Abstract
Proteasome inhibitors have shown remarkable success in the treatment of hematologic neoplasm. There has been a lot of attention to applying these drugs for solid tumor treatment. Recent preclinical study has signified the effectiveness on cell proliferation inhibition in lung adenocarcinoma treated by carfilzomib (CFZ), a second generation proteasome inhibitor. However, no insight has been gained regarding the mechanism. In this study, we have systematically investigated the CFZ functions in cell proliferation and growth, cell cycle arrest, and apoptosis in lung adenocarcinoma cells. Flow cytometry experiments showed that CFZ significantly induced G2/M cell cycle arrest and apoptosis in lung adenocarcinoma. MTS and colony formation assays revealed that CFZ substantially inhibited survival of lung adenocarcinoma cells. All results were consistently correlated to the upregulation expression of Gadd45a, which is an important gene in modulating cell cycle arrest and apoptosis in response to physiologic and environmental stresses. Here, upregulation of Gadd45a expression was observed after CFZ treatment. Knocking down Gadd45a expression suppressed G2/M arrest and apoptosis in CFZ-treated cells, and reduced cytotoxicity of this drug. The protein expression analysis has further identified that the AKT/FOXO3a pathway is involved in Gadd45a upregulation after CFZ treatment. These findings unveil a novel mechanism of proteasome inhibitor in anti-solid tumor activity, and shed light on novel preferable therapeutic strategy for lung adenocarcinoma. We believe that Gadd45a expression can be a highly promising candidate predictor in evaluating the efficacy of proteasome inhibitors in solid tumor therapy.
Keywords: Carfilzomib, Lung adenocarcinoma, Gadd45a, Cell cycle arrest, Apoptosis
1. Introduction
Lung cancer, a leading cause of cancer-related death, is a major health problem for its high incidence and mortality (Siegel et al., 2018). Adenocarcinoma is the most common type of lung cancer, comprising nearly 50% of all lung cancer cases, which are usually advanced and metastatic at the time of diagnosis. Over recent decades, the treatment of lung adenocarcinoma has been gradually dominated by molecular target drugs, such as epidermal growth factor receptor (EGFR) inhibitors and anaplastic lymphoma kinase (ALK) inhibitors. However, the treatment has marginal effect in certain types of lung adenocarcinoma. Tyrosine kinase inhibitors (TKIs)-targeted treatments have worked efficiently in patients with EGFR mutation, while few of them can escape drug resistance (van der Wekken et al., 2016). Half of the patients with lung adenocarcinoma die within one year after diagnosis and the five-year survival is below 20% (Miller et al., 2016). Therefore, it is highly desirable to develop novel therapeutic strategies for treating lung adenocarcinoma.
Inhibiting proteasome may provide an alternative promising way, as demonstrated by the success in treating relapsed and refractory multiple myeloma. Proteasome is regarded as a multi-catalytic proteinase complex via eliminating intracellular damaged, misfolded, and short half-life functional proteins. It also plays a critical role in cell cycle control, apoptosis, and response to cell stress (Orlowski and Kuhn, 2008; Ren et al., 2019). Proteasome inhibitors have shown efficient anti-tumor activity both in vitro and in vivo. Recently, there has been increasing interest in the application of proteasome inhibitors for solid malignancy treatments. Bortezomib (BTZ), the first-generation proteasome inhibitor, can significantly suppress lung adenocarcinoma cells in preclinical study (Morgillo et al., 2011). Unfortunately, BTZ has poor efficacy in clinical trials of patients with advanced lung adenocarcinoma because of lower selectivity and reversible possibility (Li et al., 2010). Carfilzomib (CFZ), a second-generation proteasome inhibitor, has been designed to achieve a higher selectivity on chymotrypsin subunit than BTZ. Both the proteasome and immune-proteasome are selectively and irreversibly inhibited by CFZ. Moreover, CFZ is active to BTZ-resistant multiple myeloma, with far fewer side-effects (Manasanch and Orlowski, 2017). For its efficacy in multiple myeloma, the US Food and Drug Administration (FDA) has approved CFZ in the treatment of multiple myeloma patients after both BTZ and immunomodulatory therapies fail (Siegel et al., 2012). Further experiments show that CFZ can also successfully inhibit proliferation and promote apoptosis in solid tumors (Baker et al., 2014; Hanke et al., 2016). However, it is still not clear what is the underlying mechanism that renders CFZ having remarkable effectiveness in solid tumor treatment.
It is well known that proteasome is crucial in regulation of cell cycle and apoptosis. Any proteasome inhibitors, including BTZ and CFZ, suppress tumor growth by directly regulating the protein expression in relation to the two processes. Gadd45a modulates cell cycle regulation, DNA replication/repair, cell proliferation, apoptosis, and genomic stability via interaction with partner proteins, such as proliferating cell nuclear antigen (PCNA), P21, cyclin-dependent kinase 1 (CDK1)/cyclin B1 complex, elongation factor-1 α (EF-1α), and mitogen-activated protein kinase kinase kinase 4 (MEKK4) (Takekawa and Saito, 1998; Jin et al., 2000, 2002; Tong et al., 2005). Upregulation of Gadd45a is observed after several anti-cancer treatments and is involved in the efficacy of these treatments (da Silva et al., 2018; Li et al., 2018). Therefore, understanding the function of Gadd45a after CFZ treatment will pave the way for unveiling the mechanism that renders CFZ its remarkable effectiveness. Gadd45a can be induced by a variety of physiological and environmental stressors (Liebermann and Hoffman, 2008; Salvador et al., 2013). Many studies unveil that Gadd45a plays a crucial role in preventing the transformation of normal cells to malignant ones. Mouse embryonic fibroblasts (MEFs), derived from Gadd45a knockouts, exhibit disturbance of cell cycle, centrosome amplification, aneuploidy, and chromosome aberrations. Mice lacking Gadd45a gene are susceptible to ionizing radiation-and chemical carcinogen-induced tumors (Hollander et al., 1999, 2001). Gadd45a can also suppress tumor invasion, metastasis, and angiogenesis by decreasing matrix metalloproteinases (MMPs), regulating signal transducer and activator of transcription 3 (STAT3) activity, and maintaining cell-to-cell adhesion (Hildesheim et al., 2004; Ji et al., 2007; Yang et al., 2013).
In this study, we show that Gadd45a expression is increased in lung adenocarcinoma after CFZ treatment. Knocking down Gadd45a successfully attenuates G2/M cell cycle arrest and apoptosis induced by CFZ. We further demonstrate that the CFZ treatment leads to Gadd45a upregulation via AKT/FOXO3a (protein kinase B/forkhead box O3a) pathway, a P53-independent mechanism. These results suggest that Gadd45a can be a promising target to enhance efficacy of proteasome inhibitor. These findings unveil a new mechanism of proteasome inhibitor in anti-solid tumor activity and provide a more viable means for deepening our understanding towards the foundation of proteasome inhibitor treatment.
2. Materials and methods
2.1. Cell culture and transient transfection
The human lung adenocarcinoma cell lines, HCC-827 and NCI-H1299, were provided by Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou, China. Both cell lines were cultured with RPMI-1640 containing 10% fetal bovine serum and antibiotics. Cells were grown on 30-mm plates in 30%–50% confluence and transfected with small interfering RNAs (siRNAs) using Lipofectamine 3000 (Invitrogen, California, USA). The transfected cells were further analyzed, after incubation at 37 °C with 5% CO2 for 48 h.
2.2. Reagents and chemicals
CFZ from Selleck Chemicals (Shanghai, China) was dissolved in dimethyl sulfoxide (DMSO) at a stock concentration of 10 mmol/L and stored at −80 °C. siRNAs for Gadd45a and FOXO3a were designed and synthetized by GenePharma (Shanghai, China). The antibodies against Gadd45a, FOXO3a, and β-actin were ordered from Proteintech Group (Chicago, USA). Anti-P38, anti-phospho-P38 (Thr180/Tyr182), anti-c-Jun N-terminal kinase (JNK), anti-phospho-JNK (Thr183/Tyr185), anti-AKT, anti-phospho-AKT, and anti-FOXO3a (Thr32) were available from Cell Signaling Technology (Danvers, MA, USA).
2.3. Quantitative real-time PCR
Total RNA was isolated from cultured cells using TRIzol reagent (Invitrogen), and complementary DNAs (cDNAs) were synthesized with 3 μg total RNA by using RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific, Waltham, MA, USA). Quantitative real-time polymerase chain reaction (qPCR) was performed to detect variation of specific gene expression using Aceq Universal SYBR qPCR Master Mix (Vazyme, Nanjing, China) on Roche LightCycler480 (Roche, Shanghai, China). Units are metric and follow international system (SI) convention.
2.4. Western blotting assay
Western blotting assay was performed as described in our earlier work (Yang et al., 2013). Specific protein signals were detected by chemiluminescence (Fude Biological Technology, Hangzhou, China) with primary antibodies and horseradish peroxidase-conjugated secondary antibodies (Proteintech Group).
2.5. Flow cytometry analysis
Cell cycle distribution was analyzed by the Cell Cycle Assay Kit (Beyotime Biotechnology, Shanghai, China). The harvested cells were fixed with 70% ethanol at 4 °C overnight. After phosphate-buffered saline (PBS) washing, the cells were stained with propidium iodide (PI), followed by cell cycle analysis with flow cytometer (BD Biosciences, California, USA) (Yao et al., 2018).
Annexin V-allophycocyanin (APC)/7-amino-actinomycin D (7-AAD) assay kit (BioGems, California, USA) was used to evaluate cell apoptosis following the protocols of manufacture. After harvest and PBS washing, the cells were labeled by Annexin V-APC and 7-AAD. Then the labeled cells were sorted and measured by flow cytometer.
2.6. Cell viability assay
3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium salt (MTS; CellTiter 96® AQueous Assay, Promega, Madison, WI, USA) was used to analyze cell viability. Transfected cells were plated at a density of 5000 cells per well in 96-well plates and cultured overnight. After treatment with increasing concentrations of CFZ for 24 h, the cultured plates were incubated with 20 μL MTS solution per well for 3–4 h. The absorbance at 490 nm was measured by a plate scanner (ThermoFisher Multiskan FC, USA).
2.7. Colony formation assay
One thousand transfected cells were seeded and treated by CFZ (25 nmol/L) for 24 h. Then the cells were incubated at 37 °C with 5% CO2 for 10 d. After incubation, the cultured plates were fixed using precooling 70% methanol and stained with crystal violet.
2.8. Statistical analysis
The data were presented as mean±standard error (SE) of three independent experiments, and the statistical test was Student’s t-test (unpaired and two-tailed) or one-way analysis of variance (ANOVA). P<0.05 was considered as statistical significance.
3. Results
3.1. G2/M cell cycle arrest in lung adenocarcinoma cells after CFZ treatment
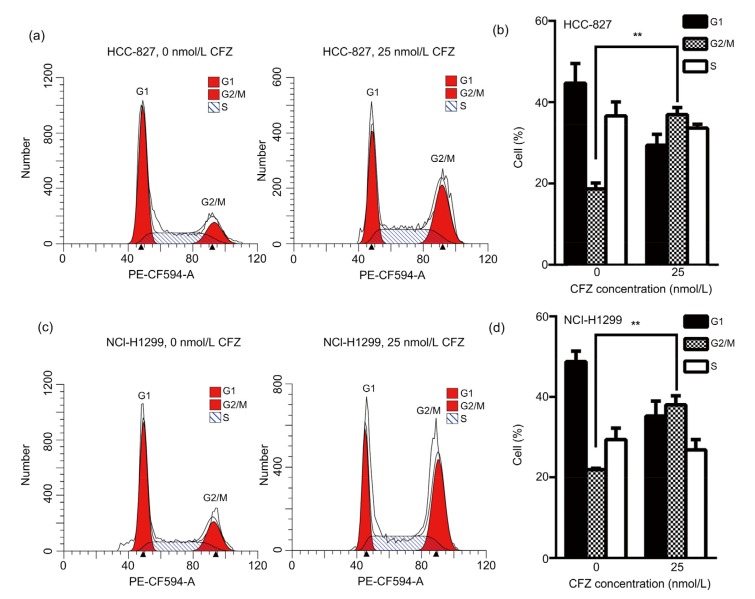
To understand the mechanism of CFZ in anti-solid tumor, the CFZ effect on the cell cycle distribution of lung adenocarcinoma cells was analyzed by flow cytometry. After CFZ (25 nmol/L) treatment for 24 h, the G2/M phage percentage was increased from (18.67±1.43)% to (36.96±1.74)% in HCC-827 cells (Figs. 1a and 1b). The same experiments were performed in NCI-H1299. The G2/M phage percentage was (38.00±2.27)% in CFZ-treated NCI-H1299 cells, higher than in those untreated cells (21.87±0.35)% (Figs. 1c and 1d). The results show that CFZ suppresses proliferation of lung adenocarcinoma cells via inducing G2/M arrest.
Fig. 1.
G2/M cell cycle arrest in lung adenocarcinoma cells after carfilzomib treatment
After treatment of carfilzomib (CFZ; 25 nmol/L) for 24 h, HCC-827 (a, b) and NCI-H1299 (c, d) cells were harvested and subjected to flow cytometry analysis. The percentage of cells in each phage was presented as mean±standard error (SE), n=3. ** P<0.01
3.2. Upregulated Gadd45a expression in CFZ-treated lung adenocarcinoma cells
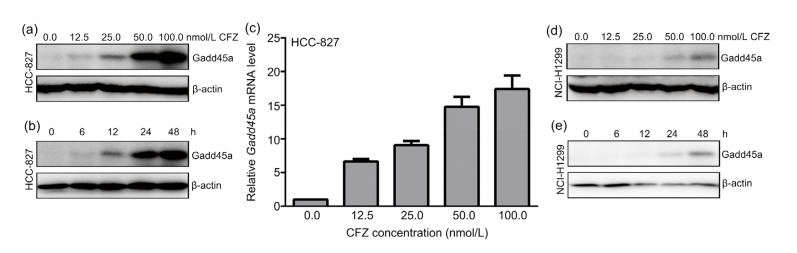
The Gadd45a expression was gradually upregulated with increasing doses of CFZ treatments for 24 h in HCC-827 cells (Fig. 2a). The Gadd45a expression was also studied by varying the treatment time at the same concentration of CFZ (25 nmol/L). In the control experiments of 6, 12, 24, and 48 h CFZ treatments, Gadd45a expression was found to increase monotonically with time (Fig. 2b). As the stability of Gadd45a protein was modulated by ubiquitin-proteasome (Gao et al., 2013), Gadd45a mRNA level were further measured in CFZ-treated cells. The results demonstrated that increasing concentrations of CFZ treatments for 24 h increased Gadd45a expression 7–18 times at transcription level in HCC-827 cells, as compared with untreated ones (Fig. 2c). Gadd45a expression was further analyzed in NCI-H1299 cells after CFZ incubation. The same dependence trend on both dose and time in CFZ-treated NCI-H1299 cells has been found (Figs. 2d and 2e). All results show that CFZ remarkably increases Gadd45a expression at both mRNA and protein levels, which suggests that Gadd45a is involved in the mechanism of solid tumor response to CFZ treatment.
Fig. 2.
Upregulated Gadd45a expression in carfilzomib-treated lung adenocarcinoma cells
(a) After incubation with indicated concentrations of carfilzomib (CFZ) for 24 h, HCC-827 cells were harvested for an immunoblotting with Gadd45a antibody. β-Actin was used as internal control. (b) Western blotting was further performed to measure the variation of Gadd45a after treatment of CFZ (25 nmol/L) for a series length of time. (c) Quantitative real-time PCR analysis showed the mRNA level of Gadd45a after increasing doses of CFZ treatment for 24 h in HCC-827. mRNA levels were normalized by glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA levels. The data were presented as mean±standard error (SE), n=3. (d, e) After the same treatments of (a) and (b), NCI-H1299 cells were lysed and subjected to immunoblot analysis with Gadd45a antibody
3.3. Decrease of G2/M cell cycle arrest in CFZ-treated lung adenocarcinoma cells after Gadd45a knockdown
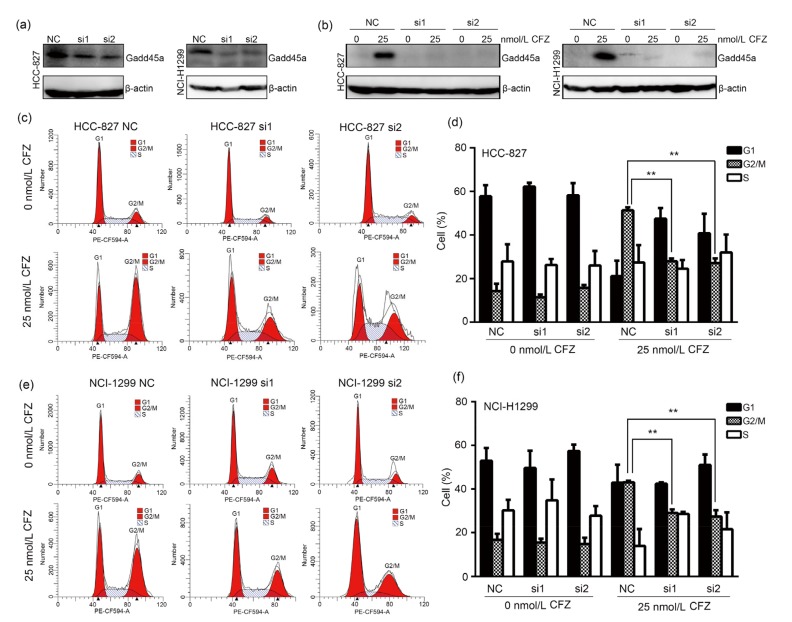
To further investigate the role that Gadd45a played in anti-tumor effect of CFZ, Gadd45a expression was transiently knocked down in lung adenocarcinoma cells by siRNAs. The control experiments showed that Gadd45a expression was efficiently knocked down in both HCC-827 and NCI-H1299, regardless of CFZ treatment (Figs. 3a and 3b). Cell cycle distribution was analyzed in Gadd45a knocked-down HCC-827 cells after CFZ treatment. As shown in Figs. 3c and 3d, the percentage of G2/M phase cells was increased to (51.40±0.73)% in the negative control cells after CFZ treatment (25 nmol/L) for 24 h. In contrast, lower percentages of G2/M phase cells were observed in Gadd45a siRNAs-transfected cells after the same treatment ((27.96±0.72)% in si1-transfected cells and (27.15±1.23)% in si2-transfected cells). These results suggest that Gadd45a expression is involved in G2/M arrest of CFZ-treated cells. Cell cycle distribution was also measured in Gadd45a siRNA-transfected NCI-H1299 cells after CFZ treatment. The percentages of the G2/M cell cycle stage were (29.03±0.96)% and (27.38±1.70)% in si1-and si2-transfected cells, respectively, after CFZ exposure (Figs. 3e and 3f), while it was (43.13±0.36)% in the negative control cells. Therefore, it is concluded that CFZ induces G2/M cell cycle arrest via upregulating Gadd45a expression in lung adenocarcinoma cells.
Fig. 3.
Decrease of G2/M cell cycle arrest in carfilzomib-treated lung adenocarcinoma cells after Gadd45a knockdown
(a) The efficiency of Gadd45a knockdown was evaluated by immunoblot analysis. (b) HCC-827 and NCI-H1299 cells were treated with carfilzomib (CFZ, 25 nmol/L, 24 h) after Gadd45a siRNA transfection. The variation of Gadd45a was quantified by western blotting. (c, e) After Gadd45a siRNA transfection, HCC-827 and NCI-H1299 cells were treated with CFZ (25 nmol/L) for 24 h. Flow cytometry was performed to detect the cell cycle distribution. (d, f) The percentage of cells in each phage was presented as mean±standard error (SE), n=3. NC: negative control. ** P<0.01
3.4. Inhibition of apoptosis in CFZ-treated lung adenocarcinoma cells after Gadd45a knockdown
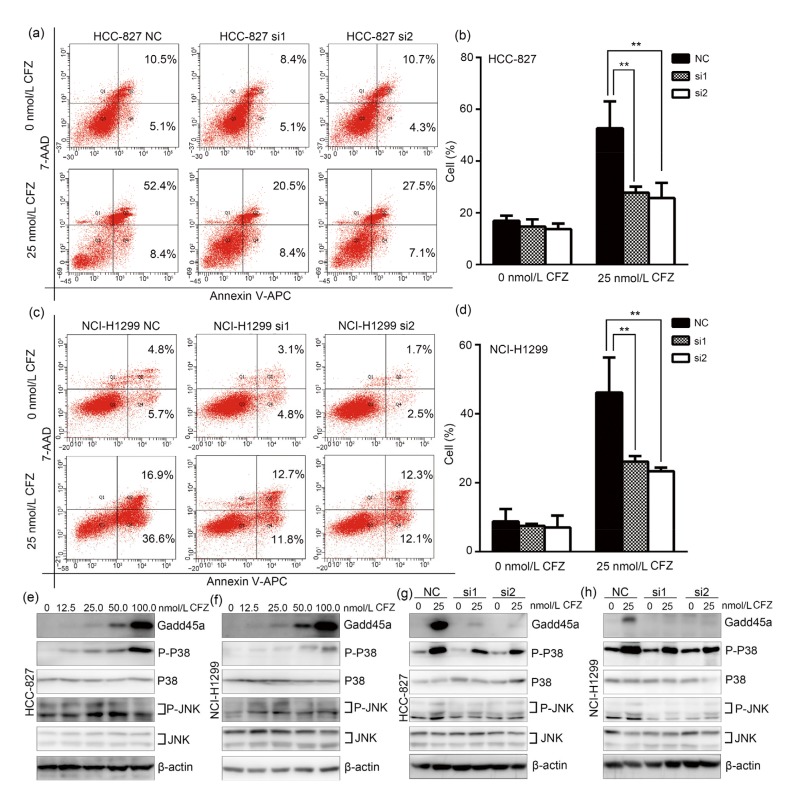
It has been shown that Gadd45a accumulation efficiently induces apoptosis in response to cell stress (Tong et al., 2005). We analyzed the relationship between Gadd45a upregulation and apoptosis in CFZ-treated lung adenocarcinoma cells. Gadd45a siRNA-transfected HCC-827 cells were firstly incubated with 25 nmol/L CFZ for 24 h. After staining with Annexin V-APC/7-AAD, the apoptotic cells were sorted by flow cytometry. The percentages of apoptotic cells including early and late apoptosis were (27.83±1.33)% and (25.70±3.37)% in both si1-and si2-transfected cells after CFZ treatment (Figs. 4a and 4b), respectively. The values are significantly lower than that in the negative control cells (52.67±5.98)%. Parallel experiments were done for Gadd45a knocked-down NCI-H1299 cells after CFZ treatment. Similarly, the percentages of apoptotic cells were observed to be lower in Gadd45a knocked-down cells, at (26.13±0.91)% and (23.33±0.60)% in si1-and si2-transfected cells, respectively. The negative control cells after CFZ (25 nmol/L) treatment for 24 h have the apoptotic cell population percentages of (46.17±5.85)% (Figs. 4c and 4d). It is, therefore, shown that CFZ triggers apoptosis in lung adenocarcinoma via upregulating Gadd45a expression.
Fig. 4.
Inhibition of apoptosis in carfilzomib-treated lung adenocarcinoma cells after Gadd45a knockdown
(a, c) HCC-827 and NCI-H1299 were treated with carfilzomib (CFZ, 25 nmol/L) for 24 h, after knocking down Gadd45a expression. NC was a negative control for siRNA. Early and late apoptotic cells were detected by flow cytometry analysis. The percentage of apoptotic cells was shown in the representative flow cytometry profiles. (b, d) The total percentages of early and late apoptotic cells were presented as mean±standard error (SE), n=3. NC: negative control. ** P<0.01. (e, f) Exponentially growing HCC-827 and NCI-H1299 cells were treated with CFZ in the same way of Fig. 2a. The variations of P-P38, total P38, P-JNK, and total JNK are measured by immunoblotting. (g, h) After Gadd45a siRNA transfection, HCC-827 and NCI-H1299 were incubated with CFZ (25 nmol/L) for 24 h. Western blotting was further performed to analyze the phosphorylation of P38 and JNK, and the total proteins of them. JNK: c-Jun N-terminal kinase; P-JNK: phosphorylated JNK protein; P-P38: phosphorylated P38 protein
It has been shown that Gadd45a upregulation promotes apoptosis via activating the JNK/P38 pathway by interaction with MEKK4/MTK1 (Takekawa and Saito, 1998). To further verify the role of Gadd45a in apoptosis and cell growth inhibition induced by CFZ, it is important to determine whether Gadd45a modulated the phosphorylation of JNK and P38 in CFZ-treated lung adenocarcinoma cells. As shown in Figs. 4e and 4f, Gadd45a expression and phosphorylation of both P38 and JNK were simultaneously elevated after the CFZ treatment at various concentrations for 24 h in both HCC-827 and NCI-H1299 cells. In addition, the phosphorylation of P38 and JNK was measured in Gadd45a knocked-down lung adenocarcinoma cells after CFZ treatment. The results showed that phosphorylation of P38 and JNK was significantly lower in Gadd45a siRNAs-transfected cells after CFZ (25 nmol/L) treatment for 24 h, than in the negative control cells (Figs. 4g and 4h). These observations indicate that Gadd45a overexpression increases the phosphorylation of P38 and JNK in CFZ-treated cells, and CFZ induces apoptosis and cell growth inhibition via activation of P38 and JNK.
3.5. Increases of viability and cell colony formation ability of CFZ-treated lung adenocarcinoma cells after Gadd45a knockdown
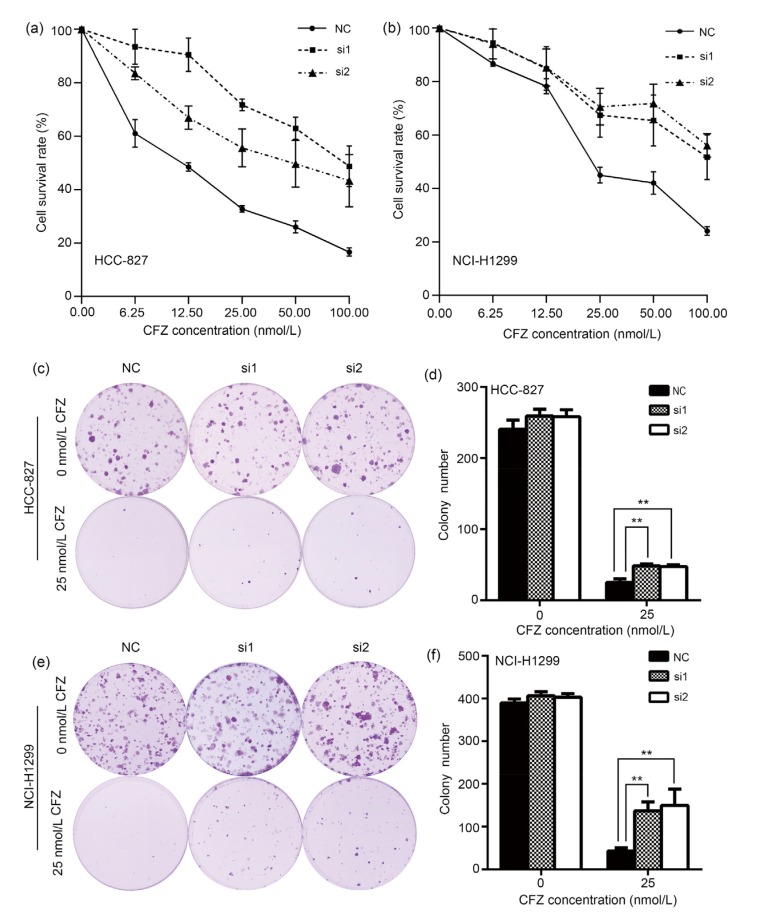
The impact of Gadd45a expression on cell viability of lung adenocarcinoma cells was evaluated after CFZ treatment. HCC-827 cells transfected with Gadd45a siRNAs were treated with various concentrations of CFZ for 24 h. Cell viability was analyzed by the MTS assay. The cell viability decreased monotonically with CFZ dose (Fig. 5a). However, Gadd45a siRNA-transfected cells showed significantly higher cell survival rates after CFZ treatment, in contrast to the negative control cells. In parallel, the cell viability after CFZ treatment was also measured in NCI-H1299 cells. Similarly, knocking down Gadd45a expression remarkably increased cell survival rates after CFZ treatment with different doses in NCI-H1299 cells (Fig. 5b). It suggests that Gadd45a expression is involved in the anti-solid tumor efficacy of CFZ. In colony formation assay, Gadd45a knocked-down HCC-827 cells had higher colony number after incubation with 25 nmol/L CFZ for 24 h, than the negative control cells (Figs. 5c and 5d). In NCI-H1299 cells, disrupting Gadd45a expression also increased the ability of colony formation after the same treatment (Figs. 5e and 5f). All the results consistently show that CFZ efficiently suppresses proliferation of lung adenocarcinoma cells via regulating Gadd45a expression.
Fig. 5.
Increases of viability and cell colony formation ability of carfilzomib-treated lung adenocarcinoma cells after Gadd45a knockdown
(a, b) MTS assay was performed to detect the cell viabilities of HCC-827 and NCI-H1299 cells transfected by Gadd45a siRNAs after carfilzomib (CFZ; 6.25, 12.50, 25.00, 50.00, 100.00 nmol/L) treatment for 24 h. (c, e) Cell colony formation characterized the role of Gadd45a in lung adenocarcinoma cells under CFZ treatment (25 nmol/L, 24 h). (d, f), Colony numbers of HCC-827 and NCI-H1299 cells transfected by Gadd45a siRNAs after CFZ treatment (25 nmol/L, 24 h). The data were presented as mean±standard error (SE), n=3. NC: negative control. ** P<0.01
3.6. Upregulated Gadd45a expression by CFZ treatment via AKT/FOXO3a pathway
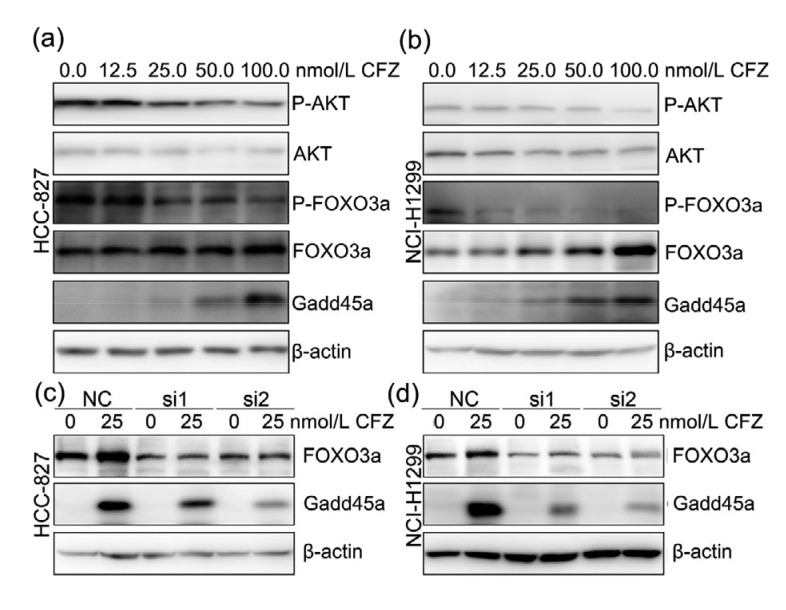
FOXO3a can directly increase Gadd45a expression by binding to the forkhead response element (FHRE)-responsive element (Tran et al., 2002). Activation of AKT leads to the phosphorylation of FOXO3a and disturbs its transcription activity by suppressing FOXO3a nuclear location and promoting FOXO3a degradation (Plas and Thompson, 2003; Bouchard et al., 2004). Here, phosphorylated FOXO3a was significantly decreased in both HCC-827 and NCI-H1299 cells after CFZ treatment (Figs. 6a and 6b). Since the phosphorylation was involved in lower stability of FOXO3a, total FOXO3a was gradually accumulated after increasing concentrations of CFZ treatments. The results also showed that CFZ treatments remarkably suppressed the phosphorylation of AKT, while the total AKT was nearly unaffected (Figs. 6a and 6b). These observations indicate that CFZ treatment elevates non-phosphorylated FOXO3a via inactivation of AKT. The non-phosphorylated FOXO3a shows higher stability and performs transcription function by translocating to nucleus. To confirm the relationship between Gadd45a expression and FOXO3a, Gadd45a expression was further measured in FOXO3a knocked-down cells after CFZ treatments. The results showed that disruption of FOXO3a expression successfully suppressed the Gadd45a upregulation induced by CFZ treatment (Figs. 6c and 6d). In summary, CFZ stimulates Gadd45a expression via the AKT/FOXO3a pathway.
Fig. 6.
Upregulated Gadd45a expression by carfilzomib treatment via AKT/FOXO3a pathway
(a, b) After incubation with indicated concentration of CFZ for 24 h, HCC-827 and NCI-H1299 cells were prepared for immunoblotting analysis with P-AKT, AKT, P-FOXO3a, FOXO3a, and Gadd45a antibodies. β-Actin was used as internal control. (c, d) HCC-827 and NCI-H1299 cells were treated with CFZ (25 nmol/L) for 24 h after FOXO3a siRNA transfection. Both of the expression of FOXO3a and Gadd45a were measured by western blotting. NC: negative control; CFZ: carfilzomib; AKT: protein kinase B; P-AKT: phosphorylated AKT protein; FOXO3a: forkhead box O3a; P-FOXO3a: phosphorylated FOXO3a protein
4. Discussion
Proteasome plays a crucial role in maintaining homeostasis of the eukaryotic cell, eliminating 80% intracellular proteins, which regulate cell cycle, apoptosis, proliferation, signal transduction, and transcription. To date, a very large number of preclinical and clinical investigations have shown that proteasome inhibitors have efficient anti-malignancy activity. The FDA has approved several proteasome inhibitors in relapsed and refractory multiple myeloma treatment because of their remarkable success in fighting hematologic neoplasm. Moreover, proteasome inhibitor is proved to successfully sensitize resistant cancer cells to chemotherapy and overcome chemoresistance. Preclinical anti-tumor efficacy has been constantly improved by protecting proteasome inhibitors from degradation (Ao et al., 2015). CFZ, a second generation proteasome inhibitor, has higher selectivity and less resistance, which irreversibly inhibits chymotrypsin-like activity of proteasome. As compared to the first generation proteasome inhibitor BTZ, CFZ has higher potent anti-tumor response (Manasanch and Orlowski, 2017; Park et al., 2018). Patients with multiple myeloma and mantle cell lymphoma are reactive to single CFZ treatment, showing superior efficacy and improved tolerability. Recent preclinical investigations have shown that CFZ inhibits cell proliferation in lung adenocarcinoma (Baker et al., 2014; Ao et al., 2015). The preliminary observations have attracted worldwide interests in applying CFZ in solid tumor treatment. Our systematic study further confirms that CFZ efficiently induces G2/M cell cycle arrest (Figs. 1 and 3), leads to apoptosis (Fig. 4), and significantly suppresses cell growth (Fig. 5) in lung adenocarcinoma cells. These results have laid solid foundation for CFZ application in solid tumor therapy. Meanwhile, our early study has unveiled the crucial role of Gadd45a, one of the most important genes in oncogenesis, in the cell cycle, apoptosis, tumor angiogenesis and metastasis (Jin et al., 2002; Tong et al., 2005; Ji et al., 2007; Yang et al., 2013). The close correlation strongly suggests that Gadd45a may be the critical key in uncovering the underlying mechanism behind the highly efficient CFZ treatment.
It has been well established that Gadd45a is a ubiquitously expressed tumor suppressor gene in relation to cell responses to various oncogenic stresses. Loss of Gadd45a expression initiates disorders of cell growth, proliferation, cytokinesis, and DNA repair. Gadd45a expression has been increased in cancer cells after several anti-tumor therapies, and is also involved in the efficiency of these treatments. Ionizing radiation treatment successfully induced Gadd45a expression in cervical cancers. Gadd45a overexpression sensitized this cancer cell to radiotherapy (Li et al., 2018). Multidrug-resistant osteosarcoma cells can regain sensitivity to doxorubicin via increasing Gadd45a expression (Yang et al., 2009). Our experiments have shown that CFZ treatment remarkably upregulates Gadd45a expression at both transcription and protein levels in lung adenocarcinoma cells (Fig. 2). This observation suggests that Gadd45a may be involved in CFZ anti-tumor activity. To verify this conjecture, we knocked down the Gadd45a expression in lung adenocarcinoma cells and measured the corresponding cell response to CZF treatment (Fig. 5). Consistently, Gadd45a expression disruption could decrease the anti-tumor activity of CFZ. Such correspondence implies that the efficiency of CFZ treatment is essentially the consequence of the Gadd45a expression upregulation. In cell viability and colony formation study, higher cell survival rates and more colony forming units were found in tumor cells transfected with Gadd45a siRNAs after CFZ treatment (Fig. 5). These results confirm our conjecture that the Gadd45a expression in lung adenocarcinoma cells directly correlates to the cell response to CFZ treatment.
Inductions of cell cycle arrest and apoptosis are the essential mechanisms of Gadd45a in suppressing tumor cell growth and proliferation. Gadd45a inhibits entry of cells into S phase via displacing PCNA from cyclin D1 complex (Smith et al., 1994). CR6-interacting factor 1 inhibits cell cycle progression at G1/S checkpoint via binding with Gadd45a (Chung et al., 2003). At the same time, interaction between Gadd45a with CDK1 suppresses the activity of CDK1/cyclin B1 complex, and arrests cells at G2/M checkpoint (Zhan et al., 1999; Jin et al., 2002). We demonstrate that CFZ arrests lung adenocarcinoma cells at G2/M phage via upregulation of Gadd45a expression (Fig. 3). Knocking down Gadd45a expression successfully suppresses G2/M cell cycle arrest induced by CFZ treatment. Gadd45a has shown critical function in oncogenesis for its pro-apoptotic activity. The interaction between Gadd45a and EF-1α has promoted apoptosis via disrupting cytoskeletal stability and translocating Bim to mitochondria (Tong et al., 2005). Gadd45a can also activate the JNK/P38 pathway to induce apoptosis via interaction with MEKK4/MTK1 (Takekawa and Saito, 1998). Gadd45a gene silencing inhibits apoptosis and senescence of skin squamous cell carcinoma (Liu LQ et al., 2018). In this study, Gadd45a expression has been shown to be involved in CFZ-induced apoptosis. Gadd45a siRNA transfection greatly attenuated apoptosis in CFZ-treated lung adenocarcinoma cells (Fig. 4). Phosphorylation of JNK and P38 is increased after CFZ treatment. This is further verified by knocking down Gadd45a expression. As expected, upregulation of phosphorylation of JNK and P38 in CFZ-treated cells has been suppressed accordingly (Fig. 4). On both sides, we show that Gadd45a activates the JNK/P38 pathway to promote apoptosis in CFZ-treated cells.
The underlying mechanism of upregulation of Gadd45a in CFZ-treated cells has been further investigated. It is well known that Gadd45a is a major downstream target gene of P53. However, P53 mutation has been identified in both HCC-827 and NCI-H1299 used in this study, excluding P53 from upregulating Gadd45a expression after CFZ treatment. Alternatively, FOXO3a, a central transcription factor, can directly upregulate Gadd45a expression. FOXO3a activity is modulated by its translation between nucleus and cytoplasm. This is achieved by phosphorylation by a series of kinases, including AKT, extracellular signal-regulated kinase (ERK), and nuclear factor-κB (NF-κB) (Liu Y et al., 2018). Phosphorylated FOXO3a can be degraded by proteasome (Plas and Thompson, 2003). Fig. 6 shows that the decrease in the phosphorylation of FOXO3a and the increase in the total FOXO3a occur simultaneously after CFZ treatment. These results indicate that CFZ treatment induces an upregulation of un-phosphorylated FOXO3a, which is able to translocate into the nucleus and exerts its transcriptional activity. On the other hand, knocking down FOXO3a expression significantly attenuates Gadd45a upregulation induced by CFZ treatment (Figs. 6c and 6d). Moreover, the phosphorylation of AKT, a suppressor of FOXO3a, was found to decrease after CFZ treatment (Figs. 6a and 6b). The above results show that CFZ increases Gadd45a expression via the AKT/FOXO3a pathway.
5. Conclusions
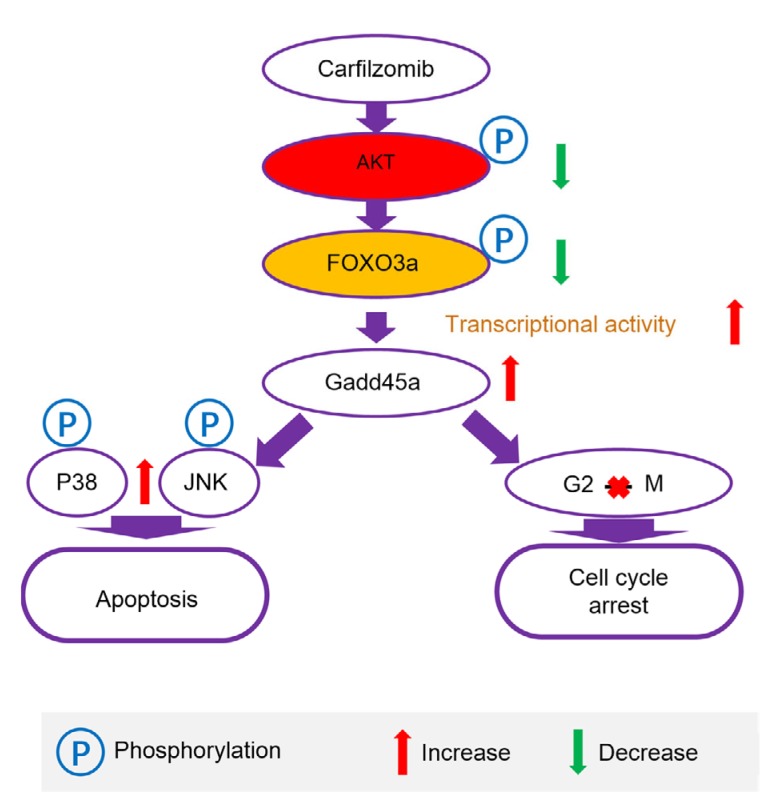
In summary, we have demonstrated that CFZ, a second generation proteasome inhibitor, efficiently suppressed proliferation and growth, and induced G2/M cell cycle arrest and apoptosis in lung adenocarcinoma cells via upregulation of Gadd45a expression. The protein expression analysis has clearly identified that the AKT/FOXO3a pathway is involved in Gadd45a upregulation after CFZ treatment (Fig. 7). These findings unveil a novel mechanism of proteasome inhibitor in anti-solid tumor activity, and suggest a novel preferable therapeutic strategy for lung adenocarcinoma. We believe that Gadd45a expression can be a highly promising candidate predictor in evaluation of the efficacy of proteasome inhibitors in solid tumor therapy.
Fig. 7.
Mechanism that carfilzomib inhibits the growth of lung adenocarcinoma
AKT: protein kinase B; FOXO3a: forkhead box O3a; JNK: c-Jun N-terminal kinase
Acknowledgments
We thank Dr. Li-feng FENG (Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou, China) for modifying the article.
Footnotes
Project supported by the National Natural Science Foundation of China (Nos. 81601992, 81802986, and 81601029), the Natural Science Foundation of Zhejiang Province (No. LQ16H160008), and the Medical and Health Program of Zhejiang Province (No. 2019338991), China
Contributors: Fang YANG conceived the research, designed the research, wrote and edited the manuscript. Fang YANG, Wang-wang LIU, Hui CHEN, Jia ZHU, Ai-hua HUANG, Fei ZHOU, Yi GAN, Yan-hua ZHANG, and Li MA performed research; Fang YANG, Wang-wang LIU, and Hui CHEN analyzed the data. All authors read and approved the final manuscript. Therefore, all authors had full access to all the data in the study and take responsibility for the integrity and security of the data.
Compliance with ethics guidelines: Fang YANG, Wang-wang LIU, Hui CHEN, Jia ZHU, Ai-hua HUANG, Fei ZHOU, Yi GAN, Yan-hua ZHANG, and Li MA declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Ao L, Reichel D, Hu D, et al. Polymer micelle formulations of proteasome inhibitor carfilzomib for improved metabolic stability and anticancer efficacy in human multiple myeloma and lung cancer cell lines. J Pharmacol Exp Ther. 2015;355(2):168–173. doi: 10.1124/jpet.115.226993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker AF, Hanke NT, Sands BJ, et al. Carfilzomib demonstrates broad anti-tumor activity in pre-clinical non-small cell and small cell lung cancer models. J Exp Clin Cancer Res, 33:111. 2014 doi: 10.1186/s13046-014-0111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouchard C, Marquardt J, Brás A, et al. Myc-induced proliferation and transformation require Akt-mediated phosphorylation of FoxO proteins. EMBO J. 2004;23(14):2830–2840. doi: 10.1038/sj.emboj.7600279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung HK, Yi YW, Jung NC, et al. CR6-interacting factor 1 interacts with Gadd45 family proteins and modulates the cell cycle. J Biol Chem. 2003;278(30):28079–28088. doi: 10.1074/jbc.M212835200. [DOI] [PubMed] [Google Scholar]
- 5.da Silva GN, Filoni LT, Salvadori MC, et al. Gemcitabine/cisplatin treatment induces concomitant SERTAD1, CDKN2B and GADD45A modulation and cellular changes in bladder cancer cells regardless of the site of TP53 mutation. Pathol Oncol Res. 2018;24(2):407–417. doi: 10.1007/s12253-017-0255-x. [DOI] [PubMed] [Google Scholar]
- 6.Gao M, Li XG, Dong W, et al. Ribosomal protein S7 regulates arsenite-induced GADD45α expression by attenuating MDM2-mediated GADD45α ubiquitination and degradation. Nucleic Acids Res. 2013;41(10):5210–5222. doi: 10.1093/nar/gkt223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanke NT, Garland LL, Baker AF. Carfilzomib combined with suberanilohydroxamic acid (SAHA) synergistically promotes endoplasmic reticulum stress in non-small cell lung cancer cell lines. J Cancer Res Clin Oncol. 2016;142(3):549–560. doi: 10.1007/s00432-015-2047-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hildesheim J, Belova GI, Tyner SD, et al. Gadd45a regulates matrix metalloproteinases by suppressing ΔNp63α and β-catenin via p38 MAP kinase and APC complex activation. Oncogene. 2004;23(10):1829–1837. doi: 10.1038/sj.onc.1207301. [DOI] [PubMed] [Google Scholar]
- 9.Hollander MC, Sheikh MS, Bulavin DV, et al. Genomic instability in Gadd45a-deficient mice. Nat Genet. 1999;23(2):176–184. doi: 10.1038/13802. [DOI] [PubMed] [Google Scholar]
- 10.Hollander MC, Kovalsky O, Salvador JM, et al. Dimethylbenzanthracene carcinogenesis in Gadd45a-null mice is associated with decreased DNA repair and increased mutation frequency. Cancer Res. 2001;61(6):2487–2491. [PubMed] [Google Scholar]
- 11.Ji J, Liu R, Tong T, et al. Gadd45a regulates β-catenin distribution and maintains cell–cell adhesion/contact. Oncogene. 2007;26(44):6396–6405. doi: 10.1038/sj.onc.1210469. [DOI] [PubMed] [Google Scholar]
- 12.Jin SQ, Antinore MJ, Lung FDT, et al. The GADD45 inhibition of Cdc2 kinase correlates with GADD45-mediated growth suppression. J Biol Chem. 2000;275(22):16602–16608. doi: 10.1074/jbc.M000284200. [DOI] [PubMed] [Google Scholar]
- 13.Jin SQ, Tong T, Fan WH, et al. GADD45-induced cell cycle G2-M arrest associates with altered subcellular distribution of cyclin B1 and is independent of p38 kinase activity. Oncogene. 2002;21(57):8696–8704. doi: 10.1038/sj.onc.1206034. [DOI] [PubMed] [Google Scholar]
- 14.Li Q, Wei X, Zhou ZW, et al. GADD45α sensitizes cervical cancer cells to radiotherapy via increasing cytoplasmic ape1 level. Cell Death Dis. 2018;9(5):524. doi: 10.1038/s41419-018-0452-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li TH, Ho L, Piperdi B, et al. Phase II study of the proteasome inhibitor bortezomib (PS-341, Velcade®) in chemotherapy-naive patients with advanced stage non-small cell lung cancer (NSCLC) Lung Cancer. 2010;68(1):89–93. doi: 10.1016/j.lungcan.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Liebermann DA, Hoffman B. Gadd45 in stress signaling. J Mol Signal, 3:15. 2008 doi: 10.1186/1750-2187-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu LQ, Tian FJ, Xiong Y, et al. Gadd45a gene silencing by RNAi promotes cell proliferation and inhibits apoptosis and senescence in skin squamous cell carcinoma through the p53 signaling pathway. J Cell Physiol. 2018;233(9):7424–7434. doi: 10.1002/jcp.26588. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Ao X, Ding W, et al. Critical role of FOXO3a in carcinogenesis. Mol Cancer, 17:104. 2018 doi: 10.1186/s12943-018-0856-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manasanch EE, Orlowski RZ. Proteasome inhibitors in cancer therapy. Nat Rev Clin Oncol. 2017;14(7):417–433. doi: 10.1038/nrclinonc.2016.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 21.Morgillo F, D'Aiuto E, Troiani T, et al. Antitumor activity of bortezomib in human cancer cells with acquired resistance to anti-epidermal growth factor receptor tyrosine kinase inhibitors. Lung Cancer. 2011;71(3):283–290. doi: 10.1016/j.lungcan.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 22.Orlowski RZ, Kuhn DJ. Proteasome inhibitors in cancer therapy: lessons from the first decade. Clin Cancer Res. 2008;14(6):1649–1657. doi: 10.1158/1078-0432.CCR-07-2218. [DOI] [PubMed] [Google Scholar]
- 23.Park JE, Miller Z, Jun Y, et al. Next-generation proteasome inhibitors for cancer therapy. Transl Res. 2018;198:1–16. doi: 10.1016/j.trsl.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plas DR, Thompson CB. Akt activation promotes degradation of tuberin and FOXO3a via the proteasome. J Biol Chem. 2003;278(14):12361–12366. doi: 10.1074/jbc.M213069200. [DOI] [PubMed] [Google Scholar]
- 25.Ren WB, Xia XJ, Huang J, et al. Interferon-γ regulates cell malignant growth via the c-Abl/HDAC2 signaling pathway in mammary epithelial cells. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2019;20(1):39–48. doi: 10.1631/jzus.B1800211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salvador JM, Brown-Clay JD, Fornace AJ., Jr . Gadd45 in stress signaling, cell cycle control, and apoptosis. In: Liebermann DA Hoffman B., editor. Gadd45 Stress Sensor Genes. Springer, New York; 2013. pp. 1–19. [DOI] [PubMed] [Google Scholar]
- 27.Siegel DS, Martin T, Wang M, et al. A phase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood. 2012;120(14):2817–2825. doi: 10.1182/blood-2012-05-425934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 29.Smith ML, Chen IT, Zhan Q, et al. Interaction of the p53-regulated protein Gadd45 with proliferating cell nuclear antigen. Science. 1994;266(5189):1376–1380. doi: 10.1126/science.7973727. [DOI] [PubMed] [Google Scholar]
- 30.Takekawa M, Saito H. A family of stress-inducible GADD45-like proteins mediate activation of the stress-responsive MTK1/MEKK4 MAPKKK. Cell. 1998;95(4):521–530. doi: 10.1016/S0092-8674(00)81619-0. [DOI] [PubMed] [Google Scholar]
- 31.Tong T, Ji JF, Jin SQ, et al. Gadd45a expression induces Bim dissociation from the cytoskeleton and translocation to mitochondria. Mol Cell Biol. 2005;25(11):4488–4500. doi: 10.1128/MCB.25.11.4488-4500.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tran H, Brunet A, Grenier JM, et al. DNA repair pathway stimulated by the forkhead transcription factor FOXO3a through the Gadd45 protein. Science. 2002;296(5567):530–534. doi: 10.1126/science.1068712. [DOI] [PubMed] [Google Scholar]
- 33.van der Wekken AJ, Saber A, Hiltermann TJN, et al. Resistance mechanisms after tyrosine kinase inhibitors afatinib and crizotinib in non-small cell lung cancer, a review of the literature. Crit Rev Oncol Hematol. 2016;100:107–116. doi: 10.1016/j.critrevonc.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 34.Yang C, Yang SH, Wood KB, et al. Multidrug resistant osteosarcoma cell lines exhibit deficiency of GADD45α expression. Apoptosis. 2009;14(1):124–133. doi: 10.1007/s10495-008-0282-x. [DOI] [PubMed] [Google Scholar]
- 35.Yang F, Zhang WM, Li D, et al. Gadd45a suppresses tumor angiogenesis via inhibition of the mTOR/STAT3 protein pathway. J Biol Chem. 2013;288(9):6552–6560. doi: 10.1074/jbc.M112.418335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yao K, Fu XF, Du X, et al. PGC-1α coordinates with Bcl-2 to control the cell cycle in U251 cells through reducing ROS. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2018;19(6):415–424. doi: 10.1631/jzus.B1700148. [DOI] [Google Scholar]
- 37.Zhan QM, Antinore MJ, Wang XW, et al. Association with Cdc2 and inhibition of Cdc2/Cyclin B1 kinase activity by the p53-regulated protein Gadd45. Oncogene. 1999;18(18):2892–2900. doi: 10.1038/sj.onc.1202667. [DOI] [PubMed] [Google Scholar]