Chronic hepatitis B (CHB) remains a global health problem, carrying a high risk for progression into cirrhosis and liver failure. Molecular chaperones are involved in diverse pathophysiological processes including viral infection. However, the role of molecular chaperones in hepatitis B virus (HBV) infection and its underlying mechanisms remain unclear. Here, we identified GRP78 as one of the molecular chaperones most strongly induced by HBV in human hepatocytes.
KEYWORDS: HBV, GRP78, AKT/mTOR signaling, mild ER stress, persistent viral infection
ABSTRACT
Chronic hepatitis B (CHB) remains a global health problem, carrying a high risk for progression into cirrhosis and liver failure. Molecular chaperones are involved in diverse pathophysiological processes including viral infection. However, the role of molecular chaperones in hepatitis B virus (HBV) infection and its underlying mechanisms remain unclear. Here, we identified GRP78 as one of the molecular chaperones most strongly induced by HBV in human hepatocytes. Gain- and loss-of-function analyses demonstrated that GRP78 exerted an inhibitory effect on HBV transcription and replication. Further study showed that GRP78 was involved in the activation of AKT/mTOR signaling in hepatocytes, which contributed to GRP78-mediated inhibition of HBV. Of note, HBV-upregulated GRP78 was found to play a crucial role in maintaining the survival of hepatocytes via facilitating a mild endoplasmic reticulum (ER) stress. Together, our findings suggest that HBV may sacrifice part of its replication for establishing a persistent infection through induction of GRP78, a master ER stress regulator. Targeting GRP78 may help develop to design novel therapeutic strategies against chronic HBV infection and the associated hepatocellular carcinoma.
INTRODUCTION
Hepatitis B virus (HBV) infection remains a serious health problem, with about 260 million people chronically infected with HBV worldwide. Every year, nearly one million people die of chronic HBV infection-related diseases, including cirrhosis and hepatocellular carcinoma (HCC) (1, 2). The therapeutic efficacy of currently used anti-HBV drugs, such as antiviral nucleoside analogues and interferon (IFN), is limited due to short-term efficacy, drug resistance or side effects, etc. (3, 4). The development of novel therapeutic approaches for chronic hepatitis B (CHB) is likely to require a more thorough understanding of the host factors that favor viral persistence in chronically infected patients. However, despite decades of intensive investigation, the host interaction with HBV and the molecular mechanisms underlying persistent viral infection remain elusive (2, 5, 6).
Molecular chaperones, including heat shock protein 70 (HSP70), HSP90, and glucose-regulated protein 78 (GRP78), are key host factors contributing to maintaining cellular homeostasis under both optimal and adverse growth conditions, and they are involved in the pathogenesis of a variety of diseases, such as cancer, neurodegenerative diseases, and cardiovascular diseases (7, 8). Accumulating evidence indicates that molecular chaperones are also closely associated with viral infection, and they have emerged as important therapeutic targets for viral diseases (9, 10). It is reported that the HSP70/DnaJ chaperone network is required at distinct steps of the dengue virus life cycle (11). HSP90 suppresses the regulatory effects of mammalian endogenous retroviruses on neighboring genes (12). Calreticulin is hijacked by the white spot syndrome virus for its own replication cycle (13). PDIA3 (ERp57) contributes to the reproduction of enterovirus 71 (14), and its expression level is associated with HBV-related HCC (15). GRP78, as a master regulator of endoplasmic reticulum (ER) stress, has received particular attention in recent years and has been proposed as a universal therapeutic target for diverse human diseases (16). It is reported that GRP78 contributes to the proper folding/assembly of HBV proteins and HBV secretion (17) and acts as a pro-HBV factor in Boehmeria nivea extract-mediated inhibition of HBV replication (18). However, Ma et al. (19) reported that GRP78 inhibited HBV replication via activation of type I IFN signaling. Zheng et al. (20) also demonstrated the anti-HBV effect of GRP78, but its antiviral activity was not due to the activation of IFN signaling. As for the effect of HBV on the expression level of GRP78, the data also appeared to be contradictory: Ma et al. (19) and Liu et al. (21) reported that HBV induced the upregulation of GRP78, whereas data from Zhang et al. (22) showed that HBV disrupted the induction of GRP78. In addition, GRP78 may also contribute to the inhibition of other hepatotropic viruses, including hepatitis A virus and hepatitis C virus (HCV) (23, 24). Of note, GRP78 may play an important role in the development of persistent infection of several viruses, including HCV and Japanese encephalitis virus (25, 26). Until now, the role of molecular chaperones in HBV infection and its underlying mechanisms have remained largely unclear.
In the present study, we found that, of selected molecular chaperones, HBV induced the upregulation of GRP78 most significantly in hepatocytes and that GRP78 exhibited an inhibitory effect on HBV replication. Further, it was found that GRP78 did not have a significant effect on the antiviral innate immune responses in HBV-replicating cells, but it was important for the activation of AKT/mTOR signaling, which was revealed to contribute to the inhibition of HBV replication by GRP78. Furthermore, our data revealed that GRP78 played a crucial role in maintaining the cell survival of HBV-replicating hepatocytes by facilitating the establishment of a mild ER stress. Together, our data suggest that HBV may sacrifice part of its replication to facilitate a persistent infection in a more favorable cellular environment through induction of the ER stress master regulator GRP78 and that targeting GRP78 may be a way to develop a potential therapeutic strategy for treating chronic HBV infection and the associated HCC.
(This study was presented in part as a poster at the 17th International Congress of Immunology, Beijing, China, 19 to 23 October 2019.)
RESULTS
HBV infection induces the upregulation of GRP78 in hepatocytes.
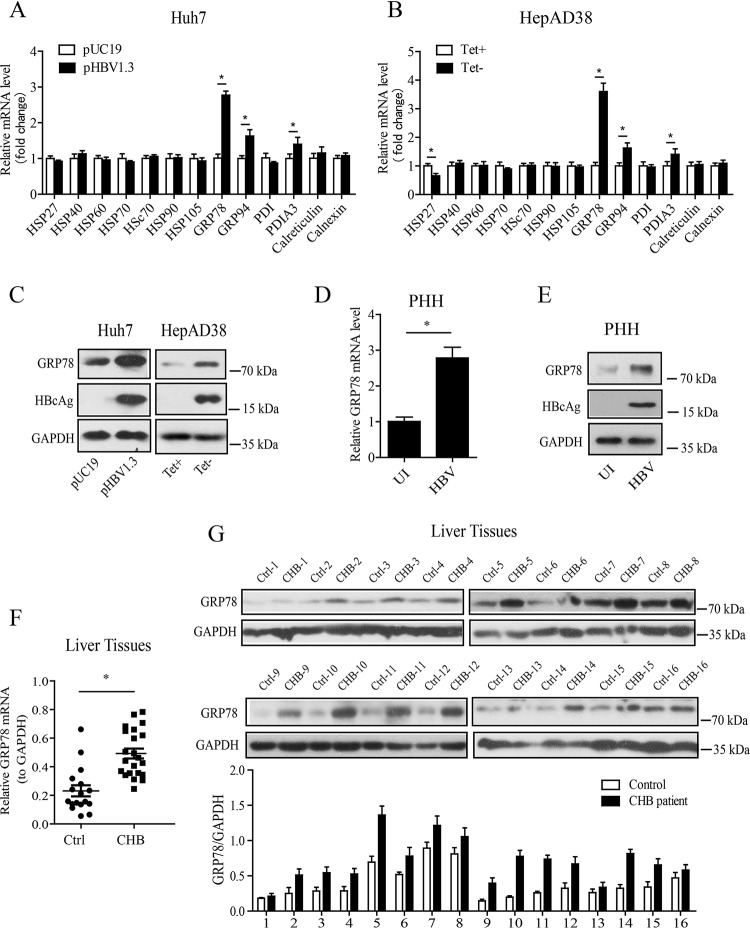
To investigate the role of molecular chaperones in HBV infection, we first transfected Huh7 cells with a replication-competent HBV plasmid (pHBV1.3) and then detected the mRNA levels of molecular chaperones, including HSP27, HSP40, HSP60, HSP70, HSC70, HSP90, GRP78, GRP94, protein disulfide isomerase (PDI), PDIA3, calreticulin, and calnexin, by quantitative reverse transcription-PCR (qRT-PCR). The results showed that, of these selected molecular chaperones, GRP78 was most strongly induced in pHBV1.3-transfected Huh7 cells (Fig. 1A). We also examined the effect of HBV on GRP78 expression in HepAD38 cells, in which the HBV production is under the control of the tetracycline-off (Tet-off) promoter, and Tet removal permits the transcription and replication of HBV (27). Similar to the data obtained from pHBV1.3-transfected Huh7 cells, GRP78 was most strongly induced by HBV in HepAD38 cells among the selected molecular chaperones. Further, we examined the effect of HBV on the expression of GRP78 at the protein level by Western blotting (Fig. 1B). The results showed that, in both Huh7 and HepAD38 cells, HBV upregulated the protein level of GRP78 significantly (Fig. 1C). Furthermore, we assessed the effect of HBV on the expression level of GRP78 in primary human hepatocytes (PHHs). We found that GRP78 expression was significantly increased by HBV infection at both mRNA and protein levels in PHHs (Fig. 1D and E). Of note, our data revealed that the expression of GRP78 was upregulated at both mRNA (Fig. 1F) and protein levels (Fig. 1G) in liver tissues from CHB patients compared to those from control individuals.
FIG 1.
HBV infection induced the upregulation of GRP78 expression in human hepatocytes. (A) Huh7 cells were transfected with pHBV1.3 or empty control vector pUC19. At 48 h posttransfection, the mRNA levels of molecular chaperones were determined by qRT-PCR. The data are means ± the SEM of four samples pooled from three independent experiments. *, P < 0.05. (B) The mRNA levels of molecular chaperones in HepAD38 cells treated with or without tetracycline were determined as in panel A. The data are means ± the SEM of five samples pooled from three independent experiments. *, P < 0.05. (C) Huh7 cells and HepAD38 cells were treated as in panels A and B, respectively. Western blotting was then performed with antibodies against GRP78, HBcAg, or GAPDH. (D) PHHs were infected with HBV at 100 vge per cell in the presence of 4% PEG-8000 for 16 h. At 7 days postinfection, the GRP78 mRNA level was determined by qRT-PCR. The data are means ± the SEM of five samples pooled from three independent experiments. *, P < 0.05. (E) PHHs were infected with HBV as in panel D, and Western blotting was performed with antibodies against GRP78, HBcAg, or GAPDH. (F) GRP78 mRNA levels in liver tissues from CHB patients (n = 22) and control individuals (n = 17) were determined by qRT-PCR. *, P < 0.05. (G) GRP78 protein levels in liver tissues from CHB patients and control individuals that were age and gender matched were determined by Western blotting (n = 16).
GRP78 exhibits an inhibitory effect on HBV transcription and replication.
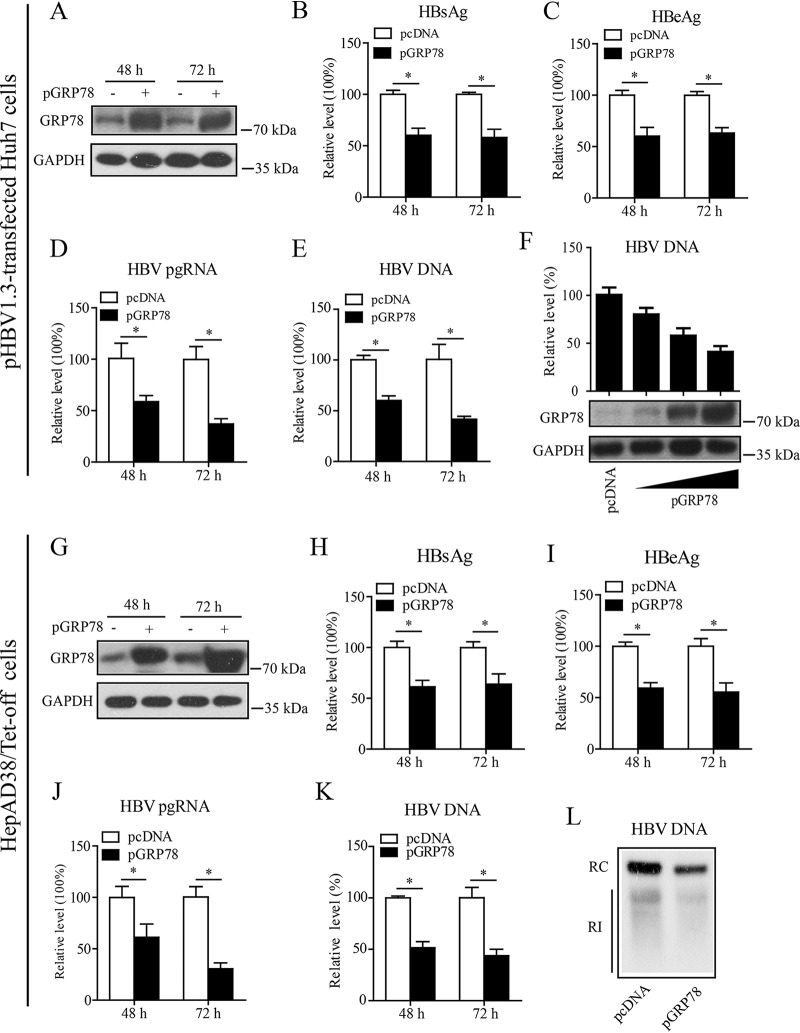
As above, the data showed that HBV induced the upregulation of GRP78 significantly, while GRP78 is emerging as an important therapeutic target for a variety of human diseases (16). We thus focused our attention on the role of GRP78 in HBV infection in the present investigation. First, we cotransfected Huh7 cells with pHBV1.3 and a GRP78 expression plasmid (pGRP78). Western blot analysis showed that pGRP78 transfection led to the efficient expression of GRP78 in Huh7 cells at the indicated time points (Fig. 2A). GRP78 overexpression was found to inhibit HBV gene expression significantly in Huh7 cells, as shown by the decreased levels of HBsAg (Fig. 2B), HBeAg (Fig. 2C), or pregenomic RNA (pgRNA) (Fig. 2D). GRP78 expression also decreased the HBV DNA level significantly (Fig. 2E), and this effect displayed a dose-dependent tendency (Fig. 2F). We further examined the effect of GRP78 on HBV gene expression and replication in HepAD38/Tet-off cells. As shown in Fig. 2G, electrotransfection with pGRP78 led to the efficient expression of GRP78 in HepAD38 cells. Consistent with the data from pHBV1.3-transfected Huh7 cells, overexpression of GRP78 significantly inhibited HBV gene expression and replication, as demonstrated by the decreased levels of HBsAg (Fig. 2H), HBeAg (Fig. 2I), pgRNA (Fig. 2J), or HBV DNA (Fig. 2K) in HepAD38 cells. Further, the inhibitory effect of GRP78 expression on HBV replication in HepAD38 cells was confirmed by Southern blotting, as indicated by the decreased level of HBV replication intermediates (RI) in pGRP78-transfected HepAD38 cells (Fig. 2L).
FIG 2.
GRP78 overexpression inhibited HBV replication. (A to E) Huh7 cells were cotransfected with pHBV1.3 and GRP78 expression plasmid (pGRP78) or control empty vector (pcDNA). At 48 or 72 h posttransfection, the cells were harvested and evaluated for effect of GRP78 on HBV replication. (A) The expression level of GRP78 was detected by Western blotting with GRP78-specific antibody. GAPDH was used as a loading control. The levels of HBsAg (B) and HBeAg (C) were examined by enzyme-linked immunosorbent assay (ELISA), the level of pgRNA was determined by qRT-PCR (D), and the intracellular HBV DNA level was determined by qPCR (E). The data are means ± the SEM of five samples pooled from three independent experiments. *, P < 0.05. (F) Huh7 cells were transfected with pHBV1.3, together with an increasing dose of pGRP78. At 48 h posttransfection, the cells were harvested and subjected to HBV DNA quantification by qPCR. The data are means ± the SEM of three samples pooled from three independent experiments. (G to K) HepAD38/Tet-off cells were electrotransfected with pGRP78 or pcDNA. At 48 or 72 h posttransfection, the cells were harvested and evaluated for effect of GRP78 on HBV replication. The levels of GRP78 (G), HBsAg (H), HBeAg (I), preC-pgRNA (J), and intracellular HBV DNA (K) were determined as in panels B and F. The data are means ± the SEM of four samples pooled from three independent experiments. *, P < 0.05. (L) HepAD38/Tet-off cells were treated as in panels G to K. At 72 h posttransfection, the cells were harvested, and HBV replicative intermediates were determined by Southern blotting. RI, replication intermediates; RC, relaxed circular DNA.
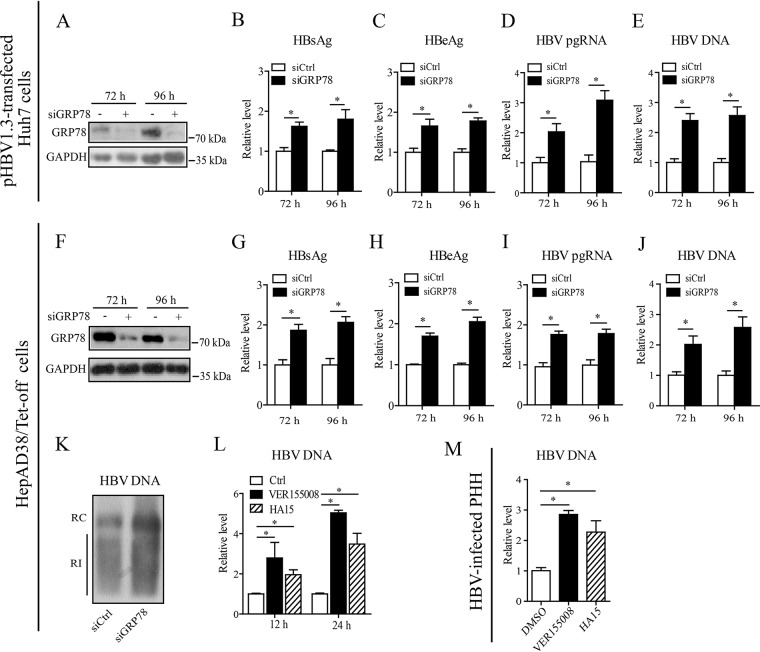
To further confirm the inhibitory effect of GRP78 on HBV gene expression and replication, we downregulated GRP78 expression in Huh7 cells by using the small interfering RNA (siRNA) technique. Western blot analysis confirmed the knockdown efficiency of GRP78-specific siRNA (siGRP78) in Huh7 cells (Fig. 3A). siRNA-mediated GRP78 downregulation significantly enhanced HBV gene expression and replication in Huh7 cells, as demonstrated by the increased levels of HBsAg (Fig. 3B), HBeAg (Fig. 3C), pgRNA (Fig. 3D), or HBV DNA (Fig. 3E). We further investigated the effect of GRP78 downregulation on HBV replication in HepAD38/Tet-off cells. As shown in Fig. 3F, transfection with GRP78-specific siRNA efficiently downregulated GRP78 protein level in HepAD38 cells. Consistent with data from Huh7 cells, knockdown of GRP78 by the siRNA approach in HepAD38 cells led to the significant increase of HBV gene expression and replication, as revealed by the upregulated levels of HBsAg (Fig. 3G), HBeAg (Fig. 3H), pgRNA (Fig. 3I), or HBV DNA (Fig. 3J). Data from Southern blotting further verified the effect of GRP78 downregulation on HBV replication (Fig. 3K). We also treated HepAD38/Tet-off cells with VER155008, a pharmacological inhibitor targeting the ATPase binding domain of GRP78. We found that inhibition of GRP78 function by VER155008 significantly enhanced HBV replication, and similar results were obtained from another structurally different GRP78 inhibitor, HA15 (Fig. 3L). Furthermore, we investigated the role of GRP78 in HBV-infected PHHs. The results showed that inhibition of GRP78 activity via either VER155008 or HA15 increased the HBV DNA level in PHHs significantly (Fig. 3M).
FIG 3.
Inhibition of GRP78 enhanced HBV replication. (A to E) Huh7 cells were transfected with GRP78 siRNA (siGRP78) or control siRNA (siCtrl), followed by the transfection of pHBV1.3. At 48 and 72 h posttransfection, the GRP78 expression level was examined by Western blotting (A), the levels of HBsAg (B) and HBeAg (C) were measured by ELISA, and the level of pgRNA (D) or intracellular HBV-DNA (E) was quantified by qRT-PCR or qPCR, respectively. The data are means ± the SEM of five samples pooled from three independent experiments. *, P < 0.05. (F to J) HepAD38/Tet-off cells were electrotransfected with siGRP78 or siCtrl. At 72 and 96 h posttransfection, the HepAD38 cells were harvested. The levels of GRP78 protein (F), HBsAg (G), HBeAg (H), pgRNA (I), and HBV DNA (J) were determined as in panels A to E. The data are means ± the SEM of four samples pooled from three independent experiments. *, P < 0.05. (K) HepAD38 cells were treated as in panels F to J. HBV replicative intermediates were detected by Southern blotting. (L) HepAD38 cells were treated with VER155008 (20 μM) or HA15 (10 μM) for 12 or 24 h, and the intracellular HBV DNA was then extracted and subjected to quantification by qPCR. The data are means ± the SEM of four samples pooled from three independent experiments. *, P < 0.05. (M) PHHs were infected with HBV as in Fig. 1D. At day 7 postinfection, the cells were treated with VER155008 (20 μM) or HA15 (10 μM) for 24 h, and the HBV DNA level was determined by qPCR. The data are means ± the SEM of three samples pooled from three independent experiments. *, P < 0.05.
Taken together, these data demonstrated that GRP78 displayed an inhibitory effect on HBV transcription and replication.
GRP78 does not have a significant effect on antiviral innate immune responses in HBV-replicating cells.
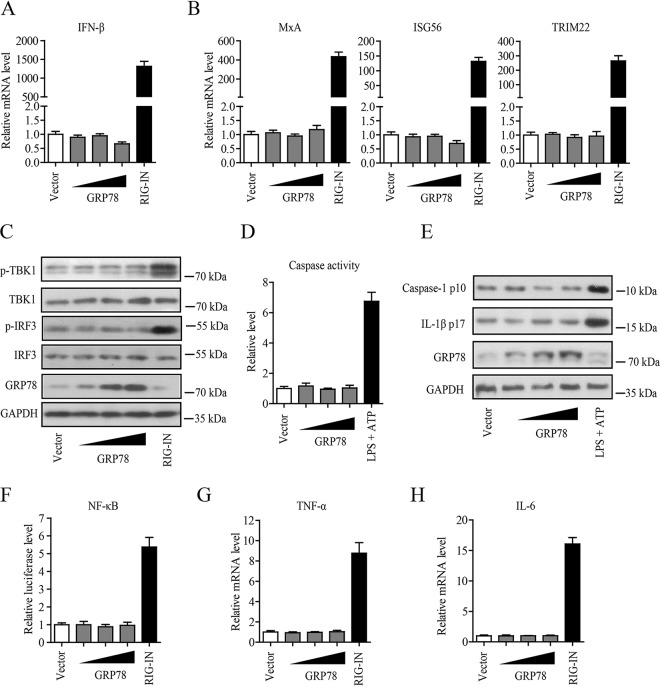
Reports indicate that GRP78 may be implicated in innate immune regulation (19, 23, 28), while it now well established that activation of innate immune response can suppress HBV replication efficiently (29). We therefore investigated whether GRP78-mediated inhibition of HBV was due to the activation of innate immune responses. We first examined the effect of GRP78 on the activation of type I IFN signaling by transfection of increasing amounts of pGRP78 into HBV-replicating cells. We found that overexpression of GRP78 did not have significant effect on the expression levels of IFN-β (Fig. 4A) and some important IFN-stimulating genes (ISGs; including the MxA, ISG56, and TRIM22 genes) (Fig. 4B) in HepAD38/Tet-off cells, as well as in pHBV1.3 transiently transfected HepG2 cells (see Fig. S1A and B in the supplemental material), although a constitutively active mutant of RIG-I (RIG-IN), as a positive control, dramatically increased the expression levels of IFN-β and ISGs in these cells (Fig. 4A and B; Fig. S1A and B). Further, Western blotting showed that GRP78 failed to enhance the phosphorylation of TBK1 and IRF3 (Fig. 4C). Our data also revealed that GRP78 expression did not lead to the activation of inflammasomes in HBV-replicating cells, as demonstrated by the small change in the level of caspase-1 activity (Fig. 4D; Fig. S1C) and that of caspase-1 p10 or IL-1β p17 (Fig. 4E). In addition, we also tested the effect of GRP78 on the NF-κB activity. We found that overexpression of GRP78 did not activate the NF-κB promoter activity (Fig. 4F) and also failed to increase the expression level of proinflammatory cytokines TNF-α (Fig. 4G; Fig. S1D) and IL-6 (Fig. 4H; Fig. S1E) in HBV-replicating cells. Collectively, our data indicated that GRP78-mediated inhibition of HBV replication might not involve the activation of innate immune signaling.
FIG 4.
GRP78-mediated inhibition of HBV replication was not due to the activation of innate immune responses. (A) HepAD38/Tet-off cells were transfected with increasing amounts of pGRP78 or a constitutively active mutant of RIG-I expression plasmid (pRIG-IN). At 48 h posttransfection, the cells were collected, and the level of IFN-β mRNA was tested by qRT-PCR. The data are means ± the SEM of five samples pooled from three independent experiments. (B) Cells were treated as in panel A. The mRNA level of MxA, ISG56, or TRIM22 was determined by qRT-PCR. The data are means ± the SEM of four samples pooled from three independent experiments. (C) Cells were treated as in panel A and then subjected to Western blotting with antibodies against p-TBK1, TBK1, p-IRF3, IRF3 GRP78, or GAPDH. (D) HepAD38/Tet-off cells were transfected with pcDNA or increasing amounts of pGRP78. At 48 h posttransfection, the caspase-1 activity was determined. The data are means ± the SEM of three samples pooled from three independent experiments. Cells stimulated with lipopolysaccharide (500 ng/ml) plus ATP (5 mM) were used as a positive control for inflammasome activation. (E) HepAD38 cells were treated as in panel D, and Western blotting was then performed with antibodies against caspase-1, IL-1β, GRP78, and GAPDH. (F) NF-κB promoter-dependent luciferase reporter plasmids (pNF-κB-Luc) were transfected into HepAD38 cells, together with pcDNA and increasing amounts of pGRP78 or pRIG-IN. After 48 h, the luciferase activity in the cell lysates was measured. The data are means ± the SEM of four samples pooled from three independent experiments. (G and H) Cells were transfected as in panel A. After 48 h, the mRNA level of TNF-α (G) or IL-6 (H) was determined by qRT-PCR. The data are means ± the SEM of four samples pooled from three independent experiments.
GRP78 contributes to the activation of AKT/mTOR signaling in HBV-replicating cells.
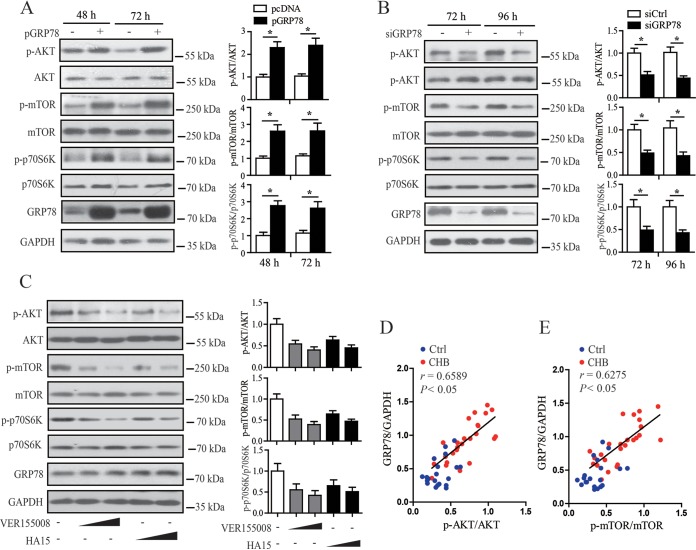
Accumulating evidence demonstrates that GRP78 may have a close relationship with the activation of AKT/mTOR signaling (30, 31), whereas AKT/mTOR have been demonstrated to be a barrier for a variety of viruses, including HBV (32–36). We thus further investigated the effect of GRP78 on AKT/mTOR signaling in HBV-replicating cells. Our results showed that GRP78 overexpression led to the activation of AKT/mTOR signaling in HBV-replicating HepAD38 cells and Huh7 cells, as revealed by the upregulated level of p-AKT, p-mTOR, or p-p70s6K (Fig. 5A; Fig. S2A), whereas the siRNA-mediated knockdown experiment showed that downregulation of GRP78 significantly decreased the phosphorylation level of AKT, mTOR, or p70s6K (Fig. 5B; Fig. S2B). Inhibition of GRP78 activity by VER155008 or HA15 also efficiently decreased the levels of p-AKT, p-mTOR, or p70s6K (Fig. 5C). Furthermore, we examined the relationship between the level of GRP78 protein and those of p-AKT and p-mTOR in human liver biopsy specimens. We found that the level of GRP78 protein was positively associated with that of p-AKT or p-mTOR in CHB patients (Fig. 5D and E; Fig. S3). Together, these data indicate that GRP78 expression might contribute to the activation of AKT/mTOR signaling in HBV infection.
FIG 5.
GRP78 contributed to the activation of AKT/mTOR signaling in HBV-replicating cells. (A) HepAD38/Tet-off cells were transfected with pcDNA or pGRP78. At 48 and 72 h posttransfection, the levels of p-AKT, AKT, p-mTOR, mTOR, p-p70s6K, p70s6K, GRP78, and GAPDH were determined by Western blotting. (Right) The relative levels of p-AKT to AKT, p-mTOR to mTOR, or p-p70s6K to p70s6K were examined by densitometric analysis, and the value from pcDNA-transfected cells was set at 1.0. The data are means ± the SEM of six samples pooled from three independent experiments. *, P < 0.05. (B) HepAD38/Tet-off cells were transfected with siGRP78 or siCtrl. At 72 and 96 h posttransfection, the level of p-AKT or p-mTOR were determined as in panel A, and value from siCtrl-transfected cells was set at 1.0. The data are means ± the SEM of five samples pooled from three independent experiments. *, P < 0.05. (C) HepAD38/Tet-off cells were treated with increasing doses of VER155008 (10 or 20 μM) or HA15 (5 or 10 μM) for 24 h. The level of p-AKT, p-mTOR, or p-p70s6K was determined as in panel A, and the value from dimethyl sulfoxide-treated cells was set at 1.0. The data are means ± the SEM of five samples pooled from three independent experiments. (D and E) The level of GRP78, p-AKT, or p-mTOR of liver tissues from CHB patients (n = 26) and control individuals (n = 20) was determined by Western blotting as in panel A. The correlation of the GRP78 protein level with the p-AKT or p-mTOR level was carried out by Pearson correlation analysis.
AKT/mTOR signaling is involved in GRP78-mediated inhibition of HBV replication.
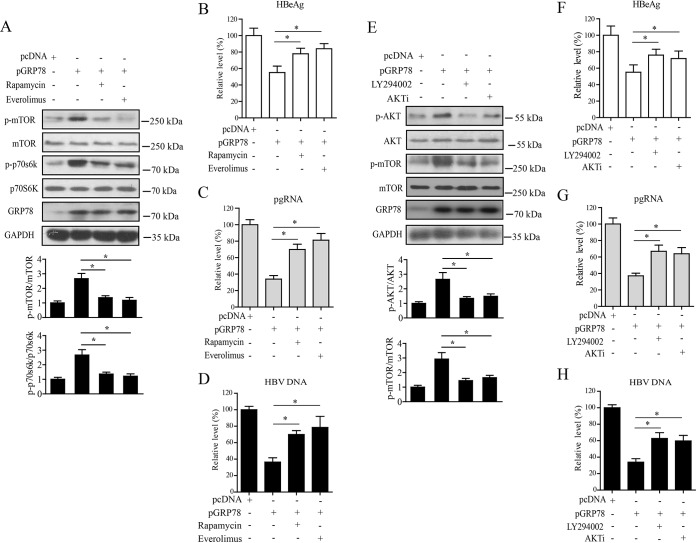
To investigate whether AKT/mTOR signaling was involved in GRP78-mediated inhibition of HBV, we first treated GRP78-transfected HepAD38/Tet-off cells with mTOR signaling-specific inhibitor rapamycin or everolimus. The results showed that treatment with rapamycin or everolimus significantly inhibited GRP78-activated mTOR signaling, as indicated by the decreased levels of p-mTOR and p-p70s6K (Fig. 6A). Inhibition of mTOR activation led to the significant attenuation of GRP78-mediated inhibition of HBV transcription and replication, as indicated by the increased levels of HBeAg (Fig. 6B), pgRNA (Fig. 6C), and HBV-DNA (Fig. 6D). We also treated GRP78-transfected HepAD38/Tet-off cells with phosphatidylinositol 3-kinase (PI3K) inhibitor LY294002 and AKT inhibitor (AKTi). It was found that the treatment of HepAD38/Tet-off cells with LY294002 and AKTi significantly inhibited AKT/mTOR signaling, as indicated by the decreased level of p-AKT or p-mTOR (Fig. 6E), and consequently attenuated GRP78-mediated suppression of HBV transcription and replication (Fig. 6F to H). We also examined the role of AKT/mTOR signaling in GRP78-mediated inhibition of HBV in Huh7 cells, and similar results were obtained (Fig. S4). Taken together, these data indicated that the AKT/mTOR signaling might be involved in GRP78-mediated inhibition of HBV.
FIG 6.
AKT/mTOR signaling was involved in GRP78-mediated inhibition of HBV replication. (A) HepAD38/Tet-off cells were transfected with pGRP78 for 48 h, followed by treatment with rapamycin (100 nM) or everolimus (100 nM) for another 24 h. The cells were then subjected to Western blotting with antibodies against p-mTOR, mTOR, p-p70S6K, p70S6K, GRP78, and GAPDH. (Lower panel) The relative levels of p-mTOR to mTOR or p-p70s6K to p70s6K were examined by densitometric analysis, and the value from empty vector-transfected cells was set at 1.0. The data are means ± the SEM of five samples pooled from three independent experiments. *, P < 0.05. (B to D) Cells were treated as in panel A. The levels of HBeAg (B), pgRNA (C), or HBV-DNA (D) were determined by ELISA, qRT-PCR, and qPCR, respectively. The data are means ± the SEM of five samples pooled from three independent experiments. *, P < 0.05. (E) HepAD38/Tet-off cells were transfected with pGRP78 for 48 h, followed by treatment with LY294002 (10 μM) or AKTi (10 μM) for another 24 h. The cells were then collected and subjected to Western blotting with antibodies against p-AKT, AKT, p-mTOR, mTOR, GRP78, and GAPDH. (Lower panel) The relative level of p-AKT to AKT or p-mTOR to mTOR was examined by densitometric analysis, and the value from empty vector-transfected cells was set at 1.0. The data are means ± the SEM of four samples pooled from three independent experiments. *, P < 0.05. (F to H) Cells were treated as for panel E. The levels of HBeAg (F), pgRNA (G), or HBV DNA (H) were determined as in panels B to D. The data are means ± the SEM of five samples pooled from three independent experiments. *, P < 0.05.
GRP78 plays a prosurvival role in HBV-replicating cells.
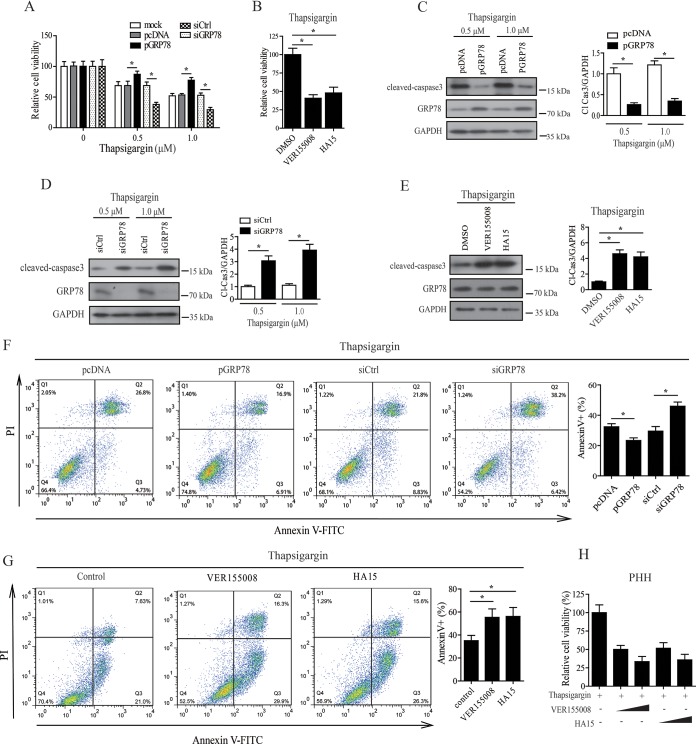
The data presented above showed that HBV induced the upregulation of GRP78, whereas GRP78 displayed an inhibitory effect on HBV replication. This led us to question why HBV should upregulate the expression level of a molecule which inhibited its own replication. Evidence suggests that inhibition of hepatocyte apoptosis may be necessary for the noncytotoxic and persistent replication of HBV (33, 37). We therefore further investigated the role of GRP78 in cell survival of HBV-replicating HepAD38 cells in response to thapsigargin treatment or serum deprivation. A CCK8 assay showed that overexpression of GRP78 decreased cell death, while knockdown of GRP78 by siRNA increased cell death significantly (Fig. 7A; Fig. S5A), and inhibition of GRP78 activity by VER155008 or HA15 was also revealed to decrease the cell viability significantly (Fig. 7B) in response to thapsigargin treatment. Western blotting showed that GRP78 overexpression decreased, whereas knockdown of GRP78 by siRNA or inhibition of GRP78 activity by VER155008 or HA15 significantly increased, the level of cleaved caspase-3 (an apoptosis marker) in response to thapsigargin treatment or serum deprivation (Fig. 7C to E; Fig. S5B and C). Furthermore, the gain- and loss-of-function analyses revealed that GRP78 contributed to the decrease of annexin V-positive cells in thapsigargin-treated or serum-starved HepAD38 cells (Fig. 7F and G; Fig. S5D). Since primary human hepatocytes have been proved to be valuable for viability studies (38), we further tested the effect of GRP78 on the cell viability of HBV-infected PHHs by CCK8 test. Our data showed that inhibition of GRP78 function by either VER155008 or HA15 significantly decreased the cell viability of HBV-infected PHHs in response to thapsigargin treatment (Fig. 7H).
FIG 7.
GRP78 acted as a prosurvival factor in HBV-replicating cells. (A) GRP78 overexpression or knockdown cells were treated with 0.5 or 1 μM thapsigargin for 24 h, and the cell viability was then determined by CCK8 assay. The data are means ± the SEM of five samples pooled from three independent experiments. *, P < 0.05. (B) HepAD38/Tet-off cells were treated with VER155008 (20 μM) or HA15 (10 μM) in the presence of 0.5 μM thapsigargin for 24 h, and the cell viability was determined as in panel A. The data are means ± the SEM of four samples pooled from three independent experiments. *, P < 0.05. (C) HepAD38/Tet-off cells with GRP78 overexpression were treated with 0.5 or 1 μM thapsigargin for 24 h. The cells were then subjected to Western blotting with antibodies against cleaved caspase-3 (Cl. Casp-3), GRP78, or GAPDH. (Right panel) The relative level of Cl. Casp-3 to GAPDH was examined by densitometric analysis, and the value from empty vector-transfected cells was set at 1.0. The data are means ± the SEM of five samples pooled from three independent experiments. *, P < 0.05. (D) GRP78 knockdown HepAD38/Tet-off cells were treated with 0.5 or 1 μM thapsigargin for 24 h, cells were then subjected to Western blotting as in panel C. The data are means ± the SEM of four samples pooled from three independent experiments. *, P < 0.05. (E) Cells were treated as in panel B and then subjected to Western blotting as in panel C. The data are means ± the SEM of three samples pooled from three independent experiments. *, P < 0.05. (F) GRP78 overexpression or knockdown cells were treated with 0.5 μM thapsigargin. The cells were then subjected to apoptosis analysis by fluorescence-activated cell sorting (FACS). The data are means ± the SEM of five samples pooled from three independent experiments. *, P < 0.05. (G) Cells were treated as in panel B and then subjected to apoptosis analysis by FACS. The data are means ± the SEM of four samples pooled from three independent experiments. *, P < 0.05. (H) PHHs were infected with HBV as in Fig. 1D and E. The cells were treated with increasing doses of VER155008 (10 or 20 μM) or HA15 (5 or 10 μM) in the presence of 0.5 μM thapsigargin. The cell viability was determined by a CCK8 test. The data are means ± the SEM of three samples pooled from three independent experiments. *, P < 0.05.
GRP78 contributes to cell survival by facilitating mild ER stress in HBV-replicating cells.
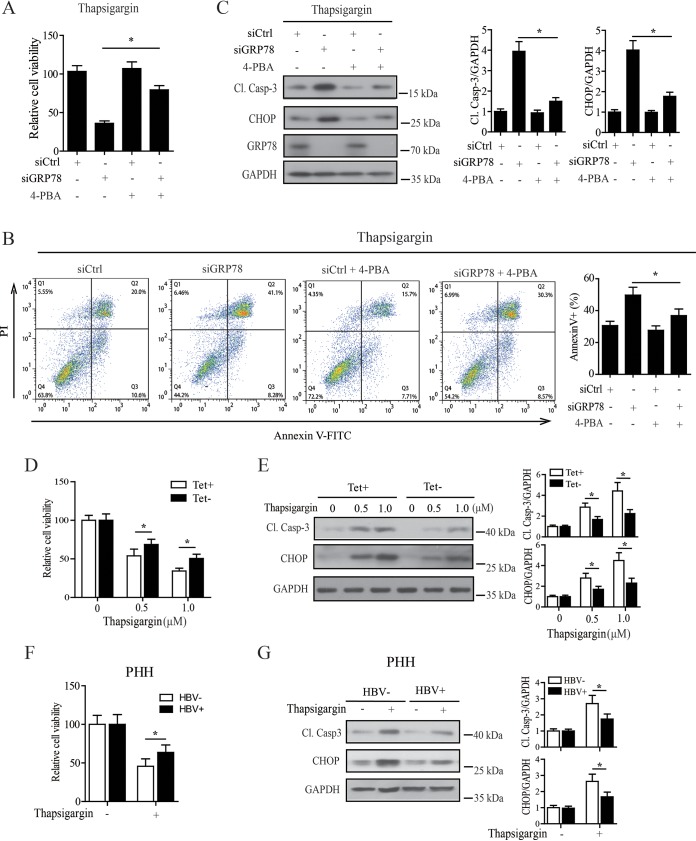
Accumulating evidence shows that regulation of ER stress plays a crucial role in controlling cell death. ER stress can simultaneously activate both adaptive and proapoptotic pathways, depending on the stress intensity. Severe and sustained ER stress will activate proapoptotic proteins, such as C/EBP homologous binding protein (CHOP), and ultimately lead to the cell death, whereas mild ER stress (characterized by the constant upregulation of GRP78 without induction of apoptosis) is often associated with the activation of an adaptive response which allows the cells to cope with various apoptotic insults (39–42). Since our data revealed that GRP78 was crucial for the regulation of ER stress in HBV-replicating cells (Fig. S6), we paid particular attention to the role of ER stress signaling in GRP78-contributed cell survival. We subjected GRP78-knockdown cells to the treatment of thapsigargin or serum deprivation in the presence or absence of 4-phenylbutyric acid (4-PBA), a chemical chaperone known to reduce ER stress. Our data showed that downregulation of GRP78-sensitized HepAD38 cells to thapsigargin- or serum deprivation-induced cell death significantly, as shown by the decreased cell viability (Fig. 8A; Fig. S7A) and the upregulation of annexin V-positive cells (Fig. 8B, Fig. S7B), as expected, whereas inhibition of ER stress by 4-PBA significantly reversed this phenomenon (Fig. 8A and B; Fig. S7A and B). Accordingly, GRP78 knockdown was found to significantly upregulate the level of ER stress-associated proapoptotic protein CHOP in response to the treatment of thapsigargin or serum deprivation, which could be significantly attenuated by the treatment with 4-PBA (Fig. 8C; Fig. S7C), indicating that GRP78 contributed to the survival of HBV-replicating cells through controlling excessive ER stress.
FIG 8.
GRP78 contributed to the survival of HBV-replicating cells via facilitating the establishment of a mild ER stress. (A) GRP78 knockdown HepAD38/Tet-off cells were pretreated with 4-PBA (5 mM) for 30 min and then treated with 0.5 μM thapsigargin for 24 h. The cell viability was then determined by a CCK8 test. The data are means ± the SEM of six samples pooled from three independent experiments. *, P < 0.05. (B) Cells were treated as in panel A and then subjected to apoptosis analysis by FACS. The data are means ± the SEM of five samples pooled from three independent experiments. *, P < 0.05. (C) Cells were treated as in panel A and then subjected to Western blotting with antibodies against cleaved caspase-3, CHOP, GRP78, or GAPDH. (Right panel) The relative level of Cl. Casp-3 or CHOP to GAPDH was examined by densitometric analysis, and the value from control siRNA-transfected cells was set at 1.0. The data are means ± the SEM of five samples pooled from three independent experiments. *, P < 0.05. (D) HepAD38 cells treated with or without tetracycline were subjected to thapsigargin treatment at the concentration of 0.5 or 1 μM for 24 h, and the cell viability was determined by a CCK8 assay. The data are means ± the SEM of five samples pooled from three independent experiments. *, P < 0.05. (E) Cells were treated as in panel D and then subjected to Western blotting with antibodies against Cl. Casp-3, CHOP, or GAPDH. (Right panel) The relative level of Cl. Casp-3 or CHOP to GAPDH was examined by densitometric analysis, and the value from siCtrl-transfected cells was set at 1.0. The data are means ± the SEM of four samples pooled from three independent experiments. *, P < 0.05. (F) PHHs infected with or without HBV were treated with 0.5 μM thapsigargin for 24 h, and the cell viability was determined by a CCK8 test. The data are means ± the SEM of three samples pooled from three independent experiments. *, P < 0.05. (G) PHHs were treated as in panel F, and levels of Cl. Casp-3 and CHOP were determined by Western blotting as in panel E. The data are means ± the SEM of four samples pooled from three independent experiments.
Our data revealed that HBV, per se, induced ER stress in hepatocytes (Fig. S8); we therefore also investigated the sensitivity of hepatocytes with or without HBV replication to thapsigargin-induced cell death. As shown in Fig. 8D, compared to HepAD38 cells without HBV replication (Tet+), cells with HBV replication (Tet–) showed greater resistance to thapsigargin-induced cell death. We also investigated whether the difference in the sensitivity to cell death was due to the activation of the ER stress-related apoptotic signal. In accordance with data of cell viability assay, our data revealed that the expression level of the ER stress-associated protein CHOP was significantly higher in cells without HBV replication than those in HBV-replicating cells after thapsigargin treatment (Fig. 8E). Further, we compared the cell viability of HBV-infected and noninfected PHHs in response to thapsigargin treatment. Similar to the data obtained from HepAD38 cells, HBV-infected PHHs were more resistant to thapsigargin-induced cell death than HBV-uninfected cells (Fig. 8F). Accordingly, the level of proapoptotic protein CHOP in HBV-infected PHHs was significantly lower than that in HBV-uninfected control cells in response to thapsigargin treatment (Fig. 8G). Combining the prosurvival activity of HBV with the data that HBV induced the upregulation of GRP78, which plays a crucial role in regulating HBV-induced ER stress, we propose that HBV induced a mild ER stress for maintaining cell survival, with GRP78 playing a key role in this process.
DISCUSSION
CHB is still a major public health issue and causes millions of deaths every year around the world. It remains a challenge to decipher the molecular mechanisms by which persistent HBV infection is established and maintained (2).
Accumulating evidence indicates that molecular chaperones are closely associated with a variety of pathophysiological processes, including persistent viral infection. In present investigation, GRP78 was identified as one of the most strongly induced molecular chaperones by HBV in hepatocytes. Of note, GRP78 expression was significantly upregulated in the liver tissues of CHB patients, suggesting the possible involvement of GRP78 in the pathogenesis of CHB. Contradictory to our data, Zhang et al. (22) reported that HBV induced the downregulation of GRP78 in HepG2.2.15 cells, as well as in human liver tissues. The reason for this discrepancy is unknown, but it may be due to different cell lines or liver biopsy samples used. Since the production of HBV particles in HepAD38 is under the control of a Tet-responsive promoter, HepAD38 has been proved to be a suitable tool to directly compare the cellular characteristics with or without HBV replication, while the different genetic backgrounds of HepG2 and HepG2.2.15 may result in an inaccurate evaluation of the effect of HBV replication on host gene expression. In the case of comparative biological analysis between HepG2.2.15 and HepG2, another previous investigation using a two-dimensional blue native/SDS-PAGE technique showed that, compared to HepG2 cells, the expression level of GRP78/Bip was upregulated in HepG2.2.15 cells (21). Consistent with our data, Ma et al. (19) also reported that HBV induced the upregulation of GRP78 at both mRNA and protein level in HBV stably transfected HepAD38 cells, as well as in HBV transiently transfected HepG2 cells, and their data also revealed that inhibition of HBV replication in CHB patients by lamivudine significantly downregulated the expression level of GRP78 in human liver tissues.
Since GRP78 is one of the most strongly induced molecular chaperones by HBV in hepatocytes and also in consideration of the important roles of GRP78 in a variety of pathophysiological processes, we focused our attention on GRP78 in the present investigation. Through genetic and pharmacological approaches, we demonstrated that GRP78 exhibited an inhibitory effect on HBV replication in hepatocytes. However, it has been reported that GRP78 may contribute to the folding of HBV protein (17) and is required for the secretion of HBV virions (18), although our results showed that GRP78 could also decrease the HBV DNA level in the culture supernatants of HepAD38 cells (see Fig. S9 in the supplemental material). In agreement with our results, Ma et al. (19) and Zheng et al. (20) reported that GRP78 expression could downregulate the HBV titers of both cytoplasm and supernatants. This discrepancy remains unclear; it may be due to the inhibitory effect of GRP78 overriding its stimulatory effect on HBV replication.
Studies indicate that GRP78 may be involved in the regulation of innate immune responses. Data from Wei et al. (23) indicated that GRP78 contributed to TLR3-mediated innate immune responses against HCV. Ma et al. (19) reported that GRP78 overexpression led to the upregulation of IFN-β and some of ISGs (including OAS1, OAS2, and RNase L) and that IFN-β in turn induced the upregulation of GRP78 in HepG2 cells. The mutual activation of GRP78 and IFN-β might contribute to the suppression of HBV. However, in contrast to the data from Ma et al, our results showed that GRP78 overexpression did not activate the IFN signaling in both HBV-replicating Huh7 and HepAD38 cells, although RIG-IN, as a positive control, activated type I IFN signaling dramatically. In agreement with our data, Zheng et al. (20) also reported that IFN signaling might not be responsible for GRP78 inhibition of HBV. In addition, since transformed cell lines like HepG2 have defects in IFN signaling (43, 44), it is also intriguing that GRP78 can inhibit HBV replication by activating type I signaling in these cell lines. Further, our data also showed that overexpression of GRP78 did not have a significant effect on the activation of inflammasome and NF-κB activity in HBV-replicating cells. Together, our data suggest that GRP78-mediated inhibition of HBV replication might not involve the activation of innate immune responses.
Accumulating evidence demonstrates that the AKT/mTOR pathway may act as a barrier for a variety of viruses, including the hepatitis viruses (32–34, 45). It is also evident that GRP78 may be closely associated with the activation AKT/mTOR signaling (30, 46). We thus investigated the effect of GRP78 on AKT/mTOR signaling in HBV-infected cells. Our data revealed that overexpression of GRP78 activated while knockdown of GRP78 suppressed AKT/mTOR signaling significantly in HBV-replicating hepatoma cells. Of note, our data revealed that there existed a positive correlation between the expression level of GRP78 and that of p-AKT or p-mTOR in clinical liver biopsy specimens, suggesting the possible role of GRP78 in the activation of AKT/mTOR under physiological conditions. Further, our data revealed that inhibiting AKT/mTOR signaling indeed significantly attenuated GRP78-mediated inhibition of HBV transcription and replication, indicating that AKT/mTOR signaling was involved in the inhibitory effect of GRP78 on HBV.
These data showed that HBV induced the upregulation of GRP78, while GRP78 displayed an inhibitory effect on HBV replication. We wondered why HBV should stimulate the expression of a molecule that would inhibit the replication of itself. It was reported that inhibition of hepatocyte apoptosis is important for HBV to release infectious progeny and might be important for the persistent replication of HBV (2, 37). We thus further evaluated the role of GRP78 in cell apoptosis in HBV-replicating cells. Our data showed that ectopic expression of GRP78 could decrease thapsigargin- or serum deprivation-induced cell death, whereas inhibition GRP78 by genetic or pharmacological approaches significantly increased it, indicating that GRP78 indeed played a protective role in HBV-replicating cells. Accumulating evidence shows that ER stress signaling plays a crucial role in the regulation of cell survival, although its role is somewhat paradoxical: mild ER stress is often favorable for cell survival, whereas excessive ER stress leads to cell death (47–51). Our data showed that knockdown of GRP78 by an siRNA approach induced severe ER stress in hepatocytes, characterized by the upregulation of the proapoptotic protein CHOP, followed by cell death, whereas inhibiting ER stress by 4-PBA significantly attenuated these processes, indicating that GRP78 contributed to cell survival of HBV-infected hepatocytes by controlling excessive ER stress. We and others demonstrated that HBV, per se, induced ER stress in hepatocytes (52–54). We thus investigated the sensitivity of human hepatocytes with or without HBV replication to thapsigargin- or serum deprivation-induced cell death and found that cells with HBV replication were more resistant to thapsigargin- or serum deprivation-induced cell death, which was correlated with the suppression of CHOP expression in HBV-replicating cells. Together, these data indicated that HBV induced a mild ER stress, which is favorable for cell survival, with GRP78 playing a central role in this process.
In conclusion, our data demonstrated that HBV induced the upregulation of the molecular chaperone GRP78 significantly in hepatocytes. GRP78 exhibited an inhibitory effect on HBV replication. Mechanistically, GRP78 did not have a significant effect on innate antiviral responses, but it contributed to the regulation of AKT/mTOR signaling, which contributed to the GRP78-mediated inhibition of HBV replication. Further, our data showed that GRP78 plays a crucial role in maintaining cell survival via facilitating a mild ER stress. We suggest that, on the one hand, GRP78 inhibits HBV replication to keep viral load to a relatively low level and that, on the other hand, it is beneficial for creating a “mild” cellular environment via regulating HBV-induced ER stress and finally contributes to persistent viral infection. These data indicate that targeting GRP78 might provide an important therapeutic strategy against chronic HBV infection and the associated HCC.
MATERIALS AND METHODS
Antibodies and reagents.
Antibodies against GRP78 (sc-13968), IL-1β (sc-7884), and caspase-1 (sc-56036) were obtained from Santa Cruz (Santa Cruz, CA). Antibodies against p-AKT (catalog no. 9271), AKT (catalog no. 9272), p-mTOR (catalog no. 2971), mTOR (catalog no. 2972), p-p70S6K (catalog no. 9234), p70S6K (catalog no. 9202), cleaved caspase-3 (catalog no. 9661), and CHOP (catalog no. 2895) were purchased from Cell Signaling Technology (Beverly, MA). Antibody against GAPDH (AP0063) was from BioWorld Biotechnology (Minneapolis, MN). Antibody against HBcAg (ab8639) was obtained from Abcam (Cambridge, MA). VER155008 (a potent inhibitor of the Hsp70 family of chaperones with 50% inhibitory concentrations of 0.5, 2.6, and 2.6 μM in cell-free assays for HSP70, HSC70, and GRP78, respectively) (catalog no. 0217), GRP78 inhibitor HA15 (catalog no. 2118), PI3K inhibitor LY294002 (catalog no. 1571), AKT inhibitor (catalog no. 124005), mTOR inhibitor rapamycin (catalog no. 553210), mTOR inhibitor everolimus (catalog no. 2282), ER stress inducer thapsigargin (catalog no. 1845), and chemical chaperone 4-phenylbutyric acid (Y0000808) were obtained from Merck (Darmstadt, Germany).
Cell culture and human samples.
Huh7 and HepAD38 cells (in which the HBV replication is under the control of a tetracycline-regulated promoter) were maintained in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum, 100 μg/ml penicillin, and 100 μg/ml streptomycin. HepAD38 cells were grown in the absence or presence of 0.3 μg/ml tetracycline and 400 μg/ml G418. Primary human hepatocytes (PHHs) were isolated from the surgically removed liver sections and cultured as described previously (38, 55). All cells were maintained in an incubator containing 5% CO2 at 37°C. Human liver tissue samples were collected from CHB patients and control individuals in Zhongshan Hospital (Fudan University, Shanghai, China). For the control group, liver tissues were obtained from patients who underwent surgical resection for benign hepatic lesions. Patients with the following criteria were excluded: infection with HBV and HCV, decompensated liver disease, liver cirrhosis, steatohepatitis, or immunologically mediated liver diseases. This study was approved by the Ethics Committee of Fudan University and performed in compliance with the Helsinki Declaration.
Plasmid or siRNA transfection and HBV infection.
Huh7 cells were cotransfected with pHBV1.3 and pGRP78 (Addgene, catalog no. 32701) or empty control vector (pcDNA3.1) by Lipofectamine 2000 (Invitrogen, Carlsbad, CA). GRP78-specific or control siRNA was transfected into Huh7 cells by HiPerFect transfection reagent (Qiagen, Hilden, Germany). For the transfection of HepAD38 cells, 106 cells were resuspended in 100 μl of Cell Line Nucleofector solution C (Amaxa GmbH, Koln, Germany) and nucleofected with 2 μg of the indicated plasmids or 100 nM siRNA. HBV virions were prepared from the stable HBV-producing HepAD38 cells. To infect PHHs, the cells were incubated with HBV inoculum for 16 h at a multiplicity of infection of 100 virus genome equivalents (vge) per cell in the presence of 4% polyethylene glycol 8000 (PEG-8000) as described previously (38, 56). The infection experiments were repeated at least three times on different days.
Quantitative RT-PCR.
qRT-PCR was performed as previously described (56, 57). The primers for qRT-PCR are listed in Table S1 in the supplemental material.
Extraction and quantitative analysis of intracellular HBV DNA.
The method for the extraction and analysis of intracellular core particle-associated HBV DNA was described previously (56–58). Briefly, HBV genomic DNA was then extracted with the Viral Genome purification kit (Cwbiotech, Beijing, China) according to the manufacturer’s protocol and subjected to quantitative PCR (qPCR) using an HBV diagnostic kit (Kehua Biotech, Shanghai, China) according to the manufacturer’s instructions. For the Southern hybridization, the intracellular core particle-associated HBV DNA was extracted and probed with a digoxigenin (DIG)-labeled full-length HBV probe synthesized from a DIG probe synthesis kit (Roche Diagnostics, Mannheim, Germany).
Western blot analysis.
Western blot analysis was performed as described previously (56, 58, 59).
Cell viability analysis by CCK8 assay.
Cells were plated on a 96-well plate in a volume of 100 μl and allowed to attach for 24 h. After indicated treatments, 20 μl of CCK-8 solution (Dojindo Laboratories, Kumamoto, Japan) was added to each well, and the cells were incubated for 2 h. The absorbance was then measured at 450 nm. Values are expressed as a percentage relative to those obtained for the control groups.
Flow cytometric analysis of cell apoptosis.
Cells were detached from the plate with 0.25% trypsin and resuspended in binding buffer at the density of 106/ml. One hundred microliters of cells was incubated with fluorescein isothiocyanate-labeled annexin V and propidium iodide (eBioscience, San Diego, CA) for 15 min on ice in the dark. Data were acquired on a BD FACSCalibur (BD Biosciences) in CellQuest (BD Biosciences) and analyzed by using FlowJo software (Tree Star).
Statistical analysis.
Results are expressed as means ± the standard errors of the mean (SEM). Statistical analyses of the data were performed using the GraphPad Prism (v5.0) statistical program. Differences between two groups were evaluated by two-tailed Student t tests, while differences between multiple groups were evaluated by analysis of variance with post hoc tests to compare differences between individual groups. A P value of <0.05 was considered statistically significant.
Supplementary Material
ACKNOWLEDGMENT
This study was supported by grants from the National Natural Science Foundation of China (31470839, 31872731, and 21334001), the Priority Academic Program Development of Jiangsu Higher Education Institutions, the Jiangsu Provincial Innovative Research Team and the Program for Changjiang Scholars and Innovative Research Team in University of Ministry of Education of China (PCSIRT-IRT1075), and the Science and Technology Innovation Commission of Shenzhen (under grant JCYJ20170818094217688).
B.G. and W.S. designed the experiments. W.S., Z.G., L.L., Z.W., Z.X., Y.Y., Y.L., M.H., and R.G. performed the experiments. W.S., Z.G., L.L., and B.G. analyzed the data. B.G. wrote the paper with W.S.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Trepo C, Chan HL, Lok A. 2014. Hepatitis B virus infection. Lancet 384:2053–2063. doi: 10.1016/S0140-6736(14)60220-8. [DOI] [PubMed] [Google Scholar]
- 2.Tsai KN, Kuo CF, Ou JJ. 2018. Mechanisms of hepatitis B virus persistence. Trends Microbiol 26:33–42. doi: 10.1016/j.tim.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okada M, Enomoto M, Kawada N, Nguyen MH. 2017. Effects of antiviral therapy in patients with chronic hepatitis B and cirrhosis. Expert Rev Gastroenterol Hepatol 11:1095–1104. doi: 10.1080/17474124.2017.1361822. [DOI] [PubMed] [Google Scholar]
- 4.Papatheodoridis GV, Manolakopoulos S, Dusheiko G, Archimandritis AJ. 2008. Therapeutic strategies in the management of patients with chronic hepatitis B virus infection. Lancet Infect Dis 8:167–178. doi: 10.1016/S1473-3099(07)70264-5. [DOI] [PubMed] [Google Scholar]
- 5.Seeger C, Mason WS. 2000. Hepatitis B virus biology. Microbiol Mol Biol Rev 64:51–68. doi: 10.1128/mmbr.64.1.51-68.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong GL, Wong VW, Chan HL. 2016. Virus and host testing to manage chronic hepatitis B. Clin Infect Dis 62(Suppl 4):S298–S305. doi: 10.1093/cid/ciw024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Almeida MB, do Nascimento JLM, Herculano AM, Crespo-López ME. 2011. Molecular chaperones: toward new therapeutic tools. Biomed Pharmacother 65:239–243. doi: 10.1016/j.biopha.2011.04.025. [DOI] [PubMed] [Google Scholar]
- 8.Feldman DE, Frydman J. 2000. Protein folding in vivo: the importance of molecular chaperones. Curr Opin Struct Biol 10:26–33. doi: 10.1016/s0959-440x(99)00044-5. [DOI] [PubMed] [Google Scholar]
- 9.Phillips AM, Ponomarenko AI, Chen K, Ashenberg O, Miao J, McHugh SM, Butty VL, Whittaker CA, Moore CL, Bloom JD, Lin YS, Shoulders MD. 2018. Destabilized adaptive influenza variants critical for innate immune system escape are potentiated by host chaperones. PLoS Biol 16:e3000008. doi: 10.1371/journal.pbio.3000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiao A, Wong J, Luo H. 2010. Viral interaction with molecular chaperones: role in regulating viral infection. Arch Virol 155:1021–1031. doi: 10.1007/s00705-010-0691-3. [DOI] [PubMed] [Google Scholar]
- 11.Taguwa S, Maringer K, Li X, Bernal-Rubio D, Rauch JN, Gestwicki JE, Andino R, Fernandez-Sesma A, Frydman J. 2015. Defining Hsp70 subnetworks in dengue virus replication reveals key vulnerability in flavivirus infection. Cell 163:1108–1123. doi: 10.1016/j.cell.2015.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hummel B, Hansen EC, Yoveva A, Aprile-Garcia F, Hussong R, Sawarkar R. 2017. The evolutionary capacitor HSP90 buffers the regulatory effects of mammalian endogenous retroviruses. Nat Struct Mol Biol 24:234–242. doi: 10.1038/nsmb.3368. [DOI] [PubMed] [Google Scholar]
- 13.Watthanasurorot A, Guo E, Tharntada S, Lo CF, Soderhall K, Soderhall I. 2014. Hijacking of host calreticulin is required for the white spot syndrome virus replication cycle. J Virol 88:8116–8128. doi: 10.1128/JVI.01014-14. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Wang M, Dong Q, Wang H, He Y, Chen Y, Zhang H, Wu R, Chen X, Zhou B, He J, Kung HF, Huang C, Wei Y, Huang JD, Xu H, He ML. 2016. Oblongifolin M, an active compound isolated from a Chinese medical herb Garcinia oblongifolia, potently inhibits enterovirus 71 reproduction through downregulation of ERp57. Oncotarget 7:8797–8808. doi: 10.18632/oncotarget.7122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu M, Du L, He Z, Yan L, Shi Y, Shang J, Tang H. 2017. Increased ERp57 expression in HBV-related hepatocellular carcinoma: possible correlation and prognosis. Biomed Res Int 2017:1252647. doi: 10.1155/2017/1252647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Booth L, Roberts JL, Cash DR, Tavallai S, Jean S, Fidanza A, Cruz-Luna T, Siembiba P, Cycon KA, Cornelissen CN, Dent P. 2015. GRP78/BiP/HSPA5/Dna K is a universal therapeutic target for human disease. J Cell Physiol 230:1661–1676. doi: 10.1002/jcp.24919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho DY, Yang GH, Ryu CJ, Hong HJ. 2003. Molecular chaperone GRP78/BiP interacts with the large surface protein of hepatitis B virus in vitro and in vivo. J Virol 77:2784–2788. doi: 10.1128/jvi.77.4.2784-2788.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang KL, Lai YK, Lin CC, Chang JM. 2009. Involvement of GRP78 in inhibition of HBV secretion by Boehmeria nivea extract in human HepG2 2.2.15 cells. J Viral Hepat 16:367–375. doi: 10.1111/j.1365-2893.2009.01072.x. [DOI] [PubMed] [Google Scholar]
- 19.Ma Y, Yu J, Chan HL, Chen YC, Wang H, Chen Y, Chan CY, Go MY, Tsai SN, Ngai SM, To KF, Tong JH, He QY, Sung JJ, Kung HF, Cheng CH, He ML. 2009. Glucose-regulated protein 78 is an intracellular antiviral factor against hepatitis B virus. Mol Cell Proteomics 8:2582–2594. doi: 10.1074/mcp.M900180-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng NQ, Zheng ZH, Xu HX, Huang MX, Peng XM. 2017. Glucose-regulated protein 78 demonstrates antiviral effects but is more suitable for hepatocellular carcinoma prevention in hepatitis B. Virol J 14:77. doi: 10.1186/s12985-017-0747-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu K, Qian L, Wang J, Li W, Deng X, Chen X, Sun W, Wei H, Qian X, Jiang Y, He F. 2009. Two-dimensional blue native/SDS-PAGE analysis reveals heat shock protein chaperone machinery involved in hepatitis B virus production in HepG2.2.15 cells. Mol Cell Proteomics 8:495–505. doi: 10.1074/mcp.M800250-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang X, Zhang R, Yang H, Xiang Q, Jiang Q, He Q, Zhang T, Chen C, Zhu H, Wang Q, Ning Q, Li Y, Lei P, Shen G. 2016. Hepatitis B virus enhances cisplatin-induced hepatotoxicity via a mechanism involving suppression of glucose-regulated protein of 78 kDa. Chem Biol Interact 254:45–53. doi: 10.1016/j.cbi.2016.05.030. [DOI] [PubMed] [Google Scholar]
- 23.Wei D, Li NL, Zeng Y, Liu B, Kumthip K, Wang TT, Huo D, Ingels JF, Lu L, Shang J, Li K. 2016. The molecular chaperone GRP78 contributes to Toll-like receptor 3-mediated innate immune response to hepatitis C virus in hepatocytes. J Biol Chem 291:12294–12309. doi: 10.1074/jbc.M115.711598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang X, Kanda T, Haga Y, Sasaki R, Nakamura M, Wu S, Nakamoto S, Shirasawa H, Okamoto H, Yokosuka O. 2017. Glucose-regulated protein 78 is an antiviral against hepatitis A virus replication. Exp Ther Med 13:3305–3308. doi: 10.3892/etm.2017.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang X, Kanda T, Wu S, Nakamoto S, Wakita T, Shirasawa H, Yokosuka O. 2014. Hepatitis C virus nonstructural protein 5A inhibits thapsigargin-induced apoptosis. PLoS One 9:e113499. doi: 10.1371/journal.pone.0113499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lyoo HR, Park SY, Kim JY, Jeong YS. 2015. Constant up-regulation of BiP/GRP78 expression prevents virus-induced apoptosis in BHK-21 cells with Japanese encephalitis virus persistent infection. Virol J 12:32. doi: 10.1186/s12985-015-0269-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ladner SK, Otto MJ, Barker CS, Zaifert K, Wang GH, Guo JT, Seeger C, King RW. 1997. Inducible expression of human hepatitis B virus (HBV) in stably transfected hepatoblastoma cells: a novel system for screening potential inhibitors of HBV replication. Antimicrob Agents Chemother 41:1715–1720. doi: 10.1128/AAC.41.8.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brenner C, Galluzzi L, Kepp O, Kroemer G. 2013. Decoding cell death signals in liver inflammation. J Hepatol 59:583–594. doi: 10.1016/j.jhep.2013.03.033. [DOI] [PubMed] [Google Scholar]
- 29.Guidotti LG, Chisari FV. 2001. Noncytolytic control of viral infections by the innate and adaptive immune response. Annu Rev Immunol 19:65–91. doi: 10.1146/annurev.immunol.19.1.65. [DOI] [PubMed] [Google Scholar]
- 30.Lin YG, Shen J, Yoo E, Liu R, Yen HY, Mehta A, Rajaei A, Yang W, Mhawech-Fauceglia P, DeMayo FJ, Lydon J, Gill P, Lee AS. 2015. Targeting the glucose-regulated protein-78 abrogates Pten-null driven AKT activation and endometrioid tumorigenesis. Oncogene 34:5418–5426. doi: 10.1038/onc.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dadey D, Kapoor V, Hoye K, Khudanyan A, Collins A, Thotala D, Hallahan DE. 2017. Antibody targeting GRP78 enhances the efficacy of radiation therapy in human glioblastoma and non-small cell lung cancer cell lines and tumor models. Clin Cancer Res 23:2556–2564. doi: 10.1158/1078-0432.CCR-16-1935. [DOI] [PubMed] [Google Scholar]
- 32.Mannova P, Beretta L. 2005. Activation of the N-Ras/PI3K/Akt/mTOR pathway by hepatitis C virus: control of cell survival and viral replication. J Virol 79:8742–8749. doi: 10.1128/JVI.79.14.8742-8749.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rawat S, Bouchard MJ. 2015. The hepatitis B virus (HBV) HBx protein activates AKT to simultaneously regulate HBV replication and hepatocyte survival. J Virol 89:999–1012. doi: 10.1128/JVI.02440-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou X, Wang Y, Metselaar HJ, Janssen HL, Peppelenbosch MP, Pan Q. 2014. Rapamycin and everolimus facilitate hepatitis E virus replication: revealing a basal defense mechanism of the PI3K/PKB/mTOR pathway. J Hepatol 61:746–754. doi: 10.1016/j.jhep.2014.05.026. [DOI] [PubMed] [Google Scholar]
- 35.Chen X, Hu Y, Zhang W, Chen K, Hu J, Li X, Liang L, Cai X, Hu J, Wang K, Huang A, Tang N. 2019. Cisplatin induces autophagy to enhance hepatitis B virus replication via activation of ROS/JNK and inhibition of the Akt/mTOR pathway. Free Radic Biol Med 131:225–236. doi: 10.1016/j.freeradbiomed.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 36.Xiang K, Wang B. 2018. Role of the PI3K/AKT/mTOR pathway in hepatitis B virus infection and replication. Mol Med Rep 17:4713–4719. doi: 10.3892/mmr.2018.8395. [DOI] [PubMed] [Google Scholar]
- 37.Arzberger S, Hosel M, Protzer U. 2010. Apoptosis of hepatitis B virus-infected hepatocytes prevents release of infectious virus. J Virol 84:11994–12001. doi: 10.1128/JVI.00653-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schulze-Bergkamen H, Untergasser A, Dax A, Vogel H, Büchler P, Klar E, Lehnert T, Friess H, Büchler MW, Kirschfink M, Stremmel W, Krammer PH, Müller M, Protzer U. 2003. Primary human hepatocytes: a valuable tool for investigation of apoptosis and hepatitis B virus infection. J Hepatol 38:736–744. doi: 10.1016/s0168-8278(03)00120-x. [DOI] [PubMed] [Google Scholar]
- 39.Rutkowski DT, Arnold SM, Miller CN, Wu J, Li J, Gunnison KM, Mori K, Sadighi AA, Raden D, Kaufman RJ. 2006. Adaptation to ER stress is mediated by differential stabilities of pro-survival and proapoptotic mRNAs and proteins. PLoS Biol 4:e374. doi: 10.1371/journal.pbio.0040374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Achard CS, Laybutt DR. 2012. Lipid-induced endoplasmic reticulum stress in liver cells results in two distinct outcomes: adaptation with enhanced insulin signaling or insulin resistance. Endocrinology 153:2164–2177. doi: 10.1210/en.2011-1881. [DOI] [PubMed] [Google Scholar]
- 41.Liang P, Zhong L, Gong L, Wang J, Zhu Y, Liu W, Yang J. 2017. Fibroblast growth factor 21 protects rat cardiomyocytes from endoplasmic reticulum stress by promoting the fibroblast growth factor receptor 1-extracellular signal-regulated kinase 1/2 signaling pathway. Int J Mol Med 40:1477–1485. doi: 10.3892/ijmm.2017.3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xie Y, Ye S, Zhang J, He M, Dong C, Tu W, Liu P, Shao C. 2016. Protective effect of mild endoplasmic reticulum stress on radiation-induced bystander effects in hepatocyte cells. Sci Rep 6:38832. doi: 10.1038/srep38832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keskinen P, Nyqvist M, Sareneva T, Pirhonen J, Melen K, Julkunen I. 1999. Impaired antiviral response in human hepatoma cells. Virology 263:364–375. doi: 10.1006/viro.1999.9983. [DOI] [PubMed] [Google Scholar]
- 44.Melen K, Keskinen P, Lehtonen A, Julkunen I. 2000. Interferon-induced gene expression and signaling in human hepatoma cell lines. J Hepatol 33:764–772. doi: 10.1016/s0168-8278(00)80308-6. [DOI] [PubMed] [Google Scholar]
- 45.Guo H, Zhou T, Jiang D, Cuconati A, Xiao GH, Block TM, Guo JT. 2007. Regulation of hepatitis B virus replication by the phosphatidylinositol 3-kinase/Akt signal transduction pathway. J Virol 81:10072–10080. doi: 10.1128/JVI.00541-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou X, Xing X, Zhang S, Liu L, Wang C, Li L, Ji Q, Liu H. 2016. Glucose-regulated protein 78 contributes to the proliferation and tumorigenesis of human colorectal carcinoma via AKT and ERK pathways. Oncol Rep 36:2723–2730. doi: 10.3892/or.2016.5097. [DOI] [PubMed] [Google Scholar]
- 47.Au-Yeung N, Mandhana R, Horvath CM. 2013. Transcriptional regulation by STAT1 and STAT2 in the interferon JAK-STAT pathway. JAKSTAT 2:e23931. doi: 10.4161/jkst.23931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gorman AM, Healy SJ, Jager R, Samali A. 2012. Stress management at the ER: regulators of ER stress-induced apoptosis. Pharmacol Ther 134:306–316. doi: 10.1016/j.pharmthera.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 49.Kaufman RJ. 2002. Orchestrating the unfolded protein response in health and disease. J Clin Invest 110:1389–1398. doi: 10.1172/JCI16886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rutkowski DT, Kaufman RJ. 2004. A trip to the ER: coping with stress. Trends Cell Biol 14:20–28. doi: 10.1016/j.tcb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 51.Rutkowski DT, Kaufman RJ. 2007. That which does not kill me makes me stronger: adapting to chronic ER stress. Trends Biochem Sci 32:469–476. doi: 10.1016/j.tibs.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 52.Li B, Gao B, Ye L, Han X, Wang W, Kong L, Fang X, Zeng Y, Zheng H, Li S, Wu Z, Ye L. 2007. Hepatitis B virus X protein (HBx) activates ATF6 and IRE1-XBP1 pathways of unfolded protein response. Virus Res 124:44–49. doi: 10.1016/j.virusres.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 53.Li J, Liu Y, Wang Z, Liu K, Wang Y, Liu J, Ding H, Yuan Z. 2011. Subversion of cellular autophagy machinery by hepatitis B virus for viral envelopment. J Virol 85:6319–6333. doi: 10.1128/JVI.02627-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li J, He J, Fu Y, Hu X, Sun LQ, Huang Y, Fan X. 2017. Hepatitis B virus X protein inhibits apoptosis by modulating endoplasmic reticulum stress response. Oncotarget 8:96027–96034. doi: 10.18632/oncotarget.21630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lecluyse EL, Alexandre E. 2010. Isolation and culture of primary hepatocytes from resected human liver tissue. Methods Mol Biol 640–82. doi: 10.1007/978-1-60761-688-7_3. [DOI] [PubMed] [Google Scholar]
- 56.Zhong L, Shu W, Dai W, Gao B, Xiong S. 2017. Reactive oxygen species-mediated c-Jun NH2-terminal kinase activation contributes to hepatitis B virus X protein-induced autophagy via regulation of the beclin-1/Bcl-2 interaction. J Virol 91:e00001-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gao B, Duan Z, Xu W, Xiong S. 2009. Tripartite motif-containing 22 inhibits the activity of hepatitis B virus core promoter, which is dependent on nuclear-located RING domain. Hepatology 50:424–433. doi: 10.1002/hep.23011. [DOI] [PubMed] [Google Scholar]
- 58.Zhong L, Hu J, Shu W, Gao B, Xiong S. 2015. Epigallocatechin-3-gallate opposes HBV-induced incomplete autophagy by enhancing lysosomal acidification, which is unfavorable for HBV replication. Cell Death Dis 6:e1770. doi: 10.1038/cddis.2015.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou L, He X, Gao B, Xiong S. 2015. Inhibition of histone deacetylase activity aggravates coxsackievirus B3-induced myocarditis by promoting viral replication and myocardial apoptosis. J Virol 89:10512–10523. doi: 10.1128/JVI.01028-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.