Clear cell renal cell carcinoma (ccRCC) is regarded as the most aggressive subtype of RCC, with high rates of metastasis and recurrence. An extensive body of studies had proved long noncoding RNAs (lncRNAs) play pivotal parts in the development and evolution of diverse malignant tumors. However, the potential of LINC01094 in ccRCC tumorigenesis is still unexplored. In the present research, with the aid of the TCGA database, we found that LINC01094 was highly expressed in ccRCC tissues.
KEYWORDS: ccRCC, LINC01094, miR-224-5p, CHSY1, FOXM1
ABSTRACT
Clear cell renal cell carcinoma (ccRCC) is regarded as the most aggressive subtype of RCC, with high rates of metastasis and recurrence. An extensive body of studies had proved long noncoding RNAs (lncRNAs) play pivotal parts in the development and evolution of diverse malignant tumors. However, the potential of LINC01094 in ccRCC tumorigenesis is still unexplored. In the present research, with the aid of the TCGA database, we found that LINC01094 was highly expressed in ccRCC tissues. Upregulation of LINC01094 was also confirmed in ccRCC cell lines, and functional experiments delineated that LINC01094 knockdown led to inhibition on ccRCC cell growth and metastasis. Moreover, LINC01094 was activated by FOXM1 at the transcriptional level. Further assay demonstrated that LINC01094 worked as a sponge of microRNA 224-5p (miR-224-5p) and CHSY1 was a miR-224-5p-targeted mRNA. Further, we verified that LINC01094 acted as a competing endogenous RNA in ccRCC to regulate CHSY1 expression via competitively bind to miR-224-5p. Lastly, our results expounded that LINC01094 exerted its tumor-promoting performance in ccRCC development through miR-224-5p/CHSY1 regulatory axis, which shed light on the molecular mechanism underlying LINC01094 in ccRCC and opened a new prospective for the treatment of ccRCC.
INTRODUCTION
Currently, renal cell carcinoma (RCC) has been identified as the second most fatal malignancy in urological system and constitutes approximately 3% of malignant tumors globally (1). RCC, deriving from the epithelium of the proximal convoluted tubules, accounts for over 90% of kidney cancers, and RCC is mainly categorized as clear cell renal cell carcinoma (ccRCC), papillary RCC, and chromophobe RCC (2, 3). As the most aggressive histological subtype, ccRCC is characterized by high metastasis and relapse rate, occupying estimated 80 to 90% of RCC cases (4, 5). Despite tremendous advances in the therapeutic regimens for ccRCC, the prognosis of metastatic ccRCC patients remains unsatisfactory and their 5-year survival rate is largely to be improved (6). Thus, clarifying the latent mechanisms governing ccRCC and discerning potential targets against ccRCC are of great significance in the improvement of ccRCC treatment.
Long noncoding RNAs (lncRNAs), a group of noncoding transcripts that are >200 nucleotides in length, possess limited or no protein-coding capability (7). Mounting evidence has elucidated the vital roles of lncRNAs in regulating the progress and evolution of multiple diseases, including cancers (8, 9). Many studies have shown that lncRNAs are engaged in malignancy progression through the mediation of diverse biological processes, such as proliferation (10), apoptosis (11), metastasis (12), autophagy (13), and differentiation (14). Abnormal lncRNAs have been illustrated in wide ranges of malignant tumors, including ccRCC (15, 16). Analysis of the TCGA database has shown that LINC01094 is upregulated in ccRCC, but the functional role of LINC01094 in the tumorigenesis of ccRCC remains unknown.
A microRNA (miRNA) is a kind of ncRNA molecule (18 to 25 nucleotides). miRNAs work as crucial regulators of genes by pairing with the 3′ untranslated region (3′-UTR) in target mRNAs to degrade mRNA or repress protein translation (17, 18). Accumulating evidence has shown that miRNAs play critical roles in the development of numerous cancers, including ccRCC (19–21). The tumor suppressor function of miRNA 224-5p (miR-224-5p) has been justified by recent research. For example, miR-224-5p inhibits cell proliferation and migration, as well as the invasion of uveal melanoma, via PIK3R3/AKT3 (22). LncRNA FTH1P3 accelerates oral squamous cell carcinoma deterioration through exhausting miR-224-5p to regulate fizzled 5 (23). Nevertheless, the exact role of miR-224-5p in ccRCC needs to be explored.
Chondroitin synthase 1 (CHSY1), located on chromosome 15q26.3, is one of glycosyltransferases responsible for the biosynthesis of glycosaminoglycan (24). Recent investigations have shown that CHSY1 exhibits carcinogenic activities in several cancers. For instance, CHSY1 activates the hedgehog signaling pathway to facilitate aggressive phenotypes of hepatocellular carcinoma cells (25). CHSY1 promotes proliferation and apoptosis resistance in colorectal cancer by targeting the NF-κB and/or the caspase-3/7 signaling pathway (26). However, explorations on the potential of CHSY1 in ccRCC are scanty.
In this study, our purpose was to unveil the actual function and regulatory mechanism of LINC01094 in the progression of ccRCC. Our results validated that LINC01094 acted as an oncogene in ccRCC via regulation of miR-224-5p/CHSY1.
RESULTS
Silencing of LINC01094 retarded cell growth and metastasis of ccRCC.
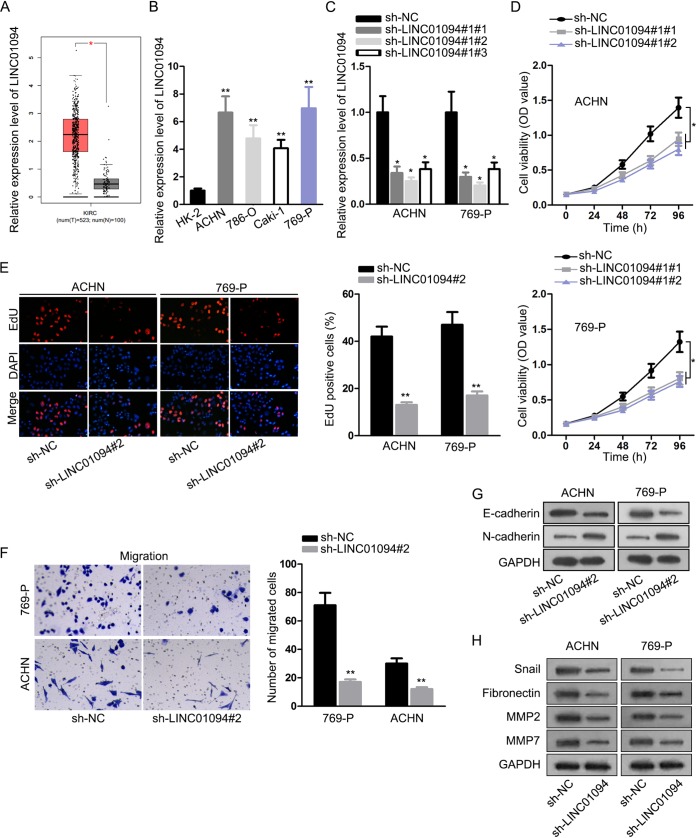
First, with the assistance of TCGA database, it was exposed that LINC01094 level was elevated in ccRCC tissues in comparison to nontumor samples (Fig. 1A). To validate the level of LINC01094 in ccRCC, we detected its expression pattern in cancerous cells. In contrast to normal renal tubular epithelial cells, upregulation of LINC01094 in ccRCC cells was demonstrated (Fig. 1B). Since ACHN and 769-P cells exhibited higher expression of LINC01094, we knocked down LINC01094 in these two cell lines by transfecting with sh-LINC01094#1/#2/#3 (Fig. 1C). This was followed by several functional experiments, and a CCK-8 assay illustrated that suppression of LINC01094 inhibited the viability of ACHN and 769-P cells (Fig. 1D). An EdU (5-ethynyl-2′-deoxyuridine) incorporation assay ascertained that depletion of LINC01094 led to the decreased percentage of EdU-positive cells, further suggesting the suppressed role of LINC01094 depletion in cell proliferation (Fig. 1E). Moreover, the results of a transwell assay showed that cell migratory capacity was restrained when LINC01094 was silenced (Fig. 1F). Consistently, knockdown of LINC01094 increased the E-cadherin level and decreased the N-cadherin level (Fig. 1G); in addition, LINC01094 insufficiency decreased the protein levels of Snail, fibronectin, MMP2, and MMP7 (Fig. 1H). To sum up, we concluded that LINC01094 downregulation hampered ccRCC progression by hindering cell proliferation, migration, and EMT process.
FIG 1.
Silencing of LINC01094 retarded cell growth and metastasis of ccRCC. (A) TCGA analysis of LINC01094 expression in ccRCC tissues and nontumor samples. (B) Levels of LINC01094 in kidney tubular epithelial HK-2 and ccRCC cells (ACHN, 786-O, Caki-1, and 769-P) determined by an RT-qPCR assay (**, P < 0.01 [one-way ANOVA]). (C) The efficiency of LINC01094 knockdown was verified by utilizing RT-qPCR (*, P < 0.05 [one-way ANOVA]). (D and E) CCK-8 (*, P < 0.05; two-way ANOVA) and EdU (**, P < 0.01 [Student t test]) experiments for examining cell proliferation after LINC01094 was silenced. (F) A transwell assay was used to assess cell migration after transfection (**, P < 0.01 [Student t test]). (G and H) The protein level of EMT markers was determined by Western blotting.
FOXM1 enhanced LINC01094 expression in ccRCC at the transcriptional level.
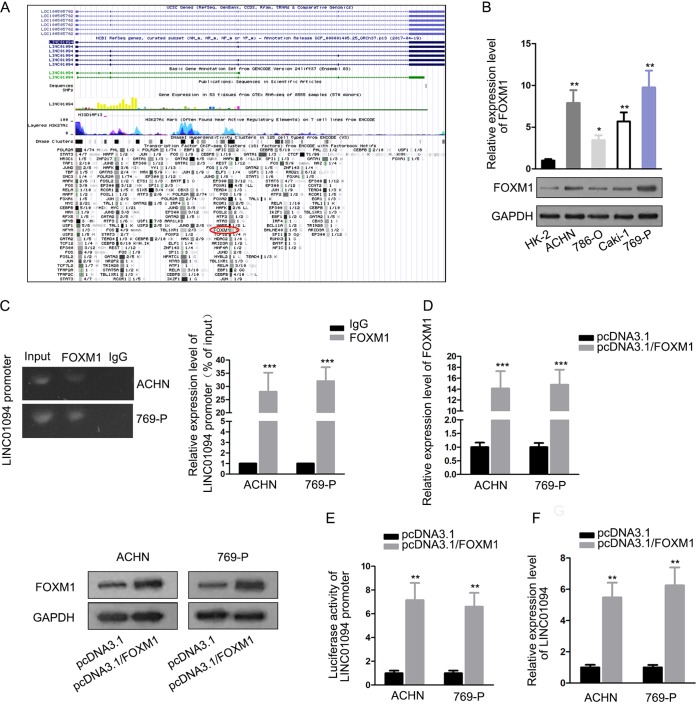
Next, by browsing the UCSC website, we found that FOXM1 potentially bound to the LINC01094 promoter region (Fig. 2A). Real-time quantitative PCR (RT-qPCR) and Western blotting showed that FOXM1 was upregulated in ccRCC cells compared to normal cells (Fig. 2B). As a result, we further explored the association between FOXM1 and LINC01094. A chromatin immunoprecipitation (ChIP) experiment illuminated that LINC01094 promoter was abundantly detected in the precipitated complex of anti-FOXM1 by RT-qPCR analysis, which reached approximately 27 to 31% compared to 1% in the IgG group (as a control), implying an interactivity of FOXM1 with the LINC01094 promoter in both ACHN and 769-P cells (Fig. 2C). After the efficiency of FOXM1 overexpression was confirmed, we found that the luciferase activity of LINC01094 promoter was fortified due to the upregulation of FOXM1 (Fig. 2D and E). In agreement with these findings, we found that forced expression of FOXM1 increased the expression of LINC01094 (Fig. 2F). In short, these results revealed the binding relationship between FOXM1 and the LINC01094 promoter and the activation-transcription role of FOXM1in LINC01094.
FIG 2.
FOXM1 enhanced LINC01094 transcriptional level through binding to its promoter. (A) Prediction of UCSC website that FOXM1 bound with LINC01094 promoter. (B) RT-qPCR and Western blotting detected FOXM1 expression in control cells and ccRCC cells (*, P < 0.05; **, P < 0.01 [one-way ANOVA]). (C) A ChIP experiment suggested that RT-qPCR detected obvious enrichment of LINC01094 promoter in the anti-FOXM1 (***, P < 0.001 [Student t test]) group. (D) The effectiveness of transfection for FOXM1 was estimated by RT-qPCR and Western blotting (***, P < 0.001 [Student t test]). (E and F) A luciferase reporter assay and RT-qPCR analysis demonstrated that FOXM1 contributed to LINC01094 expression at transcriptional level (**, P < 0.01 [Student t test]).
LINC01094 absorbed miR-224-5p.
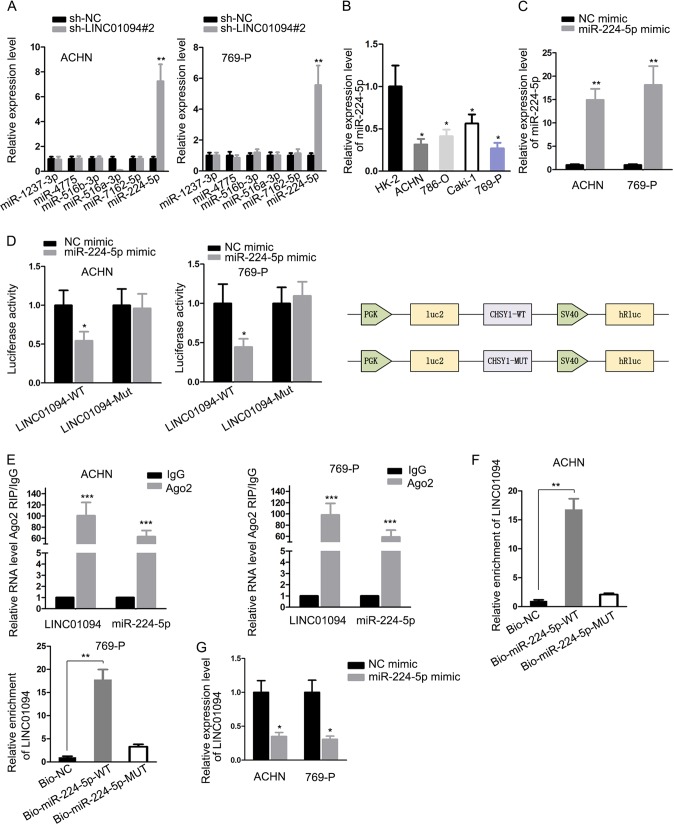
In order to unveil the regulatory mechanism of LINC01094 in ccRCC, we utilized bioinformatics tool DIANA database to find out the putative miRNAs that could bind with LINC01094. Among the top six miRNAs with the highest predicted binding ability, only the expression of miR-224-5p was increased, owing to silencing of LINC01094, and no significant alterations occurred in the levels of other miRNAs (Fig. 3A). Previous investigations justified the tumor repressor role of miR-224-5p in several malignancies (23). In addition, miR-224-5p level was weakly expressed in ccRCC cells compared to control cells (Fig. 3B). Thus, we chose miR-224-5p as the object of subsequent researches. An RT-qPCR assay showed that miR-224-5p was overexpressed in ACHN and 769-P cells using a miR-224-5p mimic (Fig. 3C). Then, a luciferase reporter experiment showed that ectopic miR-224-5p only impaired the luciferase activity of LINC01094-WT, which unraveled the interaction of miR-224-5p to LINC01094. Meanwhile, the schematic exhibited the main components of luciferase reporter assay (Fig. 3D). Concordantly, an RNA immunoprecipitation (RIP) experiment validated that miR-224-5p and LINC01094 were enriched by Ago2 antibody in contrast to IgG antibody (Fig. 3E). Moreover, an RNA pulldown assay demonstrated great enrichment of LINC01094 in a bio-miR-224-5p-WT group (Fig. 3F). In addition, we observed that the expression of LINC01094 was remarkably lessened due to miR-224-5p upregulation (Fig. 3G). These results provide strong evidence that miR-224-5p directly interacted with LINC01094 in ccRCC.
FIG 3.
LINC01094 absorbed miR-224-5p. (A) The levels of different miRNAs in sh-NC or sh-LINC01094#2-transfected cells were certified by RT-qPCR (**, P < 0.01 [Student t test]). (B) The levels of miR-224-5p in normal cells and ccRCC cells were tested by using RT-qPCR (*, P < 0.05 [one-way ANOVA]). (C) After transfection by miR-224-5p mimic or NC mimic in ACHN and 786-P cells, RT-qPCR analysis detected miR-224-5p expression (**, P < 0.01 [Student t test]). (D and E) A luciferase reporter assay (with a schematic) and an RIP assay confirmed the interplay of LINC01094 with miR-224-5p (*, P < 0.05; ***, P < 0.001 [Student t test]). (F) AN RNA pulldown assay assessed the enrichment of LINC01094 in the bio-NC, bio-miR-224-5p-WT, and bio-miR-224-5p-MUT groups (**, P < 0.01 [one-way ANOVA]). (G) An RT-qPCR was used to determine the LINC01094 levels in transfected ACHN and 786-P cells (*, P < 0.05 [Student t test]).
CHSY1 was targeted via miR-224-5p.
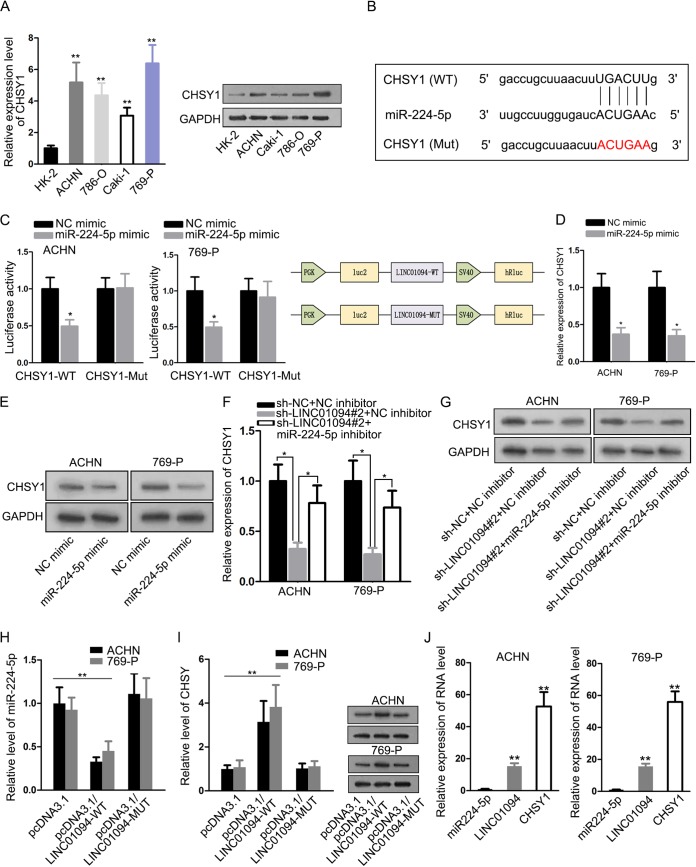
Chondroitin sulfate synthase 1 (CHSY1) was proven to work as an oncogene in colorectal cancer (26) and hepatocellular carcinoma (25), and our findings showed that the CHSY1 level in ccRCC cells was higher than in normal cells (Fig. 4A). By using the starBase database, we discovered that there were putative binding sites between CHSY1 and miR-224-5p (Fig. 4B). Hence, CHSY1 was selected for the in-depth study. A luciferase reporter experiment showed that only the CHSY1-WT luciferase activity was weakened by overexpression of miR-224-5p and that of CHSY1-Mut had no variation under miR-224-5p improvement. In addition, corresponding schematic explained the major constituents of this luciferase reporter assay (Fig. 4C). Likewise, RT-qPCR analysis and Western blotting confirmed that the CHSY1 mRNA and protein levels were diminished via upregulation of miR-224-5p (Fig. 4D and E). Furthermore, suppression of LINC01094 attenuated CHSY1 mRNA and protein expression, and miR-224-5p inhibitor reversed this repressive function of LINC01094 knockdown (Fig. 4F and G). Furthermore, as shown in Fig. 4H and I, overexpressed wild-type LINC01094 could enhance the expression of CHSY through regulating miR-224-5p, whereas mutant LINC01094 showed no significant influence on the level of CHSY. Furthermore, RT-qPCR measured the expression levels of miR-224-5p, LINC01094, and CHSY1 in ACHN and 769-P cells (1:14.45:52.73 and 1:15.62:55.94) (Fig. 4J). Taking the results together, LINC01094 mediated CHSY1 through sponging miR-224-5p in ccRCC.
FIG 4.
CHSY1 was targeted through miR-224-5p. (A) RT-qPCR and Western blot assays were conducted to determine CHSY1 expression in normal cells and ccRCC cells (**, P < 0.01 [one-way ANOVA]). (B) Possible binding sites in the 3′-UTR of CHSY1 for miR-224-5p. (C) A luciferase reporter assay (with a schematic) validated the relationship between miR-224-5p and CHSY1 (*, P < 0.05 [one-way ANOVA]). (D and E) RT-qPCR measurements (*, P < 0.05 [one-way ANOVA]) and Western blot analyses were used to examine CHSY1 mRNA and protein levels in response to addition of miR-224-5p. (F and G) The regulatory effects of LINC01094 on CHSY1 were verified by use of RT-qPCR (*, P < 0.05 [one-way ANOVA]) and Western blotting. (H) RT-qPCR measured the level of miR-224-5p in in differently transfected cells (**, P < 0.01 [one-way ANOVA]). (I) RT-qPCR (**, P < 0.01 [one-way ANOVA]) and Western blot analyses of the levels of CHSY1 in different groups. (J) RT-qPCR detected the miR-224-5p level the expression of LINC01094 and CHSY1 in ACHN and 769-P cells (**, P < 0.01 [one-way ANOVA]).
LINC01094 contributed to the development of ccRCC by sponging miR-224-5p to regulate CHSY1 expression.
Finally, rescue assays were carried out to investigate the role of LINC01094, miR-224-5p, and CHSY1 in ccRCC progression. Overexpression of CHSY1 was certified through RT-qPCR and Western blot assays after transfection in 769-P cells (Fig. 5A). The CCK-8 experiment and EdU incorporation assay demonstrated that the inhibiting influence on cell proliferation caused by LINC01094 depletion was abrogated by miR-224-5p silencing or CHSY1 overexpression (Fig. 5B and C). The transwell assay showed that cell migration hampered by LINC01094 suppression was recovered by miR-224-5p knockdown or CHSY1 augmentation (Fig. 5D). In addition, Western blot showed that miR-224-5p inhibitor or upregulation of CHSY1 abolished the impact of LINC01094 silencing on the protein levels of E-cadherin, N-cadherin, Snail, fibronectin, MMP2, and MMP7 (Fig. 5E and F). These results suggested that LINC01094 executed its carcinogenic function in ccRCC through regulation of miR-224-5p/CHSY1.
FIG 5.
LINC01094 contributed to the development of ccRCC by sponging miR-224-5p to regulate CHSY1 expression. (A) After transfection with empty vector pcDNA3.1 or pcDNA3.1/CHSY1 plasmid, RT-qPCR was used to estimate CHSY1 expression in 769-P cells (**, P < 0.01 [Student t test]). (B and C) The function of LINC01094/miR-224-5p/CHSY1 in proliferation was determined using CCK-8 (*, P < 0.05 [2-way ANOVA]) and EdU (**, P < 0.01 [two-way or one-way ANOVA]) assays. (D) The migratory capability was evaluated with a transwell assay (***, P < 0.001 [one-way ANOVA]). (E and F) Western blotting was used to measure EMT-relevant proteins. n.s., not significant.
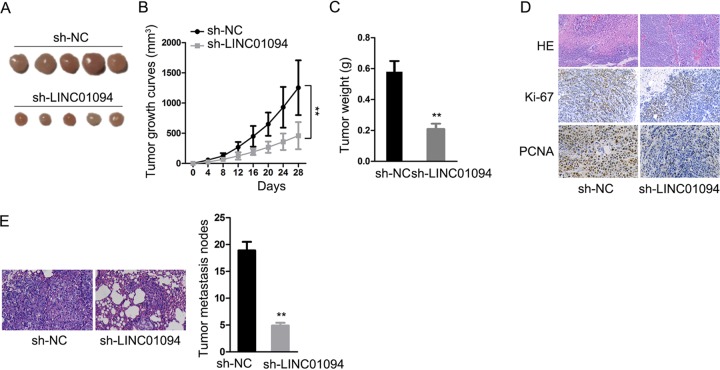
LINC01094 promoted in vivo tumor growth and metastasis.
To further research the effects of LINC01094 on ccRCC progression, we constructed an animal model to show that knockdown of LINC01094 obstructed tumor growth and metastasis in vivo (Fig. 6). These data also demonstrated that LINC01094 promoted ccRCC tumor growth and metastasis both in vitro and in vivo.
FIG 6.
(A) Four weeks after injection, tumors were dissected from nude mice and photos were taken. (B) The growth of tumors in differently transfected cells is displayed in a line chart (**, P < 0.01 [two-way ANOVA]). (C) Tumor weights were measured (**, P < 0.01 [Student t test]). (D and E) Immunohistochemical staining shows the extent of tumor metastasis under different conditions (**, P < 0.01 [Student t test]).
DISCUSSION
Renal cell carcinoma (RCC), the primary cause of cancer death worldwide, represents one of the most universal urological malignant neoplasms (27, 28). Among the prevailing RCC histological subtypes, clear cell renal cell carcinoma (ccRCC) is the most aggressive subtype and accounts for almost 80% of RCC diagnoses (29). Although surgical excision is the curative treatment for the majority of ccRCC patients, approximately 30% of new ccRCC cases present an extremely low survival rate due to local or distant metastasis (30, 31). Accordingly, it is imperative to gain a deeper understanding of the molecular mechanism underlying ccRCC and to investigate novel therapeutic strategies for the treatment of ccRCC.
In recent years, the roles of lncRNAs in various malignant tumors have drawn widespread attention. lncRNAs participate in the initiation and development of human tumors through RNA decoy, epigenetic modification, alternative splicing, and transcriptional or posttranscriptional regulation (32–34). Numerous studies have indicated that lncRNAs regulate cell processes to function as tumor boosters or repressors in a wide range of malignancies, including ccRCC (35–37). In the present study, data from the TCGA database suggested that LINC01094 was strikingly upregulated in ccRCC specimens compared to noncancer tissues. To probe the specific role of LINC01094 in ccRCC, loss-of-function assays were conducted, and our findings affirmed that depletion of LINC01094 inhibited cell proliferation, migration, metastasis, and EMT of ccRCC.
Forkhead Box M1 (FOXM1), a member of the FOX protein family, is immensely important in cell cycle progression, mitosis, and tissue homeostasis (38, 39). Recent research has shown that transcription factor FOXM1 executes oncogenic activities in a variety of cancers. For example, STAT1-inhibited FOXM1 strengthens the gemcitabine sensitivity of pancreatic cancer cells (40). FOXM1 regulates KIF4A expression to promote hepatocellular carcinoma progression (41). High expression of FOXM1 is a predictor for poor clinical outcomes of prostate cancer (42). Nevertheless, the association between LINC01094 and FOXM1 in ccRCC has not been explored yet. Our study revealed that FOXM1 was present at significantly high levels in ccRCC cells compared to normal cells and that FOXM1 activated LINC01094 expression at the transcriptional level.
A growing body of research has shown that lncRNAs may interact with miRNAs to play regulatory roles in target mRNAs by serving as competing endogenous RNAs or RNA molecular sponges (43, 44). In the present study, we found that LINC01094 sponged miR-224-5p and that CHSY1 was downstream of miR-224-5p. Mechanically, LINC01094 modulated CHSY1 by competitively binding to miR-224-5p. Furthermore, our results showed that LINC01094 accelerated ccRCC deterioration by targeting the miR-224-5p/CHSY1 pathway.
Here, we first investigated the exact function and latent mechanism of LINC01094 in ccRCC progression. We show that FOXM1-induced LINC01094 exhibited its oncogenic function in ccRCC through sponging miR-224-5p to mediate CHSY1 expression, which we used to explore the role of LINC01094 in ccRCC and which might provide a novel and additional therapeutic strategy for ccRCC patients.
MATERIALS AND METHODS
Cell treatment.
Human kidney tubular epithelial cell HK-2 and human ccRCC cells (ACHN, 786-O, Caki-1, and 769-P), acquired from Cell Bank of the Chinese Academy of Sciences (Shanghai, China), were cultivated in Dulbecco modified Eagle medium (Invitrogen, Carlsbad, CA) or RPMI 1640 (Invitrogen) complemented with 10% fetal bovine serum (FBS; Invitrogen), 100 U/ml penicillin, and streptomycin under an incubator (Thermo Scientific, Waltham, MA) in a moist environment with 5% CO2 at 37°C.
Cell transfection.
The short-hairpin RNAs (shRNAs) against LINC01094 (termed sh-LINC01094#1/2/3) were applied to silence LINC01094 with scrambled shRNAs as a negative control (termed sh-NC). The miR-224-5p mimic and the miR-224-5p inhibitor, with corresponding controls (negative control [NC] mimic and NC inhibitor), were obtained from GenePharma (Shanghai, China). For the overexpression of CHSY1 or FOXM1, full-length CHSY1 or FOXM1 was ligated into pcDNA3.1 plasmid (Clontech Laboratories, San Francisco, CA), and empty vector served as the NC. Cells were transfected with indicated plasmids, miRNA mimic or miRNA inhibitor by using Lipofectamine 2000 (Invitrogen) as recommended by the product protocol. The experiments described above for stable overexpression, including the miR-224-5p mimic, pcDNA3.1/FOXM1, were also used for the luciferase reporter assay performed in ACHN and 769-P cells. After 24 h, pools of stably transfected cells were selected with puromycin.
Animal model.
Female BALB/c-nu mice (6 weeks old) were provided by Vital River (China). The cell lines (1 × 106) with sh-LINC01094 plasmids subjected to subcutaneous injection in the right flanks of six mice in each group. We measured the tumor size every week and calculated it by using a “volume = (length × width2)/2” formula. Eventually, mice were sacrificed after 4 weeks for tumor weight detection. The animal assays were carried out with the approval of Chongming Branch, Shanghai Tenth People’s Hospital, Tongji University School of Medicine.
Real-time quantitative PCR.
Harvest of total RNA from transfected cells was conducted with the TRIzol reagent (Thermo Scientific), and reverse transcription into cDNA was performed with a Moloney murine leukemia virus reverse transcriptase kit (Invitrogen) according to the supplier’s instructions. PCR was implemented on a 7900 HT Fast real-time PCR system (Applied Biosystems, Foster City, CA) utilizing SYBR green master mix (Applied Biosystems). The main primers were as follows: LINC01094-F, 5′-TGTAAAACGACGGCCAGT-3′; LINC01094-R, 5′-CAGGAAACAGCTATGACC-3′; FOXM1-F, 5′-TGCAGCTAGGGATGTGAATCTTC-3′; FOXM1-R, 5′-GGAGCCCAGTCCATCAGAACT-3′; miR-224-5p-F, 5′-CTGGTAGGTAAGTCACTA-3′; miR-224-5p-R, 5′-TCAACTGGTGTCGTGGAG-3′; GAPDH-F, 5′-ATTCCATGGCACCGTCAAGGCTGA-3′; GAPDH-R, 5′-TTCTCCATGGTGGTGAAGACGCCA-3′; U6-F, 5′-CTCGCTTCGGCAGCACA-3′; and U6-R, 5′-AACGCTTCACGAATTTGCGT-3′. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and U6 were applied as internal controls, and data analysis was carried out using the 2−ΔΔCT method.
Western blotting.
After the extraction of total protein by employing radioimmunoprecipitation assay buffer containing protease inhibitor, the protein concentrations were determined using a BCA detection kit (Beyotime Institute of Biotechnology, Haimen, China) based on the producer’s protocol. The protein samples were detached by SDS–10% PAGE and subsequently transferred onto polyvinylidene difluoride (PVDF) membranes. After sealing using 5% nonfat milk, the PVDF membranes were incubated overnight with primary antibodies at 4°C, probed by secondary antibodies at indoor temperature for 2 h, and detected with a chemiluminescence detection kit (Donghuan Biotech, Shanghai, China). The primary antibodies (E-cadherin, N-cadherin, CHSY1, FOXM1, Snail, fibronectin, MMP2, MMP7, and GAPDH) were procured from Cell Signaling Technology, Inc. (Danvers, MA).
Cell viability.
Cell viability was evaluated with a CCK-8 detection kit (Dojindo Molecular Technologies, Inc., Kumamoto, Japan) according to the manufacturer’s protocol. A total of 3.0 × 103 transfected cells were inoculated into each pore of 96-well dishes. After incubation for 0, 24, 48, 72, or 96 h, each pore was supplemented with 10 μl of CCK-8 reagent. After an additional incubation for 2 h, the optical density at 450 nm was determined under a microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA).
EdU incorporation assay.
An EdU incorporation assay was conducted to test the proliferative capability with the EdU detection kit (RiboBio, Guangzhou, China) according to the manufacturer’s guidelines. After transfection, ACHN and 769-P cells (5 × 103 cells/well) were placed in 96-well plates and maintained with 50 μM EdU at 37°C for about 2 h. The cells were then immobilized by 4% paraformaldehyde for 30 min, permeated by using 0.5% Triton X-100 for 20 min, treated with Apollo Staining reaction reagent for 30 min, and finally stained by DAPI (4′,6′-diamidino-2-phenylindole) for nearly 10 min. Cells were visualized with an Olympus ZKX53 microscope (Olympus, Tokyo, Japan).
Cell migration.
Transwell assay was applied to estimate cell migration potential with the transwell chambers (Corning, Cambridge, MA) in light of the product manual. ACHN and 769-P cells in 200 μl of serum-free medium were placed in the upper chambers, and in the lower chambers 600 μl of medium with plus 10% FBS was added. After 24 h of incubation at 37°C, the cells on the upper surface were discarded, and migrated cells on the bottom were immobilized in 4% formaldehyde, stained by 0.1% crystal violet, and observed by using an Olympus ZKX53 microscope. Next, the cells were counted in five randomly selected fields, and each assay was repeated three times.
Luciferase reporter assay.
The full-length of LINC01094/FOXM1 containing wild-type or mutant miR-224-5p/LINC01094 promoter binding sites was cloned to pGL3 luciferase vector (Promega, Madison, WI). Similarly, to construct wild-type or mutant CHSY1 plasmids, 3′-UTR sequences with or without miR-224-5p binding sites were ligated into the pGL3 vector. ACHN and 769-P cells were cotreated with corresponding reporter plasmids and miR-224-5p mimic or NC mimic by utilizing Lipofectamine 2000 (Invitrogen). At 48 h posttransfection, the luciferase activity was tested with a dual-luciferase reporter assay system (Promega) based on the vendor’s recommendations.
RIP assay.
The RIP assay was carried out with a EZ-Magna RIP kit (Millipore, Billerica, MA) according to the product specifications. In short, harvested cells were first lysed with complete RIP lysis buffer and then treated with RIP buffer and magnetic beads coated with antibody targeting Ago2 or IgG (Millipore). Thereafter, the magnetic beads were cultured with proteinase K, and the precipitated RNA was eluted and purified for RT-qPCR.
Chromatin immunoprecipitation.
Once they reached 90% confluence, the cells were cross-linked using 1% formaldehyde, and chromatins were sheared into fragments with a Bioruptor sonicator (Diagenode, Denville, NJ). After preclearing with protein A/G beads, the samples were precipitated by using a specific FOXM1 antibody at 4°C overnight. IgG antibody was used as a negative control. After washing, the expression of LINC01094 promoter in immunoprecipitate complexes was determined by RT-qPCR.
Immunohistochemical staining.
Paraffin embedded ccRCC samples was sliced and rehydrated through a xylene and ethanol series, followed by hematoxylin (Gill’s, 1×) staining for 5 min. Slides were washed for 5 min and soaked in acid alcohol (1% HCl in 70% ethanol) two or three times until the sections became pink. The slides were then washed in water again for 3 to 5 min and slowly soaked in ammonia water (1 liter of H2O with 1 ml of NH4OH) five or six times. The slides were washed again in water, which lasted 3 to 5 min. An eosin Y solution was then used to counterstain the slides for 1 min. The samples were dehydrated with an ethanol series and washed with a xylene series. Cover slides were installed using ProLong Gold (Invitrogen, Carlsbad, CA) and held for one night at room temperature.
Statistical analysis.
SPSS 17.0 software (SPSS, Chicago, IL) was used for all statistical analyses. Each experiment was assayed in triplicate, and all results are displayed as means ± the standard deviations (SD). Comparisons between two groups were made using a Student t test, and comparison among three or more groups were estimated using one-way or two-way analysis of variance (ANOVA). A P value of <0.05 denoted a statistically significant difference.
ACKNOWLEDGMENTS
We appreciate the contributions from all of the workers in this study.
This study was supported by the Scientific Research Topic of Shanghai Municipal Health and Family Planning Commission, The Effect of BX649059’s Interaction with microRNA on the Occurrence and Development of Renal Cancer and Its Molecular Mechanism (grant 201640146).
REFERENCES
- 1.Ricketts CJ, Crooks DR, Sourbier C, Schmidt LS, Srinivasan R, Linehan WM. 2016. SnapShot: renal cell carcinoma. Cancer Cell 29:610–610.e611. doi: 10.1016/j.ccell.2016.03.021. [DOI] [PubMed] [Google Scholar]
- 2.Hsieh JJ, Purdue MP, Signoretti S, Swanton C, Albiges L, Schmidinger M, Heng DY, Larkin J, Ficarra V. 2017. Renal cell carcinoma. Nat Rev Dis Primers 3:17009. doi: 10.1038/nrdp.2017.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rini BI, Campbell SC, Escudier B. 2009. Renal cell carcinoma. Lancet 373:1119–1132. doi: 10.1016/S0140-6736(09)60229-4. [DOI] [PubMed] [Google Scholar]
- 4.Lokeshwar SD, Talukder A, Yates TJ, Hennig MJP, Garcia-Roig M, Lahorewala SS, Mullani NN, Klaassen Z, Kava BR, Manoharan M, Soloway MS, Lokeshwar VB. 2018. Molecular characterization of renal cell carcinoma: a potential three-microRNA prognostic signature. Cancer Epidemiol Biomarkers Prev 27:464–472. doi: 10.1158/1055-9965.EPI-17-0700. [DOI] [PubMed] [Google Scholar]
- 5.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. 2011. Global cancer statistics. CA Cancer J Clin 61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 6.Jing ZF, Bi JB, Li ZL, Liu XK, Li J, Zhu YY, Zhang XT, Zhang Z, Li ZH, Kong CZ. 2019. miR-19 promotes the proliferation of clear cell renal cell carcinoma by targeting the FRK-PTEN axis. Onco Targets Ther 12:2713–2727. doi: 10.2147/OTT.S199238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ponting CP, Oliver PL, Reik W. 2009. Evolution and functions of long noncoding RNAs. Cell 136:629–641. doi: 10.1016/j.cell.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Shi X, Sun M, Liu H, Yao Y, Song Y. 2013. Long noncoding RNAs: a new frontier in the study of human diseases. Cancer Lett 339:159–166. doi: 10.1016/j.canlet.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 9.Santosh B, Varshney A, Yadava PK. 2015. Non-coding RNAs: biological functions and applications. Cell Biochem Funct 33:14–22. doi: 10.1002/cbf.3079. [DOI] [PubMed] [Google Scholar]
- 10.Zhi Y, Abudoureyimu M, Zhou H, Wang T, Feng B, Wang R, Chu X. 2019. FOXM1-mediated LINC-ROR regulates the proliferation and sensitivity to Sorafenib in hepatocellular carcinoma. Mol Ther Nucleic Acids 16:576–588. doi: 10.1016/j.omtn.2019.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan YF, Yu ZP, Cui XY. 2019. lncRNA colorectal neoplasia differentially expressed (CRNDE) promotes proliferation and inhibits apoptosis in non-small cell lung cancer cells by regulating the miR-641/CDK6 axis. Med Sci Monit 25:2745–2755. doi: 10.12659/MSM.913420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu J, Dong G, Shi H, Zhang J, Ning Z, Bao X, Liu C, Hu J, Liu M, Xiong B. 2019. lncRNA MIR503HG inhibits cell migration and invasion via miR-103/OLFM4 axis in triple negative breast cancer. J Cell Mol Med doi: 10.1111/jcmm.14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu J, Li W, Ning J, Yu W, Rao T, Cheng F. 2019. Long noncoding RNA UCA1 targets miR-582-5p and contributes to the progression and drug resistance of bladder cancer cells through ATG7-mediated autophagy inhibition. Onco Targets Ther 12:495–508. doi: 10.2147/OTT.S183940. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Hu G, Tang Q, Sharma S, Yu F, Escobar TM, Muljo SA, Zhu J, Zhao K. 2013. Expression and regulation of intergenic long noncoding RNAs during T cell development and differentiation. Nat Immunol 14:1190. doi: 10.1038/ni.2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong D, Mu Z, Wei N, Sun M, Wang W, Xin N, Shao Y, Zhao C. 2019. Long non-coding RNA ZFAS1 promotes proliferation and metastasis of clear cell renal cell carcinoma via targeting miR-10a/SKA1 pathway. Biomed Pharmacother 111:917–925. doi: 10.1016/j.biopha.2018.12.143. [DOI] [PubMed] [Google Scholar]
- 16.Zheng XL, Zhang YY, Lv WG. 2019. Long noncoding RNA ITGB1 promotes migration and invasion of clear cell renal cell carcinoma by downregulating Mcl-1. Eur Rev Med Pharmacol Sci 23:1996–2002. doi: 10.26355/eurrev_201903_17238. [DOI] [PubMed] [Google Scholar]
- 17.Ambros V. 2004. The functions of animal microRNAs. Nature 431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 18.Bartel DP. 2004. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 19.Zhu J, Xu C, Ruan L, Wu J, Li Y, Zhang X. 2019. MicroRNA-146b overexpression promotes human bladder cancer invasion via enhancing ETS2-mediated mmp2 mRNA transcription. Mol Ther Nucleic Acids 16:531–542. doi: 10.1016/j.omtn.2019.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zuo Z, Ye F, Liu Z, Huang J, Gong Y. 2019. MicroRNA-153 inhibits cell proliferation, migration, invasion and epithelial-mesenchymal transition in breast cancer via direct targeting of RUNX2. Exp Ther Med 17:4693–4702. doi: 10.3892/etm.2019.7470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuan J, Dong R, Liu F, Zhan L, Liu Y, Wei J, Wang N. 2019. The miR-183/182/96 cluster functions as a potential carcinogenic factor and prognostic factor in kidney renal clear cell carcinoma. Exp Ther Med 17:2457–2464. doi: 10.3892/etm.2019.7221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J, Liu X, Li C, Wang W. 2019. miR-224-5p inhibits proliferation, migration, and invasion by targeting PIK3R3/AKT3 in uveal melanoma. J Cell Biochem doi: 10.1002/jcb.28507. [DOI] [PubMed] [Google Scholar]
- 23.Zhang CZ. 2017. Long non-coding RNA FTH1P3 facilitates oral squamous cell carcinoma progression by acting as a molecular sponge of miR-224-5p to modulate fizzled 5 expression. Gene 607:47–55. doi: 10.1016/j.gene.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 24.Wilson DG, Phamluong K, Lin WY, Barck K, Carano RA, Diehl L, Peterson AS, Martin F, Solloway MJ. 2012. Chondroitin sulfate synthase 1 (Chsy1) is required for bone development and digit patterning. Dev Biol 363:413–425. doi: 10.1016/j.ydbio.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Liu CH, Lan CT, Chou JF, Tseng TJ, Liao WC. 2017. CHSY1 promotes aggressive phenotypes of hepatocellular carcinoma cells via activation of the hedgehog signaling pathway. Cancer Lett 403:280–288. doi: 10.1016/j.canlet.2017.06.023. [DOI] [PubMed] [Google Scholar]
- 26.Zeng L, Qian J, Luo X, Zhou A, Zhang Z, Fang Q. 2018. CHSY1 promoted proliferation and suppressed apoptosis in colorectal cancer through regulation of the NF-κB and/or caspase-3/7 signaling pathway. Oncol Lett 16:6140–6146. doi: 10.3892/ol.2018.9385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mulders PF, Brouwers AH, Hulsbergen-van der Kaa CA, van Lin EN, Osanto S, de Mulder PH. 2008. Guideline “Renal cell carcinoma.” Ned Tijdschr Geneeskd 152:376–380. (In Dutch.) [PubMed] [Google Scholar]
- 28.Ramana J. 2012. RCDB: renal cancer gene database. BMC Res Notes 5:246. doi: 10.1186/1756-0500-5-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheville JC, Lohse CM, Zincke H, Weaver AL, Blute ML. 2003. Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Am J Surg Pathol 27:612–624. doi: 10.1097/00000478-200305000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Bukowski RM. 2010. Metastatic clear cell carcinoma of the kidney: therapeutic role of bevacizumab. Cancer Manag Res 2:83–96. doi: 10.2147/cmar.s7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta K, Miller JD, Li JZ, Russell MW, Charbonneau C. 2008. Epidemiologic and socioeconomic burden of metastatic renal cell carcinoma (mRCC): a literature review. Cancer Treat Rev 34:193–205. doi: 10.1016/j.ctrv.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Lee JT. 2012. Epigenetic regulation by long noncoding RNAs. Science 338:1435–1439. doi: 10.1126/science.1231776. [DOI] [PubMed] [Google Scholar]
- 33.Zhou M, Wang X, Shi H, Cheng L, Wang Z, Zhao H, Yang L, Sun J. 2016. Characterization of long non-coding RNA-associated ceRNA network to reveal potential prognostic lncRNA biomarkers in human ovarian cancer. Oncotarget 7:12598–12611. doi: 10.18632/oncotarget.7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choy M, Guo Y, Li H, Wei G, Ye R, Liang W, Xiao H, Li Y, Guan H. 2019. Long noncoding RNA LOC100129940-N is upregulated in papillary thyroid cancer and promotes the invasion and progression. Int J Endocrinol 2019:7043509. doi: 10.1155/2019/7043509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ding H, Liu J, Zou R, Cheng P, Su Y. 2019. Long non-coding RNA TPTEP1 inhibits hepatocellular carcinoma progression by suppressing STAT3 phosphorylation. J Exp Clin Cancer Res 38:189. doi: 10.1186/s13046-019-1193-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Jia X, Shi L, Wang X, Luo L, Ling L, Yin J, Song Y, Zhang Z, Qiu N, Liu H, Deng M, He Z, Li H, Zheng G. 2019. KLF5 regulated lncRNA RP1 promotes the growth and metastasis of breast cancer via repressing p27kip1 translation. Cell Death Dis 10:373. doi: 10.1038/s41419-019-1566-5. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Wang G, Zhang ZJ, Jian WG, Liu PH, Xue W, Wang TD, Meng YY, Yuan C, Li HM, Yu YP, Liu ZX, Wu Q, Zhang DM, Zhang C. 2019. Novel long noncoding RNA OTUD6B-AS1 indicates poor prognosis and inhibits clear cell renal cell carcinoma proliferation via the Wnt/β-catenin signaling pathway. Mol Cancer 18:15. doi: 10.1186/s12943-019-0942-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luo YD, Ding X, Du HM, Wu YN, Li HQ, Wu HM, Zhang XM. 2019. FOXM1 is a novel predictor of recurrence in patients with oral squamous cell carcinoma associated with an increase in epithelial‑mesenchymal transition. Mol Med Rep 19:4101–4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lam EWF, Brosens JJ, Gomes AR, Koo C-Y. 2013. Forkhead box proteins: tuning forks for transcriptional harmony. Nat Rev Cancer 13:482. doi: 10.1038/nrc3539. [DOI] [PubMed] [Google Scholar]
- 40.Liu C, Shi J, Li Q, Li Z, Lou C, Zhao Q, Zhu Y, Zhan F, Lian J, Wang B, Guan X, Fang L, Li Z, Wang Y, Zhou B, Yao Y, Zhang Y. 2019. STAT1-mediated inhibition of FOXM1 enhances gemcitabine sensitivity in pancreatic cancer. Clin Sci (Lond) 133:645–663. doi: 10.1042/CS20180816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu G, Yan Z, Zhang C, Cheng M, Yan Y, Wang Y, Deng L, Lu Q, Luo S. 2019. FOXM1 promotes hepatocellular carcinoma progression by regulating KIF4A expression. J Exp Clin Cancer Res 38:188. doi: 10.1186/s13046-019-1202-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim MY, Jung AR, Kim GE, Yang J, Ha US, Hong SH, Choi YJ, Moon MH, Kim SW, Lee JY, Park YH. 2019. High FOXM1 expression is a prognostic marker for poor clinical outcomes in prostate cancer. J Cancer 10:749–756. doi: 10.7150/jca.28099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peng WX, Koirala P, Mo YY. 2017. lncRNA-mediated regulation of cell signaling in cancer. Oncogene 36:5661. doi: 10.1038/onc.2017.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilusz JE, Sunwoo H, Spector DL. 2009. Long noncoding RNAs: functional surprises from the RNA world. Genes Dev 23:1494–1504. doi: 10.1101/gad.1800909. [DOI] [PMC free article] [PubMed] [Google Scholar]