Abstract
Wnt is a conserved family of secreted proteins that play diverse roles in tissue growth and differentiation. Identification of transcription factors that regulate wnt expression is pivotal for understanding tissue-specific signaling pathways regulated by Wnt. We identified pdm3m7, a new allele of the pdm3 gene encoding a POU family transcription factor, in a lethality-based genetic screen for modifiers of Wingless (Wg) signaling in Drosophila. Interestingly, pdm3m7 larvae showed slow locomotion, implying neuromuscular defects. Analysis of larval neuromuscular junctions (NMJs) revealed decreased bouton number with enlarged bouton in pdm3 mutants. pdm3 NMJs also had fewer branches at axon terminals than wild-type NMJs. Consistent with pdm3m7 being a candidate wg modifier, NMJ phenotypes in pdm3 mutants were similar to those of wg mutants, implying a functional link between these two genes. Indeed, lethality caused by Pdm3 overexpression in motor neurons was completely rescued by knockdown of wg, indicating that Pdm3 acts upstream to Wg. Furthermore, transient expression of Pdm3 induced ectopic expression of wg-LacZ reporter and Wg effector proteins in wing discs. We propose that Pdm3 expressed in presynaptic NMJ neurons regulates wg transcription for growth and development of both presynaptic neurons and postsynaptic muscles.
Subject terms: Morphogen signalling, Developmental neurogenesis
Introduction
Transcription factors play essential roles by inducing genes during the formation of body plans, organ development, tissue specificity, and generation of diverse cell types. Numerous transcription factors are grouped based on similarity in their sequences and domain structures. Pituitary-specific positive transcription factor 1, Octamer transcription factor-1, Uncoordinated-86 domain (POU) transcription factors1 belong to a subfamily of homeodomain transcription factors, and are highly conserved in all metazoans2–5. POU domain consists of two DNA binding domains, POU homeodomain and POU specific domain, and these two domains are linked by a flexible linker5,6. Based on sequence homology of the POU domain and the linker, POU proteins are grouped into six classes2,4,5. POU proteins are often expressed in spatiotemporally restricted patterns during development, implying that they may be specialized for differentiation of specific cells or tissues by activating required signal transduction pathways5.
The class VI Drosophila POU domain motif 3 (Pdm3) protein is reported to function in olfactory receptor neurons (ORNs) by regulating olfactory receptor gene expression and axon targeting, and in ring (R) neurons by regulating the development of ellipsoid body (EB) and axon targeting to EB in the central brain7,8. Pdm3 is also important for the axon targeting of a type of tracheal dendrite (td) neurons9. In particular, td neurons that normally form synapse in the nerve cord change their target to the central brain by ectopic expression of Pdm3. Besides the neuronal functions of Pdm3, Pdm3 also acts as a repressor of abdominal pigmentation in D. melanogaster10, and plays a role in female-limited color dimorphism in abdomen of D. montium11. Despite these studies, it is still unknown how Pdm3 performs these neuronal and non-neuronal functions.
pdm3f00828 and pdm31 homozygotes exhibit defects in axon targeting, odor perception, and locomotion7,8. pdm3f00828 allele has insertion of a piggyback element in an intron near the 3′ end of the pdm3 gene, and pdm31 has a premature stop codon in the middle of the coding region that results in the deletion of the POU domain8. We identified a new pdm3 allele, pdm3m7, as a suppressor of lethality induced by Sol narae (Sona) overexpression in a genetic screen. Sona is a fly ADAMTS (A disintegrin and metalloprotease with thrombospondin motif) whose family members are secreted metalloproteases important for cell proliferation, cell survival and development12–14. We have shown that Sona positively regulates Wingless (Wg) signaling and is essential for fly development, cell survival, and Wg processing12–14. Wg is a prototype of Wnt family that initiates signal transduction cascade as extracellular signaling proteins, and activation of Wnt signaling leads to transcriptional induction of multiple genes for regulation of cell proliferation, cell survival, cell fate decision, and cell migration15–17. Wg is important for the development of all appendages, and the wing imaginal disc has been a great tool to study Wg signaling because Wg secreted from its dorsal-ventral midline is crucial for growth and development of wings18.
Wg also plays an essential role in the development of NMJ. During larval development, NMJs continue to form synaptic boutons that are specialized structures with axon terminals of motor neurons surrounded by reticular subsynaptical reticulum (SSR) formed by the plasma membrane of postsynaptic muscle19. Among multiple types of boutons such as type Ib, Is, II, and III, Wg is secreted at a high level from the glutamatergic type Ib bouton known as the main localization site of Wg protein and Wg signaling components, and is absent or at very low levels in other types of boutons20. Type Ib boutons also have more extensive SSR compared to other bouton types, so are easily detected by the high level of Discs-Large (Dlg) as a postsynaptic marker. Type Ib boutons in NMJs of wg mutants show reduction in bouton number but increase in bouton size20–24. Components in Wg signaling such as Arrow (Arr) that positively regulates Wg signaling as a coreceptor of Wg also shows its mutant phenotype similar to wg, but Shaggy (Sgg)/GSK3β that negatively regulates Wg signaling as a kinase shows opposite phenotype to wg22,25. Thus, dynamic regulation of Wg signaling is essential for the development of NMJ.
Secreted Wg also signals to the presynaptic motor neuron to regulate Futsch, one of the microtubule-associated proteins (MAPs)26. Futsch is a homolog of mammalian MAP1B, and both Futsch and MAP1B are phosphorylated at a conserved site by Sgg/GSK3β27. The phosphorylated MAP1B does not bind microtubules, which results in reduced stability of microtubules3,28. Therefore, localization of Futsch at NMJ faithfully reflects the stability of microtubules that is dynamically regulated by Wg signaling. Loss of futsch phenotype is similar to the loss of wg phenotype in NMJ26.
We report here that pdm3 is identified as a suppressor of Sona-induced lethality. Based on the involvement of Sona in Wg signaling and the neuronal role of Pdm3, we specifically studied the roles of Pdm3 in NMJ. Similar to loss of wg, loss of pdm3 in NMJ caused decrease in number but increase in size of boutons. Lethality induced by overexpressed Pdm3 was completely rescued by the knockdown of wg in motor neurons but not vice versa. This indicated that Pdm3 functions upstream to Wg, and prompted us to test whether Pdm3 can induce wg transcription. Indeed, transient expression of Pdm3 in wing discs induced wg transcription and Wg effector proteins. Based on these data, we propose that one of the main functions of Pdm3 in NMJ is to induce wg transcription.
Results
pdm3 and sona have a positive genetic interaction
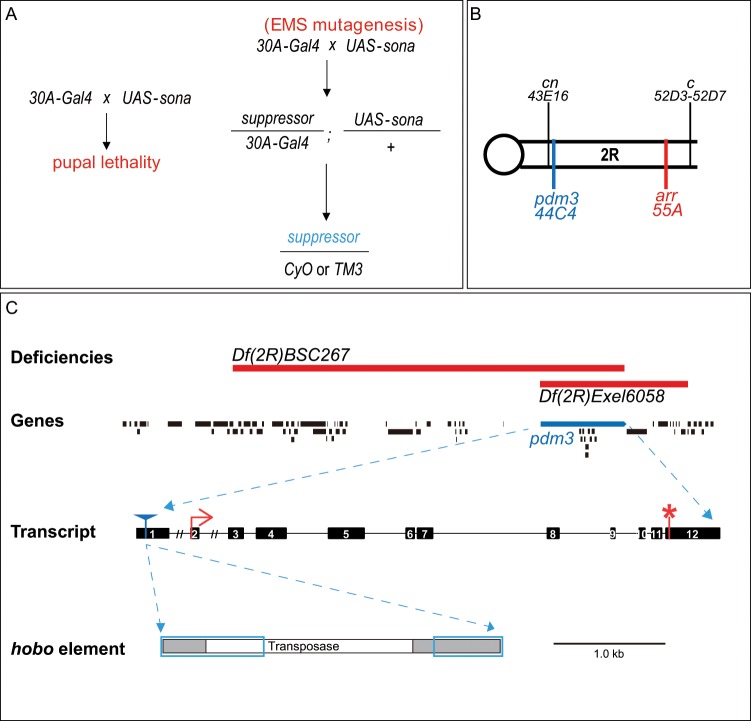
As a first step toward understanding the function of sona, we carried out a lethality-based genetic screen using ethyl methanesulfonate (EMS) as a mutagen based on the late-pupal lethality induced by Sona driven by 30A-Gal4 (Fig. 1A). 89 rare survivors were obtained among 18,000 progenies from the cross between EMS-treated 30A-Gal4 males and untreated UAS-sona females. These survivors were balanced with Sco/CyO and D/TM6 for the establishment of suppressor lines whose mutations are in the second and third chromosomes, respectively. Established lines were retested for the suppression of Sona-induced lethality, and 28 suppressors were maintained for further analysis (Fig. 1A). All suppressors showed lethality, and a few suppressors produced rare homozygous adults.
Figure 1.
A lethality-based genetic screen for sona suppressors and characterization of the m7 suppressor. (A) Scheme of a genetic screen for identifying suppressors that survive against late pupal lethality induced by Sona overexpression. EMS was used as a mutagen, and obtained suppressors from the screen were crossed with second and third chromosome balancers before further testing. (B) The m7 suppressor was mapped by meiotic mapping, deficiency mapping, and complementation test. Multiple morphological markers are present in the second chromosome of a mapping line BDRC 4347, and the two markers, cinnabar (cn) as an eye color mutation and curved (c) as a wing shape mutation were identified as sites closely located to the two independent lethal sites of m7 suppressor. Two lethal sites were separated by recombination with CS and subsequent complementation test with pdm3f00828 and arr2 identified that m7 has two lethal mutations in pdm3 and arr genes. (C) Two deficiency lines used for mapping are shown with deleted regions in red. Transheterozyogotes obtained by crossing the two deficiency lines do not have the pdm3 gene. pdm3m7 has a defective hobo element inserted in an exon that represents the 5′ untranslated region. The blue boxes indicate remaining parts of the inserted hobo element. A red arrow marks the initiation codon and a red asterisk marks the termination codon. The scale bar is for the hobo element only.
To map the position of the lethal site in suppressor m7, meiotic and deficiency mappings as well as complementation analysis were performed. Meiotic mapping was carried out by crossing the m7 suppressor with a mapping line (BDRC #4347) that contains multiple morphological markers. The meiotic mapping revealed that the lethal region in m7 is located in between the cinnabar (cn) and curved (c) (Fig. 1B). Subsequent deficiency mapping identified two different regions that are responsible for lethality, one near cn and the other near c. Complementation analysis then showed that m7 has two independent mutations in pdm3 and arrow (arr) genes on the right arm of the second chromosome (Fig. 1B). Pdm3 is a class VI POU domain transcription factor5, and Arr is a co-receptor of Wg ligand and essential for transduction of canonical Wg signaling29. The m7 suppressor was crossed with Canton-S (CS) and their progeny was checked by complementation test with pdm3f00828 and arr2 mutants in order to find flies with a single mutation, pdm3m7 and arrm7.
Genomic sequencing revealed that pdm3m7 has a defective hobo element in the first exon of the pdm3 gene that is upstream of the initiation codon (Fig. 1C) while arrm7 has a point mutation in the arr gene (in preparation). None of the other suppressors had the hobo element in the pdm3 gene, indicating that insertion of the hobo element is unique to the m7 suppressor, and occurred subsequent to the point mutation in the arr gene. We found that the level of Pdm3 is extremely low in pdm3m7 wing discs, establishing that insertion of the hobo element negatively affects the expression of Pdm3 (Fig. S1). Transheterozygotes of the two available deficiencies, Df(2 R)BSC267 and Df(2 R)Exel6058, were missing only the pdm3 gene in the entire genome, so Df(2 R)BSC267/Df(2 R)Exel6058 flies were used as a deletion mutant of pdm3 in this study (Fig. 1C).
We found that not only pdm3m7 but also pdm3f00828, pdm31 and pdm3 RNAi driven by 30A-Gal4 completely suppressed the Sona-induced pupal lethality (n > 200 each). Thus, pdm3m7 is an authentic sona suppressor, and pdm3 shows a positive genetic interaction with sona.
Boutons of pdm3 NMJs are decreased in number but increased in size, similar to wg NMJs
Further analysis of sona suppressors revealed that sona itself and most suppressors are linked to Wg signaling12,13, which raised an interesting possibility that Pdm3 is also involved in Wg signaling. We noticed that pdm3 mutant larvae are slow in locomotion (Movie 1), implying a potential role of pdm3 in NMJ. To address the relationship between pdm3 and wg in NMJ, we stained pdm31 and wgts/wgCX4 NMJs of the late 3rd instar larvae for a presynaptic marker Horseradish peroxidase (HRP) and a postsynaptic marker Dlg to detect Type Ib boutons at muscles 6 and 7 in the 2nd abdominal (A2) and the 3rd abdominal (A3) segments20.
We found that number of boutons in pdm31 NMJ was reduced by 40% and 16% compared to the wild-type counterparts in A2 and A3 segments, respectively (Fig. 2A,B,F). Bouton numbers in pdm3f00828 NMJ were reduced by 27% in A2 segment and those in pdm3m7 NMJ were only mildly reduced (Fig. S2; data not shown), so we focused our analysis on pdm31 NMJ that shows the most pronounced phenotype. Consistent with the previous report, number of boutons in wgts/wgCX4 NMJ was reduced by 12% and 11% in A2 and A3 segments, respectively (Fig. 2C–E,H).
Figure 2.
Boutons in pdm31 and wgts/wgcx4 mutant NMJs are decreased in number but increased in size. CS and pdm31 were cultured at 25 oC whereas wg mutants were cultured at 18 oC in all figures. Type Ib boutons of NMJs at muscles 6/7 were stained for HRP (green) and Dlg11. Boxed regions in (A–E) are magnified in (A’–E”). (A–E) Boutons of NMJs in the A2 segment of control (A) and pdm31. (B). Boutons of NMJs in the A2 segment of heterozygous controls (C,D) and wgts/wgCX4. (E) Shorter and thicker branches and loosely organized boutons of pdm31 and wgts/wgCX4 are marked with arrowheads (B”,E”). (F–I) Number (F) and size (G) of terminal boutons of NMJs in A2 and A3 of wild-type and pdm31. Number (H) and Size (I) of terminal boutons of NMJs in A2 and A3 of controls and wgts/wgCX4. n = 51 for A2 and 37 for A3 of CS, 37 for A2 and 40 for A3 of pdm31. n = 21 for A2 and 19 for A3 of +/wgCX4, n = 30 for A2 and 25 for A3 of +/wgts, n = 44 for A2 and 48 for A3 of wgts/wgCX4, n = 52 for A2 and 49 for A3 of +/wgCX4, and n = 50 for A2 and A3 of +/wgts and wgts/wgCX4. *Represents p < 0.05; ** represents p < 0.01; *** represents p < 0.0001. Data are presented as mean ± SEM. Scale bars: 10 µm.
We then carried out quantitative analysis on size of boutons in pdm3 and wg NMJs. To measure the size of boutons, serial images of boutons were taken, the images were combined, and then area of the most distal bouton in the combined image was measured (Fig. 2G,I). Size of pdm31 distal boutons was increased by 30% at A2 but was not increased at A3 segments compared to wild-type (Fig. 2G). Size of wgts/wgCX4 boutons was increased by 32% and 11% in A2 and A3 segments, respectively, compared to the heterozygous controls, +/wgCX4 and +/wgts (Fig. 2I). This is in line with a previous report that wgts boutons are noticeably larger20.
Some pdm31 boutons were not clearly separated from neighboring boutons (Fig. 2B”), which is also reported in wg boutons20. We defined the axon branch in which more than 50% of boutons are unseparated as ‘fused’ branch while those in which less than 50% of boutons are fused as ‘normal’ branch. We found that 30.5% of axon branches in A2 segments of pdm31 NMJs are fused (36 out of 118). Only 3% of wild-type axon branches was fused based on this definition (8 out of 282).
One unique phenotype of pdm3 boutons was an abnormally high level of Dlg in 42.9% of NMJs examined (33 out of 77, Fig. S3). Neither wild-type NMJs (0 out of 88) nor wg NMJs (0 out of 92) had high level of Dlg. This suggests that SSR is not properly developed in pdm31 NMJs. We also checked the localized pattern of a glutamate receptor GluRIIA in pdm3 and wg boutons. It has been shown that the GluRIIA pattern in wild-type bouton is cluster-like but that in wg boutons is diffused without any clusters20. Consistent with this report, cluster-like pattern of GluRIIA was found in CS, +/wgCX4, and +/wgts control boutons but wgts/wgCX4 boutons showed diffused pattern (Fig. S4C–E). Unlike wg NMJs, the pattern of GluRIIA in pdm31 NMJs was not noticeably different from control NMJs (Fig. S4A,B). Taken together, the loss of pdm3 or wg phenotype decreased number but increased size of boutons, but pdm3 and wg NMJs were dissimilar in the level of Dlg and the pattern of GluRIIA.
Number of axon branches in pdm31 NMJ is reduced
Wg signaling is required for the formation of new branches from an existing exon, and these new branches can be visualized by Futsch20,22. We found that number of axon branches in pdm31 NMJs was decreased by 25% at the A2 segment and was unchanged at the A3 segment (Fig. 3A”,B”,F). wgts/wgCX4 NMJs showed 40% and 33% reduction in number of axon branches at the A2 and A3 segments, respectively (Fig. 3C”–E”,H). Therefore, Pdm3 is important in A2 and Wg is important for both A2 and A3 for the formation of exon branches.
Figure 3.
Decreased number of axon terminals and unstable microtubules in pdm31 and wgts/wgCX4 NMJs. The boutons of NMJs in muscles 6/7 at the A2 were stained for HRP (green) and Futsch (white). The boxed regions in (A’–E’) are magnified in (A”–E”). The white arrowhead and arrows indicate bundled Futsch-positive terminals, and red arrowheads and arrows indicate unbundled Futsch-positive terminals. Number of Futsch-positive terminals represents the number of branches in NMJs. (A,B) NMJs of the control and pdm31 stained for HRP (A,B) and Futsch (A’,B’). (C–E) NMJs of the +/wgCX4 (C), +/wgts (D) and wgts/wgCX4 (E) stained for HRP. (F,G) Number of Futsch terminals (F) and percentage of unbundled axon terminals (G) in the type Ib boutons of control and pdm31. (H,I) Number of Futsch-positive terminals (H) and percentage of unbundled axon terminals (I) in the type Ib boutons of +/wgCX4, +/wgts, and wgts/wgCX4. n = 51 for A2 and 37 for A3 of CS, 37 for A2 and 40 for A3 of pdm31. n = 21 for A2 and 19 for A3 of +/wgCX4, n = 30 for A2 and 25 for A3 of +/wgts, 44 for A2 and 48 for A3 of wgts/wgCX4. *Represents p < 0.05; ** represents p < 0.01; *** represents p < 0.0001. Data are presented as mean ± SEM. Scale bars: 10 µm.
Stable microtubule-bound Futsch appears as a filamentous bundle that pass through the center of NMJ axon20,22,26. Interestingly, the distal bouton at the end of each axon branch visualized by Futsch shows four distinct shapes: a bundled shape and three types of unbundled shapes such as looped, splayed, and diffused/punctate20. Splayed or diffused/punctate axon terminals indicate that microtubules are unstable due to transition to new axonal growth, while looped axon terminals indicate paused growth cones20,30. Proportion of distal boutons with unbundled shape is increased by mutations that affect NMJ expansion such as wg and futsch20,26. Magnified images of wild-type NMJs showed that less than 10% and 20% of the axon terminals at A2 and A3 are unbundled, respectively (Fig. 3A”,A”’,G). In contrast, number of unbundled terminals was increased 7.3 and 3.4 times in A2 and A3 segments of pdm31 NMJs compared to wild-type, respectively (Fig. 3B”,B”’,G; red arrow and arrowheads). All unbundled axon terminals in pdm3 NMJs were either splayed or diffused/punctate, and looped axon terminals were not detected. Number of splayed or diffused/punctate terminals in wg NMJs was also increased about two times in A2 and A3 segments compared to wild-type (Fig. 3I). Thus, proportion of splayed or diffused/punctate terminals in A2 and A3 segments was significantly increased in both pdm3 and wg NMJs.
Increase in number of splayed or diffused/punctate terminals in pdm3 NMJs suggests that microtubules in pdm3 NMJs are unstable. To directly address this point, we visualized axon terminals with α-Tubulin and Futsch. In fact, signals from α-Tubulin and Futsch staining were much weaker in axon branches of pdm3 NMJ compared to wild-type (Fig. S5A,B). In case of wg NMJs, signal from α-Tubulin staining was substantially reduced in entire axon compared to wild-type (Fig. S5C–E). In summary, microtubules become unstable, which may lead to reduced number of axon branches in both pdm3 and wg NMJs.
Pdm3 expression in motor neuron is important for NMJ growth
Wg secreted from motor neuron and glia is important for growth and differentiation of presynaptic terminals20,24. To figure out which cell type among motor neuron and glia expresses Pdm3, we expressed pdm3 RNAi in motor neurons by the OK6-Gal4 driver and in glia by the repo-Gal4 driver (Fig. 4A,B). These two Gal4 lines have been used to show cell specificity of a given protein in numerous reports22,24,31–34. Knockdown of pdm3 by OK6-Gal4 caused 10% reduction in bouton number, suggesting that pdm3 is required in neurons for NMJ growth (Fig. 4A). Knockdown of pdm3 by repo-Gal4 did not change bouton number, suggesting that Pdm3 expression in glia is not required for NMJ growth (Fig. 4B).
Figure 4.
Pdm3 is required in motor neurons and acts upstream to Wg. (A,B) The number of boutons in OK6-Gal4/+ as a control (A) or in repo-Gal4/+ as a control (B) is set at 100% and those in OK6 > pdm3i were divided by the control bouton number and multiplied by 100 for bar graphs. n = 27 for control, 32 for OK6 > wgi, and 18 for OK6 > pdm3i. n = 23 for control, 21 for repo > wgi, and 34 for repo > pdm3i. (C) The number of boutons in the A2 and A3 segments when GFP-wg, wgi, pdm3, pdm3i are singly expressed or coexpressed by OK6-Gal4. n = 27 for A2 and 34 for A3 of CS, 18 for A2 and 17 for A3 of GFP-wg, 32 for A2 and 30 for A3 of wgi, 18 for A2 and 20 for A3 of pdm3i, 22 for A2 and A3 of GFP-wg;pdm3i, 22 for A2 and 20 for A3 of GFP-wgi;pdm3. *Represents p < 0.01; ** represents p < 0.0001. Data are presented as mean ± SEM.
We then asked whether expression of Pdm3 by OK6-Gal4 rescues pdm31 NMJ phenotype. To this end, we generated UAS-pdm3 pdm31/CyO-GFP and OK6-Gal4 pdm31/CyO-GFP flies and checked the phenotype of their progeny, UAS-pdm3 pdm31/OK6-Gal4 pdm31. Unexpectedly, pdm31 homozygotes were cold sensitive and could not grow at the temperature lower than 22 °C, but Pdm3 overexpression by OK6-Gal4 induced lethality at the temperature higher than 24 °C. Due to this temperature restraint, UAS-pdm3 pdm31/OK6-Gal4 pdm31 larvae were obtained only at 23 °C at a very low frequency. NMJs of these larvae showed increase in bouton number, and decrease in bouton size, and normalized level of Dlg compared to pdm31 NMJs (Fig. S6). Taken together, overexpressed Pdm3 in motor neurons rescued the loss of pdm3 phenotype in NMJs.
Our results so far have shown that bouton number and size of pdm3 NMJs are more severely affected in A2 than A3, so we examined the expression pattern of Pdm3 in ventral ganglion where cell bodies of motor neurons are present in order to examine the level of Pdm3 along the anterior-posterior (AP) axis. We found that Pdm3 is expressed more in the anterior part than posterior part of ventral ganglion, which is consistent with severer pdm3 phenotype in A2 than A3 (Fig. S7). Further analysis with more refined markers will help understand the effect of this AP gradient of Pdm3 on NMJ growth.
Pdm3 acts upstream to Wg in neurons
Similarity between pdm3 and wg NMJs prompted us to examine the genetic relationship between pdm3 and wg by co-expression of the two among pdm3, GFP-wg, pdm3 RNAi, and wg RNAi (Fig. 4C). As controls, bouton number of NMJs in these UAS lines was counted, which turned out to be similar to that of CS (Fig. S8). When we overexpressed pdm3 or wg by OK6-Gal4, pdm3 caused larval lethality, and wg increased bouton numbers in both A2 and A3 segments (Fig. 4C). When we knocked down pdm3 or wg by the same Gal4, the bouton number of the A2 segment was reduced by 10%, but that of the A3 segment was not changed in both cases. When GFP-wg and pdm3 RNAi were co-expressed, increase in the bouton number by GFP-Wg was not affected by pdm3i (Fig. 4C). When pdm3 and wg RNAi were co-expressed, however, lethal phenotype by overexpressed pdm3 was completely rescued by knockdown of wg. Therefore, wg is epistatically downstream to pdm3. This result raised an interesting possibility that Pdm3 may regulate wg transcription.
pdm3 adults exhibit defects in locomotion, planar cell polarity and wing posture
Pdm3 has both neuronal and non-neuronal roles in fly development (see Introduction). 100% of pdm3f00828, pdm3m7, and pdm31 adults (n = ~50 each) had other defects such as wing drooping (Fig. 5A,B), planar cell polarity (PCP) phenotype in a posterior region near the L3 vein (Fig. 5C,D), and incomplete adhesion between dorsal and ventral blades of wings (Fig. S9, Table 1 in Supplementary Information). These phenotypes of pdm3 mutants suggest that Pdm3 plays previously unidentified roles in wing development. Therefore, we decided to use wing discs to study the relationship between pdm3 and wg.
Figure 5.
Loss or gain of pdm3 phenotypes in wings, denticles and eyes. (A,B) Wing posture of pdm3 homozygotes and transheterozygotes. CS flies have normal wings. (A) pdm3m7 flies have opaque drooping wings. Opaqueness is due to the lack of adhesion between the dorsal and ventral wing blades. (C,D) The +/Df(2 R)BSC267 control wing (C) and the pdm3m7/Df(2 R)BSC267 wing (D). PCP phenotype is observed along the distal half of the L3 vein, and the most affected region is marked with the red box and magnified in (C’,D’). There are no structural defects in the hinge (arrows in C,D). (E,F) Ventral denticle bands of A5 and A6 of the control en-Gal4/+ (E) and en > pdm3 1st instar larvae. (F) Pdm3 overexpression by en-Gal4 induces embryonic lethality and defects in ventral denticles. en-Gal4 drives expression in the last row of naked cuticle (arrows) and the first row of denticle bands (arrowheads). (G,H) Adult eyes of the control GMR-Gal4/+ (G) and GMR > pdm3 (H).
Pdm3 was highly expressed in both proximal and distal hinge regions in and near where patched (ptc) is expressed (Fig. S10A,A’). This Pdm3 pattern is genuine because Pdm3 was not detected in the ptc region of ptc > pdm3i discs (Fig. S10B,B”). Expression of Pdm3 in the hinge region may be responsible for the wing drooping phenotype of pdm3 mutants although there were no visible defects in adult wing hinges (Fig. 5C,D). No change in the level of Wg was observed in the ptc > pdm3i wing discs, suggesting that loss of pdm3 does not affect wg transcription in the DV midline of the wing pouch region (Fig. S11).
Transient expression of Pdm3 induces wg transcription
To understand the role of Pdm3 in relation with Wg, we carried out gain of function analyses using multiple Gal4 lines. Overexpression of Pdm3 induced embryonic to pupal lethality with all Gal4 lines used in this study (Table 2 in supplementary information). In case of en-Gal4 driver that caused embryonic lethality, some rare larval escapers were shorter than controls and had abnormal denticle patterns in the ventral epidermis (Fig. 5E,F). Pdm3 overexpression by other tissue-specific Gal4 lines also reduced size of affected tissues. For instance, Pdm3 expression by GMR-Gal4 generated small eyes (Fig. 5G,H), and that by nub-Gal4 caused mostly pupal lethality and loss of wings in rare adults (Table 2 in supplementary information). Consistent with this phenotype of nub > pdm3 wings, size of all nub > pdm3 wing discs examined was smaller than control wing discs (n = 14 each, Fig. 6A,B).
Figure 6.
Pdm3 induces wg transcription. (A,B) nub > pdm3 wing discs (B) have smaller wing pouch than control nub-Gal4/+ (A). (C,D) Induction of Wg-LacZ (arrows) after 36 hours of pdm3 expression in ptc > pdm3 Gal80ts discs (D) compared to the control discs (C). (C’,D’) are black and white images of (C,D). (E,F) Induction of Sens (F’) and Dll (F”) marked with arrows, compared to control (E-E”).
One interesting finding was increase in the level of Wg at the DV midline of nub > pdm3 wing discs (Fig. 6A,B). To examine this phenomenon further, we transiently expressed pdm3 with Gal80ts system using ptc-Gal4 for 6, 12, 24, 36 and 48 hours at 30 °C in order to avoid lethality by Pdm3 overexpression, and checked the level of wg-LacZ as a marker for wg transcription. We found that wg-LacZ was ectopically expressed in the ptc region after 36 or 48 hours but not before 36 hours of transient Pdm3 expression (Fig. 6C,D). The downstream effector proteins of Wg signaling, Distal-less (Dll) and Sensless (Sens), were also induced at the ptc region (Fig. 6E,F). Thus, pdm3 directly or indirectly activates transcription of wg.
Discussion
We report here that Pdm3 regulates growth and development of NMJs. pdm3 mutants showed increase in bouton size and decrease in bouton number, which are similar to the phenotype of wg mutants. Lethality induced by the overexpression of Pdm3 was rescued by knockdown of wg in NMJ, indicating that Pdm3 functions upstream to Wg. Furthermore, overexpression of Pdm3 induced wg transcription in wing discs. We propose here that a major function of Pdm3 in motor neurons is to induce wg transcription, and secreted Wg from motor neurons regulates growth, development, and maturation of both pre- and post-synaptic regions of NMJ.
The mammalian homolog of Pdm3 is Brain-5 (Brn-5)/POU class 6 homeobox 1 (POU6F1) mainly expressed in brain and spinal cord. Brn-5 is heavily expressed in embryonic brain but also expressed in adult brain and multiple adult organs such as kidney, lung, testis, and anterior pituitary35. In developing brain, Brn-5 is expressed in postmitotic neurons after neuronal progenitor cells exit cell cycle in the early process of terminal neuronal differentiation36. Therefore, both Pdm3 and Brn-5 function in differentiation of neurons. Interestingly, ectopic expression of Brn-5 inhibits DNA synthesis37, which is similar to cell cycle arrest phenotype by Wg overexpression38. Given the homology between Pdm3 and Brn-5 as well as functional similarities, Brn-5 may also induce wnt transcription.
Most of Pdm3 functions identified so far are related to the maturation of neurons such as olfactory neurons, R neurons and td neurons as well as their postsynaptic partners7–9. Ectopic expression of Pdm3 induced lethality without exception, indicating that expression of Pdm3 in fly tissues is generally repressed in vivo in order to express Wg under the strict spatiotemporal control. An important question is whether Pdm3 directly transcribe wg. We found that wg transcription is induced only after 36 hours of transient overexpression of Pdm3. It is possible that the level of Pdm3 needs to be over a threshold to induce wg transcription. Alternatively, Pdm3 may need to turn on other components to indirectly induce wg transcription. DNA sequence of Brn-5 binding site has been reported39–41, so analysis on wg and wnt regulatory regions will help understand the mechanism of wnt induction by Pdm3 and Brn-5.
We consistently found more significant NMJ phenotypes in A2 than A3 in both pdm3 and wg mutants. Therefore, pdm3 and wg may play more prominent roles in the A2 than the A3 segment. In fact, the level of Pdm3 was higher in the anterior region than the posterior region of ventral ganglion, which suggests that more Wg may be present in the NMJs of anterior abdominal segments. Consistent with this idea, the number of type Ib boutons in the A2 segment was 1.8 times more than A3 segment. One difference between pdm3 and wg mutants is the lack of certain phenotypes in the A3 segment of pdm3 NMJs: the size of boutons and the number of axon terminals in A3 were not affected in pdm3 mutant. It is possible that Pdm3 turns on both common and segment-specific genes besides wg, and A3 segment-specific components may alleviate the loss of wg phenotype in the A3 segment. Similarly, other proteins induced by Pdm3 may also play important roles in NMJ growth, differentiation and maintenance. In fact, multiple signaling pathways including Glass-bottom-boat (Gbb) pathway also play roles in NMJ development42,43. Gbb is secreted from muscles and induces development of both pre- and post-synaptic structures, similar to Wg signaling.
We identified a defective hobo element in the pdm3m7 allele. The hobo element belongs to Ac family found in maize and has short inverted terminal repeats44. Laboratory and wild strains of D. melanogaster have average 28 and 22 copies of hobo elements in the genome that are either full-length or defective, respectively45,46. Because other suppressors identified in the genetic screen using Sona overexpression did not have hobo element in the pdm3 gene, the transposition of the hobo element to the pdm3 gene may have occurred subsequent to the generation of a point mutation in the arr gene by EMS. Since both arr and pdm3 are positively involved in Wg signaling, this hobo insertion may have helped the original arrm7 mutation to further decrease the activity of Wg signaling under the condition of Sona overexpression.
Besides the neuronal roles of Pdm3, all pdm3 mutants show minor but consistent defects in planar cell polarity in a restricted region of the wing as well as adhesion between the dorsal and ventral wing blades. Other phenotypes such as wing drooping and premature death were also observed in all pdm3 mutants, but these may be due to malformation of synaptic structures. Pdm3 also plays a role in female-limited color dimorphism in abdomen of D. montium11. The authors found in sexually dimorphic females that the first intron of the pdm3 gene has four tandem sets with predicted binding sites for the HOX gene Abdominal-B (Abd-B) and the sex determination gene doublesex (dsx). Interestingly, it has been shown that Wg expression is repressed by the combinatory work of Abd-B and Dsx proteins47. Taken together, it is possible that transcription of wg and pdm3 is co-repressed by Abd-B and Dsx. Such co-repression of wg and pdm3 transcription may be also required for synaptic growth and differentiation in neurons. Further studies on Pdm3 will help understand how this understudied transcription factor is involved in the final differentiation of various cell types.
Materials and Methods
Fly strains
Ok6-Gal4, BG57-Gal4 and repo-Gal448 were obtained from S.-B. Lee’s lab, and UAS-pdm37,8, UAS-GFP:wg49, pdm3f°°828 and pdm31 7,8 were obtained from the labs that produced them. UAS-sona12 is produced in our lab. UAS-wg RNAi (#4889R-3) was obtained from Fly Stocks of National Institute of Genetics. All other lines such as UAS-GFP (#1533), UAS-GFP:CD8 (#5130), UAS-pdm3 RNAi (#26749), Df(2 R)BSC267, DF(2 R)Exel6058, were obtained from Bloomington Drosophila Stock Center.
Immunohistochemistry
Immunohistochemical staining of larval wing discs was performed as described12, and that of NMJs was performed as described with slight modifications50,51. To obtain larvae for NMJ analysis, flies were cultured at 25 °C except wg mutants at 18 °C. To control the population size of larvae in each vial, eggs were harvested on grape plates and incubated at 25 °C for 24 hours. Then, 30 larvae were transferred to a food vial, and were cultured until the wandering larval stage for dissection. In case of wg larvae, 30 larvae were cultured at 18 °C until dissection. For NMJ staining, larvae were dissected with HL3.1 solution on sylgard plates and fixed for 20 minutes in 5% formaldehyde/HL3.1 solution52 or Bouin’s solution50. Fixed samples were rinsed 3 to 4 times with PBS or HL3.1 and then were blocked in 5% BSA in PBS before antibody treatment. The following antibodies were used: anti-Pdm3 (rat, 1:100)7, anti-β-Gal (chicken, 1:100; Abcam ab134435), anti-Dlg (rabbit, 1:500)53, anti-HRP-Cy3 (1:100; Jackson ImmunoResearch), anti-Wg (mouse, 1:1,000; DSHB 4D4), anti-Futsch (mouse, 50:1; DSHB 22C10), anti-α-Tubulin (mouse, 1:200; Sigma MAB1864), anti-Glutamate receptor IIA (mouse, 1:10; DSHB concentrated 8B4D2 (MH2B)). For GluRIIA staining, the samples were fixed by Bouin’s solution.
Image capture and quantitative analysis of boutons
We stained the late 3rd instar larvae for a presynaptic marker HRP and a postsynaptic marker Dlg to detect NMJs. Type Ib boutons have more extensive SSR compared to other bouton types (Is, II, and III), so are easily detected by the high level of Dlg19. Therefore, type Ib boutons are defined as round-shaped structures in NMJ branches that are stained with HRP and have high level of Dlg, and only ones that were qualified to this definition were counted as boutons. To obtain images containing boutons, type Ib boutons visualized with HRP and Dlg were taken with 1 μm interval for 7–10 Z stacks at 400X magnification by a confocal laser microscope of Carl Zeiss (NFEC-2010-09-141569) with Zen 2009 program. To manually count number of boutons, all Z stack images were then merged and type Ib boutons at muscles 6 and 7 in A2 or A3 segments in a given image were counted. The number of images used for counting for each genotype was 17–52. To measure size of boutons, images of terminal boutons were captured at 2,000X magnifications and then area of boutons in merged images was measured by Zen 2009 program.
Cuticle preparation of larvae
The cuticle preparation was performed as described with slight modifications54. Flies were put into a chamber with a grape juice-containing agar plate that has yeast paste at the center. After 4 hours of egg-laying, plates were incubated for 20 hours at 25 °C. Larvae were transferred to distilled water on cover glass, and washed again with distilled water. Water was then removed and the 1:1 mixture of lactic acid and Hoyer’s mount solution was applied. After waiting for about 1 minute, the sample on a cover glass was placed on the slide glass, and then incubated for overnight at 65 °C.
Statistical analysis
Statistical analysis was performed using ANOVA to compare different genotypes to a wild-type control within experimental groups. Data are presented as mean ± SEM. To determine statistical significance, t-test and one-way ANOVA of Microsoft Excel 2019 were used.
Supplementary information
Acknowledgements
We are grateful to Dr. K.-W. Choi and colleagues in our lab for discussion and comments on manuscript. We would like to express our gratitude to Drs. S.-B. Lee and M.-Y. Nahm for kind guidance on dissecting and staining NMJs. We are in debt to Drs. A. L. Tichy, A. Ray, J. R. Carlson, C. K. Chen, W. Y. Chen, C. T. Chien for fly strains and antibodies. We thank Bloomington Stock Center, Drosophila Genetic Resource Center, National Institute of Genetics and Developmental Studies Hybridoma Bank. This research was supported by grants from the National Research Foundation of Korea, NRF-2017R1A2B4009254 and NRF-2019R1H1A2039726.
Author contributions
Y. Kim and K.-O. Cho designed experiments, analyzed data and wrote the paper. Y. Kim conducted experiments.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-57425-9.
References
- 1.Deis, G. et al. TPX superconducting tokamak magnet system - 1995 design and status overview. Sofe ‘95 - 16th Ieee/Npss Symposium on Fusion Engineering, Vols 1 and 2, 1383–1388 (1995).
- 2.Malik Vikas, Zimmer Dennis, Jauch Ralf. Diversity among POU transcription factors in chromatin recognition and cell fate reprogramming. Cellular and Molecular Life Sciences. 2018;75(9):1587–1612. doi: 10.1007/s00018-018-2748-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gold DA, Gates RD, Jacobs DK. The early expansion and evolutionary dynamics of POU class genes. Mol. Biol. evolution. 2014;31:3136–3147. doi: 10.1093/molbev/msu243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herr W, et al. The Pou Domain - a Large Conserved Region in the Mammalian Pit-1, Oct-1, Oct-2, and Caenorhabditis-Elegans Unc-86 Gene-Products. Gene Dev. 1988;2:1513–1516. doi: 10.1101/gad.2.12a.1513. [DOI] [PubMed] [Google Scholar]
- 5.Ryan AK, Rosenfeld MG. POU domain family values: Flexibility, partnerships, and developmental codes. Gene Dev. 1997;11:1207–1225. doi: 10.1101/gad.11.10.1207. [DOI] [PubMed] [Google Scholar]
- 6.Herr W, Cleary MA. The Pou Domain - Versatility in Transcriptional Regulation by a Flexible 2-in-One DNA-Binding Domain. Gene Dev. 1995;9:1679–1693. doi: 10.1101/gad.9.14.1679. [DOI] [PubMed] [Google Scholar]
- 7.Tichy AL, Ray A, Carlson JR. A new Drosophila POU gene, pdm3, acts in odor receptor expression and axon targeting of olfactory neurons. Journal of Neuroscience. 2008;28:7121–7129. doi: 10.1523/JNEUROSCI.2063-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen CK, Chen WY, Chien CT. The POU-domain protein Pdm3 regulates axonal targeting of R neurons in the Drosophila ellipsoid body. Dev. Neurobiol. 2012;72:1422–1432. doi: 10.1002/dneu.22003. [DOI] [PubMed] [Google Scholar]
- 9.Qian CS, Kaplow M, Lee JK, Grueber WB. Diversity of Internal Sensory Neuron Axon Projection Patterns Is Controlled by the POU-Domain Protein Pdm3 in Drosophila Larvae. Journal of Neuroscience. 2018;38:2081–2093. doi: 10.1523/JNEUROSCI.2125-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rogers WA, et al. A survey of the trans-regulatory landscape for Drosophila melanogaster abdominal pigmentation. Developmental Biol. 2014;385:417–432. doi: 10.1016/j.ydbio.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 11.Yassin A, et al. The pdm3 Locus Is a Hotspot for Recurrent Evolution of Female-Limited Color Dimorphism in Drosophila. Curr. Biol. 2016;26:2412–2422. doi: 10.1016/j.cub.2016.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim, G. W. et al. Sol narae (Sona) is a Drosophila ADAMTS involved in Wg signaling. Sci Rep-Uk6, 10.1038/srep31863 (2016). [DOI] [PMC free article] [PubMed]
- 13.Won, J. H. et al. ADAMTS Sol narae cleaves extracellular Wingless to generate a novel active form that regulates cell proliferation in Drosophila. Cell Death & Disease10, 10.1038/s41419-019-1794-8 (2019). [DOI] [PMC free article] [PubMed]
- 14.Tsogtbaatar, O. et al. An ADAMTS Sol narae is required for cell survival in Drosophila. Sci Rep-Uk9, 10.1038/s41598-018-37557-9 (2019). [DOI] [PMC free article] [PubMed]
- 15.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 16.Amin N, Vincan E. The Wnt signaling pathways and cell adhesion. Front. Biosci. (Landmark Ed.) 2012;17:784–804. doi: 10.2741/3957. [DOI] [PubMed] [Google Scholar]
- 17.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annual Review of Cell and Developmental Biology. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 18.Swarup S., Verheyen E. M. Wnt/Wingless Signaling in Drosophila. Cold Spring Harbor Perspectives in Biology. 2012;4(6):a007930–a007930. doi: 10.1101/cshperspect.a007930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Menon KP, Carrillo RA, Zinn K. Development and plasticity of the Drosophila larval neuromuscular junction. Wires Dev. Biol. 2013;2:647–670. doi: 10.1002/wdev.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Packard M, et al. The drosophila wnt, wingless, provides an essential signal for pre- and postsynaptic differentiation. Cell. 2002;111:319–330. doi: 10.1016/S0092-8674(02)01047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mathew D, et al. Wingless signaling at synapses is through cleavage and nuclear import of receptor DFrizzled2. Science. 2005;310:1344–1347. doi: 10.1126/science.1117051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miech C, Pauer HU, He X, Schwarz TL. Presynaptic Local Signaling by a Canonical Wingless Pathway Regulates Development of the Drosophila Neuromuscular Junction. Journal of Neuroscience. 2008;28:10875–10884. doi: 10.1523/Jneurosci.0164-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ataman B, et al. Rapid activity-dependent modifications in synaptic structure and function require bidirectional Wnt signaling. Neuron. 2008;57:705–718. doi: 10.1016/j.neuron.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kerr KS, et al. Glial Wingless/Wnt Regulates Glutamate Receptor Clustering and Synaptic Physiology at the Drosophila Neuromuscular Junction. Journal of Neuroscience. 2014;34:2910–2920. doi: 10.1523/Jneurosci.3714-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franco B, et al. Shaggy, the homolog of glycogen synthase kinase 3, controls neuromuscular junction growth in Drosophila. Journal of Neuroscience. 2004;24:6573–6577. doi: 10.1523/Jneurosci.1580-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roos J, Hummel T, Ng N, Klambt C, Davis GW. Drosophila Futsch regulates synaptic microtubule organization and is necessary for synaptic growth. Neuron. 2000;26:371–382. doi: 10.1016/S0896-6273(00)81170-8. [DOI] [PubMed] [Google Scholar]
- 27.Gogel S, Wakefield S, Tear G, Klambt C, Gordon-Weeks PR. The Drosophila microtubule associated protein Futsch is phosphorylated by Shaggy/Zeste-white 3 at an homologous GSK3 beta phosphorylation site in MAP1B. Mol. Cell Neurosci. 2006;33:188–199. doi: 10.1016/j.mcn.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Ciani L, Krylova O, Smalley MJ, Dale TC, Salinas PC. A divergent canonical WNT-signaling pathway regulates microtubule dynamics: Dishevelled signals locally to stabilize microtubules. Journal of Cell Biology. 2004;164:243–253. doi: 10.1083/jcb.200309096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wehrli M, et al. Arrow encodes an LDL-receptor-related protein essential for Wingless signalling. Nature. 2000;407:527–530. doi: 10.1038/35035110. [DOI] [PubMed] [Google Scholar]
- 30.Dent EW, Callaway JL, Szebenyi G, Baas PW, Kalil K. Reorganization and movement of microtubules in axonal growth cones and developing interstitial branches. Journal of Neuroscience. 1999;19:8894–8908. doi: 10.1523/JNEUROSCI.19-20-08894.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freeman Marc R. DrosophilaCentral Nervous System Glia. Cold Spring Harbor Perspectives in Biology. 2015;7(11):a020552. doi: 10.1101/cshperspect.a020552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Casas-Tintó Sergio, Arnés Mercedes, Ferrús Alberto. Drosophila enhancer-Gal4 lines show ectopic expression during development. Royal Society Open Science. 2017;4(3):170039. doi: 10.1098/rsos.170039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanyal S. Genomic mapping and expression patterns of C380, OK6 and D42 enhancer trap lines in the larval nervous system of Drosophila. Gene Expr. Patterns. 2009;9:371–380. doi: 10.1016/j.gep.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 34.De Gregorio C, Delgado R, Ibacache A, Sierralta J, Couve A. Drosophila Atlastin in motor neurons is required for locomotion and presynaptic function. Journal of Cell Science. 2017;130:3507–3516. doi: 10.1242/jcs.201657. [DOI] [PubMed] [Google Scholar]
- 35.Andersen B, et al. Brn-5 Is a Divergent Pou Domain Factor Highly Expressed in Layer-Iv of the Neocortex. Journal of Biological Chemistry. 1993;268:23390–23398. [PubMed] [Google Scholar]
- 36.Cui, H. & Bulleit, R. F. Expression of the POU transcription factor Brn-5 is an early event in the terminal differentiation of CNS neurons. J Neurosci Res52, 625–632, doi:10.1002/(SICI)1097-4547(19980615)52:6<625::AID-JNR1>3.0.CO;2-A (1998). [DOI] [PubMed]
- 37.Cui H, Bulleit RF. Expression of the POU transcription factor Brn-5 inhibits proliferation of NG108-15 cells. Biochemical and Biophysical Research Communications. 1997;236:693–696. doi: 10.1006/bbrc.1997.6996. [DOI] [PubMed] [Google Scholar]
- 38.Johnston LA, Edgar BA. Wingless and Notch regulate cell-cycle arrest in the developing Drosophila wing. Nature. 1998;394:82–84. doi: 10.1038/27925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pereira JH, Kim SH. Structure of human Brn-5 transcription factor in complex with CRH gene promoter. Journal of Structural Biology. 2009;167:159–165. doi: 10.1016/j.jsb.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 40.Pereira JH, Ha SC, Kim SH. Crystallization and preliminary X-ray analysis of human Brn-5 transcription factor in complex with DNA. Acta Crystallographica Section F: Structural Biology and Crystallization Communications. 2008;64:175–178. doi: 10.1107/S1744309108003370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andersen B, et al. Brn-5 is a divergent POU domain factor highly expressed in layer IV of the neocortex. Journal of Biological Chemistry. 1993;268:23390–23398. [PubMed] [Google Scholar]
- 42.McCabe BD, et al. The BMP homolog Gbb provides a retrograde signal that regulates synaptic growth at the Drosophila neuromuscular junction. Neuron. 2003;39:241–254. doi: 10.1016/S0896-6273(03)00426-4. [DOI] [PubMed] [Google Scholar]
- 43.Bayat V, Jaiswal M, Bellen HJ. The BMP signaling pathway at the Drosophila neuromuscular junction and its links to neurodegenerative diseases. Current Opinion in Neurobiology. 2011;21:182–188. doi: 10.1016/j.conb.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Calvi BR, Hong TJ, Findley SD, Gelbart WM. Evidence for a Common Evolutionary Origin of Inverted Repeat Transposons in Drosophila and Plants - Hobo, Activator, and Tam3. Cell. 1991;66:465–471. doi: 10.1016/0092-8674(81)90010-6. [DOI] [PubMed] [Google Scholar]
- 45.Biemont C, Gautier C. Localization Polymorphism of Mdg-1, Copia, I-Mobile-Element and P-Mobile-Element in Genomes of Drosophila-Melanogaster, from Data of Inbred Lines. Heredity. 1988;60:335–346. doi: 10.1038/hdy.1988.51. [DOI] [Google Scholar]
- 46.Ruiz MT, Carareto CMA. Characterization of hobo element copy number and integrity in Brasilian populations of Drosophila melanogaster. Hereditas. 2003;138:154–157. doi: 10.1034/j.1601-5223.2003.01708.x. [DOI] [PubMed] [Google Scholar]
- 47.Wang W, Kidd BJ, Carroll SB, Yoder JH. Sexually dimorphic regulation of the Wingless morphogen controls sex-specific segment number in Drosophila. Proc. Natl Acad. Sci. U S Am. 2011;108:11139–11144. doi: 10.1073/pnas.1108431108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nahm M, et al. Spartin regulates synaptic growth and neuronal survival by inhibiting BMP-mediated microtubule stabilization. Neuron. 2013;77:680–695. doi: 10.1016/j.neuron.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pfeiffer S, Ricardo S, Manneville JB, Alexandre C, Vincent JP. Producing cells retain and recycle Wingless in Drosophila embryos. Curr. Biol. 2002;12:957–962. doi: 10.1016/S0960-9822(02)00867-9. [DOI] [PubMed] [Google Scholar]
- 50.Budnik V, Gorczyca M, Prokop A. Selected methods for the anatomical study of Drosophila embryonic and larval neuromuscular junctions. Int. Rev. Neurobiol. 2006;75:323–365. doi: 10.1016/S0074-7742(06)75015-2. [DOI] [PubMed] [Google Scholar]
- 51.Brent JR, Werner KM, McCabe BD. Drosophila larval NMJ dissection. Journal of Visualized Experiments: JoVE. 2009 doi: 10.3791/1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stewart BA, Atwood HL, Renger JJ, Wang J, Wu CF. Improved stability of Drosophila larval neuromuscular preparations in haemolymph-like physiological solutions. J. Comp. Physiol. A. 1994;175:179–191. doi: 10.1007/BF00215114. [DOI] [PubMed] [Google Scholar]
- 53.Cho KO, Chern J, Izaddoost S, Choi KW. Novel signaling from the peripodial membrane is essential for eye disc patterning in Drosophila. Cell. 2000;103:331–342. doi: 10.1016/S0092-8674(00)00124-0. [DOI] [PubMed] [Google Scholar]
- 54.Alexandre C. Cuticle preparation of Drosophila embryos and larvae. Methods Mol. Biol. 2008;420:197–205. doi: 10.1007/978-1-59745-583-1_11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.