Abstract
Multiple myeloma (MM), considered an incurable hematological malignancy, is characterized by its clonal evolution of malignant plasma cells. Although the application of autologous stem cell transplantation (ASCT) and the introduction of novel agents such as immunomodulatory drugs (IMiDs) and proteasome inhibitors (PIs) have doubled the median overall survival to eight years, relapsed and refractory diseases are still frequent events in the course of MM. To achieve a durable and deep remission, immunotherapy modalities have been developed for relapsed/refractory multiple myeloma (RRMM). Among these approaches, chimeric antigen receptor (CAR) T-cell therapy is the most promising star, based on the results of previous success in B-cell neoplasms. In this immunotherapy, autologous T cells are engineered to express an artificial receptor which targets a tumor-associated antigen and initiates the T-cell killing procedure. Tisagenlecleucel and Axicabtagene, targeting the CD19 antigen, are the two pacesetters of CAR T-cell products. They were approved by the US Food and Drug Administration (FDA) in 2017 for the treatment of acute lymphocytic leukemia (ALL) and diffuse large B-cell lymphoma (DLBCL). Their development enabled unparalleled efficacy in combating hematopoietic neoplasms. In this review article, we summarize six promising candidate antigens in MM that can be targeted by CARs and discuss some noteworthy studies of the safety profile of current CAR T-cell therapy.
Keywords: Chimeric antigen receptor (CAR) T cells, Immunotherapy, Monoclonal antibody (mAb), Target antigen, Multiple myeloma
1. Introduction
Multiple myeloma (MM) is a B-cell malignancy that displays a myriad of clinical manifestations such as hypercalcemia, anemia, renal dysfunction, and bone destruction. It leads to an overgrowth of cancerous plasma cells along with production of monoclonal protein (Kyle and Rajkumar, 2004). It has a very poor prognosis, and its occurrence increases with age, with most people being diagnosed in their mid-60s (Moreau et al., 2017).
Although MM is a relatively rare disease, it is the second most common hematological malignancy after non-Hodgkin lymphoma (Becker, 2011). The American Cancer Society (2019) estimates that in 2019, 32 110 individuals will be newly diagnosed with MM, and 12 960 deaths will be caused by this disease. Until the introduction of thalidomide—the milestone in MM treatment—melphalan in combination with prednisone (MP) had been the standard treatment regimen for decades. With the application of autologous stem cell transplantation (ASCT) and availability of novel agents such as immunomodulatory drugs (IMiDs), and subsequent proteasome inhibitors (PIs), a new therapy paradigm has led to remarkable improvements in MM (Singhal et al., 1999; Paus et al., 2005; Rajkumar et al., 2006). Notably, the median overall survival (OS) in relapsed patients has doubled from 12 to 24 months (Kumar et al., 2008). Novel strategies have significantly altered the disease trajectory such that the median survival of patients with MM has improved from three to nearly eight years (Anderson, 2012). However, relapse is inevitable in the natural course of MM, and a fraction of patients who remain unresponsive to currently available regimens, referred to as refractory individuals, have a median survival of only 13 months and progression free survival (PFS) of five months (Kumar et al., 2017). The decreasing response of relapsed/refractory multiple myeloma (RRMM) is concomitant with repetitive salvage regimens leading to clonal evolution. This has profoundly limited the benefits from treatment approaches (Cremer et al., 2005; Stewart et al., 2007), with median life expectancy ranging from six to nine months (Richardson et al., 2007). The pivotal objective of MM treatment is to achieve a durable and deep remission (Moreau et al., 2017). However, only 43% of young patients (<50 years old) and 29% of old patients (≥50 years old) have reached the goal of survival in excess of 10 years after high-dose therapy (Ludwig et al., 2008). Therefore, based on the results of previous studies which serve as a reference point, and owing to their previous success, immunotherapy modalities have been developed for RRMM, including monoclonal antibodies (mAbs) (Touzeau et al., 2017), bispecific T-cell engagers (BiTEs) (Hipp et al., 2017; Seckinger et al., 2017), and chimeric antigen receptor (CAR) T-cell therapy (Ren et al., 2019). CAR T-cell therapy involves genetically engineered T lymphocytes with CARs targeting tumor-specific antigens in the absence of the major histocompatibility complex (MHC). This new approach is increasingly being used among the different immunotherapies available (Sadelain et al., 2013), thereby aiding RRMM treatment as a salvage plan.
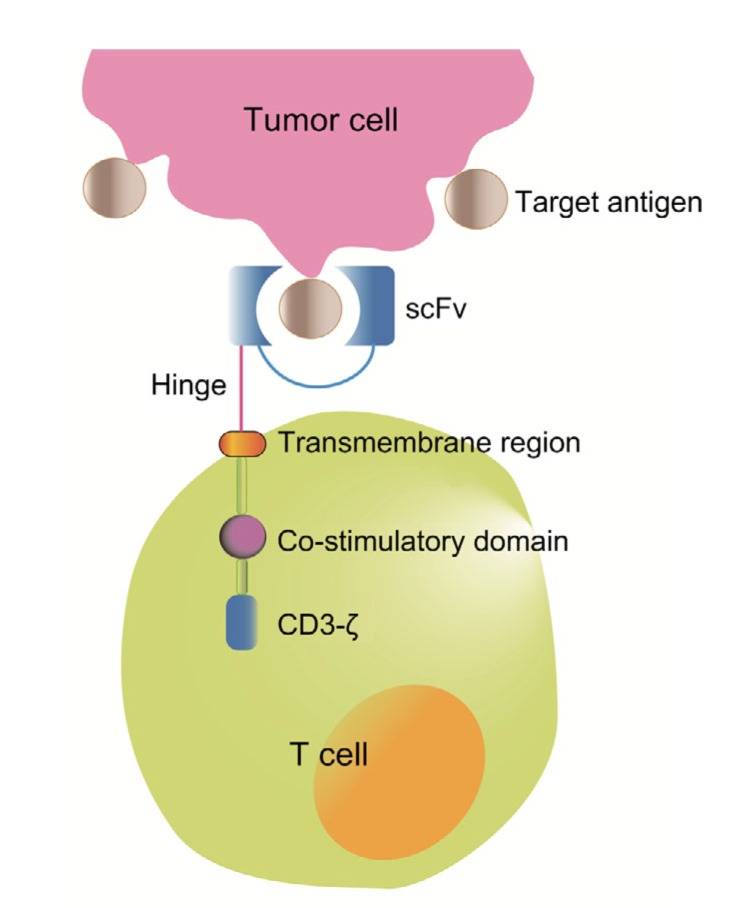
The story of CAR began in 1980s when Zelig ESHHAR introduced an extracellular target-specific single-chain variable fragment (scFv) derived from a mAb which resulted in T-cell activation (Eshhar et al., 1993). This structure was further optimized by combining it with a CD3-ζ chain of a T-cell receptor (TCR) and a co-stimulatory moiety such as 4-1BB (CD137) or CD28, which enhanced T-cell activation. T cells are equipped with a CAR structure which typically consists of a target-recognition ectodomain, a hinge region, an anchor-function transmembrane domain, and one or more signaling endodomains (Guedan et al., 2019) (Fig. 1).
Fig. 1.
Basic composition of a chimeric antigen receptor
The ectodomain of the chimeric antigen receptor (CAR) contains a single-chain variable fragment (scFv) and a hinge region. The transmembrane domain serves as a connection and a membrane anchor. The endodomain comprises the CD3-ζ signaling domain of the T-cell receptor and one or more co-stimulatory domains
The use of CAR T cells targeting CD19 is a landmark in the therapy of hematopoietic malignancies. It has been approved by the US Food and Drug Administration (FDA) for the treatment of relapsed or refractory acute lymphocytic leukemia (ALL) (Maude et al., 2015) and diffuse large B-cell lymphoma (DLBCL) (Kochenderfer et al., 2015). An ideal target is critical to develop a successful CAR with the ability to identify a tumor-associated antigen. The target antigen should have several features, such as being virtually absent from normal cells but overexpressed in malignant cells, contributing to differentiation and proliferation of malignant cells, and inducing clinical effects (Hideshima et al., 2007). For example, CD38 expressed at high epitope density on myeloma cells is involved in triggering immunosuppressive effects (Chillemi et al., 2017), enhancing T-cell activation (Quarona et al., 2013), and protecting B cells against apoptosis (Ibrahim et al., 2001). From this, CD38 antibodies such as daratumumab and isatuximab have shown considerable efficacy as a part of combination therapies with standard regimes. Currently, in the context of RRMM, CARs such as B-cell maturation antigen (BCMA), CD38, and CD138 are being investigated in clinical trials, but no CAR T-cell therapy for MM is yet licensed by authorities. In MM patients who are heavily pretreated, ineligible for transplantation or early relapsed from transplantation, CAR T-cell therapy may provide considerable improvement. In this review, we summarize current candidate antigens that are actively being investigated (Table 1), discuss important adverse effects, and provide management strategies for CAR T-cell therapy.
Table 1.
Selected CAR T-cell trials for multiple myeloma
| Antigen | Trial site/company | Phase | Accrual | n | ORR (%) | CR (%) | Identifier |
| BCMA | NCI | I | Completed | 16 | 81* | 13 | NCT02215967 |
| UPenn/Novartis | I | Completed | 25 | 48 | 8 | NCT02546167 | |
| Celgene/Bluebird | I | Completed | 33 | 85 | 45 | NCT02658929 | |
| Celgene/Bluebird | I | Ongoing | 12# | 83 | 25 | NCT03274219 | |
| Nanjing Legend | I/II | Ongoing | 57 | 88 | 68 | NCT03090659 | |
| Memorial Sloan-Kettering Cancer Center/Juno | I | Ongoing | 11 | 64 | 0 | NCT03070327 | |
| Fred Hutchinson Cancer Research Center/Juno | I | Ongoing | 11 | 100 | 36 | NCT03338972 | |
| Celgene (ex Juno) | I/II | Ongoing | 44 | 82 | 27 | NCT03430011 | |
| Poseida | I/II | Ongoing | 19 | 43 | 5 | NCT03288493 | |
| Celgene | II | Ongoing | NCT03601078 | ||||
| Celgene | III | Ongoing | NCT03651128 | ||||
| Autolus Limited | I/II | Ongoing | NCT03287804 | ||||
| Cartesian | I/II | Ongoing | NCT03448978 | ||||
| κ light chain | Baylor University | I | Completed | 7 | 0 | 0 | NCT00881920 |
| CD138 | Chinese PLA General Hospital | I/II | Completed | 5 | 20 | 0 | NCT01886976 |
| Lineberger Comprehensive Cancer Center | I | Ongoing | NCT03672318 | ||||
| CD38 | Sorrento/Celularity | I | Ongoing | NCT03464916 | |||
| Shenzhen Geno-Immune Medical Institute, China | I/II | Ongoing | NCT03271632 | ||||
| SLAMF7 | NCI | I | Ongoing | NCT03958656 | |||
| GPRC5D | Preclinical |
CAR, chimeric antigen receptor; BCMA, B-cell maturation antigen; GPRC5D, G-protein-coupled receptor, class C group 5 member D; NCI, National Cancer Institute; UPenn, University of Pennsylvania; PLA, People’s Liberation Army; ORR, overall response rate; CR, complete response.
81% (13 of 16) ORR at the highest dose of 9×106 CAR T cells/kg.
All 12 subjects were treated at the lowest dose of 1.50×108 cells/kg
2. Candidate antigens
2.1 B-cell maturation antigen
BCMA, which belongs to the tumor necrosis factor superfamily, regulates maturation and differentiation of B cells and the survival of plasma cells (O'Connor et al., 2004; Rickert et al., 2011). It is primarily expressed on normal and malignant plasma cells (Novak et al., 2004; Sanchez et al., 2012; Carpenter et al., 2013). BCMA is uniformly present on MM cells, but its expression has shown a variable intensity in clinical samples of patients (Seckinger et al., 2017). As anti-BCMA CAR T cells have exhibited robust efficacy in myeloma control in preclinical studies (Chekmasova et al., 2015), BCMA CARs have set the stage for the rapid growth of worldwide clinical trials, and they have provided the most important clinical experience to date in MM.
The anti-BCMA CAR T-cell therapy clinical trial led by Kochenderfer and colleagues in 2016—the first application in humans—recruited 12 patients with RRMM and BCMA expression (Ali et al., 2016). All patients received lymphodepleting conditioning with cyclophosphamide and fludarabine before a single dose of CAR T cells. Only one patient achieved stringent complete response (sCR) with the follow-up lasting for 17 weeks, although eight patients remained in a stable disease (SD) condition. Notably—it is not quite a matter of neglecting the unsatisfactory outcome of this trial, but of rejecting its implication—the patient with sCR received only three prior lines of therapy, whereas six out of the eight patients from the SD group received more than five prior lines of therapy. Moreover, the sCR patient received the highest anti-BCMA CAR T-cell dose level (9.0×106 cells/kg), whereas no individuals in the SD group received doses at the highest concentration. Although not particularly successful, this clinical trial demonstrated the feasibility of anti-BCMA CAR T-cell therapy, providing directions for subsequent research.
bb2121, combined with a phosphoinositide 3-kinases (PI3K) inhibitor, an anti-BCMA CAR T-cell product, has shown superior anti-myeloma activity regardless of BCMA load, and powerful recognition capability via strong surface CAR expression. It consists of autologous T cells transduced with a lentiviral vector encoding a novel anti-BCMA CAR. An ongoing multicenter phase I study of bb2121 CRB-401 enrolled 36 heavily pretreated patients with RRMM. The latest results for the first 33 patients are encouraging (Raje et al., 2019). All patients received chemo-conditioning with cyclophosphamide and fludarabine before a single dose of CAR T-cell infusion at four dose levels (50×106, 150×106, 450×106, or 800×106 cells/kg). The objective overall response rate (ORR) was 85% (median follow-up: 10.9 months), with 9% of patients achieving complete response (CR) and 36% showing sCR. Importantly, the ORR was unlikely to have been influenced by baseline serum or tumor BCMA level and previous treatments, but a better response was observed in patients with a high-risk cytogenetic profile, progressive disease (PD) or extramedullary disease before CAR T-cell therapy.
Currently, bb2121 is the most promising front runner among its analogous candidates such as JCARH125 (NCT03430011), P-BCMA-101 (NCT03 288493), and LCAR-B38M (NCT03090659) in the race for approval by authorities (Table 2). Recruitment is proceeding for a phase III study of bb2121, which should provide further reliable data (NCT 03651128).
Table 2.
Selection of ongoing and completed clinical trials of anti-BCMA CAR T cells for treatment of multiple myeloma
| CAR T cell | Lymphodepletion | CAR T cell dose (cells/kg) | CRS | NT | Comment | Identifier |
| NCI | Flu/Cy | 9×106 | 93% | 6% | Grade 3 or 4 CRS was associated with a high level of bone marrow plasma cells and NT was limited in the setting of severe CRS | NCT02215967 |
| (15/16) | (1/16) | |||||
| UPenn (Novartis) | ±Cy | Cohort 1: | 88% | 32% | The median peak fold-increases of IL-6 and several other cytokines were 1 to 2 orders of magnitude lower than that reported in the NCI BCMA CAR T cell study | NCT02546167 |
| 1×108–5×108 CAR T cells; | (22/25) | (8/25) | ||||
| Cohort 2: | ||||||
| Cy+(1×107–5×107) CAR T cells; | ||||||
| Cohort 3: | ||||||
| Cy+(1×108–5×108) CAR T cells | ||||||
| bb2121 | Flu/Cy | 50×106, 150×106, 450×6, 800×106 | 76% | 42% | CRS occurred at mostly grades 1 and 2 (70%), and NT of grade ≥3 occurred in one patient | NCT02658929 |
| (25/33) | (14/33) | |||||
| bb21217 | Flu/Cy | 150×106 | 67% | 25% | All CRS and NT events were manageable and no deaths occurred on this lowest-dose cohort | NCT03274219 |
| (8/12) | (3/12) | |||||
| LCAR-B38M/ | Cy | Median dose: 0.5×106 | 90% | 2% | CRS occurred at mostly grades 1 and 2 (83%), and NT events of grade 1 were resolved within 1 d | NCT03090659 |
| JNJ-68284528 | (51/57) | (1/57) | ||||
| MCARH171 | Cy or Flu/Cy | 1×106; 150×106, 450×106, 800×106 | 60% | 10% | CRS of grade ≥3 occurred in 20% of patients, and no NT events of grade ≥3 were observed | NCT03070327 |
| (6/10)# | (1/10)# | |||||
| FCARH143 | Flu/Cy | 50×106 | 91% | 9% | No CRS of grade ≥3 was observed | NCT03338972 |
| (10/11) | (1/11) | |||||
| JCARH125 | Flu/Cy | 50×106, 150×106, 450×106 | 80% | 25% | CRS of grade ≥3 occurred in 9% of patients, and NT events of grade ≥3 occurred in 7% of patients | NCT03430011 |
| (35/44) | (11/44) | |||||
| P-BCMA-101* | Flu/Cy | 0.75×106, 2×106, 6×106, 10×106, 15×106 | 10% | 5% | Only two cases of potential CRS were reported (grades 1 and 2) | NCT03288493 |
| (2/21) | (1/21) |
CAR, chimeric antigen receptor; NCI, National Cancer Institute; UPenn, University of Pennsylvania; Flu, fludarabine; Cy, cyclophosphamide; CRS, cytokine release syndrome; NT, neurotoxicity.
One patient required early steroids and radiation for impending cord compression and was not evaluable for toxicities;
P-BCMA-101 is manufactured using the “piggyback” approach, with a non-viral system for DNA delivery plus a small human fibronectin domain for BCMA. Favorable safety profile, high purity (>95% CAR+) and a T stem cell memory (TSCM) phenotype (gradual and prolonged activity)
2.2 κ light chain
Carlos RAMOS and colleagues were inspired to overcome the adverse effects of anti-CD19 CAR T-cell therapy, hypogammaglobulinemia, caused by ubiquitous expression of CD19 on all cells from B-cell lineage. They found that preservation of normal B cells may be achieved by targeting the light chain on the cell surface (Ramos et al., 2016). On mature B lymphocytes and mature B lymphoid malignant cells and myeloma cells, either the κ or λ light chain is expressed instead of both; thus, targeting the κ light chain can prevent complete B-cell ablation (Ramos et al., 2016). The first clinical trial, known as a blanket investigation, consisted of seven patients with MM and nine patients with non-Hodgkin lymphoma (Ramos et al., 2016). A modest outcome was observed—four patients had SD for up to 24 months—but three patients showed no response. This κ light chain-targeting CAR has proved elusive because light chains, which are generally secreted, present limited surface expression, which may block the CAR from targeting malignant cells and mediate CAR T-cell depletion.
2.3 CD138
CD138, a member of the syndecan family involved in cell adhesion, is the hallmark of plasma cells, being highly expressed on both malignant and healthy plasma cells, and predominantly expressed on epithelial cells (O'Connell et al., 2004; Palaiologou et al., 2014). Since the high plasma cell specificity of CD138 is well established, it is recommended that CD138 in combination with CD38 serves as the basis for identifying myeloma cells by flow cytometry (Frigyesi et al., 2014). As CD138 plays a crucial role in the pathophysiology of MM, such as cell proliferation, infiltration, or apoptosis, it is definitely a viable candidate antigen for CAR T-cell therapy (Yoo et al., 2015).
The first in-human trial of anti-CD138 CAR T-cell therapy led by Bo GUO and colleagues in 2013 comprised a small number of MM patients (n=5) with advanced disease. The trial aimed to investigate whether cytokine-induced killer cells with lentiviral vector-mediated transduction of an anti-CD138 CAR gene exert antitumor effects in myeloma cells (Guo et al., 2016). Four out of five patients met the criteria of SD, one of whom achieved up to 12 months of SD and another had PD within a month after infusion. In addition, certain visible changes were observed in the morphology of plasma cells in bone marrow aspirates of all patients, but these had no verified correlation with any immune response against myeloma cells exerted by anti-CD138 CAR T cells. All patients received cell infusion (an average dose of 0.7563×107 cells/kg), and only one or two grades of cytokine release syndrome (CRS) were observed. Although it is difficult to draw a firm conclusion based on the small sample size, a respectable safety profile and limited antitumor efficiency do provide the basis for further trials with this alternative target. Because of inadequate evaluation of the efficacy and adverse effects in preclinical studies, it is difficult to obtain compelling data to support clinical trials.
The latest report that strongly supports prior clinical trials was conducted by Sun et al. (2019), who developed T cells through retroviral vector-mediated transduction of anti-CD138 scFvs from BT062, the CD138 antibody. A complete elimination of CD138+ cells was observed in vitro after 3–5 d co-culture of anti-CD138 CAR T cells and MM cell lines. In a co-culture with myeloma cells from MM patients, anti-CD138 CAR T cells derived from both patients and healthy donors exhibited tumoricidal activity against not only CD138+ malignant cells but also CD138+ stem cells, and showed comparable cytokine secretion profiles of interferon-γ (IFN-γ) and interleukin-2 (IL-2). The significant killing capability of the anti-CD138 CAR T cells seemed unaffected even in the presence of high amounts of soluble CD138, which can neutralize CAR T cells by coupling with the scFv domain. Moreover, no lysis of epithelial and endothelial cells was observed, and no adverse effects such as diarrhea, mucositis, or stomatitis were noted in the BT062 treatment. A clinical trial (NCT03672318) of anti-CD138 CAR T-cell therapy targeting patients of RRMM, set up in January 2019, is in progress.
2.4 CD38
CD38 was first identified as a cell-surface structural marker on mouse B lymphocytes (Lund, 2006). Further research showed that in normal hematopoiesis, CD38 can be found on natural killer (NK) cells, monocytes, and lymphoid and myeloid cells (Quarona et al., 2013), and in the context of MM, CD38 is universally overexpressed on malignant plasma cells (van Dongen et al., 2012). In addition to its ubiquitous expression on myeloma cells, CD38 is being highlighted for its involvement in physiological and pathological conditions as a multifunctional enzyme (Howard et al., 1993; Deaglio et al., 2001; Chillemi et al., 2017).
The efficacy of CD38 as a target antigen was initially established in mAbs such as daratumumab (Lokhorst et al., 2015; Krejcik et al., 2016), which gained FDA approval for RRMM treatment in 2015. CD38 antibody was frequently suggested as a part of combination therapies in RRMM treatment. For example, daratumumab in combination with lenalidomide-dexamethasone (Rd) had an ORR of 92.9% (CR or better in 43.1%) and a PFS at 12 months of 83.2% (Lonial et al., 2016). Patients treated with daratumumab with bortezomib-dexamethasone (Vd) had an ORR of 82.9% (CR or better in 19.2%) and a PFS at 12 months of 60.7% (Palumbo et al., 2016), whereas daratumumab monotherapy showed an ORR of 29% (sCR in 2.8%), a median duration of response of 7.4 months, and a median PFS of 3.7 months (Lonial et al., 2016).
Mihara et al. (2012) transduced an anti-CD38 CAR gene into T cells obtained from healthy donors, reporting that anti-CD38 CAR T cells exert potent cytolytic effects in myeloma cell lines and primary myeloma cells from clinical patients. Most subsequent studies carried out in the light of this first trial have generated similar outcomes. Nevertheless, concerns have arisen regarding the role of CD38 in T-cell activation and regulation (Funaro et al., 1990). T cells containing CD38 and anti-CD38 CAR may lead to fratricide, resulting in abrogation of effector T cells. Drent et al. (2018) have developed several strategies to cope with these limitations. For example, generating lower-affinity CAR structures can decrease the recognition of T cells with CD38 expression at an intermediate level.
The first phase I clinical study (NCT03464916) for a USA-based anti-CD38 CAR T-cell therapy was conducted in 2018 at the University of Pennsylvania and Roger Williams Medical Center. The aim was to evaluate the efficacy and safety of CAR2 anti-CD38 A2 CAR T cells in patients with RRMM, and to shed light on further measures.
2.5 SLAMF7
SLAMF7 (aliases CD319, CRACC, CS-1) is a member of the signaling lymphocytic activation molecule (SLAM) family, first identified on NK cells (Boles and Mathew, 2001). It is expressed on a fraction of T cells, B cells, macrophages, and dendritic cells, and its expression in more than 95% of normal and malignant plasma cells of MM has been documented (Hsi et al., 2008). However, the mechanism underlying the upregulated SLAMF7 has not been entirely elucidated (Calpe et al., 2008; Schwartzberg et al., 2009; Wu and Veillette, 2016). It may be correlated with the formation of the immunosuppressive bone marrow milieu that aids the harboring of myeloma cells (Tai et al., 2008).
The introduction of elotuzumab—the first humanized SLAMF7 antibody—has laid the foundation for studies of the further utility of SLAMF7 as a target for CAR T-cell therapy (Friend et al., 2017). Gogishvili et al. (2017) designed the anti-SLAMF7 CAR T cell by obtaining scFvs from elotuzumab. CD8+ anti-SLAMF7 CAR T cells promptly exert tumoricidal effects, leading to >90% lysis of myeloma cell lines after a 20-h co-culture. As for CD38+ CD138+ SLAMF7+ malignant cells obtained from MM patients, anti-SLAMF7 CAR T cells have exhibited rapid eradication of all myeloma cells in 4 h regardless of antigen load. Furthermore, in xenograft models with extramedullary invasion, anti-SLAMF7 CAR T cells were capable of exerting anti-myeloma activity in systematic infiltration. In addition, engineered T cells derived from MM patients and healthy donors displayed similar oncolytic effects both in vivo and in vitro.
The concern surrounding anti-SLAMF7 CAR is its expression in a fraction of functional cells. CD8+ anti-SLAMF7 CARs are able to elicit fratricide via coupling with SLAMF7, which is present at a high level in T cells (Gogishvili et al., 2017). Reduction of effector T cells is observed wherein elotuzumab produces the adverse effect of lymphopenia. However, a proportion of lymphocytes with moderate SLAMF7 expression can be preserved, and the residual T cells can maintain the capability of eliciting an immune response toward certain viral pathogens (Gogishvili et al., 2017).
Furthermore, SLAMF7 serves as a pro-phagocytic signal on macrophages, which perform essential phagocytosis in hematopoietic tumors compared with other tissue-derived tumors (Chen et al., 2017). A recent study indicates that SLAMF7 is not a prerequisite for killing diffuse large B cell lymphoma (DLBCL) cells (He et al., 2019). However, macrophages are likely to mediate endogenous elimination of myeloma cells via surface SLAMF7 signaling, which may be impaired by anti-SLAMF7 CAR T cells.
A phase I clinical trial of anti-SLAMF7 CAR T-cell therapy is open for recruiting in May 2019 in the USA and CARs will be designed to express both anti-SLAMF7 antibody and a suicide gene (NCT03958656).
2.6 GPRC5D
Orphan G-protein-coupled receptor, class C group 5 member D (GPRC5D) was once a candidate for distinguishing MM cells because its elevated transcript level correlates with poor prognosis of MM (Atamaniuk et al., 2012). It is now a potential alternative option for MM treatment.
According to previous in situ hybridization studies, GPRC5D expression in MM patients is restricted to three anatomical locations—hair follicles (Inoue et al., 2004; Gao et al., 2016; Kim et al., 2017), lung tissue, and bone marrow (Atamaniuk et al., 2012; Cohen et al., 2013). Although the expression of GPRC5D mRNA was confirmed by previous studies (Atamaniuk et al., 2012), the protein product of GPRC5D could not be detected on plasma cells in samples from patients with MM (Smith et al., 2019). Gene chip profiling has demonstrated that GPRC5D expression on malignant and normal plasma cells in bone marrow is substantially higher (at least 500-fold) than that on plasma cells in the peripheral blood (Smith et al., 2019).
Previous research has revealed the likelihood that GPRC5D expression is independent of BCMA expression (Smith et al., 2019). Analysis of CD138+ plasma cells obtained from 83 clinical trial samples from patients with primary MM under the cutoff ≥50% target antigen expression showed 65% of samples with GPRC5D expression, 74% with BCMA expression, and 88% with co-expression of GPRC5D and BCMA (Smith et al., 2019).
As the most critical initial step, manufacturing the GPRC5D target scFvs provides the basis for effective and specific CAR T-cell therapy. A preclinical investigation generated anti-GPRC5D CARs via retroviral vector-mediated transduction after selecting scFvs with high GPRC5D-specific affinity from a human B-cell-derived phage display (Smith et al., 2019). Preliminary outcomes have shown that anti-GPRC5D CAR T cells exhibit anti-myeloma activity comparable to that of anti-BCMA CAR T cells in vitro and in vivo, and similar secretion profiles of cytokines such as elevated IFN-γ, tumor necrosis factor-α (TNF-α), and granulocyte-macrophage colony-stimulating factor (GM-CSF) were observed. Notably, even in relapsed xenograft models with BCMA loss after BCMA-specific CAR T-cell therapy, anti-GPRC5D CAR T cells can eliminate malignant cells, thereby inhibiting disease progression (Smith et al., 2019).
GPRC5D is highly expressed on the hair follicles of hairy animal models such as monkeys and mice, but with no alopecia or other visible damage observed, which may be caused by the immune-privileged site of hair follicles (Westgate et al., 1991; Paus et al., 2005; Wang et al., 2014). Laboratory data on GPRC5D as a potential target have provided a stepping stone for further clinical trials.
3. Controlling toxicities in CAR T-cell treatment of MM
Apart from non-specific side effects such as infusion reactions, infection, or tumor lysis syndrome, CRS and neurotoxicity (NT) are two major adverse effects observed after CAR T-cell therapy. The first sign of CRS, occurring in up to 90% of patients within the first week after infusion, is a high fever along with a cluster of other manifestations such as fatigue, nausea, myalgia, and anorexia (Lee et al., 2014; Brudno and Kochenderfer, 2016). Although most CRS is mild to moderate within a self-limiting course, severe CRS can rapidly progress and evolve into life-threatening complications including severe hypotension, dysoxia, or multiple organ dysfunction (Brentjens et al., 2010; Lee et al., 2014). CRS is driven by an excessively high level of pro-inflammatory cytokines, such as IL-6, IFN-γ, and granulocyte macrophage-colony stimulating factor (GM-CSF), and is associated with the expansion and activation of CAR T cells (Lee et al., 2014). Notably, tocilizumab, an IL-6 receptor antagonist with FDA approval for rapid CRS resolution (Le et al., 2018) based on data from anti-CD19 CAR T-cell therapy, has demonstrated potent efficiency for treating severe CRS without limiting CAR T-cell efficacy (Grupp et al., 2013). The dose of tocilizumab recommended by FDA is 8 mg/kg (12 mg/kg for patients weighing less than 30 kg) (Le et al., 2018). For patients with a poor response to the initial dose of tocilizumab, clinical situations may improve with a second administration and/or addition of corticosteroids (Neelapu et al., 2018). To date, siltuximab, which prevents IL-6 from binding to its receptor by forming an affinity complex with IL-6, is administered only under the condition of CRS being refractory to tocilizumab and corticosteroid (Mahmoudjafari et al., 2019). CAR T-cell therapy-associated NT frequently presents with a new onset of neural symptoms within three weeks after infusion (Davila et al., 2014). Although representative NT generally appears after high-grade CRS for some reasons, atypical NT events which can occur independently have also been observed. Clinical manifestations, usually self-limiting, include headache, apraxia, ataxia, dysgraphia, seizures, and myoclonus (Grupp et al., 2013; Kochenderfer et al., 2015; Maude et al., 2018). Unlike CRS, NT responds poorly to intervention with tocilizumab (Lam et al., 2016; Gauthier and Turtle, 2018). Corticosteroids are the frontline management drugs for NT to date, although no robust data are available to support this practice (Porter et al., 2018).
“On-target but off-tumor” toxicity, which refers to the adverse effects of the target antigen on normal tissue, is also a major concern. For example, CD38 is expressed on plasma cells, NK cells, monocytes, healthy T cells and B cells, and a fraction of hematopoietic progenitor cells (Terstappen et al., 1991). CD38 is upregulated with T cell activation (Dianzani et al., 1994); therefore, CD38-specific CAR T-cell therapy may result in a persistent cytopenia and T-cell fratricide.
Various approaches have been proposed to minimize the off-tumor effects of CAR T-cell therapy. The strategy of optimizing the ectodomain and then screening for CARs with a lower affinity for normal tissue allows for generation of CAR T cells that are highly specific for malignant cells owing to their excessive antigen density (Drent et al., 2017). Moreover, incorporation of the suicide gene by editing technologies such as transcription activator-like effector nuclease (TALEN) and clustered regularly interspaced palindromic repeats (CRISPR)-Cas9 mediates toxicities in a more controllable status (Straathof et al., 2005; Philip et al., 2014; Drent et al., 2016). In view of this, the doxycycline (DOX)-regulated Tet-on system can maximize antitumor activity while alleviating “off-tumor” effects. Drent et al. (2018) designed CAR T cells with a DOX-induced structure. The cells are devitalized without DOX administration and revived after a high dose of DOX.
4. Conclusions
Immunotherapy with CAR-expressing T cells has shown encouraging success in patients with RRMM; even a small nudge can yield remarkable lessons for others. In this review, we have highlighted several promising candidate antigens in RRMM treatment, including those in some stages of clinical trials, as well as a novel antigen. However, while CAR T-cell therapy has laid down a treatment pattern for RRMM, a myriad of challenges have emerged. One challenge is to reduce “off-target” toxicities. For instance, although CD56 is expressed on malignant plasma cells and not on healthy ones, it is present on major organs such as myocardial tissue, so CAR T-cell therapy may lead to heart dysfunction. Such issues can be addressed by targeting ideal antigens, optimizing CAR constructs, and incorporating the suicide gene. Another important problem to cope with is minimizing CAR T-cell therapy-related toxicities. Whereas for CRS, IL-6 blockage has been regarded as the first-line management strategy with rapid symptom control, no credible agents are available for resolving NT. Future in-depth research is needed at the pathophysiological level, aiming to facilitate management of NT and to establish a consensus on treatment regimens. To emulate traditional modalities, scientists are already setting their sights farther afield.
Footnotes
Contributors: He HUANG took the lead in writing the manuscript. Heng-wei WU wrote and edited the manuscript. Yong-xian HU contributed to shaping the tables and figures. All authors read and approved the final manuscript and, therefore, had full access to all the data in the study and take responsibility for the integrity and security of the data.
Compliance with ethics guidelines: He HUANG, Heng-wei WU, and Yong-xian HU declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Ali SA, Shi V, Maric I, et al. T cells expressing an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood. 2016;128(13):1688–1700. doi: 10.1182/blood-2016-04-711903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Cancer Society. Cancer Facts & Figures 2019. American Cancer Society, Atlanta, GA, USA; 2019. [Google Scholar]
- 3.Anderson KC. The 39th David A. Karnofsky Lecture: bench-to-bedside translation of targeted therapies in multiple myeloma. J Clin Oncol. 2012;30(4):445–452. doi: 10.1200/jco.2011.37.8919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atamaniuk J, Gleiss A, Porpaczy E, et al. Overexpression of G protein-coupled receptor 5D in the bone marrow is associated with poor prognosis in patients with multiple myeloma. Eur J Clin Invest. 2012;42(9):953–960. doi: 10.1111/j.1365-2362.2012.02679.x. [DOI] [PubMed] [Google Scholar]
- 5.Becker N. Epidemiology of multiple myeloma. In: Moehler T Goldschmidt H., editor. Multiple Myeloma. Recent Results in Cancer Research, Vol. 183. Springer, Berlin, Heidelberg; 2011. pp. 25–35. [DOI] [PubMed] [Google Scholar]
- 6.Boles KS, Mathew PA. Molecular cloning of CS1, a novel human natural killer cell receptor belonging to the CD2 subset of the immunoglobulin superfamily. Immunogenetics. 2001;52(3-4):302–307. doi: 10.1007/s002510000274. [DOI] [PubMed] [Google Scholar]
- 7.Brentjens R, Yeh R, Bernal Y, et al. Treatment of chronic lymphocytic leukemia with genetically targeted autologous T cells: case report of an unforeseen adverse event in a phase I clinical trial. Mol Ther. 2010;18(4):666–668. doi: 10.1038/mt.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood. 2016;127(26):3321–3330. doi: 10.1182/blood-2016-04-703751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calpe S, Wang NH, Romero X, et al. The SLAM and SAP gene families control innate and adaptive immune responses. Adv Immunol. 2008;97:177–250. doi: 10.1016/s0065-2776(08)00004-7. [DOI] [PubMed] [Google Scholar]
- 10.Carpenter RO, Evbuomwan MO, Pittaluga S, et al. B-cell maturation antigen is a promising target for adoptive T-cell therapy of multiple myeloma. Clin Cancer Res. 2013;19(8):2048–2060. doi: 10.1158/1078-0432.ccr-12-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chekmasova AA, Horton HM, Garrett TE, et al. A novel and highly potent CAR T cell drug product for treatment of BCMA-expressing hematological malignances. Blood. 2015;126(23):3094. doi: 10.1182/blood.V126.23.3094.3094. [DOI] [Google Scholar]
- 12.Chen J, Zhong MC, Guo HJ, et al. SLAMF7 is critical for phagocytosis of haematopoietic tumour cells via Mac-1 integrin. Nature. 2017;544(7651):493–497. doi: 10.1038/nature22076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chillemi A, Quarona V, Antonioli L, et al. Roles and modalities of ectonucleotidases in remodeling the multiple myeloma niche. Front Immunol, 8:305. 2017 doi: 10.3389/fimmu.2017.00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen Y, Gutwein O, Garach-Jehoshua O, et al. GPRC5D is a promising marker for monitoring the tumor load and to target multiple myeloma cells. Hematology. 2013;18(6):348–351. doi: 10.1179/1607845413Y.0000000079. [DOI] [PubMed] [Google Scholar]
- 15.Cremer FW, Bila J, Buck I, et al. Delineation of distinct subgroups of multiple myeloma and a model for clonal evolution based on interphase cytogenetics. Genes Chromosomes Cancer. 2005;44(2):194–203. doi: 10.1002/gcc.20231. [DOI] [PubMed] [Google Scholar]
- 16.Davila ML, Riviere I, Wang XY, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6(224):224ra25. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deaglio S, Mehta K, Malavasi F. Human CD38: a (r)evolutionary story of enzymes and receptors. Leuk Res. 2001;25(1):1–12. doi: 10.1016/S0145-2126(00)00093-X. [DOI] [PubMed] [Google Scholar]
- 18.Dianzani U, Funaro A, DiFranco D, et al. Interaction between endothelium and CD4+CD45RA+ lymphocytes: role of the human CD38 molecule. J Immunol. 1994;153(3):952–959. [PubMed] [Google Scholar]
- 19.Drent E, Groen RWJ, Noort WA, et al. Pre-clinical evaluation of CD38 chimeric antigen receptor engineered T cells for the treatment of multiple myeloma. Haematologica. 2016;101(5):616–625. doi: 10.3324/haematol.2015.137620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drent E, Themeli M, Poels R, et al. A rational strategy for reducing on-target off-tumor effects of CD38-chimeric antigen receptors by affinity optimization. Mol Ther. 2017;25(8):1946–1958. doi: 10.1016/j.ymthe.2017.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drent E, Poels R, Mulders MJ, et al. Feasibility of controlling CD38-CAR T cell activity with a Tet-on inducible CAR design. PLoS ONE. 2018;13(5):e0197349. doi: 10.1371/journal.pone.0197349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eshhar Z, Waks T, Gross G, et al. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the γ or ζ subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci USA. 1993;90(2):720–724. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friend R, Bhutani M, Voorhees PM, et al. Clinical potential of SLAMF7 antibodies–focus on elotuzumab in multiple myeloma. Drug Des Devel Ther. 2017;11:893–900. doi: 10.2147/dddt.s98053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frigyesi I, Adolfsson J, Ali M, et al. Robust isolation of malignant plasma cells in multiple myeloma. Blood. 2014;123(9):1336–1340. doi: 10.1182/blood-2013-09-529800. [DOI] [PubMed] [Google Scholar]
- 25.Funaro A, Spagnoli GC, Ausiello CM, et al. Involvement of the multilineage CD38 molecule in a unique pathway of cell activation and proliferation. J Immunol. 1990;145(8):2390–2396. [PubMed] [Google Scholar]
- 26.Gao Y, Wang XL, Yan HL, et al. Comparative transcriptome analysis of fetal skin reveals key genes related to hair follicle morphogenesis in cashmere goats. PLoS ONE. 2016;11(3):e0151118. doi: 10.1371/journal.pone.0151118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gauthier J, Turtle CJ. Insights into cytokine release syndrome and neurotoxicity after CD19-specific CAR-T cell therapy. Curr Res Transl Med. 2018;66(2):50–52. doi: 10.1016/j.retram.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gogishvili T, Danhof S, Prommersberger S, et al. SLAMF7-CAR T cells eliminate myeloma and confer selective fratricide of SLAMF7+ normal lymphocytes. Blood. 2017;130(26):2838–2847. doi: 10.1182/blood-2017-04-778423. [DOI] [PubMed] [Google Scholar]
- 29.Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368(16):1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guedan S, Calderon H, Posey AD, Jr, et al. Engineering and design of chimeric antigen receptors. Mol Ther Methods Clin Dev. 2019;12:145–156. doi: 10.1016/j.omtm.2018.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo B, Chen MX, Han QW, et al. CD138-directed adoptive immunotherapy of chimeric antigen receptor (CAR)-modified T cells for multiple myeloma. J Cell Immunother. 2016;2(1):28–35. doi: 10.1016/j.jocit.2014.11.001. [DOI] [Google Scholar]
- 32.He Y, Bouwstra R, Wiersma VR, et al. Cancer cell-expressed SLAMF7 is not required for CD47-mediated phagocytosis. Nat Commun. 2019;10(1):533. doi: 10.1038/s41467-018-08013-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hideshima T, Mitsiades C, Tonon G, et al. Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat Rev Cancer. 2007;7(8):585–598. doi: 10.1038/nrc2189. [DOI] [PubMed] [Google Scholar]
- 34.Hipp S, Tai YT, Blanset D, et al. Erratum: a novel BCMA/CD3 bispecific T-cell engager for the treatment of multiple myeloma induces selective lysis in vitro and in vivo . Leukemia. 2017;31(10):2278. doi: 10.1038/leu.2017.219. [DOI] [PubMed] [Google Scholar]
- 35.Howard M, Grimaldi JC, Bazan JF, et al. Formation and hydrolysis of cyclic ADP-ribose catalyzed by lymphocyte antigen CD38. Science. 1993;262(5136):1056–1059. doi: 10.1126/science.8235624. [DOI] [PubMed] [Google Scholar]
- 36.Hsi ED, Steinle R, Balasa B, et al. CS1, a potential new therapeutic antibody target for the treatment of multiple myeloma. Clin Cancer Res. 2008;14(9):2775–2784. doi: 10.1158/1078-0432.ccr-07-4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ibrahim S, Keating M, Do KA, et al. CD38 expression as an important prognostic factor in B-cell chronic lymphocytic leukemia. Blood. 2001;98(1):181–186. doi: 10.1182/blood.V98.1.181. [DOI] [PubMed] [Google Scholar]
- 38.Inoue S, Nambu T, Shimomura T. The RAIG family member, GPRC5D, is associated with hard-keratinized structures. J Invest Dermatol. 2004;122(3):565–573. doi: 10.1046/j.0022-202X.2004.12628.x. [DOI] [PubMed] [Google Scholar]
- 39.Kim YJ, Yoon B, Han K, et al. Comprehensive transcriptome profiling of balding and non-balding scalps in trichorhinophalangeal syndrome type I patient. Ann Dermatol. 2017;29(5):597–601. doi: 10.5021/ad.2017.29.5.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33(6):540–549. doi: 10.1200/jco.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krejcik J, Casneuf T, Nijhof IS, et al. Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood. 2016;128(3):384–394. doi: 10.1182/blood-2015-12-687749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111(5):2516–2520. doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumar SK, Dimopoulos MA, Kastritis E, et al. Natural history of relapsed myeloma, refractory to immunomodulatory drugs and proteasome inhibitors: a multicenter IMWG study. Leukemia. 2017;31(11):2443–2448. doi: 10.1038/leu.2017.138. [DOI] [PubMed] [Google Scholar]
- 44.Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med. 2004;351(18):1860–1873. doi: 10.1056/NEJMra041875. [DOI] [PubMed] [Google Scholar]
- 45.Lam L, Chin L, Halder RC, et al. Epigenetic changes in T-cell and monocyte signatures and production of neurotoxic cytokines in ALS patients. FASEB J. 2016;30(10):3461–3473. doi: 10.1096/fj.201600259RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Le RQ, Li L, Yuan WS, et al. FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome. Oncologist. 2018;23(8):943–947. doi: 10.1634/theoncologist.2018-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lokhorst HM, Plesner T, Laubach JP, et al. Targeting CD38 with daratumumab monotherapy in multiple myeloma. N Engl J Med. 2015;373(13):1207–1219. doi: 10.1056/NEJMoa1506348. [DOI] [PubMed] [Google Scholar]
- 49.Lonial S, Weiss BM, Usmani SZ, et al. Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): an open-label, randomised, phase 2 trial. Lancet. 2016;387(10027):1551–1560. doi: 10.1016/S0140-6736(15)01120-4. [DOI] [PubMed] [Google Scholar]
- 50.Ludwig H, Durie BGM, Bolejack V, et al. Myeloma in patients younger than age 50 years presents with more favorable features and shows better survival: an analysis of 10 549 patients from the international myeloma working group. Blood. 2008;111(8):4039–4047. doi: 10.1182/blood-2007-03-081018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lund FE. Signaling properties of CD38 in the mouse immune system: enzyme-dependent and -independent roles in immunity. Mol Med. 2006;12(11-12):328–333. doi: 10.2119/2006-00099.Lund. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mahmoudjafari Z, Hawks KG, Hsieh AA, et al. American Society for Blood and Marrow Transplantation Pharmacy Special Interest Group survey on chimeric antigen receptor T cell therapy administrative, logistic, and toxicity management practices in the United States. Biol Blood Marrow Transpl. 2019;25(1):26–33. doi: 10.1016/j.bbmt.2018.09.024. [DOI] [PubMed] [Google Scholar]
- 53.Maude SL, Teachey DT, Porter DL, et al. CD19-targeted chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Blood. 2015;125(26):4017–4023. doi: 10.1182/blood-2014-12-580068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mihara K, Bhattacharyya J, Kitanaka A, et al. T-cell immunotherapy with a chimeric receptor against CD38 is effective in eliminating myeloma cells. Leukemia. 2012;26(2):365–367. doi: 10.1038/leu.2011.205. [DOI] [PubMed] [Google Scholar]
- 56.Moreau P, San Miguel J, Sonneveld P, et al. Multiple myeloma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(Suppl 4):iv52–iv61. doi: 10.1093/annonc/mdx096. [DOI] [PubMed] [Google Scholar]
- 57.Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy–assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15(1):47–62. doi: 10.1038/nrclinonc.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Novak AJ, Darce JR, Arendt BK, et al. Expression of BCMA, TACI, and BAFF-R in multiple myeloma: a mechanism for growth and survival. Blood. 2004;103(2):689–694. doi: 10.1182/blood-2003-06-2043. [DOI] [PubMed] [Google Scholar]
- 59.O'Connell FP, Pinkus JL, Pinkus GS. CD138 (syndecan-1), a plasma cell marker: immunohistochemical profile in hematopoietic and nonhematopoietic neoplasms. Am J Clin Pathol. 2004;121(2):254–263. doi: 10.1309/617dwb5gnfwxhw4l. [DOI] [PubMed] [Google Scholar]
- 60.O'Connor BP, Raman VS, Erickson LD, et al. BCMA is essential for the survival of long-lived bone marrow plasma cells. J Exp Med. 2004;199(1):91–98. doi: 10.1084/jem.20031330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Palaiologou M, Delladetsima I, Tiniakos D. CD138 (syndecan-1) expression in health and disease. Histol Histopathol. 2014;29(2):177–189. doi: 10.14670/HH-29.177. [DOI] [PubMed] [Google Scholar]
- 62.Palumbo A, Chanan-Khan A, Weisel K, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(8):754–766. doi: 10.1056/NEJMoa1606038. [DOI] [PubMed] [Google Scholar]
- 63.Paus R, Nickoloff BJ, Ito T. A ‘hairy’ privilege. Trends Immunol. 2005;26(1):32–40. doi: 10.1016/j.it.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 64.Philip B, Kokalaki E, Mekkaoui L, et al. A highly compact epitope-based marker/suicide gene for easier and safer T-cell therapy. Blood. 2014;124(8):1277–1287. doi: 10.1182/blood-2014-01-545020. [DOI] [PubMed] [Google Scholar]
- 65.Porter D, Frey N, Wood PA, et al. Grading of cytokine release syndrome associated with the CAR T cell therapy tisagenlecleucel. J Hematol Oncol, 11:35. 2018 doi: 10.1186/s13045-018-0571-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Quarona V, Zaccarello G, Chillemi A, et al. CD38 and CD157: a long journey from activation markers to multifunctional molecules. Cytometry B Clin Cytom. 2013;84B(4):207–217. doi: 10.1002/cyto.b.21092. [DOI] [PubMed] [Google Scholar]
- 67.Raje N, Berdeja J, Lin Y, et al. Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N Engl J Med. 2019;380(18):1726–1737. doi: 10.1056/NEJMoa1817226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rajkumar SV, Blood E, Vesole D, et al. Phase III clinical trial of thalidomide plus dexamethasone compared with dexamethasone alone in newly diagnosed multiple myeloma: a clinical trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol. 2006;24(3):431–436. doi: 10.1200/jco.2005.03.0221. [DOI] [PubMed] [Google Scholar]
- 69.Ramos CA, Savoldo B, Torrano V, et al. Clinical responses with T lymphocytes targeting malignancy-associated κ light chains. J Clin Invest. 2016;126(7):2588–2596. doi: 10.1172/JCI86000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ren SS, Deng JW, Hong M, et al. Ethical considerations of cellular immunotherapy for cancer. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2019;20(1):23–31. doi: 10.1631/jzus.B1800421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Richardson P, Mitsiades C, Schlossman R, et al. The treatment of relapsed and refractory multiple myeloma. Hematology Am Soc Hematol Educ Program. 2007;2007(1):317–323. doi: 10.1182/asheducation-2007.1.317. [DOI] [PubMed] [Google Scholar]
- 72.Rickert RC, Jellusova J, Miletic AV. Signaling by the tumor necrosis factor receptor superfamily in B-cell biology and disease. Immunol Rev. 2011;244(1):115–133. doi: 10.1111/j.1600-065X.2011.01067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sadelain M, Brentjens R, Rivière I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013;3(4):388–398. doi: 10.1158/2159-8290.cd-12-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sanchez E, Li MJ, Kitto A, et al. Serum B-cell maturation antigen is elevated in multiple myeloma and correlates with disease status and survival. Br J Haematol. 2012;158(6):727–738. doi: 10.1111/j.1365-2141.2012.09241.x. [DOI] [PubMed] [Google Scholar]
- 75.Schwartzberg PL, Mueller KL, Qi H, et al. SLAM receptors and SAP influence lymphocyte interactions, development and function. Nat Rev Immunol. 2009;9(1):39–46. doi: 10.1038/nri2456. [DOI] [PubMed] [Google Scholar]
- 76.Seckinger A, Delgado JA, Moser S, et al. Target expression, generation, preclinical activity, and pharmacokinetics of the BCMA-T cell bispecific antibody EM801 for multiple myeloma treatment. Cancer Cell. 2017;31(3):396–410. doi: 10.1016/j.ccell.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 77.Singhal S, Mehta J, Desikan R, et al. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med. 1999;341(21):1565–1571. doi: 10.1056/nejm199911183412102. [DOI] [PubMed] [Google Scholar]
- 78.Smith EL, Harrington K, Staehr M, et al. GPRC5D is a target for the immunotherapy of multiple myeloma with rationally designed CAR T cells. Sci Transl Med. 2019;11(485):eaau7746. doi: 10.1126/scitranslmed.aau7746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stewart AK, Chang H, Trudel S, et al. Diagnostic evaluation of t(4;14) in multiple myeloma and evidence for clonal evolution. Leukemia. 2007;21(11):2358–2359. doi: 10.1038/sj.leu.2404800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Straathof KC, Pulè MA, Yotnda P, et al. An inducible caspase 9 safety switch for T-cell therapy. Blood. 2005;105(11):4247–4254. doi: 10.1182/blood-2004-11-4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sun C, Mahendravada A, Ballard B, et al. Safety and efficacy of targeting CD138 with a chimeric antigen receptor for the treatment of multiple myeloma. Oncotarget. 2019;10(24):2369–2383. doi: 10.18632/oncotarget.26792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tai YT, Dillon M, Song WH, et al. Anti-CS1 humanized monoclonal antibody Huluc63 inhibits myeloma cell adhesion and induces antibody-dependent cellular cytotoxicity in the bone marrow milieu. Blood. 2008;112(4):1329–1337. doi: 10.1182/blood-2007-08-107292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Terstappen LWMM, Huang SA, Safford M, et al. Sequential generations of hematopoietic colonies derived from single nonlineage-committed CD34+CD38− progenitor cells. Blood. 1991;77(6):1218–1227. [PubMed] [Google Scholar]
- 84.Touzeau C, Moreau P, Dumontet C. Monoclonal antibody therapy in multiple myeloma. Leukemia. 2017;31(5):1039–1047. doi: 10.1038/leu.2017.60. [DOI] [PubMed] [Google Scholar]
- 85.van Dongen JJM, Lhermitte L, Böttcher S, et al. Euroflow antibody panels for standardized n-dimensional flow cytometric immunophenotyping of normal, reactive and malignant leukocytes. Leukemia. 2012;26(9):1908–1975. doi: 10.1038/leu.2012.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang XJ, Marr AK, Breitkopf T, et al. Hair follicle mesenchyme-associated PD-L1 regulates T-cell activation induced apoptosis: a potential mechanism of immune privilege. J Invest Dermatol. 2014;134(3):736–745. doi: 10.1038/jid.2013.368. [DOI] [PubMed] [Google Scholar]
- 87.Westgate GE, Craggs RI, Gibson WT. Immune privilege in hair growth. J Invest Dermatol. 1991;97(3):417–420. doi: 10.1111/1523-1747.ep12481002. [DOI] [PubMed] [Google Scholar]
- 88.Wu N, Veillette A. SLAM family receptors in normal immunity and immune pathologies. Curr Opin Immunol. 2016;38:45–51. doi: 10.1016/j.coi.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 89.Yoo EM, Trinh KR, Tran D, et al. Anti-CD138-targeted interferon is a potent therapeutic against multiple myeloma. J Interferon Cytokine Res. 2015;35(4):281–291. doi: 10.1089/jir.2014.0125. [DOI] [PMC free article] [PubMed] [Google Scholar]