Supplemental Digital Content is available in the text
Keywords: Anti-cyclic citrullinated peptide antibody; Arthritis, Rheumatoid; Rheumatoid factor; Undifferentiated arthritis
Abstract
Background:
Clinical outcomes of undifferentiated arthritis (UA) are diverse, and only 40% of patients with UA develop rheumatoid arthritis (RA) after 3 years. Discovering predictive markers at disease onset for further intervention is critical. Therefore, our objective was to analyze the clinical outcomes of UA and ascertain the predictors for RA development.
Methods:
We performed a prospective, multi-center study from January 2013 to October 2016 among Chinese patients diagnosed with UA in 22 tertiary-care hospitals. Clinical and serological parameters were obtained at recruitment. Follow-up was undertaken in all patients every 12 weeks for 2 years. Predictive factors of disease progression were identified using multivariate Cox proportional hazards regression.
Results:
A total of 234 patients were recruited in this study, and 17 (7.3%) patients failed to follow up during the study. Among the 217 patients who completed the study, 83 (38.2%) patients went into remission. UA patients who developed RA had a higher rheumatoid factor (RF)-positivity (42.9% vs. 16.8%, χ2 = 8.228, P = 0.008), anti-cyclic citrullinated peptide (CCP) antibody-positivity (66.7% vs. 10.7%, χ2 = 43.897, P < 0.001), and double-positivity rate of RF and anti-CCP antibody (38.1% vs. 4.1%, χ2 = 32.131, P < 0.001) than those who did not. Anti-CCP antibody but not RF was an independent predictor for RA development (hazard ratio 18.017, 95% confidence interval: 5.803–55.938; P < 0.001).
Conclusion:
As an independent predictor of RA, anti-CCP antibody should be tested at disease onset in all patients with UA.
Introduction
Undifferentiated arthritis (UA) is an common type of inflammatory arthritis that cannot be diagnosed as a specific rheumatic disease.[1] Nearly half of UA patients achieve remission without treatment,[2] and less than half of them eventually develop rheumatoid arthritis (RA). Patients with a high likelihood of disease progression to RA can benefit from early intervention, whereas those with a lower likelihood of progression could also benefit from avoiding unnecessary treatment.
Several prediction models exist that evaluate people at a high risk of developing RA. The Leiden score is used widely and has been validated in many studies to have high sensitivity and specificity.[3,4] However, its utility is challenging.[5] It assesses nine parameters, on a scale of 0.5 to 2.0 points; therefore, it is not very feasible for daily clinical use. Thus, discovering baseline markers that can predict UA progression is very important.
Previous epidemiological studies on UA have reported that the incidence varies from 41 to 149 per 100,000 adults.[6–8] There are nearly 1 million UA patients in China, whereas there are only 6000 rheumatologists. It is, thus, of great value to determine reliable and feasible baseline parameters that can easily predict disease development.
Accurate prediction of RA among UA patients utilizing simple clinically relevant parameters is a key clinical advancement. Clinically actionable biomarkers for RA among UA patients remain debatable and there is yet a multi-center study to investigate novel predictors of RA among Chinese UA patients. This study aimed to identify clinically significant predictor for RA among UA patients and evaluated their respective clinical outcomes.
Methods
Ethical approval
This study protocol was approved by the Ethics Committee of Peking University People's Hospital (Beijing, China; No. FWA00001384). All individuals provided written informed consent for participation before enrollment.
Study design
We performed an observational, prospective, multi-center study. All patients were recruited between January 2013 and October 2016 to analyze the progression of UA and to determine the predictors of RA development in UA patients.
Patients
The inclusion criteria were patients aged >18 years with persistent arthritis ≥6 weeks and ≤24 weeks. None of the patients received treatment with glucocorticoids, disease-modifying anti-rheumatic drugs (DMARDs), or biologic agents.
The exclusion criteria were patients who were pregnant; those with evidence of uncontrolled hypertension, abnormal heart, liver, or renal function (≥1.5-times of the upper limit); and patients suffering from alcohol addiction.
Patients with UA were recruited from 22 tertiary-care hospitals in China [Supplementary Table 1]. All patients were evaluated by a rheumatologist upon study inclusion and were followed up every 12 weeks. Follow-up was stopped if patients developed a specific connective tissue disease (such as RA, spondyloarthritis [SpA], and reactive arthritis [ReA], etc) or if 2 years of follow-up had been completed.
The classification criteria for psoriatic arthritis (PsA) was used for the diagnosis of PsA[9]; the diagnosis of RA was made according to the 2010 American College of Rheumatology (ACR)/European League Against Rheumatism classification criteria[10]; the ACR criteria of the hand,[11] hip,[12] and knee[13] was used for the diagnosis of osteoarthritis (OA); the 2012 Systemic Lupus International Collaborating Clinics criteria was used for the diagnosis of systemic lupus erythematosus (SLE)[14]; Bohan and Peter criteria was used for the diagnosis of dermatomyositis[15]; and the Spondyloarthritis International Society criteria was used to assess SpA.[16] “Rhupus syndrome” was defined as patients who could be diagnosed both RA and SLE. Patients with clinical manifestation and exclusion of other diseases were defined as having ReA.[17] The definition of “remission” was that patients had no clinical manifestations of arthritis (soft-tissue swelling) and did not receive treatment for 3 months.
Demographic characteristics, clinical manifestations, and laboratory parameters were entered into the database by well-trained investigators in each center. We extracted the data from the electronic system for analyses.
Statistical analysis
The age of the patients followed a normal distribution and is presented as the mean ± standard deviation. The diease duration, the numbers of tender joints and swollen joints showed skewed distributions and are presented as medians and interquartile ranges. The remaining data, which are normally distributed, are presented as absolute numbers with percentages of the total.
The Student's t test was used to compare the age at arthritis onset between the two groups. The Mann-Whitney U test was used to compare the disease duration, the numbers of tender joints and swollen joints between the groups. A multivariate model, using Cox proportional hazards regression, was used to identify independent predictors of RA development. Statistical significance was set as P < 0.05. SPSS version 22.0 (SPSS, Inc., Chicago, IL, USA) was used for all analyses.
Results
Baseline characteristics of patients with UA
A total number of 234 patients were recruited in our study. Among these patients, 17 (7.3%) failed to follow-up during the study period, and 217 (92.7%) participants completed the study. Table 1 showed the baseline characteristics of the UA patients.
Table 1.
Baseline characteristics of 217 patients with undifferentiated arthritis.
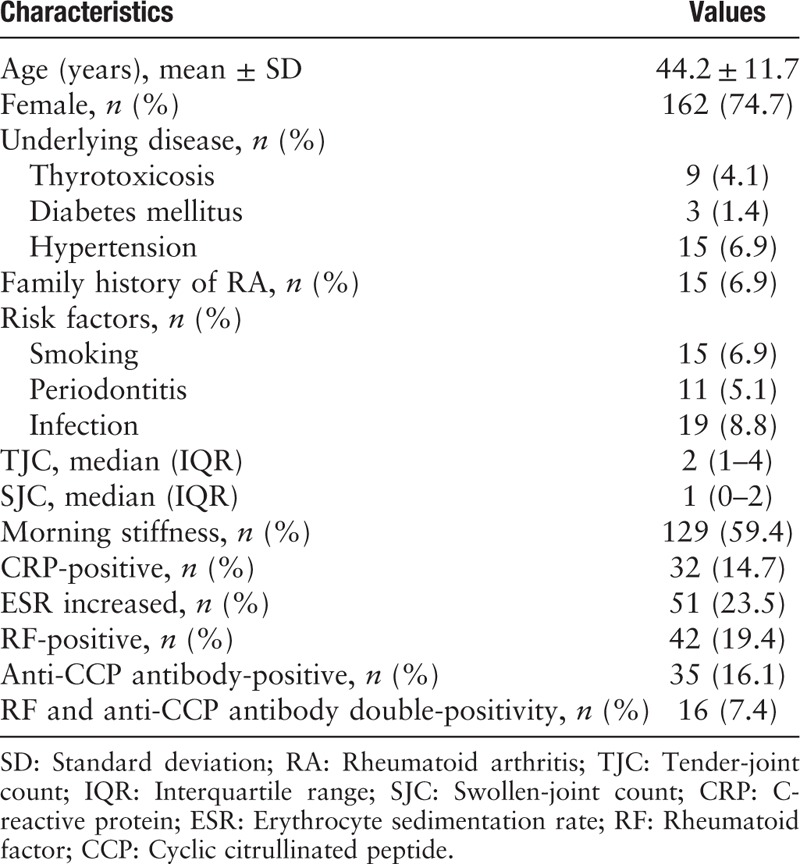
Among these patients, 59.4% had morning stiffness. The median number of tender and swollen joints were 2 (1–4) and 1 (0–2), respectively. The most implicated joint was the proximal interphalangeal, followed by wrist. The positivity rates for anti-citrullinated peptide (CCP) antibody and RF were 16.1% and 19.4%, respectively.
Hypertension (6.9%) was the most common underlying disease. Fifteen (6.9%) patients had a family history of RA. Tobacco smoking and periodontitis were documented in 15 (6.9%) and 11 (5.1%) patients, respectively. Furthermore, 19 (8.8%) patients had a history of infection.
Diagnosis after follow-up
Most patients had persistent UA and 83 (38.2%) patients went into remission after 2-years of follow-up. Among the latter, 50 (60.2%) were treated with total glucosides of paeony, 30 (36.1%) patients underwent symptomatic treatment, and 2 (2.4%) patients went into remission without treatment. Twenty (9.2%), 10 (4.6%), and 2 (0.9%) patients developed RA, OA, and PsA, respectively. SLE, dermatomyositis, Rhupus syndrome, and ReA developed in 1 (0.5%) patient each [Figure 1].
Figure 1.
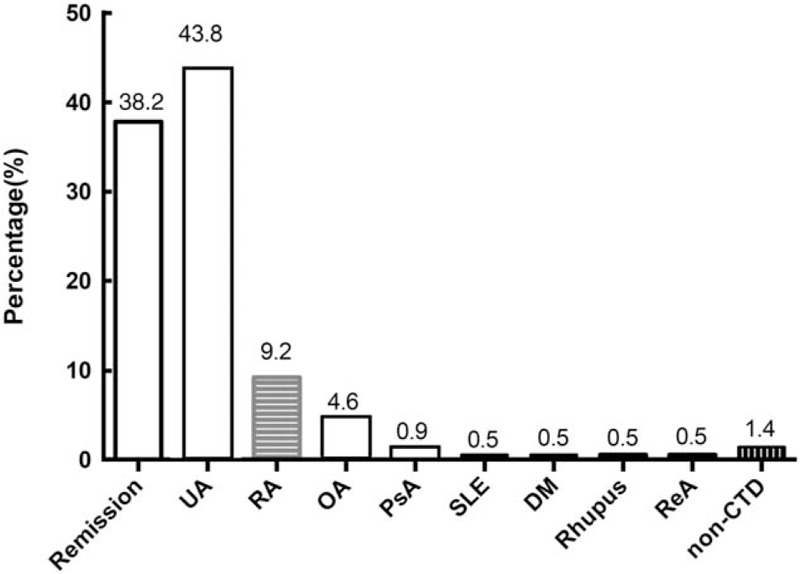
Clinical diagnosis of 217 patients with UA after 2-year follow-up. CTD: Connective tissue disease; DM: Dermatomyositis; OA: Osteoarthritis; PsA: Psoriatic arthritis; RA: Rheumatoid arthritis; ReA: Reactive arthritis; Rhupus: Rheumatoid arthritis-systemic lupus erythematosus syndrome; SLE: Systemic lupus erythematosus; UA: Undifferentiated arthritis.
Predictive factors of RA
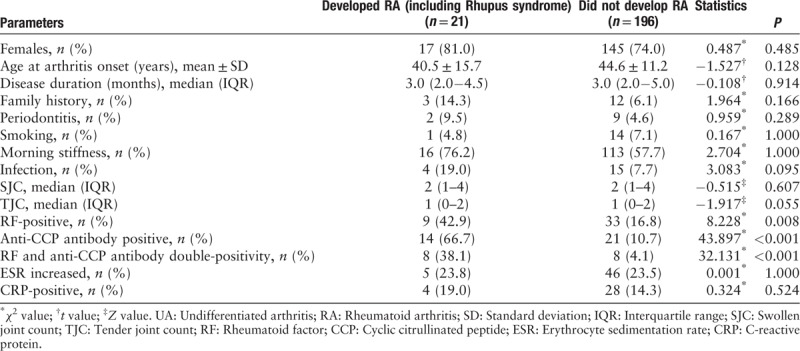
UA patients who developed RA had a significantly higher prevalence of positivity for anti-CCP antibody (66.7% vs. 10.7%, χ2 = 43.897, P < 0.001), RF (42.9% vs. 16.8%, χ2 = 8.228, P = 0.008), and a double-positivity rate of anti-CCP antibody and RF (38.1% vs. 4.1%, χ2 = 32.131, P < 0.001) [Table 2]. Anti-CCP antibody (hazard ratio 18.017, 95% confidence interval: 5.803–55.938, P < 0.001) but not RF was an independent predictive factor for RA progression.
Table 2.
Predictive factors for UA developing into RA.
Discussion
This study showed that nearly half of the patients with UA achieved spontaneous remission without DMARDs, and only a few UA patients developed RA. UA can also develop into various rheumatic diseases. Instead of using a complicated algorithm, anti-CCP antibody can be used as a good and accurate predictor for RA development.
RF positivity was a risk factor of RA development according to Jansen et al.[18] However, RF was not related to RA development in another study.[19] RF is a traditional serologic marker for inflammatory arthritis. The sensitivity of using RF positivity is the same as that using anti-CCP antibody positivity for RA diagnosis, but the specificity of using RF positivity is lower for RA diagnosis. Whether we should “abandon” the use of RF as a marker remains controversial.[20] Conversely, the positivity of RF and anti-CCP antibody positivity are independent observed in UA.[21,22] This study showed that RF was not a predictor of RA. However, it is necessary to test for RF in clinics to increase the sensitivity of UA diagnosis.
The importance of anti-CCP antibody is also controversial. Studies have shown that a high titer of anti-CCP antibody is a risk factor for radiographic progression and worsening disease activity in RA patients.[23–25] However, the titer of anti-CCP antibody is not related to the outcome in patients with early arthritis.[26] In the prediction model using the Leiden score, the titer of anti-CCP antibody is a key item for diagnosing UA. In clinical practice, it is easy to test for anti-CCP antibody. In patients with UA or arthralgia, anti-CCP antibody could predict RA development and is the strongest predictor for poor outcome.[3,27,28] Thus, all patients with UA should have their anti-CCP antibody tested to predict the development of RA.
Patients with RF and anti-CCP antibody double-positivity are likely to show to radiographic progression.[29] Although the double-positivity rate was significantly higher in patients who developed RA, but it was not an independent predictor for UA development.
Smoking is related to disease activity and RF titer in RA. Harrison et al[30] showed that in patients with inflammatory polyarthritis, smokers had a higher RF titer. The prevalence of smoking in our group was 6.9%, and we did not observe this association. The results might have been because the smoking prevalence in our study cohort was relatively low. Periodontitis is clinically associated with RA.[31]Porpyromonas gingivalis can citrullinate antigens using the peptidyl arginine deiminase enzyme. However, in patients with arthralgia, having antibodies against P. gingivalis is not a predictor for anti-CCP antibody seropositivity or RA development. There was no correlation between periodontitis and RA development.
There were two major limitations in our study. First, the number of participants was relatively small. Second, we did not include the early treatment of DMARDs in UA patients. Studies have shown that early methotrexate treatment could prevent UA development.[32,33] Initial triple-DMARD therapy can decrease disease activity and reduce the use of biologics by 50% after 3 months.[34] Biological DMARDs can slow the progression of UA, and abatacept can suppress the radiological progression.[35–37]
In conclusion, only a small proportion of UA patients progressed to RA. As a predictor for RA, anti-CCP antibody should be tested in all patients with early arthritis. UA patients who are anti-CCP antibody positive should be treated with DMARDs to prevent the onset of RA.
Acknowledgements
The authors thank all patients who are enrolled in the clinical trial. Without their active cooperation, our trial would not be possible.
Funding
The study was supported by the grants from the Ministry of Science and Technology of China (No. 2008BAI59800 and 2014BAI07B01), the National Natural Science Foundation of China (No. 81671609), and Beijing Municipal Science and Technology Project (No. Z171100000417007).
Conflicts of interest
None.
Supplementary Material
Footnotes
How to cite this article: Li C, Zhang Y, Song H, Gao J, Zhao DB, Zhu Q, He DY, Wang L, Li XP, Liu XD, Xiao WG, Wu XY, Wu HX, Tu W, Hu SX, Wang X, Li ZJ, Lu ZM, Da ZY, Liang B, Liu XM, Zhao JW, Li L, Han F, Qi WF, Wei W, Ma X, Li ZB, Zheng GM, Zhang FX, Li Y, Wang YL, Ling GH, Chen JW, Hou XQ, Zhang J, Chen QP, Liu CL, Zhang Y, Zeng JS, Zou QH, Fang YF, Su Y, Li ZG. Anti-cyclic citrullinated peptide antibody predicts the development of rheumatoid arthritis in patients with undifferentiated arthritis. Chin Med J 2019;132:2899–2904. doi: 10.1097/CM9.0000000000000570
References
- 1.Wevers-de Boer KV, Heimans L, Huizinga TW, Allaart CF. Drug therapy in undifferentiated arthritis: a systematic literature review. Ann Rheum Dis 2013; 72:1436–1444.. doi: 10.1136/annrheumdis-2012-203165. [DOI] [PubMed] [Google Scholar]
- 2.Harrison BJ, Symmons DP, Brennan P, Barrett EM, Silman AJ. Natural remission in inflammatory polyarthritis: issues of definition and prediction. Br J Rheumatol 1996; 35:1096–1100.. doi: 10.1093/rheumatology/35.11.1096. [DOI] [PubMed] [Google Scholar]
- 3.van der Helm-van Mil AHlC, van Dongen H, Breedveld FC, Toes RE, Huizinga TW. A prediction rule for disease outcome in patients with recent-onset undifferentiated arthritis: how to guide individual treatment decisions. Arthritis Rheum 2007; 56:433–440.. doi: 10.1002/art.22380. [DOI] [PubMed] [Google Scholar]
- 4.Ghosh K, Chatterjee A, Ghosh S, Chakraborty S, Chattopadhyay P, Bhattacharya A, et al. Validation of Leiden score in predicting progression of rheumatoid arthritis in undifferentiated arthritis in Indian population. Ann Med Health Sci Res 2016; 6:205–210.. doi: 10.4103/amhsr.amhsr_339_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arana-Guajardo A, Perez-Barbosa L, Vega-Morales D, Riega-Torres J, Esquivel-Valerio J, Garza-Elizondo M. Application of a prediction model for the progression of rheumatoid arthritis in patients with undifferentiated arthritis. Reumatol Clin 2014; 10:360–363.. doi: 10.1016/j.reuma.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Soderlin MK, Borjesson O, Kautiainen H, Skogh T, Leirisalo-Repo M. Annual incidence of inflammatory joint diseases in a population based study in southern Sweden. Ann Rheum Dis 2002; 61:911–915.. doi: 10.1136/ard.61.10.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Savolainen E, Kaipiainen-Seppanen O, Kroger L, Luosujarvi R. Total incidence and distribution of inflammatory joint diseases in a defined population: results from the Kuopio 2000 arthritis survey. J Rheumatol 2003; 30:2460–2468.. doi: 10.1097/00002281-200311000-00017. [PubMed] [Google Scholar]
- 8.Carbonell J, Cobo T, Balsa A, Descalzo MA, Carmona L, Group SS. The incidence of rheumatoid arthritis in Spain: results from a nationwide primary care registry. Rheumatology (Oxford) 2008; 47:1088–1092.. doi: 10.1093/rheumatology/ken205. [DOI] [PubMed] [Google Scholar]
- 9.Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H, et al. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 2006; 54:2665–2673.. doi: 10.1002/art.21972. [DOI] [PubMed] [Google Scholar]
- 10.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, 3rd, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010; 62:2569–2581.. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 11.Altman R, Alarcon G, Appelrouth D, Bloch D, Borenstein D, Brandt K, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hand. Arthritis Rheum 1990; 33:1601–1610.. doi: 10.1002/art.1780331101. [DOI] [PubMed] [Google Scholar]
- 12.Altman R, Alarcon G, Appelrouth D, Bloch D, Borenstein D, Brandt K, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hip. Arthritis Rheum 1991; 34:505–514.. doi: 10.1002/art.1780340502. [DOI] [PubMed] [Google Scholar]
- 13.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum 1986; 29:1039–1049.. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 14.Petri M, Orbai AM, Alarcon GS, Gordon C, Merrill JT, Fortin PR, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012; 64:2677–2686.. doi: 10.1002/art.34473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med 1975; 292:344–347.. doi: 10.1002/art.34473. [DOI] [PubMed] [Google Scholar]
- 16.Rudwaleit M, Landewe R, van der Heijde D, Listing J, Brandt J, Braun J, et al. The development of Assessment of SpondyloArthritis International Society classification criteria for axial spondyloarthritis (part I): classification of paper patients by expert opinion including uncertainty appraisal. Ann Rheum Dis 2009; 68:770–776.. doi: 10.1136/ard.2009.108217. [DOI] [PubMed] [Google Scholar]
- 17.Selmi C, Gershwin ME. Diagnosis and classification of reactive arthritis. Autoimmun Rev 2014; 13:546–549.. doi: 10.1016/j.autrev.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Jansen LM, van Schaardenburg D, van der Horst-Bruinsma IE, Dijkmans BA. One year outcome of undifferentiated polyarthritis. Ann Rheum Dis 2002; 61:700–703.. doi: 10.1136/ard.61.8.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen DY, Li H, Liang LQ, Xiao YJ, Xu T, Qiu Q, et al. Clinical features and independent predictors in the further development of rheumatoid arthritis in undifferentiated arthritis. Rheumatol Int 2013; 33:2827–2832.. doi: 10.1007/s00296-013-2799-8. [DOI] [PubMed] [Google Scholar]
- 20.Symmons DP. Classification criteria for rheumatoid arthritis–time to abandon rheumatoid factor. Rheumatology (Oxford) 2007; 46:725–726.. doi: 10.1093/rheumatology/kel418. [DOI] [PubMed] [Google Scholar]
- 21.Jansen AL, van der Horst-Bruinsma I, van Schaardenburg D, van de Stadt RJ, de Koning MH, Dijkmans BA. Rheumatoid factor and antibodies to cyclic citrullinated peptide differentiate rheumatoid arthritis from undifferentiated polyarthritis in patients with early arthritis. J Rheumatol 2002; 29:2074–2076.. doi:10.1016/s1297-319x10270045-1. [PubMed] [Google Scholar]
- 22.van Gaalen FA, Linn-Rasker SP, van Venrooij WJ, de Jong BA, Breedveld FC, Verweij CL, et al. Autoantibodies to cyclic citrullinated peptides predict progression to rheumatoid arthritis in patients with undifferentiated arthritis: a prospective cohort study. Arthritis Rheum 2004; 50:709–715.. doi: 10.1002/art.20044. [DOI] [PubMed] [Google Scholar]
- 23.Syversen SW, Gaarder PI, Goll GL, Odegard S, Haavardsholm EA, Mowinckel P, et al. High anti-cyclic citrullinated peptide levels and an algorithm of four variables predict radiographic progression in patients with rheumatoid arthritis: results from a 10-year longitudinal study. Ann Rheum Dis 2008; 67:212–217.. doi: 10.1136/ard.2006.068247. [DOI] [PubMed] [Google Scholar]
- 24.Ronnelid J, Wick MC, Lampa J, Lindblad S, Nordmark B, Klareskog L, et al. Longitudinal analysis of citrullinated protein/peptide antibodies (anti-CP) during 5 year follow up in early rheumatoid arthritis: anti-CP status predicts worse disease activity and greater radiological progression. Ann Rheum Dis 2005; 64:1744–1749.. doi: 10.1136/ard.2004.033571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Syversen SW, Goll GL, van der Heijde D, Landewe R, Lie BA, Odegard S, et al. Prediction of radiographic progression in rheumatoid arthritis and the role of antibodies against mutated citrullinated vimentin: results from a 10-year prospective study. Ann Rheum Dis 2010; 69:345–351.. doi: 10.1136/ard.2004.033571. [DOI] [PubMed] [Google Scholar]
- 26.Ursum JBW, van Dillen N, Dijkmans BA, van Schaardenburg D. Levels of anti-citrullinated protein antibodies and IgM rheumatoid factor are not associated with outcome in early arthritis patients: a cohort study. Arthritis Res Ther 2010; 12:R8.doi: 10.1186/ar2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi J, van de Stadt LA, Levarht EW, Huizinga TW, Toes RE, Trouw LA, et al. Anti-carbamylated protein antibodies are present in arthralgia patients and predict the development of rheumatoid arthritis. Arthritis Rheum 2013; 2013:911–915.. doi: 10.1002/art.37830. [DOI] [PubMed] [Google Scholar]
- 28.Mjaavatten MDUT, Haugen AJ, Nygaard H, Sidenvall G, Helgetveit K, Kvien TK. Positive anti-citrullinated protein antibody status and small joint arthritis are consistent predictors of chronic disease in patients with very early arthritis: results from the NOR-VEAC cohort. Arthritis Res Ther 2009; 11:R146.doi: 10.1186/ar2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mjaavatten MD, van der Heijde D, Uhlig T, Haugen AJ, Nygaard H, Sidenvall G, et al. The likelihood of persistent arthritis increases with the level of anti-citrullinated peptide antibody and immunoglobulin M rheumatoid factor: a longitudinal study of 376 patients with very early undifferentiated arthritis. Arthritis Res Ther 2010; 12:R76.doi: 10.1186/ar2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harrison BJ, Silman AJ, Wiles NJ, Scott DG, Symmons DP. The association of cigarette smoking with disease outcome in patients with early inflammatory polyarthritis. Arthritis Rheum 2001; 44:323–330.. doi: 10.1002/1529-0131(200102)44:2%3C323::AID-ANR 49% 3E3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 31.Scher JU, Ubeda C, Equinda M, Khanin R, Buischi Y, Viale A, et al. Periodontal disease and the oral microbiota in new-onset rheumatoid arthritis. Arthritis Rheum 2012; 64:3083–3094.. doi: 10.1002/art.34539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kudo-Tanaka E, Shimizu T, Nii T, Teshigawara S, Yoshimura M, Watanabe A, et al. Early therapeutic intervention with methotrexate prevents the development of rheumatoid arthritis in patients with recent-onset undifferentiated arthritis: a prospective cohort study. Mod Rheumatol 2015; 25:831–836.. doi: 10.3109/14397595.2015.1031364. [DOI] [PubMed] [Google Scholar]
- 33.de Jong PHHJ, Barendregt PJ, Barendregt PJ, Huisman M, van Zeben D, van der Lubbe PA, et al. Induction therapy with a combination of DMARDs is better than methotrexate monotherapy: first results of the tREACH trial. Ann Rheum Dis 2013; 72:72–78.. doi: 10.1136/annrheumdis-2011-201162. [DOI] [PubMed] [Google Scholar]
- 34.Li ZG, Liu Y, Xu HJ, Chen ZW, Bao CD, Gu JR, et al. Efficacy and safety of tofacitinib in Chinese patients with rheumatoid arthritis. Chin Med J 2018; 131:2683–2692.. doi: 10.4103/0366-6999.245157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saleem BMS, Quinn M, Nizam S, Hensor E, Jarrett S, Conaghan PG, et al. Does the use of tumour necrosis factor antagonist therapy in poor prognosis, undifferentiated arthritis prevent progression to rheumatoid arthritis. Ann Rheum Dis 2008; 67:1178–1180.. doi: 10.1136/ard.2007.084269. [DOI] [PubMed] [Google Scholar]
- 36.Emery PDP, Dougados M, Legerton CW, Becker JC, Vratsanos G, Genant HK, et al. Impact of T-cell costimulation modulation in patients with undifferentiated inflammatory arthritis or very early rheumatoid arthritis: a clinical and imaging study of abatacept (the ADJUST trial). Ann Rheum Dis 2010; 69:510–516.. doi: 10.1136/ard.2009.119016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buch MH, Hensor EM, Rakieh C, Freeston JE, Middleton E, Horton S, et al. Abatacept reduces disease activity and ultrasound power Doppler in ACPA-negative undifferentiated arthritis: a proof-of-concept clinical and imaging study. Rheumatology (Oxford) 2017; 56:58–67.. doi: 10.1093/rheumatology/kew357. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


