Abstract
In this study, we aimed to evaluate the toxic effects, changes in life span, and expression of various metabolism-related genes in Caenorhabditis elegans, using RNA interference (RNAi) and mutant strains, after 3-bromopyruvate (3-BrPA) treatment. C. elegans was treated with various concentrations of 3-BrPA on nematode growth medium (NGM) plates, and their survival was monitored every 24 h. The expression of genes related to metabolism was measured by the real-time fluorescent quantitative polymerase chain reaction (qPCR). Nematode survival in the presence of 3-BrPA was also studied after silencing three hexokinase (HK) genes. The average life span of C. elegans cultured on NGM with 3-BrPA was shortened to 5.7 d compared with 7.7 d in the control group. hxk-1, hxk-2, and hxk-3 were overexpressed after the treatment with 3-BrPA. After successfully interfering hxk-1, hxk-2, and hxk-3, the 50% lethal concentration (LC50) of all mutant nematodes decreased with 3-BrPA treatment for 24 h compared with that of the control. All the cyp35 genes tested were overexpressed, except cyp-35B3. The induction of cyp-35A1 expression was most obvious. The LC50 values of the mutant strains cyp-35A1, cyp-35A2, cyp-35A4, cyp-35B3, and cyp-35C1 were lower than that of the control. Thus, the toxicity of 3-BrPA is closely related to its effect on hexokinase metabolism in nematodes, and the cyp-35 family plays a key role in the metabolism of 3-BrPA.
Keywords: 3-Bromopyruvate, Caenorhabditis elegans, Hexokinase, Cytochrome P450
1. Introduction
Bromopyruvate (3-BrPA) is an intermediate of the anthelmintic thiabendazole and is considered an inhibitor of glycolysis (Yadav et al., 2017b). It can combine with the active site of hexokinase (HK) II, the XH (EXH+BrCH2-CO-COOH→EX-CH2-CO-COOH+HBr) base, and inhibit the activity of HK II during glycolysis (Kim et al., 2008). Under normal conditions, HK II is mainly expressed in the skeletal muscle, adipose and cardiac tissues, and it is not common in other tissues (Patra and Hay, 2013). HK II is highly expressed in tumor cells to enhance glycolysis (Chen GH et al., 2018; Dewaal et al., 2018). An anoxic microenvironment is easily formed in growing tumor tissues. Kim et al. (2007) demonstrated that increased mitochondrial stability is the main reason for hypoxia-induced overexpression of HK II. It has also been shown that the combination of HK II and the mitochondrial voltage-dependent anion channel protein inhibits membrane gap protein release and apoptosis and promotes tumor cell growth (Mycielska et al., 2012; Xu et al., 2017). 3-BrPA exhibits both tumoristatic and tumoricidal effects (el Sayed et al., 2017; Yadav et al., 2017a). It also possesses good antifungal properties (Dyląg et al., 2013).
Caenorhabditis elegans is a commonly studied model organism, especially in toxicology and pharmacology research, because approximately 83% of its protein sequences are homologous to those of humans (Lai et al., 2000; Qiao et al., 2014; Huang et al., 2017; García-Espiñeira et al., 2018). In this study, we aimed to evaluate the toxic effects of 3-BrPA and elucidate its metabolic pathway.
2. Materials and methods
2.1. Cultivation of C. elegans
C. elegans (N2 strain; Caenorhabditis Genetics Center, Saint Paul, MN, USA) was grown on a plate coated with Escherichia coli OP50 (Caenorhabditis Genetics Center) as nutrient at 20 °C. When the nematodes started to lay eggs, they were synchronized. The eggs hatched in the L4 stage.
2.2. Exposure of C. elegans to 3-BrPA
3-BrPA was added to the nematode growth medium (NGM) at 0, 0.7, 0.8, 0.9, and 1.0 mmol/L. Approximately 100 μL of E. coli OP50 suspension was plated on each plate and cultured overnight in an incubator at 37 °C. The nematodes (in L4 stage) were placed in each well containing the drug, and their survival was determined once daily. In another experiment, the nematodes (in L4 stage) were placed in Petri dishes and exposed to 0, 12.7, 25.4, and 50.8 mmol/L 3-BrPA, and the toxicity was observed. In an acute toxicity experiment, 3-BrPA was added to each well of a 96-well plate at 0, 2, 5, 7, 9, 11, 13, and 15 mmol/L in triplicates, and 15 L4 nematodes were placed in each well. After 24 h, the survival of nematodes was determined. Live adults were picked in a new NGM plate every day and counted until all the adults died; caution was exerted not to pick larvae, which could affect the experimental results. All experiments were repeated three times.
2.3. Construction of HK gene molecular phylogenetic tree
The FASTA sequence of HK genes was downloaded from the National Center for Biotechnology Information database (https://www.ncbi.nlm.nih.gov). The selected sequences included HK genes of Homo sapiens, Mus musculus, Danio rerio, Xenopus laevis, Drosophila melanogaster, C. elegans, and Necator americanus. An evolutionary tree was constructed using MEGA 5.05 software (https://www.megasoftware.net).
2.4. Total RNA extraction and reverse transcription of C. elegans
The synchronized nematodes (approximately 500) were homogenized in RNase-free water, centrifuged at 1788g for 2 min, and then the supernatant was discarded. After repeated freeze–thaw cycles, the pellet was centrifuged at 13 545g for 5 min at 4 °C. Chloroform was added, and the solution was emulsified and centrifuged at 13 545g for 15 min at 4 °C. Precooled isopropanol was added to the solution and mixed. Subsequently, the mixture was allowed to stand for 10 min and centrifuged at 13 545g for 15 min at 4 °C. After aspirating the supernatant, precooled (4 °C) 75% ethanol was added and centrifuged at 13 545g for 5 min at 4 °C. After discarding the supernatant and allowing the RNA extracts to dry naturally at 20 °C, the total RNA was extracted and reverse-transcribed using a reverse transcription kit (TaKaRa Bio Inc., Japan).
2.5. Determination of hxk-1, hxk-2, and hxk-3 mRNA expression
The mRNA expression of nematode HK genes hxk-1, hxk-2, and hxk-3 was determined using act-1 as the internal control gene. The results of primer 5.0 design are shown in Table 1. According to the 50% lethal concentration (LC50) of 3-BrPA in C. elegans, four concentration gradients—0, 1/10, 1/100, and 1/1000 of the lethal concentration—were set. The nematode RNA was extracted according to the procedure described earlier. The volume of the real-time fluorescent quantitative polymerase chain reaction (qPCR) system was set to 25 μL, with three sets of spacers per SYBR Premix Ex Tap II (TaKaRa Bio Inc., Japan).
Table 1.
Primer sequences of hxk-1, hxk-2, and hxk-3 for the qPCR
| Gene | WormBase ID | Forward primer (5'→3') | Reverse primer (5'→3') |
| act-1 | T04C12.6 | CGCTTCTTCCTCTTCCCTC | TGATCGTATGCAGAAGGAAA |
| hxk-1 | F14B4.2 | GGAGAGTGTGCCCGAGTTGT | ATGCTCTCTGAAGATGGATCTGG |
| hxk-2 | H25P06.1 | GCTCTTTAATGGAATTGGCTCG | CGCAATCGTTTCGAGAGTCA |
| hxk-3 | Y77E11A.1 | CAAAGCAGTGATGAACGACACA | GACACAATTTGATCGGGAAGTCG |
2.6. Silencing of the target genes hxk-1, hxk-2, and hxk-3
2.6.1 Cloning the target genes
The primers for hxk-1, hxk-2, and hxk-3 (upstream and downstream primer sequences for the XbaI and HindIII restriction sites; Table 2) were designed. The PCR products were subjected to polyacrylamide gel electrophoresis (120 V, 100 mA, 30 min). The target gene fragment was purified using the TaKaRa fragment purification kit (TaKaRa Bio Inc., Japan).
Table 2.
Primer sequences of hxk-1, hxk-2, and hxk-3 for the PCR
| Gene | WormBase ID | Forward primer (5'→3') | Reverse primer (5'→3') |
| hxk-1 | F14B4.2 | GCTCTAGATTCACTTTCTCGTTTCCA | CCCAAGCTTCGAGTTTCCACCG |
| hxk-2 | H25P06.1 | CCTCTAGATCTCTCGACAAGCT | CCCAAGCTTCAGATCCTTCAGC |
| hxk-3 | Y77E11A.1 | GCTCTAGACAACTTCAAGAAATCAG | CCCAAGCTTGTGCATTCTG |
Single colonies of DH5α were inoculated in 5 mL of Luria-Bertani (LB) liquid medium and cultured overnight in a shaking incubator. The medium was aspirated and centrifuged at 300g for 10 min at 4 °C, and then the supernatant was discarded. Precooled CaCl2-MgCl2 (5 mL) was added, and the cells were resuspended and centrifuged at 1505g for 10 min at 4 °C. The cells were recovered and the above steps were repeated. Precooled CaCl2 (0.5 mL) was added to 50 mL of the initial bacterial broth to prepare competent cells. Subsequently, 5 μL of ligation solution was added and incubated with the competent cells in ice-water mixture for 30 min. The tube was transferred to a water bath at 42 °C and allowed to stand for 1.5 min, and then transferred to the ice-water mixture and allowed to stand for 1.5 min. After the addition of 800 μL of start of cell to each tube, the tubes were allowed to stand at 37 °C for 1.5 min, and then transferred to a 37 °C incubator and incubated at 2351g for 46 min. The solution was then centrifuged at 2351g for 2 min. The supernatant was discarded, and 300 μL of the medium was removed and applied onto an LB solid plate supplemented with adenosine monophosphate (AMP) and cultured in a 37 °C incubator overnight. Five single colonies were placed in five tubes containing 5 mL of LB liquid medium supplemented with AMP and incubated overnight at 37 °C.
The cultured cells were transferred to a sterile Gene Era Biotech (GEB) microcentrifuge tube and centrifuged at 13 545g for 2 min at room temperature. To the supernatant, 250 μL of Solution I and 250 μL of Solution II were added. After mixing, 350 μL of Solution III (precooled in a 4 °C refrigerator) was added and the solution was allowed to stand at room temperature for 2 min. After centrifugation at 13 545g for 10 min at room temperature, the supernatant was collected and centrifuged at 13 545g for 1 min at room temperature, and the supernatant was discarded. Subsequently, 500 μL of Buffer WA (washing buffer A) was added and centrifuged at 13 545g for 30 s at room temperature, and then the supernatant was discarded. To the precipitate, 700 mg of Buffer WB (washing buffer B; in 56 mL of 100% ethanol) was added and centrifuged again at 13 545g for 30 s at room temperature, and then the supernatant was discarded. The upper layer was transferred into a new sterilized Eppendorf tube and 30 μL of distilled water was added to the middle layer. After standing at room temperature for 1 min, the DNA was eluted by centrifugation at 13 545g for 1 min at room temperature. The obtained plasmids were PCR-amplified. The electrophoresis-positive plasmid was sequenced for identification.
2.6.2 Expression of the target genes
According to the above steps, the plasmid in HT115-L4440 bacteria was isolated and double digested (HindIII and XbaI). The digested solution was subjected to agarose gel electrophoresis to recover the target fragment. After cutting the gel and adding three volumes of Buffer GM (gel melting buffer), the thawed mixture was transferred into a collection tube and centrifuged at 13 545g for 1 min at room temperature. To the above collection tube, 700 μL of Buffer WB was added and filtered, and the filtrate was centrifuged at 13 545g for 30 s at room temperature. This procedure was repeated and the sample was centrifuged at 13 545g for 1 min at room temperature to collect the DNA fragments. The ligation solution was prepared from the DNA fragment using a reaction system of 25 μL (L4440 plasmid, approximately 0.03 pmol; target fragment, approximately 0.3 pmol; T4 DNA ligase, 1 μL; 5×T4 DNA ligase buffer, 2.5 μL; sterilized water, up to 25 μL) at 16 °C overnight. E. coli DH5α was prepared using the CaCl2 method. The positive colony plasmid was extracted and the cloning results were analyzed.
2.6.3 Target gene silencing
HT115-positive bacteria were cultured in LB liquid (containing 100 μg/mL of ampicillin, 15 μg/mL of tetracycline, and 100 μmol/L of isopropyl-β-D -thiogalactopyranoside (IPTG)) at 37 °C for 2 h. The bacterial supernatant was centrifuged at 1581g for 10 min at room temperature. The bacterial suspension (100 μL) was applied on LB solid agar (ditto) and cultured at 37 °C overnight. Five L4 stage nematodes were placed in the LB plate and cultured at 20 °C until they laid eggs. After 24 h of culture, the adult worms were transferred onto a second plate for 24 h where they laid eggs, and then removed. After culturing in the LB plate for 24 h, a large number of interfering genes from the adult worms were obtained. The total RNA was extracted and reverse-transcribed, and then subjected to the qPCR to verify the interference efficiency.
2.6.4 Design of CYP35 family primers and determination of expression of cyp35 genes
The primers for cyp-35A1, cyp-35A2, cyp-35A4, cyp-35A5, cyp-35B3, and cyp-35C1 of the CYP35 family were designed (Table 3). The median concentrations, 1/10, 1/100, and 1/1000 of LC50 of 3-BrPA, were established to extract the total RNA from the nematodes, which was then reverse-transcribed. The qPCR system was set to 25 μL.
Table 3.
Primer sequences of cyp-35 for the qPCR
| Gene | WormBase ID | Forward primer (5'→3') | Reverse primer (5'→3') |
| cyp-35A1 | C03G6.14 | ATTGTGTCCACGCTTGATCTGT | CACACGTTCAGAGATATGGGAG |
| cyp-35A2 | C03G6.15 | GCATCCATTCTTAACGTCAGCTTC | ATCTTCGGTAACCTTCTCCTTCG |
| cyp-35A4 | C49G7.8 | ACCAAATCAAGTCTGGGAGGTA | TCTTACTGACCGTGCTTCAACTC |
| cyp-35A5 | K07C6.5 | CATCTTCACCTTGTGGGTTGG | TTAGAAATATGGGCTTCGGGAAGG |
| cyp-35B3 | K07C6.2 | GTGATTATGAAACGTCGCAAGAAG | GCGGATGCTGTAAATGGAAAGAC |
| cyp-35C1 | C06B3.3 | AAAGTGACTAACGGAGGATCTCG | CTAGCAAGAGCCGAGCTGTATTT |
2.7. Action of 3-BrPA on RNAi or mutant nematode strains
The RNA interference (RNAi) hxk-1, hxk-2, and hxk-3 gene strains and mutant nematode cyp-35A1, cyp-35A2, cyp-35A4, cyp-35A5, cyp-35B3, and cyp-35C1 gene strains (from the Caenorhabditis Genetics Center) were treated with 3-BrPA and counted when they grew to the L4 stage (see Section 2.2). The LD50 was calculated using SPSS 19.0 software (SPSS Inc., Chicago, Illinois, USA) after exposing the nematodes to 3-BrPA for 24 h.
2.8. Data analysis
The median lethal concentration of 3-BrPA for nematodes was calculated using SPSS 19.0 software. The effects of 3-BrPA on the longevity of nematodes and the expression of metabolism-related genes were analyzed using GraphPad Prism 5 (GraphPad Software, Inc., California, USA). The difference was considered statistically significant when the P value was <0.05.
3. Results
3.1. Effect of 3-BrPA on the life span of nematodes
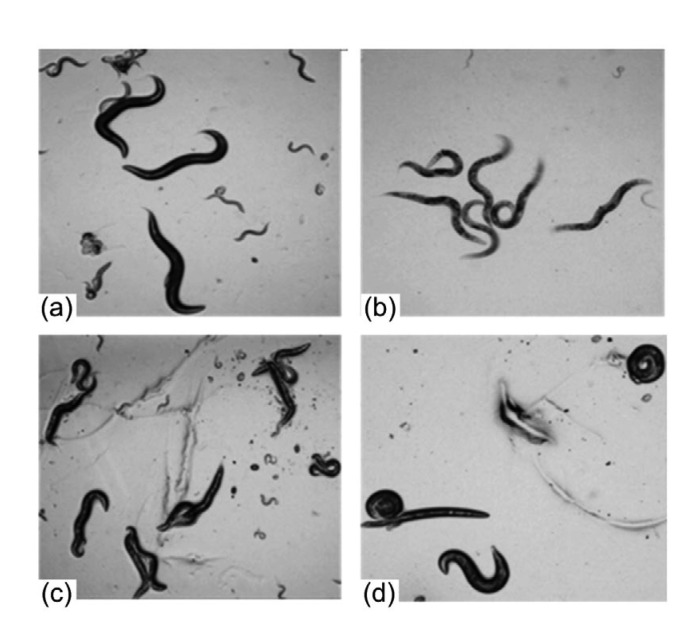
The control group exhibited a normal life span. The activity of nematodes slowed down when treated with 3-BrPA at 2.7 mmol/L. When treated with 25.4 and 50.8 mmol/L 3-BrPA, the nematodes became rigid and many died (Fig. 1).
Fig. 1.
Effect of 3-bromopyruvate (3-BrPA) on C. elegans
(a) Control; (b) 2.7 mmol/L 3-BrPA; (c) 25.4 mmol/L 3-BrPA; (d) 50.8 mmol/L 3-BrPA
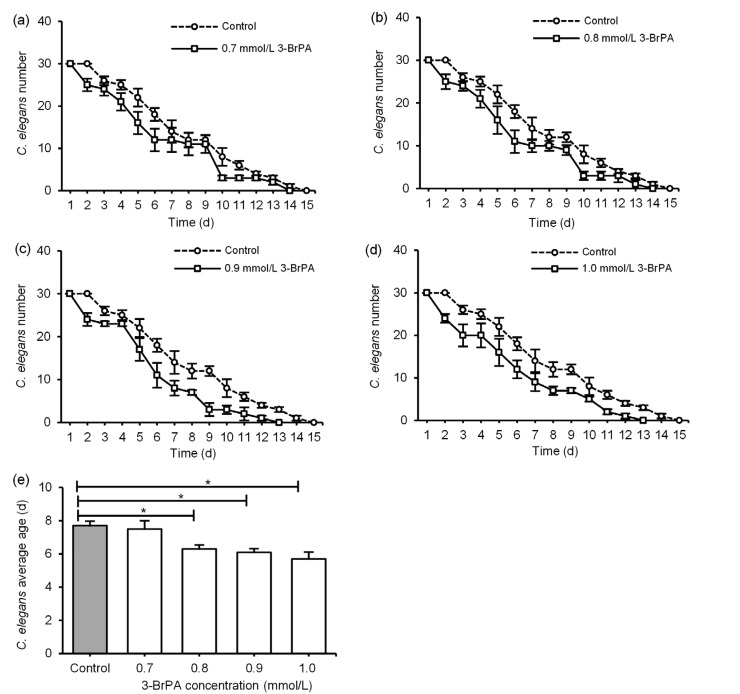
The average life span of C. elegans in the control group was 7.7 d, and all the nematodes died on Day 15 (P<0.05; Fig. 2). Compared with that of the control group, in the 0.7 mmol/L 3-BrPA group, the survival rate of the nematodes was only 30% and the average life span was 7.5 d (P<0.05; Fig. 2a). In the 0.8 mmol/L 3-BrPA group, the survival rate of the nematodes was 0 and the average life span was 6.3 d (P<0.05; Fig. 2b). In the 0.9 mmol/L 3-BrPA group, the survival rate of nematodes was 0 and the average life span was 6.1 d (P<0.05; Fig. 2c). In the 1.0 mmol/L 3-BrPA group, the average life span of nematodes was 5.7 d (P<0.05; Fig. 2d). Except for 0.7 mmol/L 3-BrPA group, the average age of other 3-BrPA groups was significantly lower than that of the control group (P<0.05; Fig. 2e).
Fig. 2.
Analysis of C. elegans life span after 3-bromopyruvate (3-BrPA) treatment
The survival numbers of the nematodes in the 0.7 (a), 0.8 (b), 0.9 (c), and 1.0 mmol/L (d) 3-BrPA groups. (e) Average age of C. elegans in different 3-BrPA concentrations. Data are expressed as mean±standard deviation (SD) with n=3. * P<0.05
3.2. Evolutionary genetic comparison of the HK genes
The phylogenetic tree in Fig. 3 reveals that the hxk-1 genes of C. elegans and N. americanus belonged to a branch with high genetic similarity; they belonged to the branch of D. melanogaster HK genes. The hxk-2 and hxk-3 genes of C. elegans presented higher approximate degree and lower genetic approximation to hxk-1, whereas those of humans and domestic mice have a higher degree of approximation to HK II. The hxk-2 and hxk-3 genes of C. elegans might be closely related to HK II in mammals, whereas hxk-1 might be distant from HK II in mammals. The average evolutionary distance between the HK gene of C. elegans and that of other species was 0.6811 (Table 4).
Fig. 3.
Phylogenetic analysis of HK in C. elegans
Table 4.
Pairwise comparison of the phylogenetic analysis of HK in C. elegans
| No. | Genus | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
| 1 | gi|915288356|ref|XM 013451975.1| Necator americanus hexokinase | ||||||||||||||
| 2 | gil392886668|ref|NM 001025935.2| Caenorhabditis elegans hexokinase 1 | 0.472 | |||||||||||||
| 3 | gil392887224|ref|NM 060504.5| C. elegans hexokinase 2 | 0.821 | 0.697 | ||||||||||||
| 4 | gil392898332|ref|NM 067687.5| C. elegans hexokinase 3 | 1.056 | 0.850 | 0.767 | |||||||||||
| 5 | gi|688992265|emb|LL507639.1| Cylicostepiens goldi genome | 1.127 | 1.087 | 1.351 | 1.454 | ||||||||||
| 6 | gi|4809268|gb|AF148513.1| Homo sapiens hexokinase 2 | 0.672 | 0.606 | 0.670 | 1.013 | 1.077 | |||||||||
| 7 | gi|1840208|gb|M775126.1| H. sapiens hexokinase 1 | 0.660 | 0.545 | 0.669 | 0.964 | 1.098 | 0.254 | ||||||||
| 8 | gi|194097329|ref|NM_002115.2| H. sapiens hexokinase 3 | 0.726 | 0.674 | 0.736 | 1.033 | 1.165 | 0.337 | 0.404 | |||||||
| 9 | gi|152211826|gb|EU008605.1| H. sapiens hexokinase | 0.703 | 0.640 | 0.753 | 1.011 | 1.166 | 0.398 | 0.435 | 0.411 | ||||||
| 10 | gi|163965442|ref|NM_013820.3| Mus musculus hexokinase 2 | 0.648 | 0.607 | 0.663 | 1.022 | 1.067 | 0.107 | 0.256 | 0.358 | 0.384 | |||||
| 11 | gi|47085786|ref|NM_213066.1| Danio rerio hexokinase 2 | 0.662 | 0.631 | 0.740 | 0.938 | 1.213 | 0.262 | 0.327 | 0.453 | 0.421 | 0.251 | ||||
| 12 | gi|45383695|ref|NM_204212.1| Gallus gallus hexokinase 2 | 0.665 | 0.627 | 0.707 | 0.943 | 1.119 | 0.188 | 0.256 | 0.341 | 0.326 | 0.198 | 0.268 | |||
| 13 | gi|148230648|ref|NM_001097134.1| Xenopus laevis hexokinase 2 | 0.664 | 0.607 | 0.679 | 0.955 | 1.187 | 0.478 | 0.427 | 0.489 | 0.524 | 0.447 | 0.465 | 0.474 | ||
| 14 | gi|11837781|gb|AF2008201| Drosophila melanogaster hexokinase | 0.700 | 0.586 | 0.738 | 1.085 | 1.231 | 0.619 | 0.634 | 0.635 | 0.581 | 0.618 | 0.671 | 0.636 | 0.667 |
3.3. mRNA expression of the HK genes in C. elegans treated with 3-BrPA
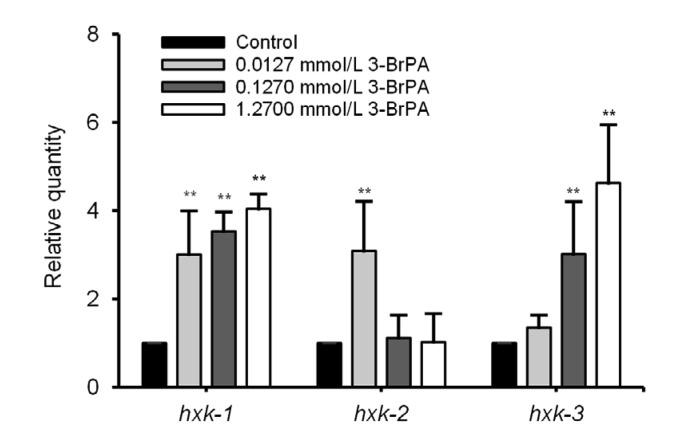
The expression of the HK genes in the control group was set to 1 (Fig. 4); the mRNA expression of the objective gene with ΔC T>1 was considered upregulated and that with ΔC T<1 was considered downregulated. It can be seen from Fig. 4 that the mRNA expression of hxk-1, hxk-2, and hxk-3 was upregulated. The mRNA expression of hxk-1 and hxk-3 was concentration-dependent, and the expression of hxk-2 exhibited the highest change at 0.0127 mmol/L 3-BrPA.
Fig. 4.
Relative mRNA expression of hxk-1, hxk-2, and hxk-3 after 3-bromopyruvate (3-BrPA) treatment
The expression of the HK genes in the control group was set to 1. Data are expressed as mean±standard deviation (SD), with n=3. ** P<0.01 vs. control
3.4. Effect of 3-BrPA on the HK genes in RNAi nematodes
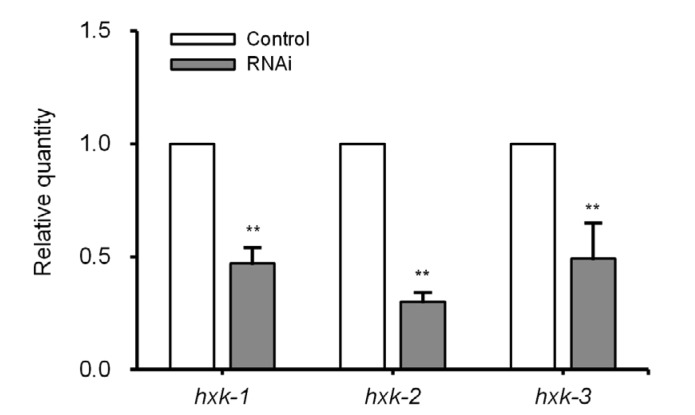
Fig. 5 shows the effects of silencing the hxk-1, hxk-2, and hxk-3 genes, determined using the qPCR. The interference effect of hxk-2 was better than those of hxk-1 and hxk-3 after silencing the hxk-1, hxk-2, and hxk-3 genes (P<0.01).
Fig. 5.
Relative mRNA expression after hxk-1, hxk-2, and hxk-3 gene interference
The expression of the HK genes in the control group was set to 1. Data are expressed as mean±standard deviation (SD), with n=3. ** P<0.01 vs. control
The LD50 was calculated using SPSS 19.0 software after exposing the hxk gene RNAi nematode to 3-BrPA for 24 h. Compared with that of the control group, the LC50 of nematodes decreased after silencing the hxk-1, hxk-2, and hxk-3 genes (P<0.05; Table 5).
Table 5.
3-Bromopyruvate (3-BrPA) LC50 of the HK gene RNAi nematodes
| Gene | Strain | 24 h LC50 (mmol/L) | 95% CI (mmol/L) |
| hxk-1 | N2 | 7.10** | 6.37–7.98 |
| hxk-2 | N2 | 9.10** | 8.29–9.99 |
| hxk-3 | N2 | 10.10* | 9.74–10.87 |
| Control | N2 | 12.70 | 4.58–26.38 |
P<0.05,
P<0.01 vs. control. LC50: 50% lethal concentration; CI: confidence interval
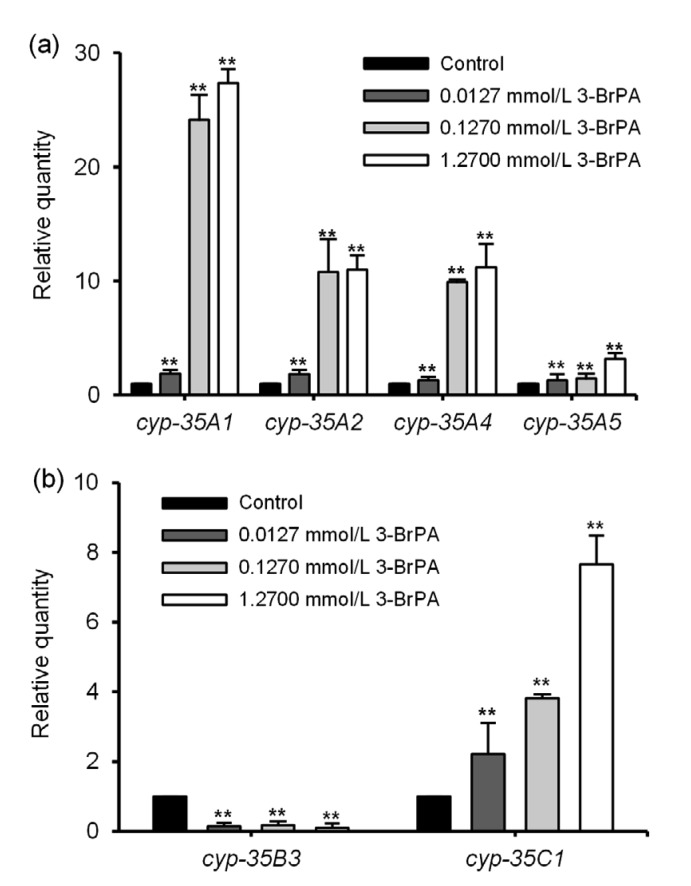
3.5. Effect of 3-BrPA on the expression of the cyp-35 gene in mutant strains
Fig. 6 shows the results of qPCR of the cyp-35A1, cyp-35A2, cyp-35A4, cyp-35A5, cyp-35B3, and cyp-35C1 gene mutant strains; the mRNA expression was set as 1 in the control group. The expression of the target gene with −ΔΔC T>1 was considered upregulated and that with −ΔΔC T<1 was considered downregulated. The upregulation of cyp-35A1 mRNA expression was the most obvious with 1.2700 mmol/L 3-BrPA, and it was concentration-dependent (Fig. 6a). When the concentration of 3-BrPA was increased from 0.0127 to 0.1270 mmol/L, the increase in the mRNA expression of cyp-35A1, cyp-35A2, and cyp-35A4 was obvious; however, the increase in cyp-35A5 mRNA expression was relatively moderate. Fig. 6b shows that the downregulation of cyp-35B3 mRNA expression and upregulation of cyp-35C1 mRNA expression were concentration-dependent.
Fig. 6.
Effect of 3-bromopyruvate (3-BrPA) on the mRNA expression of cyp-35 genes
(a) cyp-35A1, cyp-35A2, cyp-35A4, and cyp-35A5; (b) cyp-35B3 and cyp-35C1. The mRNA expression was set as 1 in the control group. Data are expressed as mean±standard deviation (SD), with n=3. ** P<0.01 vs. control
3.6. LC50 of 3-BrPA on the cyp-35 gene mutant strains
The LD50 was calculated using SPSS 19.0 software after exposing the cyp-35 gene mutant strains to 3-BrPA for 24 h. Compared with that of the control group, the LC50 of the cyp-35A1, cyp-35A2, cyp-35A4, cyp-35A5, cyp-35B3, and cyp-35C1 gene mutant strains decreased (P<0.05; Table 6).
Table 6.
3-Bromopyruvate (3-BrPA) LC50 of the cyp-35 gene mutant strains
| Gene | Strain | Variation type | Variation genomic position | 24 h LC50 (mol/L) | 95% CI (mol/L) |
| cyp-35A1 (ok1414) | VC875 | Allele: insertion/deletion | V:7359918..7361311 | 2.30** | 0.15–4.75 |
| cyp-35A2 (gk317) | VC710 | Allele: deletion | V:7362185..7363429 | 4.88** | 3.11–6.66 |
| cyp-35A4 (ok1393) | RB1294 | Allele | Unknown | 9.69** | 7.90–19.83 |
| cyp-35A5 (ok1985) | KO7C6.2 | Allele: deletion | V:3937831..3938637 | 5.26** | 4.27–6.85 |
| cyp-35B3 (gk663902) | K07C6.2 | Allele: substitution | V:3945462..3945462 | 3.10** | 1.27–4.30 |
| cyp-35C1 (ok2628) | RB1993 | Allele | Unknown | 4.67** | 3.22–5.68 |
| Control | N2 | 12.70 | 4.58–26.38 |
P<0.01, vs. control. LC50: 50% lethal concentration; CI: confidence interval
4. Discussion
HK is an enzyme that phosphorylates hexoses (six-carbon sugars), forming hexose phosphate. It is the first rate-limiting enzyme in glycolysis, which catalyzes almost all hexasaccharides (Cárdenas et al., 1998). It has been proved that 3-BrPA can bind to the active site of HK and inhibit its activity during glycolysis (Chen FZ et al., 2018). To the best of our knowledge, the present study is the first to use C. elegans to study 3-BrPA toxicity. The results showed that three different concentrations of 3-BrPA shortened the life span of nematodes. In the acute toxicity test for 24 h, the LD50 of 3-BrPA was 12.7 mmol/L, which indicated that 3-BrPA at concentrations higher than this can kill nematodes, and that at concentrations lower than this can effectively inhibit the growth and development of C. elegans.
HK was first identified in yeast, where it transferred an inorganic phosphate group from adenosine triphosphate (ATP) to the substrate (Slein et al., 1950). There are four important HK isozymes in mammals, which are designated as HK I, II, III, and IV (Wilson, 2003). In this study, through the comparison of genetic evolutionary tree, the genes hxk-2 and hxk-3 of C. elegans were found to be highly related to human HK2. As hxk-1 did not belong to the same branch as HK2 and HK3 of C. elegans, and the genetic and evolutionary similarities were low, we speculated that HK encoded by HK1 was different from that encoded by the HK2 and HK3 genes.
HK can catalyze almost all hexasaccharides and produce energy through glycolysis (Wilson, 2003). In normal tissues, its activity is limited by low energy demand and the level of its metabolite glucose-6-phosphate (de Meis et al., 1992). However, its activity is not restricted in tumor cells (Alcántar-Aguirre et al., 2013; Olsen et al., 2019). HK II is closely associated with carcinogenesis. Therefore, when tumor cells need more energy, the expression of HK II is high (Patra et al., 2013). In this study, we found that the expression of hxk-1, hxk-2, and hxk-3 in C. elegans exposed to 3-BrPA was significantly higher than that in the control group, and the expression of hxk-2 increased significantly. The results showed that 3-BrPA does not inhibit the expression of the HK genes, but inhibits the activity of HK by interacting with the protein. With decrease in the HK level, the production of ATP decreased; however, the HK pathway feedback increased HK gene expression, resulting in a further increase in the HK level. These results suggest that 3-BrPA mainly inhibits the activity of HK I and HK III. Furthermore, the high expression of the HK genes might be due to the consumption of a large amount of ATP during drug metabolism.
The cytochromes P450 (CYP) are a heme-containing superfamily of proteins. They are the major enzymes involved in drug metabolism, accounting for approximately 75% of the total metabolism (Guengerich, 2008). Menzel et al. (2001) used 18 human CYP inducers to induce cyp gene expression in nematodes and found that naphthalene flavones and lansoprazole induced CYP35 expression, revealing that the CYP35 family is closely related to drug metabolism. Roh and Choi (2011) found that nematodes with the RNAi gene cyp-35A2 exhibited a low metabolism of thiophosphate. In this study, 1.2700 mmol/L 3-BrPA upregulated the expression of cyp-35A1>cyp-35A4>cyp-35A2>cyp-35C1>cyp-35A5 in the nematodes, and when these genes were mutated, the LD50 of 3-BrPA was lower than that of the control. The results elucidate the drug metabolism and detoxification effect of CYP.
5. Conclusions
This study showed that 3-BrPA at a certain concentration shortens the average life span of nematodes. The toxicity target of 3-BrPA is closely related to its effect on hexokinase metabolism of C. elegans. The cyp-35 family plays a key role in the metabolism of 3-BrPA in nematodes. These genes can act as a reference for further studies on the toxicity of 3-BrPA and metabolism of drugs.
Acknowledgments
We thank technical guidance of Lihsia CHEN, PhD from the College of Biological Sciences, University of Minnesota, USA.
Footnotes
Project supported by the National Natural Science Foundation of China (Nos. 31172174 and 81460677), the Fundamental Research Funds for the Central Universities of China (No. 31920170039), and the Natural Science Found of Gansu Province (No. 18JR3RA283), China
Contributors: Conceptualization: Qiao-ling GU, Yan ZHANG, Gen CHEN; Methodology: Qiao-ling GU, Yan ZHANG, Xi-mei FU, Zhao-lian LU; Formal analysis: Qiao-ling GU, Yan ZHANG, Xi-mei FU, Zhao-lian LU; Resources: Gen CHEN, Rong MA, Wei KOU, Yong-mei LAN; Data curation: Qiao-ling GU, Yan ZHANG, Xi-mei FU, Yao YU; Writing original draft: Qiao-ling GU, Yan ZHANG, Xi-mei FU, Yao YU; Supervision: Gen CHEN; Funding acquisition: Qiao-ling GU, Yan ZHANG, Gen CHEN.
Compliance with ethics guidelines: Qiao-ling GU, Yan ZHANG, Xi-mei FU, Zhao-lian LU, Yao YU, Gen CHEN, Rong MA, Wei KOU, and Yong-mei LAN declared that they have no conflict of interest.
All institutional and national guidelines for the care and use of laboratory animals were followed.
References
- 1.Alcántar-Aguirre FC, Chagolla A, Tiessen A, et al. ATP produced by oxidative phosphorylation is channeled toward hexokinase bound to mitochondrial porin (VDAC) in beetroots (Beta vulgaris) Planta. 2013;237(6):1571–1583. doi: 10.1007/s00425-013-1866-4. [DOI] [PubMed] [Google Scholar]
- 2.Cárdenas ML, Cornish-Bowden A, Ureta T. Evolution and regulatory role of the hexokinases. Biochim Biophys Acta. 1998;1401(3):242–264. doi: 10.1016/S0167-4889(97)00150-X. [DOI] [PubMed] [Google Scholar]
- 3.Chen FZ, Wang H, Lai JD, et al. 3-Bromopyruvate reverses hypoxia-induced pulmonary arterial hypertension through inhibiting glycolysis: in vitro and in vivo studies. Int J Cardiol. 2018;266:236–241. doi: 10.1016/j.ijcard.2018.03.104. [DOI] [PubMed] [Google Scholar]
- 4.Chen GH, Zhang YD, Liang JF, et al. Deregulation of hexokinase II is associated with glycolysis, autophagy, and the epithelial-mesenchymal transition in tongue squamous cell carcinoma under hypoxia. BioMed Res Int, 2018:8480762. 2018 doi: 10.1155/2018/8480762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Meis L, Grieco MAB, Galina A. Reversal of oxidative phosphorylation in submitochondrial particles using glucose 6-phosphate and hexokinase as an ATP regenerating system. FEBS Lett. 1992;308(2):197–201. doi: 10.1016/0014-5793(92)81273-O. [DOI] [PubMed] [Google Scholar]
- 6.Dewaal D, Nogueira V, Terry AR, et al. Hexokinase-2 depletion inhibits glycolysis and induces oxidative phosphorylation in hepatocellular carcinoma and sensitizes to metformin. Nat Commun, 9:446. 2018 doi: 10.1038/s41467-017-02733-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dyląg M, Lis P, Niedźwiecka K, et al. 3-Bromopyruvate: a novel antifungal agent against the human pathogen Cryptococcus neoformans . Biochem Biophys Res Commun. 2013;434(2):322–327. doi: 10.1016/j.bbrc.2013.02.125. [DOI] [PubMed] [Google Scholar]
- 8.el Sayed SM, Baghdadi H, Zolaly M, et al. The promising anticancer drug 3-bromopyruvate is metabolized through glutathione conjugation which affects chemoresistance and clinical practice: an evidence-based view. Med Hypotheses. 2017;100:67–77. doi: 10.1016/j.mehy.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 9.García-Espiñeira MC, Tejeda-Benítez LP, Olivero-Verbel J. Toxic effects of bisphenol A, propyl paraben, and triclosan on Caenorhabditis elegans . Int J Environ Res Public Health. 2018;15(4):684. doi: 10.3390/ijerph15040684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guengerich FP. Cytochrome P450 and chemical toxicology. Chem Res Toxicol. 2008;21(1):70–83. doi: 10.1021/tx700079z. [DOI] [PubMed] [Google Scholar]
- 11.Huang RT, Huang Q, Wu GL, et al. Evaluation of the antioxidant property and effects in Caenorhabditis elegans of Xiangxi flavor vinegar, a Hunan local traditional vinegar. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2017;18(4):324–333. doi: 10.1631/jzus.B1600088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim JS, Ahn KJ, Kim JA, et al. Role of reactive oxygen species-mediated mitochondrial dysregulation in 3-bromopyruvate induced cell death in hepatoma cells: ROS-mediated cell death by 3-BrPA. J Bioenerg Biomembr. 2008;40(6):607–618. doi: 10.1007/s10863-008-9188-0. [DOI] [PubMed] [Google Scholar]
- 13.Kim W, Yoon JH, Jeong JM, et al. Apoptosis-inducing antitumor efficacy of hexokinase II inhibitor in hepatocellular carcinoma. Mol Cancer Ther. 2007;6(9):2554–2562. doi: 10.1158/1535-7163.MCT-07-0115. [DOI] [PubMed] [Google Scholar]
- 14.Lai CH, Chou CY, Ch'ang LY, et al. Identification of novel human genes evolutionarily conserved in Caenorhabditis elegans by comparative proteomics. Genome Res. 2000;10(5):703–713. doi: 10.1101/gr.10.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menzel R, Bogaert T, Achazi R. A systematic gene expression screen of Caenorhabditis elegans cytochrome P450 genes reveals CYP35 as strongly xenobiotic inducible. Arch Biochem Biophys. 2001;395(2):158–168. doi: 10.1006/abbi.2001.2568. [DOI] [PubMed] [Google Scholar]
- 16.Mycielska ME, Moser C, Wagner C, et al. Abstract 3211: inhibition of Hsp90 impairs expression of VDAC in plasma and mitochondrial membrane influencing cancer cell metabolism. Cancer Res. 2012;72(S8):3211. doi: 10.1158/1538-7445.AM2012-3211. [DOI] [Google Scholar]
- 17.Olsen BB, Gjedde A, Vilstrup MH, et al. Linked hexokinase and glucose-6-phosphatase activities reflect grade of ovarian malignancy. Mol Imaging Biol. 2019;21(2):375–381. doi: 10.1007/s11307-018-1247-2. [DOI] [PubMed] [Google Scholar]
- 18.Patra KC, Hay N. Hexokinase 2 as oncotarget. Oncotarget. 2013;4(11):1862–1863. doi: 10.18632/oncotarget.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patra KC, Wang Q, Bhaskar PT, et al. Hexokinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer. Cancer Cell. 2013;24(2):213–228. doi: 10.1016/j.ccr.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qiao Y, Zhao YL, Wu QL. Full toxicity assessment of Genkwa Flos and the underlying mechanism in nematode Caenorhabditis elegans . PLoS ONE. 2014;9(3):e91825. doi: 10.1371/journal.pone.0091825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roh JY, Choi J. Cyp35a2 gene expression is involved in toxicity of fenitrothion in the soil nematode Caenorhabditis elegans . Chemosphere. 2011;84(10):1356–1361. doi: 10.1016/j.chemosphere.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 22.Slein MW, Cori GT, Cori CF. A comparative study of hexokinase from yeast and animal tissues. J Biol Chem. 1950;186(2):763–780. [PubMed] [Google Scholar]
- 23.Wilson JE. Isozymes of mammalian hexokinase: structure, subcellular localization and metabolic function. J Exp Biol. 2003;206:2049–2057. doi: 10.1242/jeb.00241. [DOI] [PubMed] [Google Scholar]
- 24.Xu D, Jin JZ, Yu H, et al. Chrysin inhibited tumor glycolysis and induced apoptosis in hepatocellular carcinoma by targeting hexokinase-2. J Exp Clin Cancer Res. 2017;36(1):44. doi: 10.1186/s13046-017-0514-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yadav S, Pandey SK, Kumar A, et al. Antitumor and chemosensitizing action of 3-bromopyruvate: implication of deregulated metabolism. Chem Biol Interact. 2017;270:73–89. doi: 10.1016/j.cbi.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 26.Yadav S, Pandey SK, Singh VK, et al. Molecular docking studies of 3-bromopyruvate and its derivatives to metabolic regulatory enzymes: implication in designing of novel anticancer therapeutic strategies. PLoS ONE. 2017;12(5):e0176403. doi: 10.1371/journal.pone.0176403. [DOI] [PMC free article] [PubMed] [Google Scholar]