Abstract
Objective:
To review the latest progress on the pathogenic mechanism and management of rheumatoid arthritis (RA)-associated coronary artery disease (CAD), and propose advice on future management optimization as well as prospects for research and development of new therapeutic regimen.
Data sources:
This study was based on data obtained from PubMed up to May 2019 using various search terms and their combinations, including coronary artery disease, myocardial ischemia, cardiovascular diseases, RA, rheumatic diseases, treatment, therapy, strategies, immunotherapy, inflammation, and anti-inflammation.
Study selection:
All retrieved literature was scrutinized, most relevant articles about the pathogenic mechanism and clinical management, especially anti-inflammatory therapy of RA-associated CAD were reviewed.
Results:
RA is an immune-mediated chronic inflammatory disease which has a great social disease burden. In addition to typical arthritic manifestations, RA also affects extra-articular tissues and organs, within which the involvement of the cardiovascular system, especially incorporating CAD, is the leading cause of death for patients with RA. Recently, numerous basic and clinical studies have been carried out on the mechanism of CAD development and progression under the inflammatory cascade of RA. The effect of traditional RA drugs on CAD risk management has been gradually clarified, and more emerging biologic agents are being explored and studied, which have also achieved satisfactory outcomes. Furthermore, with the success of the CANTOS clinical trial, novel anti-inflammatory therapy for the prevention of cardiovascular disease is believed to have a broad prospect.
Conclusions:
RA is an independent risk factor for CAD, which mainly results from the underlying inflammatory cascade; therefore, anti-inflammatory therapy, especially the emerging novel biologic drugs, is important for CAD management in patients with RA and may also be a promising approach among the general population.
Keywords: Cardiovascular disease, Coronary artery disease, Rheumatoid arthritis
Introduction
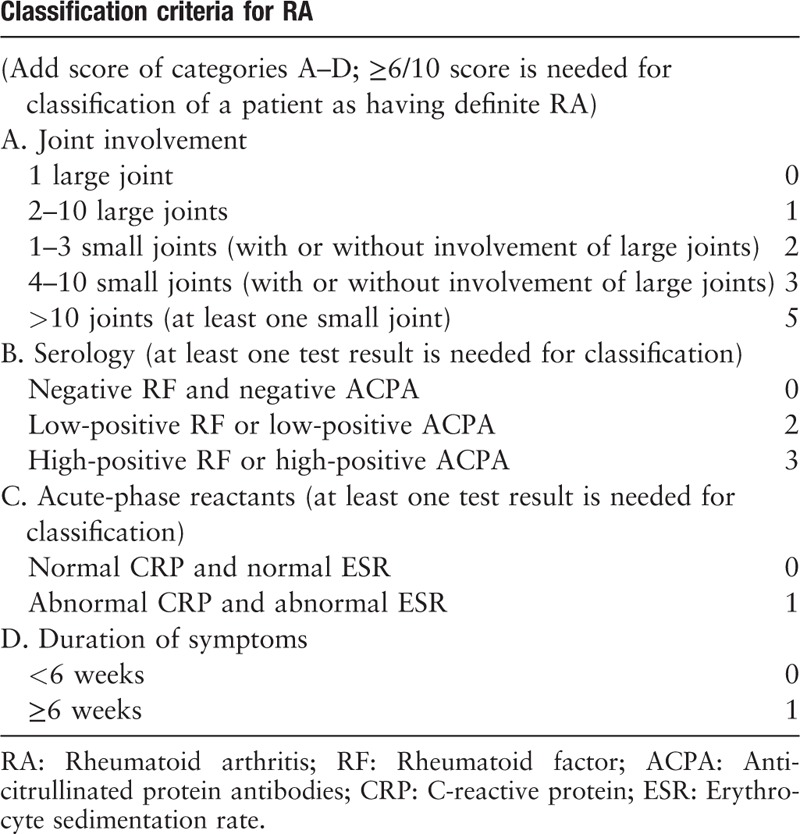
Rheumatoid arthritis (RA) is an immune-mediated chronic inflammatory disease with an annual incidence of approximately 40/100,000 and a global prevalence of 0.24%.[1] In addition to suffering from typical arthritic manifestations [Table 1],[2] approximately 40% of patients with RA suffer from extra-articular tissues and organ involvement. Patients with RA have a significantly higher incidence and mortality of cardiovascular disease (CVD) than the general population does, with increases of 50% and 60%, respectively.[3,4] Several high-profile reviews covering the theme of RA-associated CVD mainly focused on only part of the topic, either the novel anti-inflammatory strategies[5,6] or the underlying mechanisms.[7] Our review focuses on the research progress regarding both the pathogenic mechanism and management, especially the emerging biologic drugs of RA-associated coronary artery disease (CAD), and provides advice on management optimization and prospects for future studies about novel therapeutic regimen design based on current limitations.
Table 1.
The 2010 American College of Rheumatology/European League Against Rheumatism classification criteria for rheumatoid arthritis.[2]
All the retrieved literature was obtained from PubMed up to May 2019 using various search terms and their combinations, including coronary artery disease, myocardial ischemia, CVDs, RA, rheumatic diseases, treatment, therapy, strategies, immunotherapy, inflammation, and anti-inflammation. The literature was reviewed one by one, and only the most relevant articles about the pathogenic mechanism and clinical management, especially anti-inflammatory therapy of RA-associated CAD were extracted.
RA and CAD
In addition to the considerable impact on social disease burden due to loss of productive capacity,[8] patients with RA also have a 54% higher mortality than the general population, mainly due to the increased risk of CVD, respiratory dysfunction, and infections.[9]
Common RA-associated CVD includes CAD, congestive heart failure, cerebrovascular disease, peripheral arterial disease, and aortic atherosclerosis. In particular, CAD occurs early and accounts for approximately half of all deaths in RA.[10,11]
The presence of various types of coronary plaques was higher in terms of incidence, the number of involved segments and severity in patients with RA,[10] and a high rate of unstable coronary plaque and increased local inflammation were observed based on autopsy results.[12] Accordingly, the risk of myocardial infarction (MI) was significantly increased in terms of both incidence and case fatality, and the recurrence rate after acute coronary syndrome (ACS) was also elevated.[13–15] The above-mentioned studies consistently indicate that coronary plaques are more prone to occur and tend to progress to a more severe status in RA.
Common risk factors of RA and CAD
Two main common risk factors are smoking and genetic background [Figure 1]. Studies have illustrated that smoking is associated with RA disease activity, response to treatment and the prognosis of associated CAD.[16,17]
Figure 1.
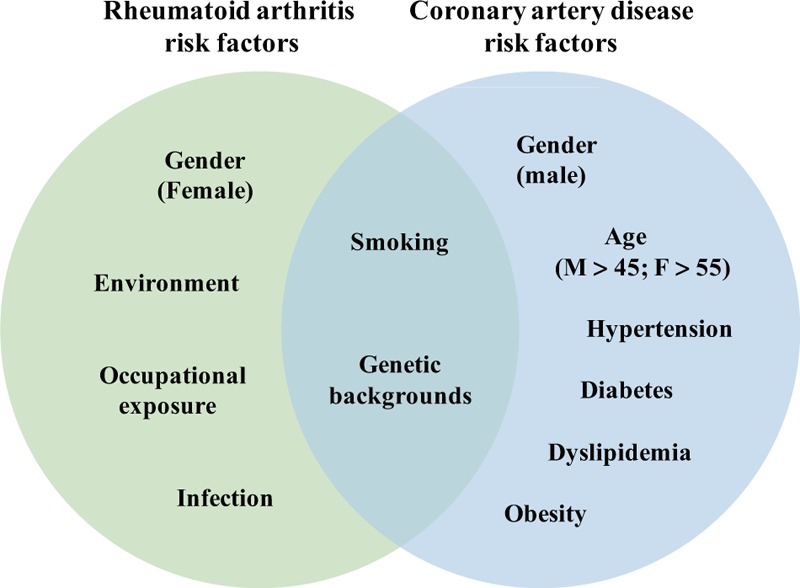
Risk factors for rheumatoid arthritis (RA) and coronary artery disease (CAD). CAD and RA have specific risk factors and also share certain common risk factors. The high risk of CAD in RA might result from both their common risk factors and the high prevalence of CAD risk factors in the RA population.
For genetic susceptibility, the HLA-DR4 phenotype has consistently been associated with RA, and the homozygous patients have significantly increased CAD incidence and mortality.[18] However, studies found that those RA-associated single-nucleotide polymorphisms were not associated with the occurrence of CAD in the general population,[19] and increased risk of coronary artery calcification (CAC) in RA is not associated with the genetically regulated atherogenic mechanisms in general population.[20] Thus, the exact shared genetic predisposition remains unclear.
For patients with newly diagnosed RA, the prevalence of CAD was not different from that of the general population before the onset of RA-related symptoms,[21] suggesting that common risk factors might play a relatively limited role in RA-associated CAD.
RA contributes to CAD-related risk factors
Traditional risk factors for CAD are shown in Figure 1.[22] RA might contribute to these factors,[23] including hypertension,[24] decreased nocturnal blood pressure fall,[25] abnormal lipid metabolism,[26] and diabetes.[27]
High C-reactive protein (CRP) level can promote hypertension through up-regulating the expression of angiotensin type-1 receptor[28] and vasoactive factor endothelin-1,[29] reducing endothelial nitric oxide (NO) production,[30] and inducing plasminogen activator inhibitor-1 which increases in hypertension population and contribute to impaired fibrinolysis and atherosclerosis.[30,31] Besides, higher disease activity was associated with lower nocturnal blood pressure fall, and accompanied by a disease activity improvement; the nocturnal blood pressure fall also increased.[25]
Increased levels of tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and interleukin-1 (IL-1), which can induce muscle atrophy, and mesenchymal cell migration and differentiation into adipocytes, and hence might lead to the concentric obesity observed in RA.[26] However, instead of hyperlipidemia, decreased levels of low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and total cholesterol (TC) were observed,[32] and accompanied by the inflammation control with Janus kinase (JAK) inhibitors[33,34] or other biologic agent treatments, such as tocilizumab (TCZ),[35] cholesterol levels were elevated simultaneously. The mechanisms behind such phenomenon remain obscure, but studies have shown that inflammation under RA has a pro-atherogenic change on lipid metabolism profile, including lipoprotein proteome composition alteration[36] and dysfunction,[37] scavenger receptor expression up-regulation,[38] impaired cholesterol efflux,[39] and amplification of foam cell formation.[40] Such changes can, in turn, exacerbate the inflammatory process.
Insulin resistance is related to the extent of inflammation in RA.[41] High levels of IL-6 and CRP will increase the risk of diabetes, while TNF-α can induce insulin resistance by down-regulating the activity of tyrosine kinase of insulin receptor, and after the treatment of infliximab, insulin resistance was improved significantly.[42] Certain disease-modifying anti-rheumatic drugs (DMARDs), including hydroxychloroquine (HCQ), methotrexate (MTX), and IL-1 antagonists, also appear to improve glucose metabolism markers to some extent,[43] and cases have been reported on the successful remission of RA and simultaneous control of diabetes after IL-1 inhibition.[44]
RA as an independent risk factor for CAD
Clinical evidence
After the adjustment of known CAD risk factors, the incidence of CAD in RA is still 2 to 3 times higher than that in the general population, indicating that RA should be considered as an independent risk factor for CAD.[45,46]
Pathogenic mechanism
Direct promotion of coronary atherosclerosis, together with the induction of coronary microvascular dysfunction, leads to the inadequate supply of myocardial oxygen in patients with RA,[47] and the main incipient steps for these two changes are endothelial dysfunction and immune system dysregulation.
Endothelial dysfunction not only undermines the barrier function of endothelial cells, causing enhancement of local inflammation and atherosclerosis, but also promotes the production of reactive oxygen species (ROS), which affects vasomotor function, leading to microvascular dysfunction. The immune system dysregulation is mainly characterized by the abnormality of immunocytes, which promotes the alteration of vascular wall matrix, the migration, and proliferation of smooth muscle cells and the formation of foam cells.[1,47] Furthermore, anomalous immunocytes, especially T and natural killer (NK) cells might inhibit the vasodilating effects of adenosine and acetylcholine while facilitate the vasoconstriction effects of the renin-angiotensin system, leading to microvascular dysfunction.[48,49]
The endothelial dysfunction and immune system dysregulation are most likely due to inflammatory cascade.[50,51] The relationship between inflammation and CAD can be most intuitively described by the positive correlation between CRP level or DAS28 score and CAD risk.[52,53] Patients with RA with high CAC tend to have a higher erythrocyte sedimentation rate as well as more pronounced joint swelling,[54] reflecting the important role of inflammation in the progression of CAD in RA.
However, CRP appears to be only a marker for inflammation rather than a direct participant in CAD development.[55] The exact mechanism may include the following pathways [Figure 2].
Figure 2.
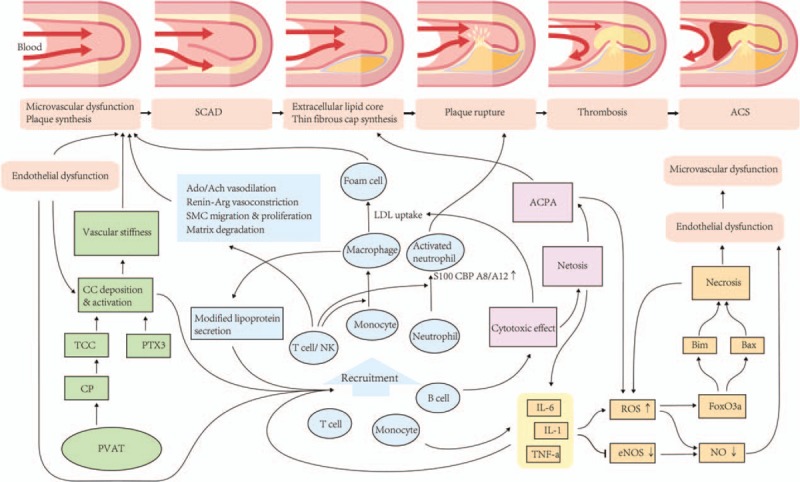
Mechanism summary of rheumatoid arthritis-associated coronary artery disease. Four pathways are mainly involved, including the effect of cytokines (yellow), abnormal immune cells activation (blue), complement activation (purple) and autoantibodies synthesis (green). Crosstalk between various pathways can continuously stabilize the inflammatory cascades, inducing two major subsequent pathophysiology changes, endothelial dysfunction and immune system dysregulation, which eventually leads to microvascular dysfunction and atherosclerosis. These pathways can also directly affect the evolution of plaque, leading to the conversion from SCAD to a more unstable and hazardous form, ACS. Ach: Acetylcholine; ACPA: Anticitrullinated protein antibody; ACS: Acute coronary syndrome; Ado: Adenosine; Bax: A pro-apoptotic Bcl-2 protein with several BH domains; Bim: A pro-apoptotic Bcl-2 protein with only BH3 domain; CC: Circulating complement; CP: Complement protein; eNOS: Endothelial nitric oxide synthase; FoxO3a: Forkhead box O3a; IL-1: Interleukin 1; IL-6: Interleukin 6; NO: Nitric oxide; PTX3: Pentraxin 3; PVAT: Perivascular adipose tissue; ROS: Reactive oxygen species; SCAD: Stable coronary artery disease; TCC: Terminal complement complexes; TNF-α: Tumor necrosis factor alpha.
Cytokines
Cytokines are believed to be one of the most important participants in inflammation, playing a central role in both atherosclerotic plaque formation and progression.[50] For RA, three cytokines, IL-6, IL-1, and TNF-α, are typically considered to be the major players in vascular inflammation.[56] Biopharmaceuticals targeting cytokines, such as IL-6 receptor monoclonal antibody,[57] IL-1β monoclonal antibody,[58] and TNF inhibitor,[59] have been used in the treatment of RA and CAD, and have achieved significant efficacy.[1] These inflammatory factors can cause endothelial dysfunction by reducing the synthesis and tissue utilization of NO. TNF-α can reduce the expression of endothelial cell NO synthase by inhibiting the activity of its promoter, thereby reducing NO synthesis and tissue utilization, promoting CAD.[60] Additionally, cytokines can promote the production of ROS, while ROS can reduce tissue NO utilization by its NO oxidization effect and further increase the production of pro-apoptotic proteins, such as Bim and Bax, by increasing activity of FoxO3a, which together lead to endothelial dysfunction.[61] The hypoxic state of tissue caused by endothelial dysfunction can induce oxidative stress, which in turn produces more ROS. The mechanism of such positive feedback continuously consolidates endothelial dysfunction and promotes the occurrence and deterioration of CAD.[47]
Abnormal activation of immunocytes
RA is associated with a series of changes in the expression of CAD-related genes, particularly S100 calcium-binding proteins A8 and A12, associated with neutrophil activation.[62] Besides, the composition of peripheral blood mononuclear cell subgroups as well as the degree of T-cell activation and differentiation of patients with RA are related to the formation of atherosclerotic plaques.[1] Patients with RA high CAC were found to have significantly increased levels of CD4+ and CD8+ T-cell activation and effector memory subpopulations (CD28−CD57+CD56+) in peripheral blood. At the same time, the level of CD14high CD16+ monocyte subsets in these patients was also increased.[63] Macrophages also play an important role in both inflammatory circuits and atherosclerotic plaque destabilization, and certain functional abnormalities have been illustrated. The re-organization of metabolism in macrophages differentiated from peripheral blood CD14+ monocytes collected from patients with both RA and CAD was observed, which presented unopposed oxygen consumption and excessive production of tissue-destructive enzymes through a molecular defect relating to GSK3b deactivation.[64] The role of NK T (NKT)-cell dysfunction in stimulating inflammatory cascade in RA[65–67] and atherosclerosis[68] has been separately studied, both mainly through the mechanism of abnormal activation, subsequent rapid production of a massive amount of cytokines and cytotoxic proteins, and regulatory effect on the differentiation and activation of other T cells. The above-mentioned results confirm the commonality of RA and CAD regarding the abnormal activation of immunocytes, suggesting the role of immunocytes in RA-associated CAD.
Autoantibodies
Anti-citrullinated protein antibodies are present specifically in RA, and citrullinated proteins have also been found to deposit in the atherosclerotic plaque.[69] Although the association of antibodies for citrullinated fibrinogen and citrullinated vimentin with CAD are still controversial,[70] other studies have clearly shown that high levels of anti-citrullinated H2B antibodies are associated with high CAC; moreover, the potential mechanism of autoantibodies in atherosclerosis development might include increased uptake of LDL by foam cells as well as enhanced NETosis, the activation of neutrophil extracellular trap,[71] which together promote inflammation, cytotoxicity, thrombosis and neutrophil activation.[72] In addition to the citrullinated proteins, other forms of autoantibodies with different targets, such as carbamylated proteins,[73] oxidized LDL,[74] apolipoprotein A-1[75] and malondialdehyde-acetaldehyde[76] were also found or speculated to be associated with atherosclerosis in RA. However, the specific mechanisms behind remain to be further revealed.
Complement activation
Perivascular adipose tissue can produce complement proteins, and visceral adipose tissue has been closely associated with CAD.[77] Theoretically, the complement activation product can target structural proteins of the blood vessel wall as well as circulate immunocytes, thereby inducing local inflammation and further formation of atherosclerotic plaques.[78] As patients RA tend to have more visceral adipose, complement synthesis and activation were assumed to be involved in CAD development in RA. The previous study indicated that in the CAD population, patients with RA have higher levels of terminal complement complexes; Pentraxin 3, which mediates the alternative pathway of complement activation; and mononuclear cell infiltration in the vessel wall compared with patients with non-RA.[79] Complement activation can also, in turn, interfere with the normal metabolism, leading to insulin resistance and obesity.[80] The complement activation theory needs further validation, and complement might be used as both a biomarker for early identification and a new target for treatment of RA-associated CAD.[79]
Prevention and Treatment
The management of RA-associated CAD aims to reduce the incidence/recurrence, progress, and mortality of CAD, and can thus be partitioned into primary and secondary prevention management, and acute event treatment management of angina and ACS. The principle is roughly the same as that for the general population, including traditional risk factors control, platelet inhibition, coronary expansion and myocardial revascularization,[81] but because RA serves as an independent risk factor, the importance for disease activity reduction must be emphasized as well, which has been highlighted in the European League Against Rheumatism (EULAR) recommendations.[82]
Currently available treatments for RA are composed of the following categories[83]: (1) conventional synthetic DMARDs (csDMARDs); (2) glucocorticoids (GC); (3) biologic DMARDs (bDMARDs); (4) biosimilar DMARDs (bsDMARDs); and (5) targeted synthetic DMARDs (tsDMARDs). Non-steroidal anti-inflammatory drugs (NSAIDs) are also acceptable for symptom remission. Numerous studies have been carried out to demonstrate the effect of anti-inflammatory drugs on the management of RA-associated CAD which is herein reviewed individually [Table 2]. It should be emphasized that some of the studies mentioned below did not focus on CAD risk alone, but rather took CVD as an integrity, which is also reasonable and of great significance as much of the risk factors and mechanism underlying CVD is similar, and the holistic decrease in CVD risk is always a hint for the improvement on coronary artery condition and associated with better long-term overall outcomes.
Table 2.
The effects of various categories of rheumatoid arthritis medications on the cardiovascular system.

csDMARDs
Commonly used csDMARDs include MTX, HCQ, leflunomide, and sulfasalazine. As a first-line medication for active-stage treatment of RA, according to a meta-analysis of observational and controlled trials, MTX can significantly reduce all cardiovascular events (CVEs) risk and MI, and also shows a trend to reduce the risk of major adverse cardiovascular events (MACEs) (no statistics significance due to the small number of events). Generally, MTX has a positive effect on CAD risk control in RA.[84] However, in a recently completed randomized, controlled trial (RCT), CIRT (NCT01594333), compared with placebo, low-dose MTX (15–20 mg weekly) did not result in lower levels of IL-1β, IL-6, or CRP, and also fewer cardiovascular events among stable atherosclerosis patients with previous MI or multi-vessel coronary disease; thus, the beneficial effect of MTX on inflammation control and cardiovascular prevention may only apply to patients with existing systemic inflammatory conditions.[85]
According to meta-analysis, HCQ users have significantly lower overall cholesterol and LDL-C levels while higher HDL-C levels than non-users among patients with RA, and the incidence of diabetes is also lower than those who have never been treated with HCQ. Although the amount of data is not sufficient to support a meta-analysis for direct effect on CVD, HCQ has shown a tendency to reduce the incidence of insulin resistance as well as CVD risk,[86] and in a nationwide cohort study, HCQ use was associated with decreased CAD risk in RA.[87] Therefore, although relatively low efficient for disease activity control, HCQ is well tolerated and thereby has high safety, which endows a potential advantage for combination with other DMARDs to better reduce the CAD risk while avoiding severe side effects. An RCT, OXI trial (NCT02648464) was conducted and currently underway to study the effect of HCQ on the prevention of recurrent cardiovascular events among patients with MI.[88]
For sulfasalazine, although well studied in RA, the effect on CAD remains controversial. According to a multinational cross-sectional cohort in the QUEST-RA program, prolonged exposure of sulfasalazine was associated with a reduced risk of CVD in RA.[89] For the general population, an RCT has shown the control of endothelial dysfunction and carotid arterial remodeling with sulfasalazine in stable patients with CAD[90] while the findings of another RCT (NCT00554203) suggested that long-term treatment was poorly tolerated and did not improve endothelial function in patients with CAD.[91]
Prolonged exposure of leflunomide was associated with a reduction of CVD morbidity in patients with RA in an observational study[89]; however, in a retrospective study of leflunomide treatment based on patients with early stage RA, the treatment group showed an even higher incidence of hypertension.[92] It has also been observed in the TEAR trial that the use of triple therapy (MTX plus sulfasalazine plus HCQ) in early aggressive patients with RA during 2-year follow-up was associated with higher HDL-C, lower LDL-C, and lower TC/HDL-C ratio compared to those who received MTX monotherapy or MTX plus etanercept therapy.[93] These results suggest that a more optimized treatment might require consideration of the disease activity grading and stage evaluation to facilitate the selection of more appropriate drugs or combination therapy.
NSAIDs and GC
There is still much controversy about the use of NSAIDs and corticosteroids, mainly because of their negative effect on the cardiovascular system.
According to meta-analysis, NSAIDs did increase the risk of all CVE risk and cerebral infarction in RA, mainly due to the use of cyclooxygenase-2 selective inhibitors (COX-2i), while non-selective NSAIDs did not contribute to the increased risk of CVE. Considering that the COX-2i, rofecoxib, used in most studies had been nearly eliminated, further analysis of rofecoxib and celecoxib was performed and showed that rofecoxib would increase all CVE risk while celecoxib would not.[84] In a cohort study based on the population in Taiwan of China, the risk of CAD when taking two different COX-2i (celecoxib and etoricoxib) in patients with RA was significantly lower than that of non-users during a 10-year follow-up. Within the first 4 years, the use of celecoxib was associated with a reduced risk of CAD; however, this positive effect gradually disappeared and even increased the risk in a high-dose group after 4 years. In contrast, not only did etoricoxib reduce the risk of CAD, but this protective effect could also remain unchanged over a 10-year follow-up.[94] According to the EULAR guidelines, Naproxen, a non-selective NSAID, is perhaps the safest NSAID in CAD risk for patients with RA currently.[82]
Corticosteroids are generally considered hazardous to glucose and lipid metabolism and can increase the risk of hypertension. The results of meta-analysis have confirmed that corticosteroids significantly increase the risk of all CVE risk, MI, heart failure, cerebral infarction, and MACE in RA.[84] However, studies have also shown that all-cause mortality of corticosteroids is associated with treatment dosage as well as exposure time that above a threshold dose of 8 mg/day, all-cause mortality shows a positive correlation with daily dosage[95]; and analogously, that the risk of CVD is elevated with increasing exposure time.[82] These results prompt clinicians to choose the lowest effective dose and reduce the exposure time of corticosteroids in RA management.
As NSAIDs and corticosteroids are essential medications for symptom relief and replacement therapy, better clinical decisions might necessitate an appropriate adjustment in the type, dosage, and exposure time, which is also supported by the EULAR recommendation, highlighting the assessment at an individual level and under the instruction of certain treatment-specific guidelines.[82]
tsDMARDs (JAK inhibitors)
Certain cytokines associated with RA, including the IL-6, IL-23, and IL-2 cytokine families and type I and type II interferon, transduce cellular activation signals through the JAK signaling pathway. JAK inhibitors have been applied to the anti-inflammatory treatment of RA due to their function in cytokine activation blockage.[96,97] There are still no studies demonstrating the effects of JAK inhibitors on CAD risk in patients with RA, but two clinical trials (NCT01185353, NCT01262118) have shown that patients with RA presented elevated LDL-C, HDL-C, and TC levels after treatment with two JAK inhibitors, baricitinib and tofacitinib.[33,34] Although current results indicate that JAK inhibitors appear to increase the risk of CAD, the exact overall clinical impact remains to be confirmed by further long-term follow-up.
bDMARDs
The application of biologic medications has brought physicians a new methodology for regulating inflammatory reactions in a more precise, efficient and safe way, and thus better control the disease condition. Whether biologic medications can reduce the incidence, recurrence, and mortality of CVD, especially CAD while controlling RA disease activity has become the main focus of current research.
Tumor necrosis factor inhibitor
As one of the most intensively studied and widely used biologic medications, tumor necrosis factor inhibitor (TNFi) has displayed positive effects on CVD prevention according to a large-scale meta-analysis, with a significant reduction in the risk of all CVE risk, MI, cerebral infarction, and MACE and no elevation of heart failure risk in RA.[82] Moreover, the risk of CAD can be further reduced in the case of long-term treatment.[98] Other studies have shown that patients treated with TNFi have a lower risk of MI compared with patients receiving csDMARD treatments, which may be attributed to a more direct effect of TNFi on atherosclerosis and thus better disease activity control.[59] TNFi also has beneficial effects on traditional CAD risk factors, such as insulin resistance and metabolic syndrome.[99] Though increases in all lipid components can be observed in researches on different TNFi,[81] the result of meta-analysis shows only increased TC and TG, but increased HDL-C and no effect on LDL-C or atherogenic index after long-term treatment,[100] indicating a potential protective effect on lipid metabolism.
Although treatment with TNFi reduces the overall risk of CAD, it remains unclear whether such a protective effect is appropriate for all types of patients.[17] Previous studies have shown that in RA population, good responders to TNFi treatment, as defined by EULAR, experience a significantly lower risk of ACS compared with non-responders/moderate responders, and the risk for ACS of good responders is not significantly different from that of the general population, while the risk of non-responders/moderate responders can be up to more than twice as high.[101] Therefore, it should be realized that therapeutic assessment might be even more important than the type of medication selected.
Canakinumab (IL-1β monoclonal antibody)/anakinra (IL-1 receptor antagonist)
It is particularly gratifying that, in a large phase III clinical trial covering 10,000 people over 4 years, the CANTOS trial (NCT01327846), patients with a history of previous MI and high high-sensitivity CRP (hs-CRP) levels were randomized to receive three doses of canakinumab or placebo. Compared with the placebo group, those receiving canakinumab showed significantly reduced hsCRP levels; moreover, for those receiving a dosage of 150 mg/3 months, in particular, significantly lower recurrence rates for CVEs were observed in the absence of a change in lipid levels.[58,102] This is the first large-scale clinical trial to directly demonstrate the association between anti-inflammatory therapy and CVD risk reduction.
Another RCT (NCT01566201) has revealed that inhibition of IL-1 activity with anakinra can reduce oxidative stress in RA, characterized by lower levels of apoptosis and oxidation-related markers, thereby improving both the histology structure and physiology function of the cardiovascular system. In addition, a greater improvement in vascular function, LV myocardial deformation, and twisting has been observed, resulting in a significantly higher left ventricular ejection fraction after treatment with anakinra in patients with RA-associated CAD compared to those without[103]; these findings indicate a potential advantage in secondary prevention of IL-1 pathway inhibition and propose further requirement for more delicate clarification of the relative superiority of different drugs under different clinical demands.
Tocilizumab (TCZ, IL-6 receptor monoclonal antibody)
A prospective study showed that TCZ treatment significantly improved endothelial function in flow-mediated dilation percentage in RA, while such improvement was not observed in either the csDMARDs or TNFi treatment groups. However, the treatment of TCZ and csDMARDs was also associated with an increased level of TC and LDL-C but a decreased level of HDL-C, which was not found in patients after TNFi treatment.[57] Such a tendency to affect lipid levels negatively was observed in many other trails, such as MEASURE (NCT00535782)[104] and ADACTA (NCT01119859).[105] However, the risk of MACE while receiving TCZ was found to be associated with disease activity control but not lipid changes.[106] In a multi-database cohort study, the cardiovascular risk associated with TCZ vs. TNFi treatment was similar[107]; besides, the preliminary results posted online of an RCT (NCT01331837) comparing the cardiovascular risk in patients with RA receiving TCZ or etanercept also showed no differences between treatments. When compared with anakinra in another prospective observational trial (NCT03288584) based on the RA population, TCZ was only associated with pulse wave velocity and brachial blood pressure improvement, while anakinra treatment resulted in an improvement of coronary flow reserve.[108] A TOCRIVAR clinical trial (NCT01752335) is currently underway, attempting to comprehensively illustrate the effects of TCZ on cardiovascular risk in RA. For use in the general CAD population, current results from an RCT (NCT01491074) showed that TCZ had no or only minor effects on the cytokines and did not improve coronary flow reserve in non-ST elevation patients with MI.[109,110]
Rituximab (CD20 monoclonal antibody)
Rituximab is capable of inflammatory inhibition through rapid and sustained B lymphocyte consumption by binding specifically to their surface antigen, CD20. A recent study showed that carotid intima-media thickness was reduced after rituximab treatment,[111] indicating a potential protective benefit to the cardiovascular system. Results from a clinical trial (NCT00844714) showed that despite a slight increase in TC and triglyceride levels, the depletion of B cells did improve the endothelial function of both the macrovascular and microvascular systems in terms of brachial artery flow-mediated dilation and reactive hyperemia velocity.[112] According to an observational prospective study, rituximab treatment showed a reduced pulse wave velocity after 12-month use while such improvement occurred only after 3 months of TCZ treatment.[113] However, no difference in long-term outcomes and the risk of MACE between these two treatments were observed in another prospective study based on patients with inadequate response to TNFi.[114] The exact effect of rituximab on CAD needs to be further studied.
Clinical Management Optimization
(1) Emphasize the awareness of prevention management and increase compliance: Highlighted by EULAR, the awareness of risk and ensuring subsequent risk control are the foundation of all effective medical treatments of RA-associated disease. However, the current situation is still suboptimal that implementation of cardiovascular risk management remains a challenge[115]; thus, better clinical awareness should be urgently emphasized. Besides, for chronic diseases, it is always necessary to increase compliance, enabling continuous risk control, which is also in accordance with the EULAR recommendations, highlighting “alignment of the patient's and provider's considerations and aims” and “enhancing adherence.”[83]
(2) Optimize the dose and course of treatment: In addition to the category, changing the dosage and duration of treatment can also increase efficiency and safety. As some studies mentioned earlier, drugs might present a protective effect within a certain dose and exposure time but become detrimental when exceeding the threshold. Side effects can be minimized to make benefits more prominent through either dosage or treatment course optimization.
(3) Risk stratification and disease stage-based medications: For patients with RA after percutaneous coronary intervention (PCI), the risk of repeating PCI would greatly increase,[116,117] indicating that the CAD risk is changing dynamically and needs stratification. However, no validated RA-specific CAD risk evaluation model has currently been established.[118,119] Besides, drugs can display various effects depending on the disease stage,[92] and treatment in early stage can take a greater account of the impact of the overall outcomes.[120] Therefore, CAD risk stratification and disease staging under RA is demanded to assist more appropriate clinical decisions.
(4) Drug response monitor: As one above-mentioned study about the TNFi treatment, different drug responses resulting from the heterogeneity in genetic background, disease activity, lifestyle, and concomitant diseases and medications[17] can lead to disparate outcomes. Therefore, drug response should be highlighted and promptly monitored to avoid the imbalance of medical dual effects.
Prospects
(1) Precision and personalized medicine: Heterogeneity among the RA population, which might lead to differences in drug response and clinical outcomes can be further disclosed by cluster analysis and machine learning techniques integrating genetic information, clinical manifestations, laboratory findings, and imaging performance. Various omics results obtained from deep sequencing, immunoassays, and mass spectrometry can be further incorporated to explore the relationship between genotypes, phenotypes, drug responses and prognosis, facilitating precision, and personalized medicine.
(2) Appropriate evaluation of the drug efficacy: According to a large-scale cohort study, the inflammatory states have improved over time; however, the overall prevalence of comorbidity has increased significantly, mainly due to CAD.[120] Therefore, simply pursuing disease activity control and serologic or imaging remission, while ignoring the long-term negative cardiovascular effect should be questioned. The central issue is to find a balance between efficiency and safety.[99] To clarify the exact drug effect, studies should not be limited to individual cases or changes in a single indicator. Ultimate outcomes should be emphasized in terms of occurrence and morbidity of CAD or more credible progression-free survival and overall survival, rather than certain laboratory tests, surrogate markers or a single risk factor.
(3) Immune regulation therapy: Most existing studies are restricted to the effect of single drugs. However, as a specific type of drug is unique in mechanism, the efficacy of mono-drug immune inhibition can be limited. Combining drugs to conduct a multifaceted intervention might better display the advantages of each drug,[93] and lead to comprehensive immune regulation and better outcomes.
(4) Target exploration: It is necessary to clarify central participants in the complex inflammatory system as potential targets for immune regulation therapy.[121] Firstly, it is indispensable to construct animal models that conform to pathologic and pathophysiologic changes resembling those under RA-associated CAD to provide reliable study materials. Additionally, molecules and pathways shared by RA and CAD can be further excavated to reveal targets involved in the common pathway. It might also be helpful to take advantage of the single-cell sequencing to reveal specific cell subpopulations and cell evolution through principal component analysis, pseudotime analysis, and TCR analysis. Lastly, current research has merely focused on therapy targeting immunocytes, such as T lymphocytes, macrophages, and NK T cells. Considering the central regulatory and effector role of these cells, the efficacy of existing immunocyte-targeted drugs, such as abatacept should be tested, and more novel therapy should be explored.
(5) Extension of medical indications: RA provides a natural model for exploring the commonality shared by CAD and inflammation. Current studies have provided notable ideas and basis for innovative pharmaceutical R&D in anti-inflammatory treatments of CAD, such as the completed CANTOS,[102] CIRT,[85] NCT01491074[110] trials, and the ongoing OXI trial.[88] Researches are needed to extend the indications under more clinical conditions, such as subclinical atherosclerosis or the acute phase of ACS.
Conclusions
RA is an independent risk factor for CAD, which predominantly results from the underlying inflammatory cascade; therefore, anti-inflammatory therapy, especially the emerging novel biologic drugs, is indispensable for CAD risk management in patients with RA and may also be efficient among general population. In this review, we provide a comprehensive overview of the research progress on the pathogenic mechanism and the management of RA-associated CAD, and further provide advice on management optimization and prospects for future study.
Acknowledgements
The authors thank Dr. Zhuang Tian, Dr. Xiaofeng Zeng, and Dr. Xinping Tian for providing valuable suggestions.
Funding
This work was supported by the National Key R&D Program of China (2016YFC0901500 and 2016YFC0901502; 2017YFC0907601 and 2017YFC0907604); CAMS Innovation Fund for Medical Sciences (CIFMS) (2016-I2M-1-002).
Conflicts of interest
None.
Footnotes
How to cite this article: Wang L, Zhang Y, Zhang SY. Immunotherapy for the rheumatoid arthritis-associated coronary artery disease: promise and future. Chin Med J 2019;132:2972–2983. doi: 10.1097/CM9.0000000000000530
References
- 1.Klingenberg R, Luscher TF. Rheumatoid arthritis and coronary atherosclerosis: two cousins engaging in a dangerous liaison. Eur Heart J 2015; 36:3423–3425.. doi: 10.1093/eurheartj/ehv489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, 3rd, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010; 62:2569–2581.. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 3.Meune C, Touze E, Trinquart L, Allanore Y. Trends in cardiovascular mortality in patients with rheumatoid arthritis over 50 years: a systematic review and meta-analysis of cohort studies. Rheumatology (Oxford) 2009; 48:1309–1313.. doi: 10.1093/rheumatology/kep252. [DOI] [PubMed] [Google Scholar]
- 4.Avina-Zubieta JA, Thomas J, Sadatsafavi M, Lehman AJ, Lacaille D. Risk of incident cardiovascular events in patients with rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis 2012; 71:1524–1529.. doi: 10.1136/annrheumdis-2011-200726. [DOI] [PubMed] [Google Scholar]
- 5.Burmester GR, Pope JE. Novel treatment strategies in rheumatoid arthritis. Lancet 2017; 389:2338–2348.. doi: 10.1016/s0140-6736(17)31491-5. [DOI] [PubMed] [Google Scholar]
- 6.McInnes IB, Schett G. Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet 2017; 389:2328–2337.. doi: 10.1016/s0140-6736(17)31472-1. [DOI] [PubMed] [Google Scholar]
- 7.Romano S, Salustri E, Ruscitti P, Carubbi F, Penco M, Giacomelli R. Cardiovascular and metabolic comorbidities in rheumatoid arthritis. Curr Rheumatol Rep 2018; 20:81.doi: 10.1007/s11926-018-0790-9. [DOI] [PubMed] [Google Scholar]
- 8.Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet 2016; 388:2023–2038.. doi: 10.1016/s0140-6736(16)30173-8. [DOI] [PubMed] [Google Scholar]
- 9.van den Hoek J, Boshuizen HC, Roorda LD, Tijhuis GJ, Nurmohamed MT, van den Bos GA, et al. Mortality in patients with rheumatoid arthritis: a 15-year prospective cohort study. Rheumatol Int 2017; 37:487–493.. doi: 10.1007/s00296-016-3638-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karpouzas GA, Malpeso J, Choi TY, Li D, Munoz S, Budoff MJ. Prevalence, extent and composition of coronary plaque in patients with rheumatoid arthritis without symptoms or prior diagnosis of coronary artery disease. Ann Rheum Dis 2014; 73:1797–1804.. doi: 10.1136/annrheumdis-2013-203617. [DOI] [PubMed] [Google Scholar]
- 11.Stevens RJ, Douglas KM, Saratzis AN, Kitas GD. Inflammation and atherosclerosis in rheumatoid arthritis. Expert Rev Mol Med 2005; 7:1–24.. doi: 10.1017/s1462399405009154. [DOI] [PubMed] [Google Scholar]
- 12.Aubry MC, Maradit-Kremers H, Reinalda MS, Crowson CS, Edwards WD, Gabriel SE. Differences in atherosclerotic coronary heart disease between subjects with and without rheumatoid arthritis. J Rheumatol 2007; 34:937–942.. [PubMed] [Google Scholar]
- 13.Maradit-Kremers H, Crowson CS, Nicola PJ, Ballman KV, Roger VL, Jacobsen SJ, et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum 2005; 52:402–411.. doi: 10.1002/art.20853. [DOI] [PubMed] [Google Scholar]
- 14.Sodergren A, Stegmayr B, Lundberg V, Ohman ML, Wallberg-Jonsson S. Increased incidence of and impaired prognosis after acute myocardial infarction among patients with seropositive rheumatoid arthritis. Ann Rheum Dis 2007; 66:263–266.. doi: 10.1136/ard.2006.052456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Douglas KM, Pace AV, Treharne GJ, Saratzis A, Nightingale P, Erb N, et al. Excess recurrent cardiac events in rheumatoid arthritis patients with acute coronary syndrome. Ann Rheum Dis 2006; 65:348–353.. doi: 10.1136/ard.2005.037978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papadopoulos NG, Alamanos Y, Voulgari PV, Epagelis EK, Tsifetaki N, Drosos AA. Does cigarette smoking influence disease expression, activity and severity in early rheumatoid arthritis patients? Clin Exp Rheumatol 2005; 23:861–866.. [PubMed] [Google Scholar]
- 17.Hyrich KL, Watson KD, Silman AJ, Symmons DP. Predictors of response to anti-TNF-alpha therapy among patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register. Rheumatology (Oxford) 2006; 45:1558–1565.. doi: 10.1093/rheumatology/kel149. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez-Gay MA, Gonzalez-Juanatey C, Lopez-Diaz MJ, Pineiro A, Garcia-Porrua C, Miranda-Filloy JA, et al. HLA-DRB1 and persistent chronic inflammation contribute to cardiovascular events and cardiovascular mortality in patients with rheumatoid arthritis. Arthritis Rheum 2007; 57:125–132.. doi: 10.1002/art.22482. [DOI] [PubMed] [Google Scholar]
- 19.Jansen H, Willenborg C, Lieb W, Zeng L, Ferrario PG, Loley C, et al. Rheumatoid arthritis and coronary artery disease: genetic analyses do not support a causal relation. J Rheumatol 2017; 44:4–10.. doi: 10.3899/jrheum.151444. [DOI] [PubMed] [Google Scholar]
- 20.Ferraz-Amaro I, Winchester R, Gregersen PK, Reynolds RJ, Wasko MC, Oeser A, et al. Coronary artery calcification and rheumatoid arthritis: lack of relationship to risk alleles for coronary artery disease in the general population. Arthritis Rheumatol 2017; 69:529–541.. doi: 10.1002/art.39862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holmqvist ME, Wedren S, Jacobsson LT, Klareskog L, Nyberg F, Rantapaa-Dahlqvist S, et al. No increased occurrence of ischemic heart disease prior to the onset of rheumatoid arthritis: results from two Swedish population-based rheumatoid arthritis cohorts. Arthritis Rheum 2009; 60:2861–2869.. doi: 10.1002/art.24855. [DOI] [PubMed] [Google Scholar]
- 22.Hajar R. Risk factors for coronary artery disease: historical perspectives. Heart Views 2017; 18:109–114.. doi: 10.4103/heartviews.heartviews_106_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kerekes G, Nurmohamed MT, Gonzalez-Gay MA, Seres I, Paragh G, Kardos Z, et al. Rheumatoid arthritis and metabolic syndrome. Nat Rev Rheumatol 2014; 10:691–696.. doi: 10.1038/nrrheum.2014.121. [DOI] [PubMed] [Google Scholar]
- 24.Panoulas VF, Metsios GS, Pace AV, John H, Treharne GJ, Banks MJ, et al. Hypertension in rheumatoid arthritis. Rheumatology (Oxford) 2008; 47:1286–1298.. doi: 10.1093/rheumatology/ken159. [DOI] [PubMed] [Google Scholar]
- 25.Hamamoto K, Yamada S, Yasumoto M, Yoda M, Yoda K, Tsuda A, et al. Association of nocturnal hypertension with disease activity in rheumatoid arthritis. Am J Hypertens 2016; 29:340–347.. doi: 10.1093/ajh/hpv119. [DOI] [PubMed] [Google Scholar]
- 26.Giles JT, Allison M, Blumenthal RS, Post W, Gelber AC, Petri M, et al. Abdominal adiposity in rheumatoid arthritis: association with cardiometabolic risk factors and disease characteristics. Arthritis Rheum 2010; 62:3173–3182.. doi: 10.1002/art.27629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boyer JF, Gourraud PA, Cantagrel A, Davignon JL, Constantin A. Traditional cardiovascular risk factors in rheumatoid arthritis: a meta-analysis. Joint Bone Spine 2011; 78:179–183.. doi: 10.1016/j.jbspin.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 28.Wang CH, Li SH, Weisel RD, Fedak PW, Dumont AS, Szmitko P, et al. C-reactive protein upregulates angiotensin type 1 receptors in vascular smooth muscle. Circulation 2003; 107:1783–1790.. doi: 10.1161/01.cir.0000061916.95736.e5. [DOI] [PubMed] [Google Scholar]
- 29.Verma S, Li SH, Badiwala MV, Weisel RD, Fedak PW, Li RK, et al. Endothelin antagonism and interleukin-6 inhibition attenuate the proatherogenic effects of C-reactive protein. Circulation 2002; 105:1890–1896.. doi: 10.1161/01.CIR.0000015126.83143.B4. [DOI] [PubMed] [Google Scholar]
- 30.Devaraj S, Xu DY, Jialal I. C-reactive protein increases plasminogen activator inhibitor-1 expression and activity in human aortic endothelial cells: implications for the metabolic syndrome and atherothrombosis. Circulation 2003; 107:398–404.. doi: 10.1161/01.CIR.0000052617.91920.FD. [DOI] [PubMed] [Google Scholar]
- 31.Poli KA, Tofler GH, Larson MG, Evans JC, Sutherland PA, Lipinska I, et al. Association of blood pressure with fibrinolytic potential in the Framingham offspring population. Circulation 2000; 101:264–269.. doi: 10.1161/01.CIR.101.3.264. [DOI] [PubMed] [Google Scholar]
- 32.Choy E, Sattar N. Interpreting lipid levels in the context of high-grade inflammatory states with a focus on rheumatoid arthritis: a challenge to conventional cardiovascular risk actions. Ann Rheum Dis 2009; 68:460–469.. doi: 10.1136/ard.2008.101964. [DOI] [PubMed] [Google Scholar]
- 33.Kremer JM, Genovese MC, Keystone E, Taylor PC, Zuckerman SH, Ruotolo G, et al. Effects of baricitinib on lipid, apolipoprotein, and lipoprotein particle profiles in a phase IIb study of patients with active rheumatoid arthritis. Arthritis Rheumatol 2017; 69:943–952.. doi: 10.1002/art.40036. [DOI] [PubMed] [Google Scholar]
- 34.Charles-Schoeman C, Fleischmann R, Davignon J, Schwartz H, Turner SM, Beysen C, et al. Potential mechanisms leading to the abnormal lipid profile in patients with rheumatoid arthritis versus healthy volunteers and reversal by tofacitinib. Arthritis Rheumatol 2015; 67:616–625.. doi: 10.1002/art.38974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Souto A, Salgado E, Maneiro JR, Mera A, Carmona L, Gomez-Reino JJ. Lipid profile changes in patients with chronic inflammatory arthritis treated with biologic agents and tofacitinib in randomized clinical trials: a systematic review and meta-analysis. Arthritis Rheumatol 2015; 67:117–127.. doi: 10.1002/art.38894. [DOI] [PubMed] [Google Scholar]
- 36.Watanabe J, Charles-Schoeman C, Miao Y, Elashoff D, Lee YY, Katselis G, et al. Proteomic profiling following immunoaffinity capture of high-density lipoprotein: association of acute-phase proteins and complement factors with proinflammatory high-density lipoprotein in rheumatoid arthritis. Arthritis Rheum 2012; 64:1828–1837.. doi: 10.1002/art.34363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim JY, Lee EY, Park JK, Song YW, Kim JR, Cho KH. Patients with rheumatoid arthritis show altered lipoprotein profiles with dysfunctional high-density lipoproteins that can exacerbate inflammatory and atherogenic process. PLoS One 2016; 11:e0164564.doi: 10.1371/journal.pone.0164564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hashizume M, Mihara M. Atherogenic effects of TNF-alpha and IL-6 via up-regulation of scavenger receptors. Cytokine 2012; 58:424–430.. doi: 10.1016/j.cyto.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 39.Charles-Schoeman C, Lee YY, Grijalva V, Amjadi S, FitzGerald J, Ranganath VK, et al. Cholesterol efflux by high density lipoproteins is impaired in patients with active rheumatoid arthritis. Ann Rheum Dis 2012; 71:1157–1162.. doi: 10.1136/annrheumdis-2011-200493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wen W, He M, Liang X, Gao SS, Zhou J, Yuan ZY. Accelerated transformation of macrophage-derived foam cells in the presence of collagen-induced arthritis mice serum is associated with dyslipidemia. Autoimmunity 2016; 49:115–123.. doi: 10.3109/08916934.2015.1118761. [DOI] [PubMed] [Google Scholar]
- 41.Giles JT, Danielides S, Szklo M, Post WS, Blumenthal RS, Petri M, et al. Insulin resistance in rheumatoid arthritis: disease-related indicators and associations with the presence and progression of subclinical atherosclerosis. Arthritis Rheumatol 2015; 67:626–636.. doi: 10.1002/art.38986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gonzalez-Gay MA, Gonzalez-Juanatey C, Vazquez-Rodriguez TR, Miranda-Filloy JA, Llorca J. Insulin resistance in rheumatoid arthritis: the impact of the anti-TNF-alpha therapy. Ann N Y Acad Sci 2010; 1193:153–159.. doi: 10.1111/j.1749-6632.2009.05287.x. [DOI] [PubMed] [Google Scholar]
- 43.Nicolau J, Lequerre T, Bacquet H, Vittecoq O. Rheumatoid arthritis, insulin resistance, and diabetes. Joint Bone Spine 2017; 84:411–416.. doi: 10.1016/j.jbspin.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 44.Ruscitti P, Cipriani P, Cantarini L, Liakouli V, Vitale A, Carubbi F, et al. Efficacy of inhibition of IL-1 in patients with rheumatoid arthritis and type 2 diabetes mellitus: two case reports and review of the literature. J Med Case Rep 2015; 9:123.doi: 10.1186/s13256-015-0603-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Solomon DH, Karlson EW, Rimm EB, Cannuscio CC, Mandl LA, Manson JE, et al. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation 2003; 107:1303–1307.. doi: 10.1161/01.CIR.0000054612.26458.B2. [DOI] [PubMed] [Google Scholar]
- 46.del Rincon ID, Williams K, Stern MP, Freeman GL, Escalante A. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum 2001; 44:2737–2745.. doi: 10.1002/1529-0131(200112)44:12<2737::AID-ART460>3.0.CO;2-%23. [DOI] [PubMed] [Google Scholar]
- 47.Faccini A, Kaski JC, Camici PG. Coronary microvascular dysfunction in chronic inflammatory rheumatoid diseases. Eur Heart J 2016; 37:1799–1806.. doi: 10.1093/eurheartj/ehw018. [DOI] [PubMed] [Google Scholar]
- 48.Jurewicz M, McDermott DH, Sechler JM, Tinckam K, Takakura A, Carpenter CB, et al. Human T and natural killer cells possess a functional renin-angiotensin system: further mechanisms of angiotensin II-induced inflammation. J Am Soc Nephrol 2007; 18:1093–1102.. doi: 10.1681/asn.2006070707. [DOI] [PubMed] [Google Scholar]
- 49.Leonard S, Croy BA, Murrant CL. Arteriolar reactivity in lymphocyte-deficient mice. Am J Physiol Heart Circ Physiol 2011; 301:H1276–H1285.. doi: 10.1152/ajpheart.00346.2011. [DOI] [PubMed] [Google Scholar]
- 50.Raggi P, Genest J, Giles JT, Rayner KJ, Dwivedi G, Beanlands RS, et al. Role of inflammation in the pathogenesis of atherosclerosis and therapeutic interventions. Atherosclerosis 2018; 276:98–108.. doi: 10.1016/j.atherosclerosis.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 51.Lauper K, Gabay C. Cardiovascular risk in patients with rheumatoid arthritis. Semin Immunopathol 2017; 39:447–459.. doi: 10.1007/s00281-017-0632-2. [DOI] [PubMed] [Google Scholar]
- 52.Zhang J, Chen L, Delzell E, Muntner P, Hillegass WB, Safford MM, et al. The association between inflammatory markers, serum lipids and the risk of cardiovascular events in patients with rheumatoid arthritis. Ann Rheum Dis 2014; 73:1301–1308.. doi: 10.1136/annrheumdis-2013-204715. [DOI] [PubMed] [Google Scholar]
- 53.Arts EE, Fransen J, den Broeder AA, Popa CD, van Riel PL. The effect of disease duration and disease activity on the risk of cardiovascular disease in rheumatoid arthritis patients. Ann Rheum Dis 2015; 74:998–1003.. doi: 10.1136/annrheumdis-2013-204531. [DOI] [PubMed] [Google Scholar]
- 54.Wahlin B, Meedt T, Jonsson F, Henein MY, Wallberg-Jonsson S. Coronary artery calcification is related to inflammation in rheumatoid arthritis: a long-term follow-up study. Biomed Res Int 2016; 2016:1261582.doi: 10.1155/2016/1261582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zacho J, Tybjaerg-Hansen A, Jensen JS, Grande P, Sillesen H, Nordestgaard BG. Genetically elevated C-reactive protein and ischemic vascular disease. N Engl J Med 2008; 359:1897–1908.. doi: 10.1056/NEJMoa0707402. [DOI] [PubMed] [Google Scholar]
- 56.Mason JC, Libby P. Cardiovascular disease in patients with chronic inflammation: mechanisms underlying premature cardiovascular events in rheumatologic conditions. Eur Heart J 2015; 36:482–489.. doi: 10.1093/eurheartj/ehu403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bacchiega BC, Bacchiega AB, Usnayo MJ, Bedirian R, Singh G, Pinheiro GD. Interleukin 6 inhibition and coronary artery disease in a high-risk population: a prospective community-based clinical study. J Am Heart Assoc 2017; 6: doi: 10.1161/jaha.116.005038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin-1beta inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS). Am Heart J 2011; 162:597–605.. doi: 10.1016/j.ahj.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 59.Low AS, Symmons DP, Lunt M, Mercer LK, Gale CP, Watson KD, et al. Relationship between exposure to tumour necrosis factor inhibitor therapy and incidence and severity of myocardial infarction in patients with rheumatoid arthritis. Ann Rheum Dis 2017; 76:654–660.. doi: 10.1136/annrheumdis-2016-209784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Steyers CM, 3rd, Miller FJ., Jr Endothelial dysfunction in chronic inflammatory diseases. Int J Mol Sci 2014; 15:11324–11349.. doi: 10.3390/ijms150711324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang S, Zhao Y, Xu M, Yu L, Zhao Y, Chen J, et al. FoxO3a modulates hypoxia stress induced oxidative stress and apoptosis in cardiac microvascular endothelial cells. PLoS One 2013; 8:e80342.doi: 10.1371/journal.pone.0080342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jessee R, Peart E, Beineke P, Rosenberg S, Wingrove JA, Kraus WE, et al. Rheumatoid arthritis complicates noninvasive whole blood gene expression testing for coronary artery disease. Am Heart J 2017; 192:13–18.. doi: 10.1016/j.ahj.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 63.Winchester R, Giles JT, Nativ S, Downer K, Zhang HZ, Bag-Ozbek A, et al. Association of elevations of specific T cell and monocyte subpopulations in rheumatoid arthritis with subclinical coronary artery atherosclerosis. Arthritis Rheumatol 2016; 68:92–102.. doi: 10.1002/art.39419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zeisbrich M, Yanes RE, Zhang H, Watanabe R, Li Y, Brosig L, et al. Hypermetabolic macrophages in rheumatoid arthritis and coronary artery disease due to glycogen synthase kinase 3b inactivation. Ann Rheum Dis 2018; 77:1053–1062.. doi: 10.1136/annrheumdis-2017-212647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jin HM, Kee SJ, Cho YN, Kang JH, Kim MJ, Jung HJ, et al. Dysregulated osteoclastogenesis is related to natural killer T cell dysfunction in rheumatoid arthritis. Arthritis Rheumatol 2015; 67:2639–2650.. doi: 10.1002/art.39244. [DOI] [PubMed] [Google Scholar]
- 66.Chen J, Yang J, Qiao Y, Li X. Understanding the regulatory roles of natural killer T cells in rheumatoid arthritis: T helper cell differentiation dependent or independent? Scand J Immunol 2016; 84:197–203.. doi: 10.1111/sji.12460. [DOI] [PubMed] [Google Scholar]
- 67.Miellot-Gafsou A, Biton J, Bourgeois E, Herbelin A, Boissier MC, Bessis N. Early activation of invariant natural killer T cells in a rheumatoid arthritis model and application to disease treatment. Immunology 2010; 130:296–306.. doi: 10.1111/j.1365-2567.2009.03235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Getz GS, Reardon CA. Natural killer T cells in atherosclerosis. Nat Rev Cardiol 2017; 14:304–314.. doi: 10.1038/nrcardio.2017.2. [DOI] [PubMed] [Google Scholar]
- 69.Sokolove J, Brennan MJ, Sharpe O, Lahey LJ, Kao AH, Krishnan E, et al. Brief report: citrullination within the atherosclerotic plaque: a potential target for the anti-citrullinated protein antibody response in rheumatoid arthritis. Arthritis Rheum 2013; 65:1719–1724.. doi: 10.1002/art.37961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Montes A, Corrales A, Calaza M, Lopez-Mejias R, Parra JA, Gonzalez-Gay MA, et al. Brief report: lack of replication of an association between anti-citrullinated fibrinogen and subclinical atherosclerosis in patients with rheumatoid arthritis. Arthritis Rheumatol 2015; 67:2861–2865.. doi: 10.1002/art.39302. [DOI] [PubMed] [Google Scholar]
- 71.Borissoff JI, Joosen IA, Versteylen MO, Brill A, Fuchs TA, Savchenko AS, et al. Elevated levels of circulating DNA and chromatin are independently associated with severe coronary atherosclerosis and a prothrombotic state. Arterioscler Thromb Vasc Biol 2013; 33:2032–2040.. doi: 10.1161/atvbaha.113.301627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Geraldino-Pardilla L, Giles JT, Sokolove J, Zartoshti A, Robinson WH, Budoff M, et al. Association of anti-citrullinated peptide antibodies with coronary artery calcification in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2017; 69:1276–1281.. doi: 10.1002/acr.23106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Spinelli FR, Pecani A, Ciciarello F, Colasanti T, Di Franco M, Miranda F, et al. Association between antibodies to carbamylated proteins and subclinical atherosclerosis in rheumatoid arthritis patients. BMC Musculoskelet Disord 2017; 18:214.doi: 10.1186/s12891-017-1563-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nowak B, Madej M, Luczak A, Malecki R, Wiland P. Disease activity, oxidized-LDL fraction and anti-oxidized LDL antibodies influence cardiovascular risk in rheumatoid arthritis. Adv Clin Exp Med 2016; 25:43–50.. doi: 10.17219/acem/29847. [DOI] [PubMed] [Google Scholar]
- 75.Vuilleumier N, Bratt J, Alizadeh R, Jogestrand T, Hafstrom I, Frostegard J. Anti-apoA-1 IgG and oxidized LDL are raised in rheumatoid arthritis (RA): potential associations with cardiovascular disease and RA disease activity. Scand J Rheumatol 2010; 39:447–453.. doi: 10.3109/03009741003742755. [DOI] [PubMed] [Google Scholar]
- 76.Mikuls TR, Duryee MJ, England BR, Anderson DR, Hearth-Holmes M, Su K, et al. Malondialdehyde-acetaldehyde antibody concentrations in rheumatoid arthritis and other rheumatic conditions. Int Immunopharmacol 2018; 56:113–118.. doi: 10.1016/j.intimp.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 77.Vlaicu SI, Tatomir A, Boodhoo D, Vesa S, Mircea PA, Rus H. The role of complement system in adipose tissue-related inflammation. Immunol Res 2016; 64:653–664.. doi: 10.1007/s12026-015-8783-5. [DOI] [PubMed] [Google Scholar]
- 78.Moreno-Navarrete JM, Fernandez-Real JM. The complement system is dysfunctional in metabolic disease: evidences in plasma and adipose tissue from obese and insulin resistant subjects. Semin Cell Dev Biol 2019; 85:164–172.. doi: 10.1016/j.semcdb.2017.10.025. [DOI] [PubMed] [Google Scholar]
- 79.Shields KJ, Mollnes TE, Eidet JR, Mikkelsen K, Almdahl SM, Bottazzi B, et al. Plasma complement and vascular complement deposition in patients with coronary artery disease with and without inflammatory rheumatic diseases. PLoS One 2017; 12:e0174577.doi: 10.1371/journal.pone.0174577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Matsunaga H, Iwashita M, Shinjo T, Yamashita A, Tsuruta M, Nagasaka S, et al. Adipose tissue complement factor B promotes adipocyte maturation. Biochem Biophys Res Commun 2018; 495:740–748.. doi: 10.1016/j.bbrc.2017.11.069. [DOI] [PubMed] [Google Scholar]
- 81.England BR, Thiele GM, Anderson DR, Mikuls TR. Increased cardiovascular risk in rheumatoid arthritis: mechanisms and implications. BMJ 2018; 361:k1036.doi: 10.1136/bmj.k1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Agca R, Heslinga SC, Rollefstad S, Heslinga M, McInnes IB, Peters MJ, et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis 2017; 76:17–28.. doi: 10.1136/annrheumdis-2016-209775. [DOI] [PubMed] [Google Scholar]
- 83.Smolen JS, Landewe R, Bijlsma J, Burmester G, Chatzidionysiou K, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 2017; 76:960–977.. doi: 10.1136/annrheumdis-2016-210715. [DOI] [PubMed] [Google Scholar]
- 84.Roubille C, Richer V, Starnino T, McCourt C, McFarlane A, Fleming P, et al. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Ann Rheum Dis 2015; 74:480–489.. doi: 10.1136/annrheumdis-2014-206624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ridker PM, Everett BM, Pradhan A, MacFadyen JG, Solomon DH, Zaharris E, et al. Low-dose methotrexate for the prevention of atherosclerotic events. N Engl J Med 2019; 380:752–762.. doi: 10.1056/NEJMoa1809798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rempenault C, Combe B, Barnetche T, Gaujoux-Viala C, Lukas C, Morel J, et al. Metabolic and cardiovascular benefits of hydroxychloroquine in patients with rheumatoid arthritis: a systematic review and meta-analysis. Ann Rheum Dis 2018; 77:98–103.. doi: 10.1136/annrheumdis-2017-211836. [DOI] [PubMed] [Google Scholar]
- 87.Hung YM, Wang YH, Lin L, Wang PYP, Chiou JY, Wei JC. Hydroxychloroquine may be associated with reduced risk of coronary artery diseases in patients with rheumatoid arthritis: a nationwide population-based cohort study. Int J Clin Pract 2018; 72:e13095.doi: 10.1111/ijcp.13095. [DOI] [PubMed] [Google Scholar]
- 88.Hartman O, Kovanen PT, Lehtonen J, Eklund KK, Sinisalo J. Hydroxychloroquine for the prevention of recurrent cardiovascular events in myocardial infarction patients: rationale and design of the OXI trial. Eur Heart J Cardiovasc Pharmacother 2017; 3:92–97.. doi: 10.1093/ehjcvp/pvw035. [DOI] [PubMed] [Google Scholar]
- 89.Naranjo A, Sokka T, Descalzo MA, Calvo-Alen J, Horslev-Petersen K, Luukkainen RK, et al. Cardiovascular disease in patients with rheumatoid arthritis: results from the QUEST-RA study. Arthritis Res Ther 2008; 10:R30.doi: 10.1186/ar2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vohra K, Krishan P, Varma S, Kalra HS. Exploring the potential of low-dose sulfasalazine in stable coronary artery disease patients: randomized, double-blind, placebo-controlled study. Eur Heart J Cardiovasc Pharmacother 2015; 1:214–216.. doi: 10.1093/ehjcvp/pvv021. [DOI] [PubMed] [Google Scholar]
- 91.Tabit CE, Holbrook M, Shenouda SM, Dohadwala MM, Widlansky ME, Frame AA, et al. Effect of sulfasalazine on inflammation and endothelial function in patients with established coronary artery disease. Vasc Med 2012; 17:101–107.. doi: 10.1177/1358863x12440117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kellner H, Bornholdt K, Hein G. Leflunomide in the treatment of patients with early rheumatoid arthritis--results of a prospective non-interventional study. Clin Rheumatol 2010; 29:913–920.. doi: 10.1007/s10067-010-1425-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Charles-Schoeman C, Wang X, Lee YY, Shahbazian A, Navarro-Millan I, Yang S, et al. Association of triple therapy with improvement in cholesterol profiles over two-year followup in the treatment of early aggressive rheumatoid arthritis trial. Arthritis Rheumatol 2016; 68:577–586.. doi: 10.1002/art.39502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hung YM, Lin L, Chen CM, Chiou JY, Wang YH, Wang PY, et al. The effect of anti-rheumatic medications for coronary artery diseases risk in patients with rheumatoid arthritis might be changed over time: a nationwide population-based cohort study. PLoS One 2017; 12:e0179081.doi: 10.1371/journal.pone.0179081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.del Rincon I, Battafarano DF, Restrepo JF, Erikson JM, Escalante A. Glucocorticoid dose thresholds associated with all-cause and cardiovascular mortality in rheumatoid arthritis. Arthritis Rheumatol 2014; 66:264–272.. doi: 10.1002/art.38210. [DOI] [PubMed] [Google Scholar]
- 96.Hodge JA, Kawabata TT, Krishnaswami S, Clark JD, Telliez JB, Dowty ME, et al. The mechanism of action of tofacitinib - an oral Janus kinase inhibitor for the treatment of rheumatoid arthritis. Clin Exp Rheumatol 2016; 34:318–328.. doi:. [PubMed] [Google Scholar]
- 97.Maeshima K, Yamaoka K, Kubo S, Nakano K, Iwata S, Saito K, et al. The JAK inhibitor tofacitinib regulates synovitis through inhibition of interferon-gamma and interleukin-17 production by human CD4+ T cells. Arthritis Rheum 2012; 64:1790–1798.. doi: 10.1002/art.34329. [DOI] [PubMed] [Google Scholar]
- 98.Bili A, Tang X, Pranesh S, Bozaite R, Morris SJ, Antohe JL, et al. Tumor necrosis factor alpha inhibitor use and decreased risk for incident coronary events in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2014; 66:355–363.. doi: 10.1002/acr.22166. [DOI] [PubMed] [Google Scholar]
- 99.Agca R, Heslinga SC, van Halm VP, Nurmohamed MT. Atherosclerotic cardiovascular disease in patients with chronic inflammatory joint disorders. Heart 2016; 102:790–795.. doi: 10.1136/heartjnl-2015-307838. [DOI] [PubMed] [Google Scholar]
- 100.Daien CI, Duny Y, Barnetche T, Daures JP, Combe B, Morel J. Effect of TNF inhibitors on lipid profile in rheumatoid arthritis: a systematic review with meta-analysis. Ann Rheum Dis 2012; 71:862–868.. doi: 10.1136/annrheumdis-2011-201148. [DOI] [PubMed] [Google Scholar]
- 101.Ljung L, Rantapaa-Dahlqvist S, Jacobsson LT, Askling J. Response to biological treatment and subsequent risk of coronary events in rheumatoid arthritis. Ann Rheum Dis 2016; 75:2087–2094.. doi: 10.1136/annrheumdis-2015-208995. [DOI] [PubMed] [Google Scholar]
- 102.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017; 377:1119–1131.. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 103.Ikonomidis I, Tzortzis S, Andreadou I, Paraskevaidis I, Katseli C, Katsimbri P, et al. Increased benefit of interleukin-1 inhibition on vascular function, myocardial deformation, and twisting in patients with coronary artery disease and coexisting rheumatoid arthritis. Circ Cardiovasc Imaging 2014; 7:619–628.. doi: 10.1161/circimaging.113.001193. [DOI] [PubMed] [Google Scholar]
- 104.McInnes IB, Thompson L, Giles JT, Bathon JM, Salmon JE, Beaulieu AD, et al. Effect of interleukin-6 receptor blockade on surrogates of vascular risk in rheumatoid arthritis: MEASURE, a randomised, placebo-controlled study. Ann Rheum Dis 2015; 74:694–702.. doi: 10.1136/annrheumdis-2013-204345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gabay C, McInnes IB, Kavanaugh A, Tuckwell K, Klearman M, Pulley J, et al. Comparison of lipid and lipid-associated cardiovascular risk marker changes after treatment with tocilizumab or adalimumab in patients with rheumatoid arthritis. Ann Rheum Dis 2016; 75:1806–1812.. doi: 10.1136/annrheumdis-2015-207872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rao VU, Pavlov A, Klearman M, Musselman D, Giles JT, Bathon JM, et al. An evaluation of risk factors for major adverse cardiovascular events during tocilizumab therapy. Arthritis Rheumatol 2015; 67:372–380.. doi: 10.1002/art.38920. [DOI] [PubMed] [Google Scholar]
- 107.Kim SC, Solomon DH, Rogers JR, Gale S, Klearman M, Sarsour K, et al. Cardiovascular safety of tocilizumab versus tumor necrosis factor inhibitors in patients with rheumatoid arthritis: a multi-database cohort study. Arthritis Rheumatol 2017; 69:1154–1164.. doi: 10.1002/art.40084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ikonomidis I, Pavlidis G, Katsimbri P, Andreadou I, Triantafyllidi H, Tsoumani M, et al. Differential effects of inhibition of interleukin 1 and 6 on myocardial, coronary and vascular function. Clin Res Cardiol 2019; 108:1093–1101.. doi: 10.1007/s00392-019-01443-9. [DOI] [PubMed] [Google Scholar]
- 109.Kleveland O, Ueland T, Kunszt G, Bratlie M, Yndestad A, Broch K, et al. Interleukin-6 receptor inhibition with tocilizumab induces a selective and substantial increase in plasma IP-10 and MIP-1beta in non-ST-elevation myocardial infarction. Int J Cardiol 2018; 271:1–7.. doi: 10.1016/j.ijcard.2018.04.136. [DOI] [PubMed] [Google Scholar]
- 110.Holte E, Kleveland O, Ueland T, Kunszt G, Bratlie M, Broch K, et al. Effect of interleukin-6 inhibition on coronary microvascular and endothelial function in myocardial infarction. Heart 2017; 103:1521–1527.. doi: 10.1136/heartjnl-2016-310875. [DOI] [PubMed] [Google Scholar]
- 111.Benucci M, Saviola G, Manfredi M, Sarzi-Puttini P, Atzeni F. Factors correlated with improvement of endothelial dysfunction during rituximab therapy in patients with rheumatoid arthritis. Biologics 2013; 7:69–75.. doi: 10.2147/btt.s39182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hsue PY, Scherzer R, Grunfeld C, Imboden J, Wu Y, Del Puerto G, et al. Depletion of B-cells with rituximab improves endothelial function and reduces inflammation among individuals with rheumatoid arthritis. J Am Heart Assoc 2014; 3:e001267.doi: 10.1161/jaha.114.001267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Provan SA, Berg IJ, Hammer HB, Mathiessen A, Kvien TK, Semb AG. The impact of newer biological disease modifying anti-rheumatic drugs on cardiovascular risk factors: a 12-month longitudinal study in rheumatoid arthritis patients treated with rituximab, abatacept and tociliziumab. PLoS One 2015; 10:e0130709.doi: 10.1371/journal.pone.0130709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gottenberg JE, Morel J, Perrodeau E, Bardin T, Combe B, Dougados M, et al. Comparative effectiveness of rituximab, abatacept, and tocilizumab in adults with rheumatoid arthritis and inadequate response to TNF inhibitors: prospective cohort study. BMJ 2019; 364:167.doi: 10.1136/bmj.l67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.van den Oever IAM, Heslinga M, Griep EN, Griep-Wentink HRM, Schotsman R, Cambach W, et al. Cardiovascular risk management in rheumatoid arthritis patients still suboptimal: the Implementation of Cardiovascular Risk Management in Rheumatoid Arthritis project. Rheumatology (Oxford) 2017; 56:1472–1478.. doi: 10.1093/rheumatology/kew497. [DOI] [PubMed] [Google Scholar]
- 116.Liao KP. Cardiovascular disease in patients with rheumatoid arthritis. Trends Cardiovasc Med 2017; 27:136–140.. doi: 10.1016/j.tcm.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sintek MA, Sparrow CT, Mikuls TR, Lindley KJ, Bach RG, Kurz HI, et al. Repeat revascularisation outcomes after percutaneous coronary intervention in patients with rheumatoid arthritis. Heart 2016; 102:363–369.. doi: 10.1136/heartjnl-2015-308634. [DOI] [PubMed] [Google Scholar]
- 118.Lai CH, Lai WW, Chiou MJ, Lin WC, Yang YJ, Li CY, et al. Outcomes of percutaneous coronary intervention in patients with rheumatoid arthritis and systemic lupus erythematosus: an 11-year nationwide cohort study. Ann Rheum Dis 2016; 75:1350–1356.. doi: 10.1136/annrheumdis-2015-207719. [DOI] [PubMed] [Google Scholar]
- 119.Ozen G, Sunbul M, Atagunduz P, Direskeneli H, Tigen K, Inanc N. The 2013 ACC/AHA 10-year atherosclerotic cardiovascular disease risk index is better than SCORE and QRisk II in rheumatoid arthritis: is it enough? Rheumatology (Oxford) 2016; 55:513–522.. doi: 10.1093/rheumatology/kev363. [DOI] [PubMed] [Google Scholar]
- 120.Nikiphorou E, Norton S, Carpenter L, Dixey J, Andrew Walsh D, Kiely P, et al. Secular changes in clinical features at presentation of rheumatoid arthritis: increase in comorbidity but improved inflammatory states. Arthritis Care Res (Hoboken) 2017; 69:21–27.. doi: 10.1002/acr.23014. [DOI] [PubMed] [Google Scholar]
- 121.Khambhati J, Engels M, Allard-Ratick M, Sandesara PB, Quyyumi AA, Sperling L. Immunotherapy for the prevention of atherosclerotic cardiovascular disease: Promise and possibilities. Atherosclerosis 2018; 276:1–9.. doi: 10.1016/j.atherosclerosis.2018.07.007. [DOI] [PubMed] [Google Scholar]


