 95% CI 0.34–4.79, P = 0.72) and low heterogeneity in time to sputum smear conversion (I2 = 42%, RR 1.15, 95% CI 0.93–1.41, P = 0.20). A sensitivity analysis was performed to evaluate the stability of the results, by excluding each study one by one and recalculating the combined RR or HR on the remaining studies. This analysis confirmed the stability of the results: the overall effects did not show statistically significant reversal and recalculated pooled RR and HR were consistent and without apparent fluctuation (data not shown).
Primary outcomes
Time to sputum culture conversion
Four trials[21,23–25] with 982 cases were included in the meta-analysis [Figure 3]. Overall, there was no significant effect of vitamin D supplementation on time to sputum culture conversion (HR 1.04, 95% CI 0.89–1.23, P = 0.60, I2 = 14%).
Figure 3.
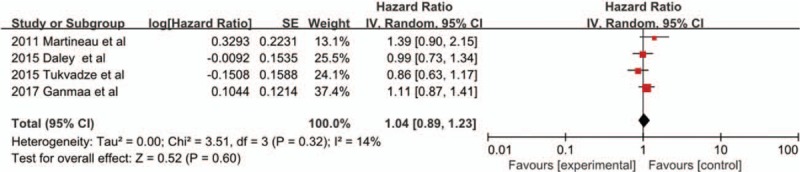
Hazard ratios for time to sputum culture conversion from four included studies. CI: Confidence interval; IV: Inverse variance; SE: Standard error.
Time to sputum smear conversion
Four trials[21,23,25,26] examined the time to sputum smear conversion between vitamin D supplementation and placebo [Figure 4]. The pooled analysis including 927 adults showed that there was no significant association between time to sputum smear conversion and vitamin D supplementation in PTB treatment (HR 1.15, 95% CI 0.93–1.41, P = 0.20, I2 = 42%).
Figure 4.
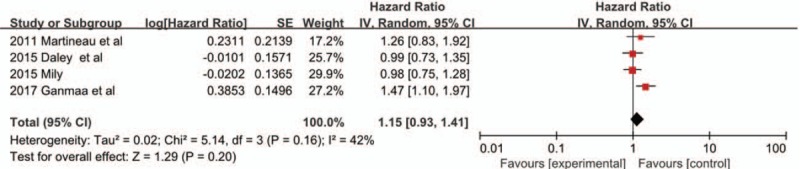
Hazard ratios for time to sputum smear conversion from four included studies. CI: Confidence interval; IV: Inverse variance; SE: Standard error.
Time to sputum culture conversion in the sub-group of participants with TaqI genotype
Two trials[21,24] were included in the meta-analysis [Figure 5]. The pooled analysis including 237 adults showed that there was a statistically significant difference in time to sputum culture conversion in participants with tt genotype (HR 8.09, 95% CI 1.39–47.09, P = 0.02, I2 = 0%). However, there was no effect in TT and Tt genotypes (HR 1.25, 95% CI 0.82–1.89, P = 0.29; HR 0.96, 95% CI 0.64–1.44, P = 0.86, respectively).
Figure 5.
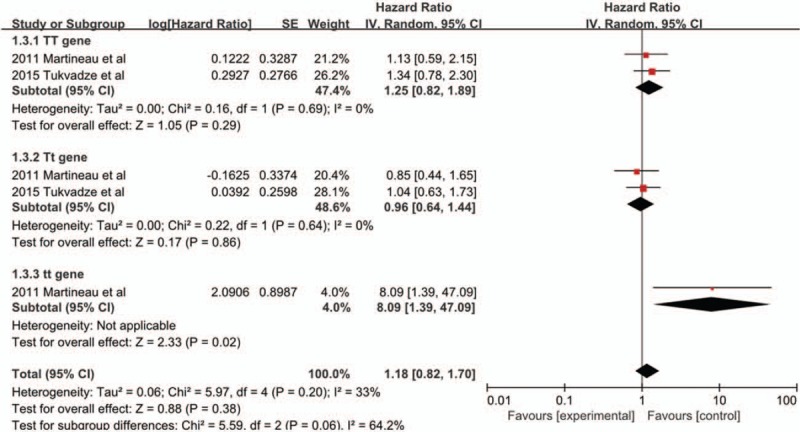
Hazard ratios for time to sputum culture conversion in the sub-group of participants with TaqI genotype. CI: Confidence interval; IV: Inverse variance; SE: Standard error.
MDR TB sputum culture conversion rate
Two trials[24,25] examined the effect of vitamin D supplementation on MDR sputum culture conversion rate. The pooled analysis including 39 adults showed a significant difference MDR TB sputum culture conversion rate at 8 weeks between the vitamin D supplementation and placebo groups (RR 2.40, 95% CI 1.11–5.18, P = 0.03, I2 = 0%) [Figure 6].
Figure 6.
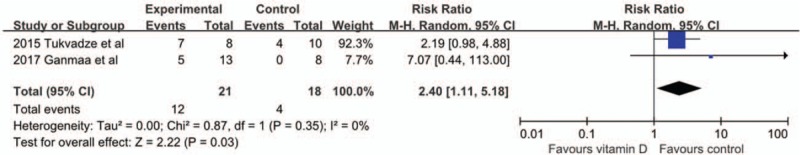
Forest plot of MDR TB sputum culture conversion rate. CI: Confidence interval; MDR TB: Multidrug-resistant tuberculosis; M-H: Mantel-Haenszel.
Proportion of participants with negative sputum culture
Five trials[21,23–26] evaluated the relationship between vitamin D supplementation and PTB therapy. Our pooled analysis included 1126 adults and found that there were no significant differences in the proportion of participants with negative sputum culture at 8 weeks (RR 1.05, 95% CI 0.99–1.10, P = 0.08, I2 = 0%) [Figure 7]. The result is consistent with those in the ITT analysis (RR 1.04, 95% CI 0.97–1.11, P = 0.32, I2 = 0%) [Supplementary Figure 1].
Figure 7.
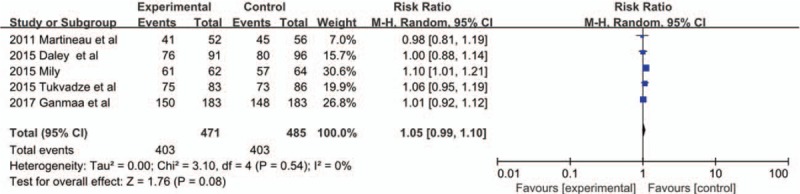
Forest plot of the proportion of participants with negative sputum culture in the per-protocol analysis. CI: Confidence interval; M-H: Mantel-Haenszel.
Secondary outcomes
BMI
Two trials[21,25] reported BMI after anti-TB therapy with vitamin D supplementation. The pooled analysis including 516 adults showed no significant differences in the BMI (MD −0.31, 95% CI −0.86 to 0.25, P = 0.28, I2 = 9%) [Supplementary Figure 2].
Chest radiography
Two trials[21,25] with 486 patients were included in the meta-analysis. The analysis showed that there was no significant difference in chest radiography findings (MD −0.02, 95% CI −0.41 to 0.36, P = 0.90, I2 = 0%) [Supplementary Figure 3].
Hypercalcemia
Five trials[21,23–26] examined the effect of vitamin D supplementation on the incidence of hypercalcemia. The pooled analysis including 1126 adults showed no significant difference in the incidence of hypercalcemia between the vitamin D supplementation and placebo groups (RR 1.28, 95% CI .0.34–4.79, P = 0.72, I2 = 54%) [Supplementary Figure 4].
Mortality
Four trials[21,23–25] examined the effect of vitamin D supplementation on mortality. The pooled analysis including 977 adults showed no significant difference in mortality between the vitamin D supplementation and placebo groups (RR 0.86, 95% CI 0.20–3.74, P = 0.85, I2 = 19%) [Supplementary Figure 5].
Quality of evidence
We used the GRADE system to determine the quality of evidence in our meta-analysis. The time to sputum culture conversion, proportion of participants with negative culture, incidence of hypercalcemia, and mortality had “moderate” quality with no inconsistency, no indirectness, no imprecision, and undetected publication bias but with a serious risk of bias. The time to sputum smear conversion with a serious risk of bias and inconsistency and MDR TB sputum culture conversion, BMI, and chest radiography findings with a serious risk of bias and imprecision had “low quality.” The sub-group with TaqI genotype had “very low” quality with a serious risk of bias, inconsistency, and imprecision.
Discussion
To the best of our knowledge, this might be the first meta-analysis to investigate the effect of adjunctive vitamin D on adults with PTB. We found that vitamin D supplementation does not shorten the time to sputum culture and smear conversion and does not lead to an increase in the proportion of participants with negative sputum culture. We also found that vitamin D had no impact on BMI, chest radiography findings, incidence of hypercalcemia, and mortality. However, it may reduce time to sputum culture conversion in patients with tt genotype of the VDR polymorphism and improve MDR TB sputum culture conversion rate.
Three meta-analyses by Riazv et al,[37] Xia et al,[28] and Wang et al[29] showed that vitamin D supplementation did not improve sputum conversion in patients with TB. A recent meta-analysis by Wu et al[30] reported that vitamin D supplementation was unable to shorten the time to sputum smear and culture conversion. Jolliffe et al[38] also reported that adjunctive vitamin D did not influence time to sputum culture conversion and the proportion of participants with negative sputum culture at 8 weeks but could accelerate sputum culture conversion in patients with MDR TB. These results were consistent with those of our meta-analysis, showing that vitamin D supplementation had no beneficial effects on time to sputum conversion and clinical signs but may improve MDR TB sputum culture conversion rate. However, Wu et al[30] reported that adjunctive vitamin D could increase the proportion of sputum smear and culture conversion and also improve chest radiography findings. Jolliffe et al[38] also reported that vitamin D could accelerate sputum smear conversion. There are several points to explain the difference in the results of our meta-analysis and the studies of Wu et al[30] and Jolliffe et al.[38] First, the inclusion criteria were different. Our meta-analysis included only adult patients, while the studies of Wu et al[30] and Jolliffe et al[38] included participants aged ≥15 years. Jolliffe et al[38] added the results of Wejse et al[20] and Ralph et al,[27] which did not meet our inclusion criteria, which may affect the results of time to sputum smear conversion. Second, Wu et al[30] pooled the results of the proportion of sputum smear or culture conversion using PP analysis, while we extracted data on the proportion of sputum culture conversion at 8 weeks and conducted PP and ITT analysis separately. ITT analysis more closely represents clinical practice and can be considered as a de facto standard for analysis of clinical trials.[39] Third, our meta-analysis investigated chest radiography findings at 8 weeks, while Wu et al[30] added the results of Salahuddin et al[22] (which did not meet our inclusion criteria) at 12 weeks. Lastly, Wu et al[30] used fixed-effects model to pool the results. Considering studies included in this review varied markedly in terms of population, doses of vitamin D, baseline 25(OH)D concentration, sun exposure time, type of diet, and reported outcome measure, we; therefore, used the random-effects model, which not only weighs each study by its inverse variance but also considers both within- and between study variations to calculate pooled RRs and 95% CIs, yielding a more global and conservative estimate.[40] All meta-analyses showed that vitamin D supplementation was safe and not associated with adverse effects.
Jolliffe et al[41] suggested that special single nucleotide polymorphisms (SNPs) in the vitamin D pathway affect disease outcomes. A review by Sutaria et al[42] reported that some VDR gene polymorphisms were associated with increased susceptibility to TB and vitamin D supplementation led to improved clinical outcomes. Our study found that vitamin D supplementation may accelerate time to sputum culture conversion in the sub-group of participants with tt VDR polymorphism or carrying the minor allele of each polymorphism. Although Tukvadze et al[24] reported that there was no benefit on sputum culture conversion with TaqI genotype, Martineau et al[21] reported that vitamin D significantly accelerated sputum culture conversion in PTB with the tt genotype. Ganmaa et al[25] also identified two other SNPs in VDR (rs4334089 and rs11568820) and one SNP in CYP27B1 (rs4646536) that significantly modified the effect of vitamin D on sputum culture conversion. Both studies reported that vitamin D supplementation was beneficial for patients with TB with particular genotypes. Considering our included trials were few and extracted data were limited and the pooled quality evidence is “extremely low,” further experimental studies are needed to explore the mechanism of vitamin D pathway gene, and additional RCTs are warranted to investigate the effect of vitamin D supplementation in patients with TB with certain VDR polymorphism.
In our study, we found that vitamin D may be beneficial in patients with MDR TB. Tukvadze et al[24] and Ganmaa et al[25] both showed a strong trend toward improving outcomes with adjunctive vitamin D3 in patients with MDR TB. Rathored et al[43] showed that, in patients with MDR TB, low serum 25(OH)D level was inversely correlated with sputum smear negativity. Jolliffe et al[38] reported that vitamin D supplementation accelerated sputum culture conversion in patients with MDR TB. Consistently, our meta-analysis showed that vitamin D supplementation may improve MDR TB sputum culture conversion rate. However, our analysis was based on a relatively small number of participants, which has insufficient evidence to justify a clinical recommendation to supplement vitamin D in MDR TB treatment. Further trials of investigating adjunctive vitamin D in patients with MDR TB are warranted to confirm this.
This study has several strengths. First, to the best of our knowledge, our meta-analysis might be the first to investigate adult PTB, and most of the studies are of high quality. Second, we conducted analyses based on ITT and PP data separately where possible, making our findings robust. Lastly, we performed a sub-group analysis of time to sputum culture conversion in participants with TaqI genotype and MDR TB sputum culture conversion rate, which may help guide future research direction.
This study also has several limitations. First, we were unable to assess the publication bias using a funnel plot due to the small number of studies included in this analysis. Therefore, publication bias cannot be excluded. Second, the number of patients with MDR-TB was small and the result of MDR-TB sputum culture conversion rate had low evidence with a serious risk of bias and imprecision. Thus, there was insufficient evidence to justify a clinical recommendation of vitamin D supplementation in MDR TB treatment. More clinical trials with larger sample size are needed to explore this issue. Third, time to sputum culture conversion in the sub-group of participants with TaqI genotype also had a small sample size and limited data, and the quality of evidence is extremely low. Further trials of adjunctive vitamin D in patients with VDR polymorphism are warranted. Finally, the administration doses of vitamin D and duration of treatment and follow-up were different across studies. We attempted to consider this by adopting the random-effects model, and no significant statistical heterogeneity was found in most of our analyses.
In conclusion, vitamin D supplementation had no beneficial effect in anti-TB treatment, but it accelerated time to sputum culture conversion in participants with tt genotype of the TaqI VDR polymorphism and improved the MDR TB sputum culture conversion rate. However, because of limited data and the varied methodological quality of the trials, which could possibly arouse a spurious result, the evidence should be viewed with caution. More high-quality and adequately powered RCTs investigating vitamin D supplementation in patients with TB with VDR gene polymorphisms and MDR TB are warranted.
Conflicts of interest
None.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
How to cite this article: Zhang J, Chen C, Yang J. Effectiveness of vitamin D supplementation on the outcome of pulmonary tuberculosis treatment in adults: a meta-analysis of randomized controlled trials. Chin Med J 2019;132:2950–2959. doi: 10.1097/CM9.0000000000000554
References
- 1.Abel L, Fellay J, Haas DW, Schurr E, Srikrishna G, Urbanowski M, et al. Genetics of human susceptibility to active and latent tuberculosis: present knowledge and future perspectives. Lancet Infect Dis 2018; 18:e64–e75.. doi: 10.1016/s1473-3099(17)30623-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Global Tuberculosis Report 2017. Geneva: World Health Organization, 2017. Available from: https://apps.who.int/iris/handle/10665/259366. [Last accessed on December 1, 2019] [Google Scholar]
- 3.Wilkinson RJ, Llewelyn M, Toossi Z, Patel P, Pasvol G, Lalvani A, et al. Influence of vitamin D deficiency and vitamin D receptor polymorphisms on tuberculosis among Gujarati Asians in west London: a case-control study. Lancet 2000; 355:618–621.. doi: 10.1016/s0140-6736(99)02301-6. [DOI] [PubMed] [Google Scholar]
- 4.Arnedo-Pena A, Juan-Cerdan JV, Romeu-Garcia A, Garcia-Ferrer D, Holguin-Gomez R, Iborra-Millet J, et al. Latent tuberculosis infection, tuberculin skin test and vitamin D status in contacts of tuberculosis patients: a cross-sectional and case-control study. BMC Infect Dis 2011; 11:349.doi: 10.1186/1471-2334-11-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martineau AR, Nhamoyebonde S, Oni T, Rangaka MX, Marais S, Bangani N, et al. Reciprocal seasonal variation in vitamin D status and tuberculosis notifications in Cape Town, South Africa. Proc Natl Acad Sci U S A 2011; 108:19013–19017.. doi: 10.1073/pnas.1111825108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dini C, Bianchi A. The potential role of vitamin D for prevention and treatment of tuberculosis and infectious diseases. Ann Ist Super Sanita 2012; 48:319–327.. doi: 10.4415/ann_12_03_13. [DOI] [PubMed] [Google Scholar]
- 7.Coussens AK, Martineau AR, Wilkinson RJ. Anti-inflammatory and antimicrobial actions of vitamin D in combating TB/HIV. Scientifica 2014; 2014:903680.doi: 10.1155/2014/903680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Talat N, Perry S, Parsonnet J, Dawood G, Hussain R. Vitamin D deficiency and tuberculosis progression. Emerg Infect Dis 2010; 16:853–855.. doi: 10.3201/eid1605.091693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deluca HF, Cantorna MT. Vitamin D: its role and uses in immunology. FASEB J 2001; 15:2579–2585.. doi: 10.1096/fj.01-0433rev. [DOI] [PubMed] [Google Scholar]
- 10.Fabri M, Stenger S, Shin DM, Yuk JM, Liu PT, Realegeno S, et al. Vitamin D is required for IFN-gamma-mediated antimicrobial activity of human macrophages. Sci Transl Med 2011; 3:104ra2.doi: 10.1126/scitranslmed.3003045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prietl B, Treiber G, Pieber TR, Amrein K. Vitamin D and immune function. Nutrients 2013; 5:2502–2521.. doi: 10.3390/nu5072502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C. Vitamin D: modulator of the immune system. Curr Opin Pharmacol 2010; 10:482–496.. doi: 10.1016/j.coph.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006; 311:1770–1773.. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 14.Ramanathan B, Davis EG, Ross CR, Blecha F. Cathelicidins: microbicidal activity, mechanisms of action, and roles in innate immunity. Microbes Infect 2002; 4:361–372.. doi: 10.1016/S1286-4579(02)01549-6. [DOI] [PubMed] [Google Scholar]
- 15.Martineau AR, Wilkinson RJ, Wilkinson KA, Newton SM, Kampmann B, Hall BM, et al. A single dose of vitamin D enhances immunity to mycobacteria. Am J Respir Crit Care Med 2007; 176:208–213.. doi: 10.1164/rccm.200701-007OC. [DOI] [PubMed] [Google Scholar]
- 16.National Collaborating Centre for Chronic Conditions National Institute for Health and Clinical Excellence: Guidance. Tuberculosis: Clinical Diagnosis and Management of Tuberculosis, and Measures for Its Prevention and Control. London: Royal College of Physicians (UK) Royal College of Physicians of London; 2006. [Google Scholar]
- 17.Selvaraj P, Chandra G, Jawahar MS, Rani MV, Rajeshwari DN, Narayanan PR. Regulatory role of vitamin D receptor gene variants of Bsm I, Apa I, Taq I, and Fok I polymorphisms on macrophage phagocytosis and lymphoproliferative response to mycobacterium tuberculosis antigen in pulmonary tuberculosis. J Clin Immunol 2004; 24:523–532.. doi: 10.1023/B:JOCI.000-0040923.07879.31. [DOI] [PubMed] [Google Scholar]
- 18.Roth DE, Soto G, Arenas F, Bautista CT, Ortiz J, Rodriguez R, et al. Association between vitamin D receptor gene polymorphisms and response to treatment of pulmonary tuberculosis. J Infect Dis 2004; 190:920–927.. doi: 10.1086/423212. [DOI] [PubMed] [Google Scholar]
- 19.Nursyam EW, Amin Z, Rumende CM. The effect of vitamin D as supplementary treatment in patients with moderately advanced pulmonary tuberculous lesion. Acta Med Indones 2006; 38:3–5.. [PubMed] [Google Scholar]
- 20.Wejse C, Gomes VF, Rabna P, Gustafson P, Aaby P, Lisse IM, et al. Vitamin D as supplementary treatment for tuberculosis: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med 2009; 179:843–850.. doi: 10.1164/rccm.200804-567OC. [DOI] [PubMed] [Google Scholar]
- 21.Martineau AR, Timms PM, Bothamley GH, Hanifa Y, Islam K, Claxton AP, et al. High-dose vitamin D3 during intensive-phase antimicrobial treatment of pulmonary tuberculosis: a double-blind randomised controlled trial. Lancet 2011; 377:242–250.. doi: 10.1016/s0140-6736(10)61889-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salahuddin N, Ali F, Hasan Z, Rao N, Aqeel M, Mahmood F. Vitamin D accelerates clinical recovery from tuberculosis: results of the SUCCINCT study [Supplementary Cholecalciferol in recovery from tuberculosis]. A randomized, placebo-controlled, clinical trial of vitamin D supplementation in patients with pulmonary tuberculosis’. BMC Infect Dis 2013; 13:22.doi: 10.1186/1471-2334-13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daley P, Jagannathan V, John KR, Sarojini J, Latha A, Vieth R, et al. Adjunctive vitamin D for treatment of active tuberculosis in India: a randomised, double-blind, placebo-controlled trial. Lancet Infect Dis 2015; 15:528–534.. doi: 10.1016/s1473-3099(15)70053-8. [DOI] [PubMed] [Google Scholar]
- 24.Tukvadze N, Sanikidze E, Kipiani M, Hebbar G, Easley KA, Shenvi N, et al. High-dose vitamin D3 in adults with pulmonary tuberculosis: a double-blind randomized controlled trial. Am J Clin Nutr 2015; 102:1059–1069.. doi: 10.3945/ajcn.115.113886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ganmaa D, Munkhzul B, Fawzi W, Spiegelman D, Willett WC, Bayasgalan P, et al. High-dose vitamin D3 during tuberculosis treatment in Mongolia. A randomized controlled trial. Am J Respir Crit Care Med 2017; 196:628–637.. doi: 10.1164/rccm.201705-0936OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mily A, Rekha RS, Kamal SM, Arifuzzaman AS, Rahim Z, Khan L, et al. Significant effects of oral phenylbutyrate and vitamin D3 adjunctive therapy in pulmonary tuberculosis: a randomized controlled trial. PloS One 2015; 10:e0138340.doi: 10.1371/journal.pone.0138340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ralph AP, Waramori G, Pontororing GJ, Kenangalem E, Wiguna A, Tjitra E, et al. L-arginine and vitamin D adjunctive therapies in pulmonary tuberculosis: a randomised, double-blind, placebo-controlled trial. Plos One 2013; 8:e70032.doi: 10.1371/journal.pone.0070032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xia J, Shi L, Zhao L, Xu F. Impact of vitamin D supplementation on the outcome of tuberculosis treatment: a systematic review and meta-analysis of randomized controlled trials. Chin Med J 2014; 127:3127–3134.. doi: 10.3760/cma.j.issn.0366-6999.20140702. [PubMed] [Google Scholar]
- 29.Wang J, Feng M, Ying S, Zhou J, Li X. Efficacy and safety of vitamin D supplementation for pulmonary tuberculosis: a systematic review and meta-analysis. Iran J Public Health 2018; 47:466–472.. [PMC free article] [PubMed] [Google Scholar]
- 30.Wu HX, Xiong XF, Zhu M, Wei J, Zhuo KQ, Cheng DY. Effects of vitamin D supplementation on the outcomes of patients with pulmonary tuberculosis: a systematic review and meta-analysis. BMC Pulm Med 2018; 18:108.doi: 10.1186/s12890-018-0677-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343:d5928.doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327:557–560.. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629–634.. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kota SK, Jammula S, Kota SK, Tripathy PR, Panda S, Modi KD. Effect of vitamin D supplementation in type 2 diabetes patients with pulmonary tuberculosis. Diabetes Metab Syndr 2011; 5:85–89.. doi: 10.1016/j.dsx.m2012. 02.021. [DOI] [PubMed] [Google Scholar]
- 35.Mathyssen C, Gayan-Ramirez G, Bouillon R, Janssens W. Vitamin D supplementation in respiratory diseases: evidence from randomized controlled trials. Polish Arch Intern Med 2017; 127:775–784.. doi: 10.20452/pamw.4134. [DOI] [PubMed] [Google Scholar]
- 36.Ganmaa D, Giovannucci E, Bloom BR, Fawzi W, Burr W, Batbaatar D, et al. Vitamin D, tuberculin skin test conversion, and latent tuberculosis in Mongolian school-age children: a randomized, double-blind, placebo-controlled feasibility trial. Am J Clin Nutr 2012; 96:391–396.. doi: 10.3945/ajcn.112.034967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riazv H, Riaz I, Abir T, Badshah M, Milton A. Vitamin D as a supplementary agent in the treatment of pulmonary tuberculosis: a systematic review and meta-analysis of randomized controlled trials. Eur Respir J 2013; 42:4623. [Google Scholar]
- 38.Jolliffe DA, Ganmaa D, Wejse C, Raqib R, Haq MA, Salahuddin N, et al. Adjunctive vitamin D in tuberculosis treatment: meta-analysis of individual participant data. Eur Respir J 2019; 53:1802003.doi: 10.1183/13993003.02003-2018. [DOI] [PubMed] [Google Scholar]
- 39.2016; Ranganathan P, Pramesh C, Aggarwal R. Common pitfalls in statistical analysis: intention-to-treat versus per-protocol analysis. 7:144–146.. doi: 10.4103/2229-3485.184823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pelucchi C, Galeone C, Bach JF, La Vecchia C, Chatenoud L. Pet exposure and risk of atopic dermatitis at the pediatric age: a meta-analysis of birth cohort studies. J Allergy Clin Immunol 2013; 132:616–22e7.. doi: 10.1016/j.jaci.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 41.Jolliffe DA, Walton RT, Griffiths CJ, Martineau AR. Single nucleotide polymorphisms in the vitamin D pathway associating with circulating concentrations of vitamin D metabolites and non-skeletal health outcomes: review of genetic association studies. J Steroid Biochem Mol Biol 2016; 164:18–29.. doi: 10.1016/j.jsbmb.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 42.Sutaria N, Liu CT, Chen TC. Vitamin D status, receptor gene polymorphisms, and supplementation on tuberculosis: a systematic review of case-control studies and randomized controlled trials. J Clin Transl Endocrinol 2014; 1:151–160.. doi: 10.1016/j.jcte.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rathored J, Sharma SK, Singh B, Banavaliker JN, Sreenivas V, Srivastava AK, et al. Risk and outcome of multidrug-resistant tuberculosis: vitamin D receptor polymorphisms and serum 25(OH)D. Int J Tuberc Lung Dis 2012; 16:1522–1528.. doi: 10.5588/ijtld.12.0122. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


