Abstract
Background:
The pathogenesis of multiple sclerosis (MS) is mediated primarily by T cells, but most studies of MS and its animal model, experimental autoimmune encephalomyelitis (EAE), have focused on CD4+ T cells. The aims of the current study were to determine the pathological interrelationship between CD4 and CD8 autoreactive T cells in MS/EAE.
Methods:
Female C57BL/6 mice (n = 20) were induced by myelin oligodendrocyte glycoprotein (MOG)35–55 peptide. At 14 days after immunization, T cells were isolated from the spleen and purified as CD4+ and CD8+ T cells by using CD4 and CD8 isolation kits, and then the purity was determined by flow cytometric analysis. These cells were stimulated by MOG35–55 peptide and applied to proliferation assays. The interferon-gamma (IFN-γ) and interleukin (IL)-4 secretion of supernatant of cultured CD4+ and CD8+ T cells were measured by enzyme-linked immunosorbent assays (ELISA). For adoptive transfer, recipient mice were injected with MOG35–55-specific CD8+ or CD4+ T cells. EAE clinical course was measured by EAE score at 0–5 scale and spinal cord was examined by staining with hematoxylin and eosin and Luxol fast blue staining.
Results:
CD8+CD3+ and CD4+CD3+ cells were 86% and 94% pure of total CD3+ cells after CD8/CD4 bead enrichment, respectively. These cells were stimulated by MOG35–55 peptide and applied to proliferation assays. Although the CD8+ T cells had a generally lower response to MOG35–55 than CD4+ T cells, the response of CD8+ T cells was not always dependent on CD4. CD8+ T cell secreted less IFN-γ and IL-4 compared with CD4+ T cells. EAE was induced in wildtype B6 naïve mice by adoptive transfer of MOG35–55-specific T cells from B6 active-induced EAE (aEAE) mice. A similar EAE score and slight inflammation and demyelination were found in naive B6 mice after transferring of CD8+ T cells from immunized B6 mice compared with transfer of CD4+ T cells.
Conclusion:
Our data suggest that CD8+ autoreactive T cells in EAE have a lower encephalitogenic function but are unique and independent on pathogenic of EAE rather than their CD4+ counterparts.
Keywords: Autoimmune, CD8-Positive T-Lymphocytes, Encephalomyelitis, Experimental, Multiple Sclerosis, Myelin-Oligodendrocyte Glycoprotein
Introduction
Multiple sclerosis (MS) is an autoimmune disease that occurs in central nervous system (CNS).[1] Unfortunately, current therapies of MS are based on non-specific immunosuppressive drugs, such as natalizumab, fingolimod, mitoxantrone, and dimethyl fumarate, which have many side effects.[1,2] The pathogenesis of MS is mediated primarily by T cells that are activated by CNS auto-antigens and finally damage the myelin sheath to generate active lesions in the CNS.[3] Typically, studies of MS and its animal model, experimental autoimmune encephalomyelitis (EAE), have focused on CD4+ T cells, which is induced by myelin antigens (aEAE) or adoptive transfer of myelin-reactive T cells (tEAE).[3] However, other T cells subsets such as CD8+ T cells are involved in MS pathogenesis.[4,5] CD8+ T cells are present in MS tissue at all stages of the disease. There are more CD8+ T cells than CD4+ T cells in lesions, perivascular cuffs, normal-appearing white matter, and CNS parenchymal lesions.[4] CD8+ T cells in MS are characterized by their antigenic repertoire.[5] Furthermore, studies of CD8+ T cells in MS are rarer than those of CD4+ T cells, but it is indisputable that CD8+ T cells play a critical role in MS pathogenesis in addition to CD4+ T cells.[6]
Our previous studies have demonstrated that in experimental autoimmune uveitis (EAU) CD8 autoreactive T cells are major participants in the autoimmune response.[7–10] We have previously reported that the commonly used uveitogenic peptide, interphotoreceptor retinoid-binding protein (IRBP)1–20, generates both CD4+ and CD8+ autoreactive T cells in the C57BL/6 (B6) mouse, and showed that CD8+ autoreactive T cells are uveitogenic.[7,10] We also characterized proteolipid protein (PLP)56–70-specific CD4+ autoreactive T cells of EAE in Biozi AB/H mice,[11] and glutamic acid decarboxylase65-specific CD4+ autoreactive T cells of type I diabetes in non-obese diabetic (NOD) mice.[12]
The aims of the current study were to determine the pathological interrelationship between CD4 and CD8 autoreactive T cells in MS/EAE. As we reported previously, IRBP1–20-specific CD4+ and CD8+ autoreactive T cells from B6 EAU mouse were uvietogenic, while CD8+ autoreactive T cells have weaker proliferation response, and less interferon-gamma (IFN-γ) secretion and uveitis.[7,8,10]. Because the pathological activity of autoreactive T cells is closely associated with their degree of activation, we focused on cell proliferation, secretion of Th1 cytokines, such as IFN-γ, and T helper 2 (Th2) cytokines, such as IL-4, as well as their encephalitogenic functions upon the myelin oligodendrocyte glycoprotein (MOG)35–55-specific response.
Methods
Ethical approval
All the experiments were performed at School of Life Sciences, Central South University, and all experimental steps were examined and approved by the Ethics Committee of Central South University (No. 2019sydw0005). The treatments for animal were in accordance with the National Institute of Health's Guide for the Care and Use of Laboratory Animals. We have taken all steps to minimize the animal's pain and suffering.
Animals
Specific pathogen-free female C57BL/6 mice (10–14 weeks, n = 20) were purchased from Hunan Slake Jingda Laboratory Animal Co., Ltd. (Changsha, Hunan, China) (License No. SCXK [Hunan] 2016–0002) and maintained at the Center of Experimental Animal, Xiangya Medical College, Central South University (License No. SCXK [Hunan] 2015–0017). The mice were housed in a single cage with an independent ventilation system at a temperature of 18 to 22°C and relative humidity of 40% to 60% with free access to food and water. The sample size prediction matched the requirements of the experimental design.
Active-induced and adoptive-transferred EAE models
The models were established according to the study of LaMothe et al[13] and Zhong et al[3] with modifications described in our previous studies.[10] For active induction of disease, aEAE was induced by subcutaneous injection of 200 μL of an emulsion containing 200 μg MOG35–55 peptide purified by high performance liquid chromatography (HPLC) with a purity of >95% (amino acids 35–55 of bovine MOG; Sigma-Aldrich, St. Louis, MO, USA) and complete Freund's adjuvant (Sigma-Aldrich) distributed over six sites at the tail base and on the flank. At 0 and 24 h after immunization, mice were intraperitoneally injected with pertussis toxin (List Biological, Campbell, CA). For adoptive transfer, unless stated otherwise, recipient mice were injected via the caudal vein with 5 × 106 MOG35–55-specific CD8+ or CD4+ enriched T cells prepared as described previously in 0.2 mL phosphate buffer saline (PBS).[10] EAE was scored by a 0–5 scale as follows: 0, no obvious changes in motor functions of the mouse in comparison with non-immunized mice; 1, limp tail; 2, limp tail and weakness of hind limbs; 3, limp tail and complete paralysis of hind limbs (most common) or limp tail with paralysis of one front and one hind limb; 4, complete hind limb and partial front limb paralysis; 5, death or euthanized because of severe paralysis.[13]
Preparation of MOG35–55-specific T cells
The procedure was modified from our previously published protocol.[10] At 14 days after immunization, EAE mice were sacrificed by sodium pentobarbital (Sinopharm Chemical Reagent Co., Beijing, China, 50 mg/kg), and T cells were isolated from the spleen by passage through a nylon wool column (Kisker, Steinfurt, Germany). Then, 1 × 107 cells in 2 mL Roswell Park Memorial Institute (RPMI) 1640 medium per well in a six-well plate (Costar; Corning, Corning, NY, USA) were stimulated with 20 μg/mL MOG35–55 in the presence of 1 × 107 mitomycin C (Med Chem Express, NJ, USA)-treated syngeneic spleen cells as antigen presenting cells (APCs). After 2 days, the activated lymphoblasts were isolated by density gradient centrifugation (Lymphocyte Separation, Tianjin, China) and cultured in RPMI 1640 medium containing IL-2 (USCN Co., Wuhan, Hubei, China, 10 ng/mL).
Proliferation assay
As modified from our previously published protocol,[10] CD8 or CD4 enriched T cells from MOG35–55-immunized wildtype B6 mice were prepared and seeded at 4 × 105 cells/well in 96-well plates. The cells were then cultured at 37°C for 48 h in 200 μL medium with or without MOG35–55 in the presence of mitomycin C-treated syngeneic spleen antigen presenting cells (1 × 105). [3H] thymidine incorporation during the last 8 h was assessed by a microplate scintillation counter (Packard; PerkinElmer, Meriden, CT, USA). The proliferative response is expressed as the mean counts per minute ± standard deviation (SD) of triplicate determinations.
Purification of CD4+ and CD8+ T cells
As modified from our previously published protocol,[10] purified CD4+ and CD8+ T cells were prepared from spleens using CD4 and CD8 isolation kits (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). The spleen cells were first incubated in 10 μL of CD8 MicroBeads and 90 μL buffer per 1 × 107 cells for 15 min in a refrigerator (2−8°C). Buffer was added to a final volume of 500 μL for up to 5 × 107 cells. The cells were then separated into bound and unbound cells on a magnetic separator column (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) and washed with 15 mL medium, according to the manufacturer's protocol. The flow through fraction containing CD4- or CD8-enriched cells was collected, and the purity of the isolated cell fraction was determined by flow cytometric analysis.
Flow cytometry analysis
As modified from our previously published protocol,[10] aliquots of 2 × 105 cells were double stained with combinations of allophycocyanin (APC)-, fluorescein isothiocyanate (FITC)- or phycoerythroprotein (PE)-conjugated monoclonal antibodies against mouse CD3, CD4, or CD8 (Biolegend, San Diego, CA, USA). Data collection and analysis were performed on a flow cytometer Dxp Athena (Cytek Biosciences Inc., Fremont, CA, USA). The data were analyzed by FlowJo software (FlowJo Co., San Diego, CA, USA).
Enzyme-linked immunosorbent assays
As modified from our previously published protocol,[10] 1 × 106 cells in 1 mL RPMI 1640 medium per well in a 24-well plate (Costar; Corning, Corning, NY, USA) were stimulated with 20 μg/mL MOG35–55 in the presence of mitomycin C-treated syngeneic spleen cells as antigen presenting cells. After 24 h, the IFN-γ and IL-4 secretion of supernatant of cultured CD4+ and CD8+ T cells were measured by commercially available enzyme-linked immunosorbent assays (ELISA) kits (USCN Co., Wuhan, Hubei, China).
Histology
As modified from our previously published protocol,[10] brain and spinal cord sections were prepared from tissue collected from tEAE animals that were adoptively transferred with MOG35–55-specific CD8 or CD4 enriched T cells from aEAE animals. Brain and spinal cord tissues were fixed in ice-cold 4% paraformaldehyde/methanol and embedded in paraffin before microtome sections (5 μm thick) were prepared for staining with hematoxylin and eosin (H&E), and Luxol fast blue (LFB) to stain myelin.
Statistical analysis
The statistical analysis was achieved by SPSS 21.0 statistical software (IBM, Almonk, NY, USA). All data were analyzed by the unpaired Student's t test. Data are expressed as the mean ± standard deviation (SD), including proliferation assay, ELISA, EAE score. Each experiment was repeated at least three times. A P value of ≤0.05 was considered statistically significant.
Results
MOG35–55 specificity of purified CD8+ T cells
As shown in Figure 1, there was 86% CD8+ CD3+ cells of total CD3+ cells in the fraction of T cells after CD8 bead enrichment and 94% CD4+CD3+ cells of total CD3+ cells in the fraction of T cells after CD4 bead enrichment. These CD8+CD3+ and CD4+CD3+ cells were highly pure and appropriate for the following functional experiments.
Figure 1.
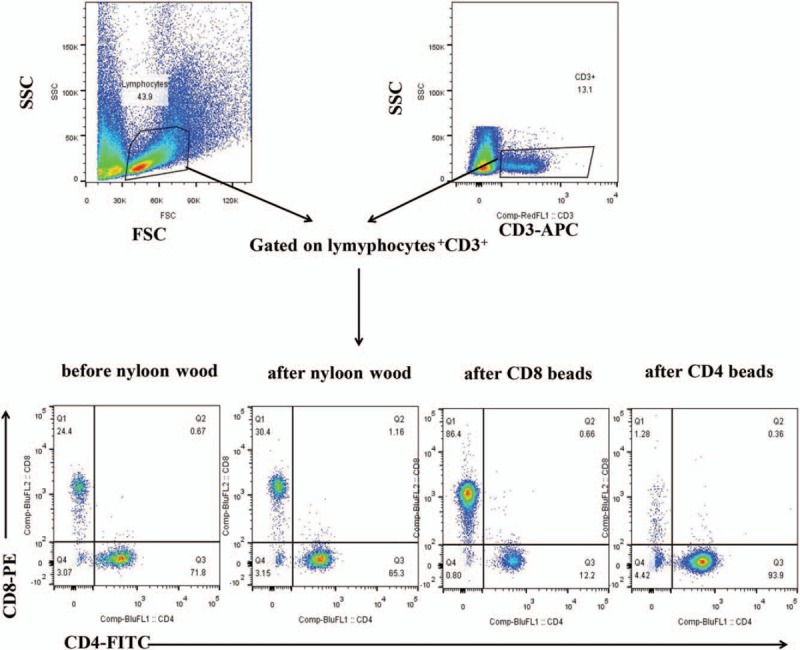
Beads-enriched CD8 T Cells are high purified. T cells were isolated from spleen cells of aEAE mice by passage through a nylon wool column, then purified by CD4 and CD8 isolation kits, finally the purity of the isolated cell fraction was determined by flow cytometric analysis with APC-conjugated anti-CD3 antibody, FITC-conjugated anti-CD4 antibody, and PE-conjugated antibody anti-CD8. aEAE: Activated EAE; APC: Allophycocyanin; FSC: Forward scatter; SSC: Side scatter; EAE: Experimental autoimmune encephalomyelitis; PE: Phycoerythroprotein; FITC: Fluorescein isothiocyanate.
The purified CD8+ and CD4+ T cells were examined for antigen-specific functions by a proliferation assay. The representative results shown in Figure 2 indicated that the MOG35–55 peptide had a strong stimulatory effect on both CD8+ and CD4+ T cells at the highest dose (20 μg/mL) of MOG35–55 peptide, but not at low dose (0.2 or 2 μg/mL) or no peptide (0 μg/mL). Although at the highest dose (20 μg/mL) of MOG35–55 peptide, purified CD8+ T cells had a lower response (21,526 ± 1236 counts per minute [cpm] vs. CD4+ T cell supernatants: 63,953 ± 3284 cpm, t = 13.34, P = 0.006) to MOG35–55 than CD4+ T cells, it was apparent that the response of CD8+ T cells was not always dependent on CD4.
Figure 2.
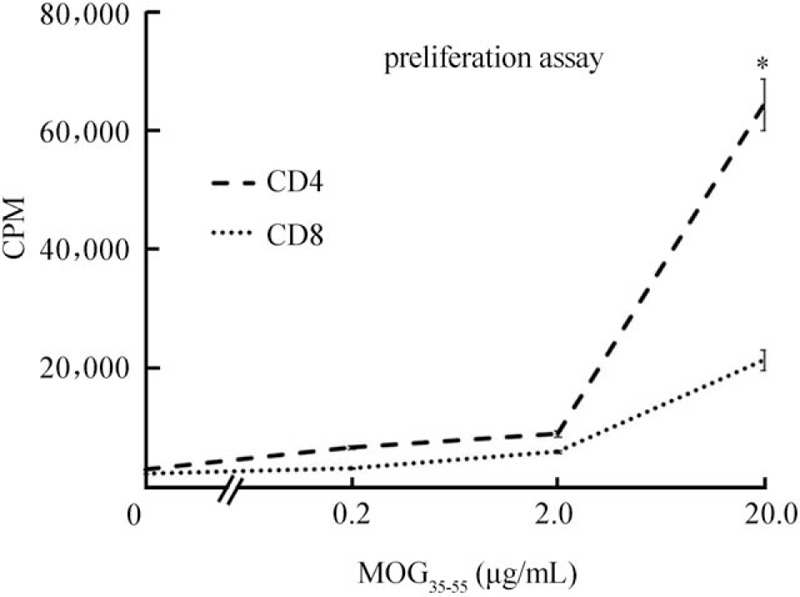
Purified CD8+ T Cells are MOG35–55-specific. To compare of the response of the purified CD4 and CD8 T cell populations to MOG35–55, CD8 or CD4 enriched T cells from MOG35–55-immunized wild-type B6 mice were prepared and seeded at 4 × 105 cells/well in 96-well plates and cultured at 37°C for 48 h in a total volume of 200 μL of medium, with or without MOG35–55, in the presence of mytomycin C-treated syngeneic spleen APCs (1 × 105), and [3H] thymidine incorporation during the last 8 h was assessed. The proliferative response is expressed as the mean counts per minute (cpm) ± SD of triplicate determinations. ∗P < 0.01. APC: antigen presenting cell; CPM: Counts per minute; MOG: Myelin oligodendrocyte glycoprotein; SD: Standard deviation.
Cytokine profiles of MOG35–55-specific CD8+ T and CD4+ T cells
To determine the cytokine profiles of activated CD4+ and CD8+ autoreactive T cells, we used ELISAs to measure cytokine levels in the culture supernatants of activated CD4+ and CD8+ T cells at 24 hours post-stimulation. As shown in Figure 3, at stimulation by the highest dose (20 μg/mL) of MOG35–55 peptide, the CD8+ T cell supernatants contained lower levels of IFN-γ (803.3 ± 39.2 pg/mL vs. CD4+ T cell supernatants: 1451.3 ± 59.4 pg/mL, t = 45.10, P = 0.001) and IL-4 (245.5 ± 11.8 pg/mL vs. CD4+ T cell supernatants: 298.6 ± 7.7 pg/mL, t = 5.75, P = 0.029) compared with CD4+ T cell supernatants.
Figure 3.
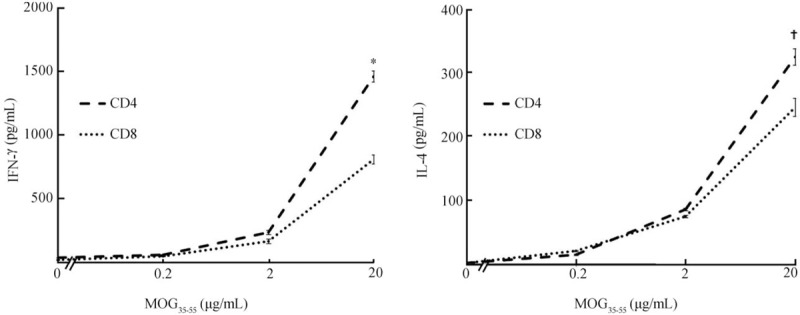
Cytokine profiles of MOG35–55-specific CD8+ T Cells are similar to those of MOG35–55-specific CD4+ T Cells. Cytokine levels in the culture medium of activated CD4+ and CD+8 T cells at 24 to 48 h poststimulation in vitro are measured by ELISA. Stimulation means that CD4 enriched T cells from MOG35–55-immunized wild-type B6 mice were prepared and seeded at 8 × 105 cells/well in 24-well plates and cultured at 37°C for 24 to 48 h in a total volume of 500 μL of medium, with or without MOG35–55, in the presence of mytomycin C-treated syngeneic spleen APCs (2 × 105). The data are the mean ± SD from three separate experiments. ∗P < 0.01, †P < 0.05. APC: Antigen presenting cell; IFN-γ: Interferon-gamma; IL-4: Interleukin-4; MOG: Myelin oligodendrocyte glycoprotein; SD: Standard deviation.
Adoptive transfer of MOG35–55-specific CD8+ T Cells to naïve mice induces tEAE
To determine the role of MOG35–55-specific CD8+ T cells in the pathogenesis of EAE, we induced the disease in wild-type B6 naïve mice by adoptive transfer of MOG35–55-specific T cells from B6 aEAE mice and then measured the clinical signs using the EAE score assessed by daily checks and histopathology.[13] As shown in Figure 4A, the EAE score after transferring CD8+ T cells from immunized B6 mice to naive B6 mice was similar to that after transferring CD4+ T cells.
Figure 4.
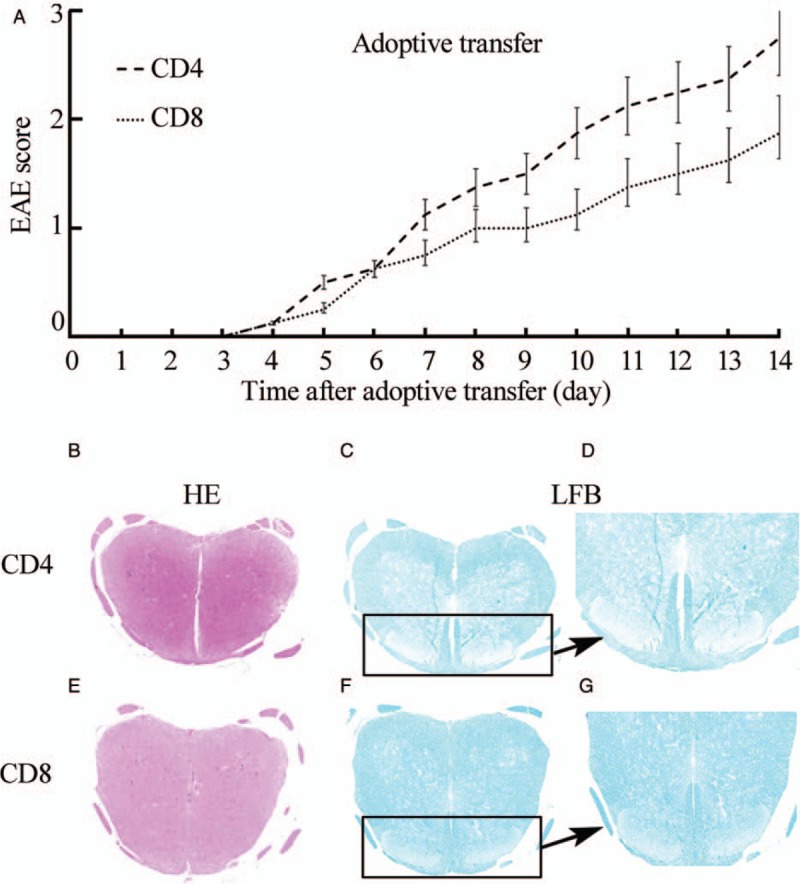
Adoptive transfer of MOG35–55-specific CD8 T Cells or CD4 T cells to naïve mice is able to induce tEAE. Attempts were made to induce disease in wild-type B6 naïve mice by adoptive transfer of CD8 MOG35–55-specific T cells or CD8 MOG35–55-specific T cells from B6 aEAE mice, and measurement of clinical signs of EAE score was assessed by daily check (A) and histopathology (B-G; H&E, original magnification ×50 [B and E]; LFB, original magnification ×50 [C and F]; LFB, original magnification ×200 [D and G]). The data are the mean±SD from three separate experiments. aEAE: Activated EAE; H&E: Hematoxylin and eosin; LFB: Luxol fast blue; MOG: Myelin oligodendrocyte glycoprotein; SD: Standard deviation; LFB: Luxol fast blue.
Pathological examination revealed slight inflammation [H&E, Figure 4B] and obvious demyelination [LFB; Figure 4C and 4D] in naive B6 mice transferred with CD8+ T cells from immunized B6 mice, which is consistent with previous observations.[14] Interestingly, the histology of mice transferred with CD8+ T cells revealed slightly worse pathology compared with mice transferred with CD4+ T cells [Figure 4B–G].
Discussion
In the present study, we used the EAE model that is very close to the EAU model with similar CD4+ autoreactive T cell-dominated autoimmune disease. The differences between the two models are the target organ and autoantigen. We used our previous strategy in the EAU model to study the role of MOG35–55-specific T cells in the pathogenesis of EAE and MS. Our current data are consistent with our previous data in the EAU model and PLP56–70-specific CD4+ autoreactive T cells of EAE in Biozi AB/H mice.[7–11]
There were similar functional profiles of CD4+ and CD8+ autoreactive T cells in the closely related autoimmune models. We believe that our data are reasonable and reliable, because MS, uveitis, and even type I diabetes are Th1-dominate autoimmune diseases, suggesting an intrinsic relationship between MS, uveitis, and type I diabetes. A possible disease mechanism is that the autoreactive T cells generated in the thymus are released to the periphery, contact with different autoantigens, and then mature and attack various organs.[15] Therefore, the local microenvironment, including dendritic cells (DC), macrophages, and non-professional parenchymal cells, is critical for development of autoimmune disease.[15] For example, different DC subsets are dependent on stimulation by a lot of pathogen, and also play critical role on the differential of T cell, of course, on MOG35–55-specific T cells.[16] Hence, there is two good research directions in the future, one is to compare the similar CD8+ autoreactive T cells in different model such as EAE, EAU and type I diabetes; another is to determine how the different DC subsets such as cDC1, cDC2, and pDC effect on the differential of MOG35–55-specific T cells, such as to Th1, Th2, Th17, regulatory T cells (Treg) etc.[16]
By the way, mast cells and neutrophil infiltration were also contributed to EAE pathogenesis.[17,18] The lower encephalitogenic functions of CD8+ autoreactive T cells in EAE than their CD4+ counterparts might be the reason for less research of CD8+ autoreactive T cells in EAE. There may be more regulatory CD8+ autoreactive T cells than their CD4+ counterparts, which is the case in EAU as we reported previously,[8] and thus this might be another good future research direction. Recently, Zhong et al[3] reported that Th1, Th17, and CD4+CD25+ regulatory T cells were found in EAE mice, and supported our future hypothesis.
Wagner et al[19] just reported a “two-side sword” publication about myelin-specific CD8+ T cells in EAE. First, it was shown that CD8+ T cells enhanced atypical but no classic EAE like CD4+ T cells by using myelin basic protein (MBP) on TCR transgenic 8.8 mice, and it supported our findings. Second, it indicated the limitations of EAE: (1) EAE is a classic animal model for MS, which is induced by activation of myelin-specific CD4+ T cells not myelin-specific CD8+ T cells[19]; (2) EAE is not able to mimic the all features of MS. There is more lesion in brain of MS rather than major lesion in spinal cord of EAE.[19] It suggest myelin-specific CD8+ T cells activated atypical EAE is unique, and also independed on myelin-specific CD4+ T cells activated classic EAE.[19] If we want to see the whole picture of MS in animal model, we have to combine at least these two EAE models: myelin-specific CD8+ and CD4+ T cells,[19] might need more EAE models such as myelin antigen-specific TCR transgenic mice EAE models, spontanous EAE models, humanized mouse EAE models, Non-human primates EAE models[22] and Zebrafish EAE models.[20–23]
Our data suggest that CD8+ autoreactive T cells in EAE have a lower encephalitogenic function but are unique and independent on pathogenic of EAE rather than their CD4+ counterparts. The pathogenesis of MS is still unclear, there are a lot of factor such as different cells and their subsets, and different kinds of EAE models which need to be investigated.
Funding
This work is supported by the grants from the Natural Science Foundation of Hunan Province, China (No. 2018JJ6043), the Health and Family Plans commission of Hunan Province, China (No. B20180815), the Science and Technology Plan Project of Zhuzhou City, Hunan Province, China (No. 20160104).
Conflicts of interest
None.
Footnotes
How to cite this article: Peng Y, Zhu FZ, Chen ZX, Zhou JX, Gan L, Yang SS, Gao S, Liu QQ. Characterization of myelin oligodendrocyte glycoprotein (MOG)35–55-specific CD8+ T cells in experimental autoimmune encephalomyelitis. Chin Med J 2019;132:2934–2940. doi: 10.1097/CM9.0000000000000551
Yong Peng and Fei-Zhou Zhu contributed equally to the work.
References
- 1.Turobov VI, Danilkovich AV, Shevelev AB, Yulia K, Biryukovaet YK, Pozdniakova NV, et al. Efficacy of synthetic peptide corresponding to the ACTH-Like sequence of human immunoglobulin G1 in experimental autoimmune encephalomyelitis. Front Pharmacol 2018; 9:1–6.. doi: 10.3389/fphar.2018.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qiu W, Huang DH, Hou SF, Zhang MN, Jin T, Dong HQ, et al. Efficacy and safety of teriflunomide in Chinese patients with relapsing forms of multiple sclerosis: a subgroup analysis of the phase 3 TOWER study. Chin Med J 2018; 131:2776–2784.. doi: 10.4103/0366-6999.246067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhong SS, Xiang YJ, Liu PJ, He Y, Yang TT, Wang YY, et al. Effect of cordyceps sinensis on the treatment of experimental autoimmune encephalomyelitis: a pilot study on mice model. Chin Med J 2017; 130:2296–2301.. doi: 10.4103/0366-6999.215335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larochelle C, Metz I, Lécuyer MA, Terouz S, Roger M, Arbour N, et al. Immunological and pathological characterization of fatal rebound MS activity following natalizumab withdrawal. Mult Scler 2017; 23:72–81.. doi: 10.1177/1352458516641775. [DOI] [PubMed] [Google Scholar]
- 5.Rangachari M, Kerfoot SM, Arbour N, Alvarez JI. Editorial: lymphocytes in MS and EAE: more than just a CD4+ world. Front Immunol 2017; 8:133.doi: 10.3389/fimmu.2017.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nieuwkamp DJ, Murk JL, van Oosten BW. PML in patients treated with dimethyl fumarate. N Engl J Med 2015; 373:584.doi: 10.1056/NEJMc1506151. [DOI] [PubMed] [Google Scholar]
- 7.Shao H, Peng Y, Liao T, Wang M, Song M, Kaplan HJ, et al. A shared epitope of the interphotoreceptor retinoid-binding protein recognized by the CD4+ and CD8+ autoreactive T cells. J Immunol 2005; 175:1851–1857.. doi: 10.4049/jimmunol.175.3.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peng Y, Shao H, Ke Y, Zhang P, Han G, Kaplan HJ, et al. Minimally activated CD8 autoreactive T cells specific for IRBP express a high level of Foxp3 and are functionally suppressive. Invest Ophthalmol Vis Sci 2007; 48:2178–2184.. doi: 10.1167/iovs.06-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han G, Shao H, Peng Y, Zhang P, Ke Y, Kaplan HJ, et al. Suppressor role of rat CD8+CD45RClow T cells in experimental autoimmune uveitis (EAU). J Neuroimmunol 2007; 183:81–88.. doi: 10.1016/j.jneuroim.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peng Y, Shao H, Ke Y, Zhang P, Xiang J, Kaplan HJ, et al. In vitro activation of CD8 interphotoreceptor retinoid-binding protein-specific T cells requires not only antigenic stimulation but also exogenous growth factors. J Immunol 2006; 176:5006–5014.. doi: 10.4049/jimmunol.176.8.5006. [DOI] [PubMed] [Google Scholar]
- 11.Peng Y, Liu CP. Characterization of proteolipid protein-peptide-specific CD(4)(+) T cell of experimental allergic encephalomyelitis in Biozzi AB/H mice. Chin Med J 2002; 115:521–524.. PMID: 12133288 No DOI. [PubMed] [Google Scholar]
- 12.Peng Y, Liu CP. Inhibition of autoimmune diabetes in NOD mice by GAD-specific regulatory T cells (in Chinese). Chin J Endocrinol Metab 2003; 19:486–490.. [Google Scholar]
- 13.LaMothe RA, Kolte PN, Vo T, Ferrari JD, Gelsinger TC, Wong J, et al. Tolerogenic nanoparticles induce antigen-specific regulatory T cells and provide therapeutic efficacy and transferrable tolerance against experimental autoimmune encephalomyelitis. Front Immunol 2018; 9:281.doi: 10.3389/fimmu.2018.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levy H, Assaf Y, Frenkel D. Characterization of brain lesions in a mouse model of progressive multiple sclerosis. Exp Neurol 2010; 226:148–158.. doi: 10.1016/j.expneurol.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 15.Waisman A, Johann L. Antigen-presenting cell diversity for T cell reactivation in central nervous system autoimmunity. J Mol Med (Berl) 2018; 96:1279–1292.. doi: 10.1007/s00109-018-1709-7. [DOI] [PubMed] [Google Scholar]
- 16.Macri C, Pang ES, Patton T, O’Keeffe M. Dendritic cell subsets. Semin Cell Dev Biol 2018; 84:11–21.. doi: 10.1016/j.semcdb.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 17.Yin JJ, Hu XQ, Mao ZF, Bao J, Wei Qiu, Lu ZQ, et al. Neutralization of interleukin-9 decreasing mast cells infiltration in experimental autoimmune encephalomyelitis. Chin Med J 2017; 130:964–971.. doi: 10.4103/0366-6999.204110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He Y, Yang FY, Sun EW. Neutrophil extracellular traps in autoimmune diseases. Chin Med J 2018; 131:1513–1519.. doi: 10.4103/0366-6999.235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wagner CA, Roqué PJ, Mileur TR, Liggitt D, Goverman JM. Myelin-specific CD8 T cells exacerbate brain inflammation in CNS autoimmunity. J Clin Invest 2019; ii:132531.doi: 10.1172/JCI132531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burrows DJ, McGown A, Jain SA, De Felice M, Ramesh TM, Sharrack B, et al. Animal models of multiple sclerosis: From rodents to zebrafish. Mult Scler 2019; 25:306–324.. doi: 10.1177/1352458518805246. [DOI] [PubMed] [Google Scholar]
- 21.Tao L, Reese TA. Making mouse models that reflect human immune responses. Trends Immunol 2017; 38:181–193.. doi: 10.1016/j.it.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 22.Stimmer L, Fovet CM, Serguera C. Experimental models of autoimmune demyelinating diseases in nonhuman primates. Vet Pathol 2018; 55:27–41.. doi: 10.1177/0300985817712794. [DOI] [PubMed] [Google Scholar]
- 23.Karttunen MJ, Czopka T, Goedhart M, Early JJ1, Lyons DA. Regeneration of myelin sheaths of normal length and thickness in the zebrafish CNS correlates with growth of axons in caliber. PLoS One 2017; 12:e0178058.doi: 10.1371/journal.pone.0178058. [DOI] [PMC free article] [PubMed] [Google Scholar]


