To the Editor: Spindle cell carcinoma (SpCC) is a rare histological type of sarcomatoid carcinoma consisting of spindle-shaped tumor cells. SpCC can occur in many different organs, including the lungs. There is limited data on the clinical presentation, genetic mutant phenotype, treatment, and outcome of lung SpCC. The efficacy of molecular targeted therapy for this tumor is still unclear.[1] Here, we report an adult male with metastatic SpCC harboring a Tropomyosin 3-ROS proto-oncogene 1 (TPM3-ROS1) fusion who showed a long-term response to targeted therapy by crizotinib, a tyrosine kinase inhibitor (TKI).
A 72-year-old Chinese male was admitted to our hospital in November 2017 with progressing cough, suffocation, and intermittent fever. He had no history of smoking. There was no family history of cancer. A chest computed tomography (CT) scan revealed an 18 × 15 mm irregular mass in the apical segment of the right upper lobe with enlargement of mediastinal lymph nodes [Figure 1A], pleura invasion [Figure 1B], and left adrenal gland metastasis [Figure 1C]. Bilateral pulmonary effusion was not apparent. Magnetic resonance imaging of the head showed right cerebellar hemisphere metastasis. A CT-guided needle biopsy of the right lung mass was performed. Three specimens were obtained by CT-guided core needle biopsy. Histologically, in all three specimens, the tumor contained mostly diffuse proliferated spindle-shaped tumor cells with partial necrosis and scattered infiltration of lymphocytes [Figure 1D and 1E]. Immunohistochemical staining showed that the tumor cells were diffusely positive for pan-cytokeratin AE1/AE3, vimentin, thyroid transcription factor 1, and Ki-67 (70%) but negative for smooth muscle action, desmin, anaplastic lymphoma kinase (ALK), calretinin, melanocyte antigen, P40, P63, and Cytokeratin5/6. Tumor cells were also found in the pleural effusion [Figure 1F]. Therefore, the patient was diagnosed with stage IV right upper lung SpCC based on the World Health Organization criteria. The Eastern Cooperative Oncology Group (ECOG) performance score was 3. Gene sequencing of plasma circulating tumor DNA (ctDNA) and tissue biopsy were performed. Next-generation sequencing revealed the harboring of a TPM3-ROS1 (E7:E31) fusion (abundance, 37.9%) but no ALK rearrangement. No other targeted gene mutations were identified.
Figure 1.
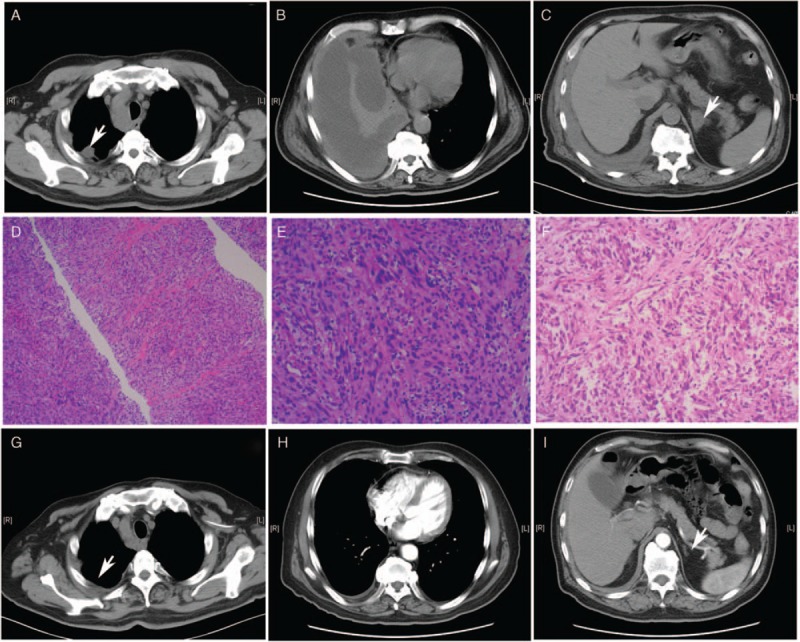
CT and histopathological findings. (A–C) Chest CT before treatment. (A) An 18 mm × 15 mm irregular mass in the apical segment of the right upper lobe with pleura invasion, as indicated by the arrow. (B) Massive right pleura effusion. (C) Metastasis of the left adrenal gland, as indicated by the arrow. (D and E) Histopathological findings of tissue biopsy. The tumor contains mostly diffuse proliferated spindle-shaped tumor cells with partial necrosis and scattered infiltration of lymphocytes. HE staining, original magnification ×100 and HE original magnification ×200, respectively. (F) Spindle-shaped tumor cells were found in the paraffin section of the pleural effusion cell collections, HE staining, original magnification ×200. (G–I) Chest CT findings at the 4-month follow-up after crizotinib treatment. (G) Lung lesions shrank. (H) The pleura effusion nearly vanished. (I) the metastasis of the left adrenal gland eliminated. CT: Computed tomography; HE: Hematoxylin and eosin.
This patient was then treated with 250 mg crizotinib twice daily from December 2017. Approximately 1 month after the initiation of treatment, the patient's symptoms were significantly relieved. He displayed no obvious side effects during the treatment of crizotinib. Four months after the initiation of crizotinib, he achieved a partial response (PR) in lung lesions, and the pleura effusion was nearly absorbed. A chest CT scan revealed both right upper lung lesions and mediastinal lymph nodes shrinkage [Figure 1G], decreased number of pleura nodules and pleura effusion solved [Figure 1H], and left adrenal gland metastasis eliminated [Figure 1I]. However, the lesions of the right cerebellum progressed mildly, and the serum carcinoembryonic antigen elevated, at the 6-month follow-up. After a multidisciplinary discussion, we implemented total brain radiation therapy and added bevacizumab (750 mg repeated every 3 weeks), to control the brain metastasis and to prevent the progress of primary lesions in his lung. He achieved PR for brain metastasis. He has been treated with crizotinib for 13 months and bevacizumab for six cycles since April 2018. Regular CT follow-up showed that the lesion of the upper right lung continued to shrink, and the pleura effusion persistently declined. The quality of life significantly improved, and the ECOG score improved to 1.
At the 13-month follow-up, we found that the tumor marker cytokeratin fragment 21-1 (CYFRA 21-1) was slightly elevated. We performed a ctDNA gene mutation test, and no meaningful genomic alterations or ROS1 resistance mutations were found. Based on the patient's wishes and his economic considerations, he then participated in a clinical trial of lorlatinib, a third-generation ALK/ROS1 TKI agent. The efficacy is still being determined.
The literature on the molecular pathology and targeted therapy of SpCCs are relatively limited. In previous studies on sarcomatoid carcinoma, including SpCCs, researchers found that these patients harbored epidermal growth factor receptor (EGFR), K-ras, B-Raf proto-oncogene (Braf), HER2 (erb-b2 receptor tyrosine kinase 2), phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA), and MET proto-oncogene (c-MET) amplifications, while EGFR mutations were rare. Neither a ROS1 fusion nor an ALK fusion have been reported to be positive in SpCCs.[2]
Molecular target therapy has been shown to be effective in patients with driver mutation-positive non-small cell lung cancers (NSCLCs). A ROS1 gene rearrangement is the third most common genomic alteration in patients with NSCLC for which TKI therapy has been proven to be effective and approved by the United States Food and Drug Administration (FDA). Crizotinib, an ALK/ROS1/MET triple inhibitor, was the first targeted agent approved by the FDA for the treatment of advanced ROS1-rearranged NSCLC. Crizotinib demonstrated prominent anti-cancer activity in patients with advanced ROS1-rearranged NSCLC (objective response rate (ORR) 72%, mean progression free survival (mPFS) 19.2 m).[3] Therefore, we speculate that SpCC with a ROS1 rearrangement may benefit from crizotinib. In the present case, the patient with SpCC showed a long response to crizotinib with a shrinkage in the tumor and an improvement in the quality of life.
TPM3, an actin-binding protein, is a member of tropomyosin family, which has been reported to be related with tumor cell movement or invasion.[4] The ROS1 gene, has a similar structure as ALK, encodes for an tyrosine receptor kinase which is involved in chromosomal translocation in lung cancer. ROS1 fusion with different partners, resulting in the constitutive activation of ROS1 and subsequently activate abnormal cell growth and division. Such oncogenic capacity has been described in a number of cancers, including NSCLC. Prior studies identified that the ROS1 gene has a total of 15 different fusion partners in NSCLC, including but not limited to Cluster of differentiation 74 (CD74), syndecan 4 (SDC4), TPM3, ezrin (EZR), and leucine rich repeats and immunoglobulin like domains 3 (LRIG3). The most common fusion partner is CD74.[5] Little is known if there are any differences regarding the fusion partner types between SpCC and other NSCLCs. The activation signal of the ROS1 fusion protein has also not yet been elucidated; however, no correlation has been observed between clinical efficacy and the type of fusion partner.
Intracranial progression is more common in patients treated with a TKI than in those who received chemotherapy. The reason might be that TKIs such as crizotinib are less able to cross the blood-brain barrier. In our patient, the brain metastases were controlled by radiation, and crizotinib was still efficacious to peripheral lesions combined with angiogenesis inhibitors.
Although crizotinib showed marked efficacy on ROS1 fusion, acquired resistance will eventually appear. Previous studies have shown the potential mechanisms of crizotinib resistance, including missense point mutations within the ROS1 kinase domain and bypass signaling. A ROS1 G2032R mutation is the most common alterations causing an acquired resistance to crizotinib.[5] In this report, we did not find any meaningful genomic alterations or ROS1 resistance mutations in the second gene sequencing at the last follow-up. This might be explained by the limitation of the accuracy of liquid ctDNA detection, but the patient rejected to another tissue biopsy. Whether other ROS1 inhibitors, such as ceritinib or lorlatinib, are effective is still not well known, especially when used as second-line therapy for crizotinib-resistant patients. Ceritinib showed no obvious benefits in crizotinib-pre-treated patients.[3] Although 2/7 of ORR was observed in crizotinib-pre-treated patients in a Phase 1 trial of lorlatinib,[3] more data remain to be confirmed in further studies.
In summary, we suggest that SpCC with a ROS1 fusion may benefit from crizotinib. Further studies are needed to determine if one TKI has an advantage over other TKIs and to determine optimal strategies for when TKI resistance emerges.
Declaration of patient consent
The authors certify that they have obtained all appropriate consents for this study. This consent includes consent by the patient/patient's guardians for their images and other clinical information to be reported in this study. The patient/patient's guardians understood that their names and initials would not be published, and efforts would be made to conceal their identity, but anonymity could not be guaranteed.
Conflicts of interest
None.
Footnotes
How to cite this article: Cai CL, Zhang MQ, Guo J, Wang LW, Zhao JQ, Guo WJ, Mu XD. Response to crizotinib in a patient with metastatic lung spindle cell carcinoma harboring TPM3-ROS1 fusion. Chin Med J 2019;132:3003–3005. doi: 10.1097/CM9.0000000000000556
References
- 1.Weissferdt A. Pulmonary sarcomatoid carcinomas: a review. Adv Anat Pathol 2018; 25:304–313.. doi: 10.1097/PAP.0000000000000202. [DOI] [PubMed] [Google Scholar]
- 2.Lee S, Kim Y, Sun JM, Choi YL, Kim JG, Shim YM, et al. Molecular profiles of EGFR, K-ras, c-met, and FGFR in pulmonary pleomorphic carcinoma, a rare lung malignancy. J Cancer Res Clin Oncol 2011; 137:1203–1211.. doi: 10.1007/s00432-011-0986-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sgambato A, Casaluce F, Maione P, Gridelli C. Targeted therapies in non-small cell lung cancer: a focus on ALK/ROS1 tyrosine kinase inhibitors. Expert Rev Anticancer Ther 2018; 18:71–80.. doi: 10.1080/14737140.2018.1412260. [DOI] [PubMed] [Google Scholar]
- 4.Choi HS, Yim SH, Xu HD, Jung SH, Shin SH, Hu HJ, et al. Tropomyosin3 overexpression and a potential link to epithelial-mesenchymal transition in human hepatocellular carcinoma. BMC Cancer 2010; 10:122–133.. doi: 10.1186/1471-2407-10-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davare MA, Vellore NA, Wagner JP, Eide CA, Goodman JR, Drilon A, et al. Structural insight into selectivity and resistance profiles of ROS1 tyrosine kinase inhibitors. Proc Natl Acad Sci U S A 2015; 29:E5381–E5390.. doi: 10.1073/pnas.1515281112. [DOI] [PMC free article] [PubMed] [Google Scholar]


