Abstract
Background
The use of drugs with anticholinergic properties (DAPs) is common among older adults despite their known adverse effects, such as cognitive decline. Professionals should pay attention to DAPs, since evidence on their adverse effects has been accumulating during the last decade. However, to our knowledge previous studies exploring temporal trends in the use of DAPs are scarce.
Objective
The aim of this study was to assess temporal trends in the use of DAPs from 2003 to 2017 in long-term care facilities in Helsinki.
Methods
Four cross-sectional studies were conducted in 2003, 2007, 2011, and 2017. Participants included older people (≥ 65 years) living in nursing homes (NHs) in 2003 (n = 1979), 2011 (n = 1568), and 2017 (n = 750), and in assisted living facilities (ALFs) in 2007 (n = 1336), 2011 (n = 1556), and 2017 (n = 1673) in Helsinki, Finland. Data on demographics, medication use, and diagnoses were collected by structured questionnaires. The assessments were conducted as a point prevalence over 1 day. The use of DAPs and the total anticholinergic burden were defined by the Anticholinergic Risk Scale (ARS).
Results
In ALFs, there has been an increasing trend in the use of DAPs over a 10-year period (41.2% in 2007 and 53.7% in 2017). In NHs, by contrast, the use of DAPs remained quite stable (52.3% in 2003 and 52.4% in 2017). The burden of DAPs measured by ARS score decreased in NHs and remained stable in ALFs. Marked changes occurred in the DAPs used; antidepressants, especially mirtazapine, increased in both settings, whereas the use of hydroxyzine and urinary antispasmodics nearly disappeared. The proportion of users of DAP antipsychotics increased in ALFs. Participants with dementia had a lower anticholinergic burden than those without dementia, in both settings.
Conclusions
Despite increased knowledge of the harms of DAPs, they remain widely used. Physicians seem to be aware of the harms of DAPs among people with dementia, and some other favorable trends in prescribing were also observed. Clinicians should especially consider the indications behind the use of DAP antidepressants and antipsychotics, and carefully weigh their potential benefits and harms.
Key Points
| Despite their well-known adverse effects, drugs with anticholinergic properties (DAPs), especially antidepressants and antipsychotics, remain widely used among older, frail people living in residential care. |
| Long-term care residents with dementia are being prescribed less DAPs compared with those without cognitive decline. |
| As a favorable trend, the use of some of the high-burden DAPs, such as hydroxyzine, has practically disappeared over the years. |
Introduction
According to recent literature, the use of drugs with anticholinergic properties (DAPs) among older people living in long-term care varies between 12 and 77%, depending on various populations and criteria for anticholinergic drugs [1–5]. The use of DAPs is common, despite adverse effects such as dryness of mouth and constipation [6], confusion, and even cognitive decline [7, 8]. There is also evidence that using DAPs worsens psychological well-being [5, 9]. Furthermore, a greater anticholinergic load may lead to worse outcomes than a moderate load [6, 10, 11]. Much effort has been directed at defining potentially inappropriate medications (PIMs) for older people, and various lists and criteria have been created, such as the Beers list [12] or STOPP/START [13]. One of the important properties of PIMs is the anticholinergic effect. There are also various lists defining DAPs [6, 14, 15]; however, no consensus has been reached on which of the developed criteria would be most useful in research or in clinical settings.
Some studies have investigated time trends in the prescribing and use of PIMs in older people [16–21]. However, to our knowledge, there are only a few studies examining temporal trends in prescribing anticholinergic drugs, and their results are controversial. An American register-based study investigated DAP use among older NH residents with Alzheimer’s disease-related dementia from 2007 to 2008 [2]. The use of DAPs was common, although there was a small but significant decrease in DAP use, from 77.1% in 2007 to 76.6% in 2008. Another American study explored trends of prescribing high-risk anticholinergic medications between 2006 and 2015 to older adults during outpatient visits [22]. Of medications, 35 were determined as high-risk anticholinergics based on the Beers criteria and the Anticholinergic Risk Scale (ARS) [6]. The prevalence of high-risk anticholinergic prescriptions remained stable during the study period. A Scottish register-based study [23] examined the trends of anticholinergic drug prescriptions to older people, from 1995 to 2010, according to the modified ARS. A significantly higher proportion of the study population received anticholinergic medication in 2010 (23.7%) than in 1995 (20.7%). Only one of the studies mentioned above focused on a long-term care population, and in that study the study period was only 2 years. Thus, to our knowledge, our study is the first to investigate temporal trends of DAP use in long-term care over a relatively long period and with several assessment points.
The main objective of our study was to investigate 14-year temporal trends in the use and burden of DAPs according to ARS in long-term care facilities in Helsinki. Another aim was to clarify temporal trends in various drug groups of DAPs. Furthermore, we explored how dementia was associated with DAP use.
Methods
Study Design and Population
Participants of this study were all older people permanently living in nursing homes (NHs) in 2003 (n =1987), 2011 (n =1576), and 2017 (n =791), and in assisted living facilities (ALFs) in 2007 (n =1377), 2011 (n =1586), and 2017 (n =1673) in Helsinki. Written informed consent was obtained from every participant or their proxy in case of moderate–severe dementia [Clinical Dementia Rating (CDR) scale 2] [24]. Residents with severe dementia (CDR 2) [24] and not having a close proxy to give informed consent were excluded. The response rate in NHs was 94% in 2003, 81% in 2011, and 68% in 2017, while the response rate in ALFs was 66% in 2007, 64% in 2011, and 62% in 2017.
Residents < 65 years of age, those without medication charts, and those not using any drugs were excluded. After refusals and exclusions, 4297 subjects remained in the NH cohorts (2003, 2011, and 2017) and 4565 in the ALF cohorts (2007, 2011, and 2017).
In Finland, there are two kinds of long-term care facilities—NHs and ALFs. ALFs provide care around the clock, but the environment is more home-like. ALFs also include group homes for dementia patients. Compared with ALFs, residents in NHs have more comorbidities and poorer cognition and functioning. However, differences between the general state of health of residents in the two different settings have recently diminished [25]. The number of registered nurses is higher in NHs than in ALFs. The current trend in Finland favors living at home with home-care assistance. Simultaneously, the number of beds has shifted from NHs towards ALFs, and long-term care beds in total have decreased.
In every participating unit, the data were gathered by trained registered nurses, and each resident was assessed over 1 day using a standardized protocol. Demographic data, diagnoses, and medication lists were retrieved from medical charts. Possible comorbid conditions were assessed using the Charlson Comorbidity Index (CCI) [26], and residents’ nutritional status was assessed using the Mini Nutritional Assessment (MNA) [27]. The severity of dementia was graded using the CDR scale [24] and its memory item. The ability to move was assessed using the MNA item (0 = bed- or wheelchair-bound; 1 = able to get out of bed, but does not go outside; 2 = able to go outside). The anticholinergic potential of medications was determined using the ARS [6], and the anticholinergic burden was determined using the ARS score. The ARS ranks medications according to their anticholinergic potential on a scale ranging from 0 (no or low risk) to 3 (high anticholinergic potential), and the ARS score is created by summing up the points of each individual medication. Only medications used regularly were taken into consideration in counting the ARS score. All DAP medications were coded according to the Anatomical Therapeutic Chemical (ATC) classification system [28] as follows: antipsychotics (N05A), antidepressants (N06A), anti-Parkinson drugs (N04B), urinary antispasmodics (G04BD), muscle relaxants (M03BX), and antihistamines for systemic use (R06A). Hydroxyzine (N05BB01) was studied by itself, and medicines for the gastrointestinal tract (metoclopramide, A03FA01; loperamide, A07DA03; and ranitidine, A02BA02) were studied together as a group.
The study protocol was approved by the Ethics Committee of Helsinki University Hospital.
Statistical Analysis
The descriptive statistics are presented as means with standard deviation (SD) or counts with percentages. The unadjusted hypothesis of linearity was tested using the Cochran–Armitage test or analysis of variance with an appropriate contrast. Adjusted hypothesis of linearity (orthogonal polynomial) was evaluated using generalized linear models (e.g. analysis of covariance and logistic models) with appropriate distribution and link function. Models included age, sex, and CCI as covariates. The bootstrap method was used when the theoretical distribution of the test statistics was unknown or in the case of violation of the assumptions (e.g. non-normality). The normality of variables was evaluated using the Shapiro–Wilk W test. The Stata 15.1 statistical package (StataCorp LP, College Station, TX, USA) was used for the analysis.
Results
In both NHs (Table 1) and ALFs (Table 2), the proportion of female residents has decreased over the years. The mean age of residents was almost 85 years and remained stable over the study period in both settings. In recent cohorts, the diagnosis of dementia became more prevalent in both settings and the residents were more dependent on another person’s assistance in mobility.
Table 1.
Characteristics of residents in nursing homes in 2003, 2011, and 2017
| 2003 (n =1979) | 2011 (n =1568) | 2017 (n =750) | p for trend | p for trend* | |
|---|---|---|---|---|---|
| Female | 1597 (80.7) | 1209 (77.1) | 580 (77.3) | 0.013 | |
| Age, years [mean (SD)] | 84 (8) | 85 (8) | 84 (8) | 0.34 | |
| Education < 8 years | 1056 (53.4) | 634 (40.4) | 271 (36.1) | < 0.001 | |
| Prior stroke | 539 (27.2) | 450 (28.7) | 215 (28.7) | 0.57 | |
| Dementia | 1374 (69.4) | 1188 (76.6) | 581 (77.5) | < 0.001 | |
| Cancer | 204 (10.3) | 127 (8.1) | 74 (9.9) | 0.048 | |
| Bed- or wheelchair-bound | 598 (30.2) | 947 (60.4) | 427 (56.9) | < 0.001 | |
| MNA [27] | 0.37 | ||||
| Malnourished, < 17 points | 560 (28.3) | 494 (31.5) | 139 (18.5) | ||
| At risk for malnutrition, 17–23 points | 1196 (60.4) | 969 (61.8) | 432 (57.6) | ||
| Well-nourished, ≥ 23.5 points | 223 (11.3) | 103 (6.6) | 77 (10.3) | ||
| CCI [mean (SD)] [26] | 2.1 (1.2) | 2.4 (1.5) | 2.1 (1.3) | 0.39 | |
| No. of medications used regularly [mean (SD)] | 7.9 (3.5) | 7.3 (3.3) | 8.3 (3.3) | 0.94 | |
| Users of DAPsa | 1036 (52.3) | 720 (45.9) | 393 (52.4) | 0.19 | 0.29 |
| ARS score [mean (SD)] [6] | 1.1 (1.5) | 0.8 (1.2) | 0.8 (1.1) | < 0.001 | < 0.001 |
| Antipsychoticsa | 633 (32.0) | 419 (26.7) | 245 (32.7) | 0.47 | 0.47 |
| Antidepressantsa | 331 (16.7) | 309 (19.7) | 169 (22.5) | < 0.001 | < 0.001 |
| Anti-Parkinson drugsa | 99 (5.0) | 57 (3.6) | 19 (2.5) | 0.002 | 0.003 |
| Urinary antispasmodicsa | 60 (3.0) | 2 (0.1) | 0 (0) | < 0.001 | < 0.001 |
| Skeletal muscle relaxantsa | 22 (1.1) | 43 (2.7) | 15 (2.0) | 0.013 | 0.014 |
| Antihistamines for systemic usea | 7 (0.4) | 20 (1.3) | 2 (0.3) | 0.21 | 0.30 |
| Hydroxyzine | 142 (7.2) | 26 (1.7) | 4 (0.5) | < 0.001 | < 0.001 |
| Gastrointestinal drugsa,b | 44 (2.2) | 13 (0.8) | 7 (0.9) | < 0.001 | 0.003 |
Data are expressed as n (%) unless otherwise specified
SD standard deviation, MNA Mini Nutritional Assessment, CCI Charlson Comorbidity Index, DAPS drugs with anticholinergic properties, ARS Anticholinergic Risk Scale
*p for trend; adjusted for age, sex, and CCI
aDefined by the ARS [6]
bIncluding metoclopramide, loperamide, and ranitidine
Table 2.
Characteristics of residents living in assisted living facilities in 2007, 2011, and 2017
| 2007 (n =1336) | 2011 (n =1556) | 2017 (n =1673) | p for trend | p for trend* | |
|---|---|---|---|---|---|
| Female | 1041 (77.9) | 1217 (78.2) | 1210 (72.3) | < 0.001 | |
| Age, years [mean (SD)] | 83 (7) | 84 (7) | 84 (8) | 0.006 | |
| Education < 8 years | 666 (49.9) | 744 (47.8) | 655 (39.2) | < 0.001 | |
| Prior stroke | 343 (25.7) | 404 (26.0) | 362 (21.6) | 0.002 | |
| Dementia | 798 (59.7) | 1090 (70.1) | 1302 (77.8) | < 0.001 | |
| Cancer | 187 (14.0) | 140 (9.0) | 189 (11.3) | 0.19 | |
| Bed- or wheelchair-bound | 195 (14.6) | 446 (28.7) | 508 (30.4) | < 0.001 | |
| MNA [27] | 0.63 | ||||
| Malnourished, < 17 points | 167 (12.5) | 311 (20.0) | 243 (14.5) | ||
| At risk for malnutrition, 17–23 points | 880 (65.9) | 960 (61.7) | 979 (58.5) | ||
| Well-nourished, ≥ 23.5 points | 289 (21.6) | 277 (17.8) | 318 (19.0) | ||
| CCI [mean (SD)] [26] | 2.1 (1.4) | 2.4 (1.5) | 2.0 (1.3) | 0.001 | |
| No. of medications used regularly [mean (SD)] | 8.3 (3.5) | 8.8 (3.8) | 9.0 (3.7) | < 0.001 | |
| Users of DAPsa | 551 (41.2) | 788 (50.6) | 899 (53.7) | < 0.001 | < 0.001 |
| ARS score [mean (SD)] | 0.8 (1.2) | 0.8 (1.1) | 0.8 (1.0) | 0.23 | 0.081 |
| Antipsychoticsa | 344 (25.7) | 489 (31.4) | 566 (33.8) | < 0.001 | < 0.001 |
| Antidepressantsa | 193 (14.4) | 350 (22.5) | 400 (23.9) | < 0.001 | < 0.001 |
| Anti-Parkinson drugsa | 58 (4.3) | 51 (3.3) | 57 (3.4) | 0.30 | 0.20 |
| Urinary antispasmodicsa | 31 (2.3) | 10 (0.6) | 0 (0) | < 0.001 | < 0.001 |
| Skeletal muscle relaxantsa | 17 (1.3) | 13 (0.8) | 25 (1.5) | 0.31 | 0.21 |
| Antihistamines for systemic usea | 1 (0.1) | 3 (0.2) | 6 (0.4) | 0.091 | 0.10 |
| Hydroxyzine | 30 (2.2) | 26 (1.7) | 6 (0.4) | < 0.001 | < 0.001 |
| Gastrointestinal drugsa,b | 11 (0.8) | 16 (1.0) | 12 (0.7) | 0.56 | 0.69 |
Data are expressed as n (%) unless otherwise specified
SD standard deviation, MNA Mini Nutritional Assessment, CCI Charlson Comorbidity Index, DAPs drugs with anticholinergic properties, ARS Anticholinergic Risk Scale
*p for trend; adjusted for age, sex, and CCI
aDefined by the ARS [6]
bIncluding metoclopramide, loperamide, and ranitidine
In NHs, no significant trend was observed in the mean number of all drugs used or in the proportion of DAP users over the years; however, there was a slightly decreasing trend in the ARS score (p <0.001). In ALFs, the mean number of medications increased over time (p <0.001). In addition, the proportion of residents using DAPs showed an increasing trend (p <0.001). In contrast to NHs, the mean ARS score did not show a decreasing trend in ALFs (p =0.23). We further tested the temporal trends of the use of ARS-defined DAPs with high anticholinergic properties (drugs with an ARS score of 2 or 3). In NHs, a significant decreasing trend was observed from 2003 (mean score 0.59, SD 1.2) to 2011 (mean score 0.33, SD 0.9), and to 2017 (mean score 0.29, SD 0.8) [p < 0.001]. In ALFs, no such trend was observed (data not shown).
The most widely used DAP drug groups defined by the ARS were antipsychotics and antidepressants. In NHs, an increasing trend in the proportion of residents using antidepressants was observed during 2003–2017. In antipsychotics users, no significant differences were seen between different years in NHs. In ALFs, an increasing trend was observed in the users of antipsychotics and antidepressants during a 10-year period. The increased use of antidepressants was mostly explained by the increased use of mirtazapine, which increased from 15.7 to 22.7% in NHs, and from 14.0 to 23.8% in ALFs. The use of tricyclic antidepressants practically disappeared, diminishing from 2.3 to 0.3% in NHs, and from 2.0 to 0.5% in ALFs.
The use of anti-Parkinson drugs and gastrointestinal drugs declined over the years in NHs, whereas use remained stable in ALFs. In both settings, there was a considerable decline in the number of users of urinary antispasmodics (oxybutynin and tolterodine) and hydroxyzine over the years studied. As a result, these medications were virtually phased out in 2017. Adjusting for age, sex, and CCI did not change the results.
To further investigate how dementia affects the use of DAPs, we stratified the DAP users according to diagnosis of dementia. In NHs, the ARS score decreased from 2003 to 2017 (p < 0.001 for years). Residents with dementia had lower ARS scores than those without dementia (p < 0.001 for dementia) and there was an interaction (p =0.003). Among residents in ALFs, the ARS score showed a slightly increasing trend during 2007–2017 for the whole cohort (p < 0.001 for years). Similarly, as in NHs, residents in ALFs with dementia had lower ARS scores (p < 0.001 for dementia) and there was an interaction (p =0.003), thus indicating that the direction of the trend differs significantly between those with and without dementia (Fig. 1).
Fig. 1.
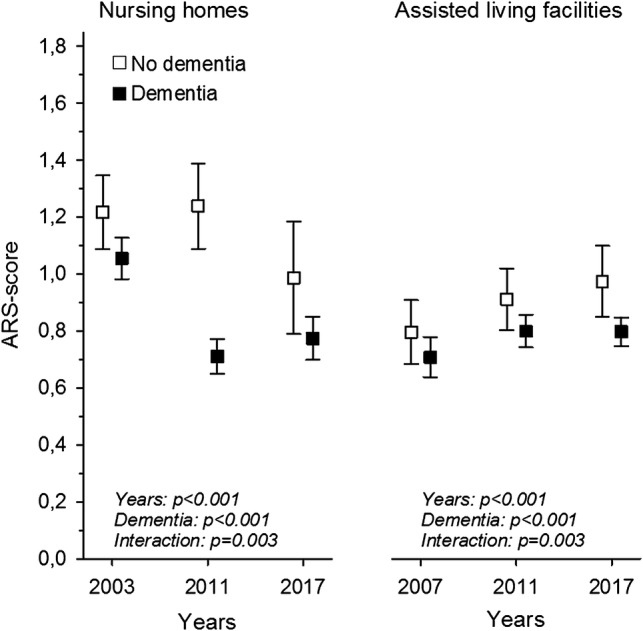
Mean ARS [6] score in NH and ALF residents with and without dementia, from 2003 to 2017, adjusted for age and sex. ARS Anticholinergic Risk Scale, NH nursing home, ALF assisted living facilities
We then stratified users of antipsychotics according to diagnosis of dementia. In NHs, there was no difference between the years (p =0.43) or between those with or without dementia (p =0.42 for dementia); however, there was an interaction (p =0.002). In ALFs, by contrast, the proportion of users of antipsychotics increased over time (p <0.001 for years), the use was more prevalent among those with dementia (p <0.001 for dementia), and there was an interaction (p =0.003), suggesting that there was a faster increase of antipsychotic use among people without dementia than among those with dementia (Fig. 2). Similarly, users of antidepressants were stratified according to diagnosis of dementia. In NHs, among residents with dementia, an increasing trend in users of antidepressants was observed, whereas among those not having a dementia diagnosis, users of antidepressants first increased from 2003 to 2011 and then decreased towards 2017 (p =0.002 for years, p =0.012 for dementia, p =0.005 for interaction). In ALFs, users of antidepressants increased over the years (p <0.001 for years), no significant difference was seen between those with and without dementia, and no interaction was present (p =0.55 for dementia, p =0.95 for interaction) (Fig. 3).
Fig. 2.
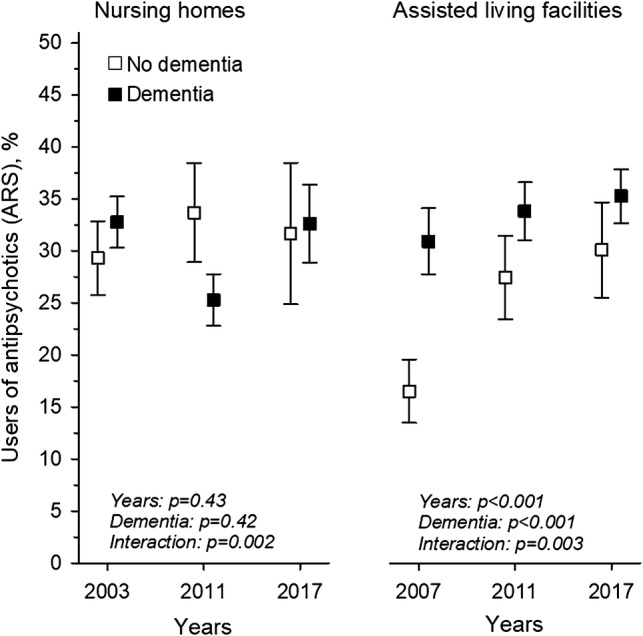
Users (%) of the ARS [6] defined antipsychotics in NH and ALF residents with and without dementia, from 2003 to 2017, adjusted for age and sex. ARS Anticholinergic Risk Scale, NH nursing home, ALF assisted living facilities
Fig. 3.
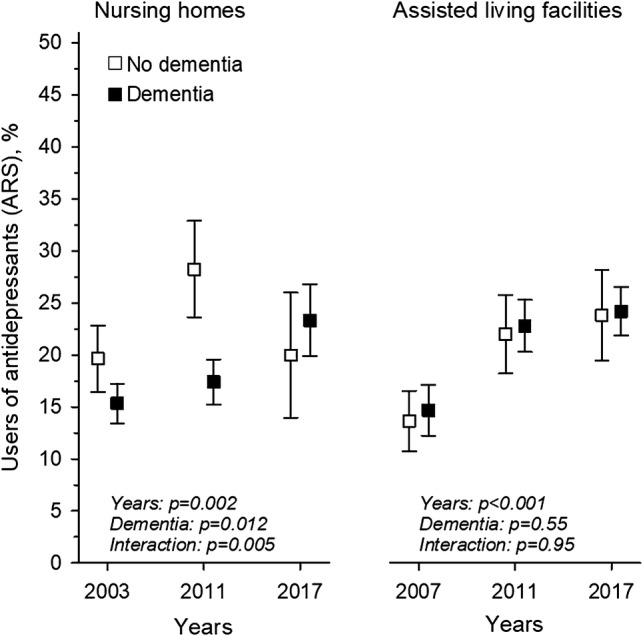
Users (%) of the ARS [6] defined antidepressants in NH and ALF residents with and without dementia, from 2003 to 2017, adjusted for age and sex. ARS Anticholinergic Risk Scale, NH nursing home, ALF assisted living facilities
Discussion
Despite the well-known harms of DAPs, approximately half of the residents in both long-term care settings were regularly administered DAPs. In ALFs, there was even an increasing trend in DAP users. Although the ARS score did not increase in ALFs, and even decreased in NHs, there was a worrisome increasing trend in the users of ARS-defined antidepressants. This was explained by the increased use of mirtazapine, while the use of tricyclics nearly disappeared over the years. In both settings, residents with dementia tended to have a smaller anticholinergic load than those without a diagnosis of dementia, indicating that physicians are likely to avoid prescribing DAPs to residents with cognitive decline. Some other positive changes were also seen, as the use of ARS-defined urinary antispasmodics and hydroxyzine had virtually been phased out during the study period.
The main strength of this study is the large sample size of different cohorts within the time period examined. The study population is representative of the aged population with cognitive decline and deficits in functioning living in long-term care facilities. The data were collected from participants’ medical charts by trained registered nurses using the same protocols, resulting in high internal validity. The ARS [6] is an internationally widely used set of criteria for describing DAPs, and it also allows calculation of the total anticholinergic burden. To our knowledge, this is the first study to explore time trends in DAP use over such a long period in long-term care facilities.
One limitation of the study is that the participants were coded, thus it was not possible to follow particular persons and the real changes in their medications or health status. However, it is known that, on average, residents live less than 2 years in long-term care, and thus the follow-up of a particular person would not be possible in any case in such a study. The response rate decreased over the years and is markedly lower in ALFs than in NHs, possibly resulting in some bias. There was a marked increase in the proportion of patients with dementia in both settings, indicating problems in acquiring informed consent. In addition, organizing the study demanded extra work from the nursing staff, which is the most likely reason for the lower response rates in ALFs than in NHs.
There is a difference in the competence of nursing staff between NHs and ALFs. Whereas in NHs there was a significant decrease in the use of DAPs with high anticholinergic properties, no such trend was observed in ALFs. During the years 2003–2017, there has been a transition in institutionalized care in Helsinki as the amount of beds in NHs have decreased and increased in ALFs. Simultaneously, the aim has been to enable older people to live in their homes longer, and the criteria to enter long-term care facilities have somewhat tightened. As a result, residents entering ALFs currently have more often cognitive decline than in the earlier years. During this transition, the nursing staff in ALFs, already being relatively smaller per resident than in NHs, might not have received enough education regarding non-pharmaceutical approaches in the care of dementia patients. In NHs, the proportion of residents with dementia diagnosis has also increased, although not as sharply as in ALFs. In NHs, the nursing staff are likely to have experience and better know-how in taking care of residents with cognitive problems and associated psychological and behavior problems.
Our findings regarding a stable or increasing trend of users of DAPs is in line with previous studies [22, 23]. Contrary to the Scottish study [23] exploring time trends in the use of DAPs, the ARS score did not increase in our settings, and even slightly decreased in NHs. In the Scottish study, the residents in care homes had a higher anticholinergic burden than home-dwelling older people [23].
The most prevalent DAP groups in our study were antipsychotics and antidepressants, which is consistent with previous studies [2, 4, 22]. Among ARS-defined antidepressants, mirtazapine was mostly responsible for the increase in antidepressant use. In addition to its antidepressant effect, mirtazapine has recently been used as a sedative agent for the treatment of insomnia, although strong evidence is lacking for such off-label use, especially among people with dementia [29, 30]. In fact, in 2017, 86.9% of our total mirtazapine users in NHs and ALFs received a dose of 15 mg or less. According to the ARS, mirtazapine is rated as having moderate anticholinergic potential. Therefore, as the ARS burden does not consider the dose [6], the anticholinergic burden arising from mirtazapine use among our participants may be an overestimation of the true load. Nevertheless, physicians tend to consider mirtazapine a safer alternative to traditional hypnotics, and it has obviously replaced some of these traditional hypnotics. While mirtazapine use has increased, a decreasing trend in anxiolytics and hypnotics was observed in a Finnish study investigating the trends regarding the use of psychotropics [31].
The use of ARS-defined antipsychotics remained high compared with previous studies [4, 18, 32–34]. However, some DAP scales do not consider, for example, antipsychotics, such as haloperidol, perphenazine, or risperidone, having anticholinergic properties [14]. A positive point is that the traditional neuroleptics have been replaced totally by atypical antipsychotics, which have less anticholinergic properties and overall are considered to have less extrapyramidal adverse effects.
Some favorable trends in DAP use in our samples were observed. For example, the use of oxybutynin and tolterodine ceased during the study period. In an American study, the prevalence of urinary antispasmodics was 5.2–6% among the aged community-dwelling population with impaired cognition [35], and, in a Norwegian NH population, antispasmodics were used by 2.4% of the study population [18]. Similarly, the use of hydroxyzine has been discontinued. In addition, the use of gastrointestinal DAPs (metoclopramide, loperamide, and ranitidine) was lower than in earlier studies [36, 37]. However, our study did not include medications administered pro re nata, as did the Norwegian study in the case of metoclopramide [36]. Furthermore, in recent years, the prescribing of histamine H2-receptor antagonists has been replaced mainly by proton pump inhibitors.
Over the study period, the total anticholinergic burden decreased in NHs, but increased slightly in ALFs. The total DAP burden was lower among residents with dementia than among those without dementia. This is in line with a Norwegian study in an NH population [38]. However, there are also controversial results, depending on the study population and the DAP scale used [39]. In an American study in NHs, dementia was negatively associated with low-level anticholinergic burden, but, instead, dementia increased the risk of high-level anticholinergic burden [4].
Our hypothesis was that the use of DAPs would have decreased during the 14-year follow-up due to increased knowledge regarding the harms of these medications. In some drug classes, this was the case, perhaps because there are well-known alternatives with less anticholinergic properties, e.g. urinary antispasmodics such as mirabegron. In other classes, such as antidepressants and antipsychotics, there might be fewer alternative medications for older people.
Conclusions
Despite well-known harms of DAPs, approximately half of the residents in long-term care are administered these medications. A large proportion of old, high-burden DAPs have disappeared over the years, whereas mirtazapine has become increasingly prevalent in institutional settings. In NHs, the anticholinergic burden has decreased, but has remained stable in ALFs. A positive finding is that less DAPs were prescribed to residents with dementia than to those without dementia, suggesting that physicians are aware of the cognitive adverse effects of DAPs.
Acknowledgements
Open access funding provided by University of Helsinki including Helsinki University Central Hospital.
Compliance with Ethical Standards
Funding
This study was supported by the Sohlberg Foundation and the Finnish Medical Foundation (1995).
Conflict of interest
Ulla Aalto, Hanna-Maria Roitto, Harriet Finne-Soveri, Hannu Kautiainen and Kaisu Pitkälä declare they have no conflicts of interest that are directly relevant to the content of this article.
Contributor Information
Ulla L. Aalto, Email: ulla.aalto@fimnet.fi
Kaisu H. Pitkälä, Email: kaisu.pitkala@helsinki.fi
References
- 1.Haasum Y, Fastbom J, Johnell K. Institutionalization as a risk factor for inappropriate drug use in the elderly: a Swedish nationwide register-based study. Ann Pharmacother. 2012;46:339–346. doi: 10.1345/aph.1Q597. [DOI] [PubMed] [Google Scholar]
- 2.Palmer JB, Albrecht JS, Park Y, et al. Use of drugs with anticholinergic properties among nursing home residents with dementia: a national analysis of Medicare beneficiaries from 2007 to 2008. Drugs Aging. 2015;32:79–86. doi: 10.1007/s40266-014-0227-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wawruch M, Macugova A, Kostkova L, et al. The use of medications with anticholinergic properties and risk factors for their use in hospitalised elderly patients. Pharmacoepidemiol Drug Saf. 2012;21:170–176. doi: 10.1002/pds.2169. [DOI] [PubMed] [Google Scholar]
- 4.Niznik J, Zhao X, Jiang T, et al. Anticholinergic prescribing in Medicare part D beneficiaries residing in nursing homes: results from a retrospective cross-sectional analysis of Medicare data. Drugs Aging. 2017;34:925–939. doi: 10.1007/s40266-017-0502-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aalto UL, Roitto HM, Finne-Soveri H, Kautiainen H, Pitkala K. Use of anticholinergic drugs and its relationship with psychological well-being and mortality in long-term care facilities in Helsinki. J Am Med Dir Assoc. 2018;19:511–515. doi: 10.1016/j.jamda.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 6.Rudolph JL, Salow MJ, Angelini MC, McGlinchey RE. The anticholinergic risk scale and anticholinergic adverse effects in older persons. Arch Intern Med. 2008;168:508–513. doi: 10.1001/archinternmed.2007.106. [DOI] [PubMed] [Google Scholar]
- 7.Tune LE. Anticholinergic effects of medication in elderly patients. J Clin Psychiatry. 2001;62(21):11–14. [PubMed] [Google Scholar]
- 8.Fox C, Smith T, Maidment I, et al. Effect of medications with anti-cholinergic properties on cognitive function, delirium, physical function and mortality: a systematic review. Age Ageing. 2014;43:604–615. doi: 10.1093/ageing/afu096. [DOI] [PubMed] [Google Scholar]
- 9.Teramura-Grönblad M, Muurinen S, Soini H, Suominen M, Pitkälä KH. Use of anticholinergic drugs and cholinesterase inhibitors and their association with psychological well-being among frail older adults in residential care facilities. Ann Pharmacother. 2011;45:596–602. doi: 10.1345/aph.1P650. [DOI] [PubMed] [Google Scholar]
- 10.Cardwell K, Hughes CM, Ryan C. The association between anticholinergic medication burden and health related outcomes in the ‘oldest old’: a systematic review of the literature. Drugs Aging. 2015;32:835–848. doi: 10.1007/s40266-015-0310-9. [DOI] [PubMed] [Google Scholar]
- 11.Salahudeen MS, Duffull SB, Nishtala PS. Anticholinergic burden quantified by anticholinergic risk scales and adverse outcomes in older people: a systematic review. BMC Geriatr. 2015;15:31. doi: 10.1186/s12877-015-0029-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. By the American geriatrics society beers criteria update expert panel. J Am Geriatr Soc. 2019;2019(67):674–694. doi: 10.1111/jgs.15767. [DOI] [PubMed] [Google Scholar]
- 13.Gallagher P, Ryan C, Byrne S, Kennedy J, O’Mahony D, STOPP (Screening Tool of Older Person’s Prescriptions) START (Screening Tool to Alert doctors to Right Treatment) Consensus validation. Int J Clin Pharmacol Ther. 2008;46:72–83. doi: 10.5414/CPP46072. [DOI] [PubMed] [Google Scholar]
- 14.Chew ML, Mulsant BH, Pollock BG, et al. Anticholinergic activity of 107 medications commonly used by older adults. J Am Geriatr Soc. 2008;56:1333–1341. doi: 10.1111/j.1532-5415.2008.01737.x. [DOI] [PubMed] [Google Scholar]
- 15.Carnahan RM, Lund BC, Perry PJ, Pollock BG, Culp KR. The anticholinergic drug scale as a measure of drug-related anticholinergic burden: associations with serum anticholinergic activity. J Clin Pharmacol. 2006;46:1481–1486. doi: 10.1177/0091270006292126. [DOI] [PubMed] [Google Scholar]
- 16.Jirón M, Pate V, Hanson LC, Lund JL, Jonsson Funk M, Stürmer T. Trends in prevalence and determinants of potentially inappropriate prescribing in the United States: 2007 to 2012. J Am Geriatr Soc. 2016;64:788–797. doi: 10.1111/jgs.14077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bruin-Huisman L, Abu-Hanna A, van Weert HCPM, Beers E. Potentially inappropriate prescribing to older patients in primary care in the Netherlands: a retrospective longitudinal study. Age Ageing. 2017;46:614–619. doi: 10.1093/ageing/afw243. [DOI] [PubMed] [Google Scholar]
- 18.Halvorsen KH, Selbaek G, Ruths S. Trends in potentially inappropriate medication prescribing to nursing home patients: comparison of three cross-sectional studies. Pharmacoepidemiol Drug Saf. 2017;26:192–200. doi: 10.1002/pds.4142. [DOI] [PubMed] [Google Scholar]
- 19.Hovstadius B, Petersson G, Hellstrom L, Ericson L. Trends in inappropriate drug therapy prescription in the elderly in Sweden from 2006 to 2013: assessment using national indicators. Drugs Aging. 2014;31:379–386. doi: 10.1007/s40266-014-0165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koyama A, Steinman M, Ensrud K, Hillier TA, Yaffe K. Ten-year trajectory of potentially inappropriate medications in very old women: importance of cognitive status. J Am Geriatr Soc. 2013;61:258–263. doi: 10.1111/jgs.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hosia-Randell HM, Muurinen SM, Pitkala KH. Exposure to potentially inappropriate drugs and drug-drug interactions in elderly nursing home residents in Helsinki, Finland: a cross-sectional study. Drugs Aging. 2008;25:683–692. doi: 10.2165/00002512-200825080-00005. [DOI] [PubMed] [Google Scholar]
- 22.Rhee TG, Choi YC, Ouellet GM, Ross JS. National prescribing trends for high-risk anticholinergic medications in older adults. J Am Geriatr Soc. 2018;66:1382–1387. doi: 10.1111/jgs.15357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sumukadas D, McMurdo ME, Mangoni AA, Guthrie B. Temporal trends in anticholinergic medication prescription in older people: repeated cross-sectional analysis of population prescribing data. Age Ageing. 2014;43:515–521. doi: 10.1093/ageing/aft199. [DOI] [PubMed] [Google Scholar]
- 24.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 25.Finne-Soveri H. City preparing for ageing. Case study Helsinki. [Finnish] National Institute for Health and Welfare. Report 31/2012. Helsinki, 2012.
- 26.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 27.Vellas B, Guigoz Y, Garry PJ, et al. The mini nutritional assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition. 1999;15:116–122. doi: 10.1016/S0899-9007(98)00171-3. [DOI] [PubMed] [Google Scholar]
- 28.WHO Collaborating Centre for Drug Statistics Methodology. The Anatomical Therapeutic Chemical Classification index with DDDs, 2019. Oslo, 2018.
- 29.Wiegand MH. Antidepressants for the treatment of insomnia: a suitable approach? Drugs. 2008;68:2411–2417. doi: 10.2165/0003495-200868170-00001. [DOI] [PubMed] [Google Scholar]
- 30.Scoralick FM, Louzada LL, Quintas JL, Naves JO, Camargos EF, Nobrega OT. Mirtazapine does not improve sleep disorders in Alzheimer’s disease: results from a double-blind, placebo-controlled pilot study. Psychogeriatrics. 2017;17:89–96. doi: 10.1111/psyg.12191. [DOI] [PubMed] [Google Scholar]
- 31.Roitto HM, Kautiainen H, Aalto UL, Öhman H, Laurila J, Pitkälä KH. Fourteen-year trends in the use of psychotropic medications, opioids, and other sedatives among institutionalized older people in Helsinki, Finland. J Am Med Dir Assoc. 2019;20:305–311. doi: 10.1016/j.jamda.2018.12.022. [DOI] [PubMed] [Google Scholar]
- 32.Bathena SPR, Leppik IE, Kanner AM, Birnbaum AK. Antiseizure, antidepressant, and antipsychotic medication prescribing in elderly nursing home residents. Epilepsy Behav. 2017;69:116–120. doi: 10.1016/j.yebeh.2017.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phillips LJ, Birtley NM, Petroski GF, Siem C, Rantz M. An observational study of antipsychotic medication use among long-stay nursing home residents without qualifying diagnoses. J Psychiatr Ment Health Nurs. 2018;25:464–474. doi: 10.1111/jpm.12488. [DOI] [PubMed] [Google Scholar]
- 34.Westaway K, Sluggett J, Alderman C, Moffat A, Procter N, Roughead E. The extent of antipsychotic use in Australian residential aged care facilities and interventions shown to be effective in reducing antipsychotic use: a literature review. Dementia (London). 2018 doi: 10.1177/1471301218795792. [DOI] [PubMed] [Google Scholar]
- 35.Green A, Segal J, Tian J, et al. Use of bladder antimuscarinics in older adults with impaired cognition. J Am Ger Soc. 2017;65:390–394. doi: 10.1111/jgs.14498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fog AF, Kvalvaag G, Engedal K, Straand J. Drug-related problems and changes in drug utilization after medication reviews in nursing homes in Oslo, Norway. Scand J Prim Health Care. 2017;35:329–335. doi: 10.1080/02813432.2017.1397246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reppas-Rindlisbacher CE, Fischer HD, Fung K, et al. Anticholinergic drug burden in persons with dementia taking a cholinesterase inhibitor: the effect of multiple physicians. J Am Geriatr Soc. 2016;64:492–500. doi: 10.1111/jgs.14034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kersten H, Molden E, Willumsen T, Engedal K, Bruun Wyller T. Higher anticholinergic drug scale (ADS) scores are associated with peripheral but not cognitive markers of cholinergic blockade. Cross sectional data from 21 Norwegian nursing homes. Br J Clin Pharmacol. 2012;75:842–849. doi: 10.1111/j.1365-2125.2012.04411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mate KE, Kerr KP, Pond D, et al. Impact of multiple low-level anticholinergic medications on anticholinergic load of community-dwelling elderly with and without dementia. Drugs Aging. 2015;32:159–167. doi: 10.1007/s40266-014-0230-0. [DOI] [PubMed] [Google Scholar]


