Introduction
Acanthosis nigricans (AN) is a common condition seen in individuals as a consequence of insulin resistance states, other metabolic disorders, malignancy, genetic syndromes, and drugs. This article presents a severe, childhood-onset case of AN that was of unclear etiology. Whole-genome sequencing identified a variant of epidermal growth factor receptor (EGFR), a receptor involved in keratinocyte proliferation and fibroblast migration.1
Case report
A 23-year-old white woman was referred to the dermatology clinic for evaluation of severe AN. The plaques first appeared around the age of 6 years on the posterolateral neck. They had progressively gotten darker and thicker, involving additional areas, particularly during menarche and pregnancy. The plaques were neither painful nor pruritic. The patient described having a normal weight throughout her childhood and adolescence but reported a 60-pound weight gain that she maintained after her 2 recent pregnancies. She had regular menses since menarche at the age of 12 years. Findings from a complete review of systems were negative except for progressive worsening of AN.
Past medical history was significant for reported hyperinsulinemia diagnosed at age 4 years after a corticosteroid injection for contact dermatitis caused by poison ivy. She did not have any chronic medical conditions. She was not taking any medications at the time of presentation except for a Mirena intrauterine device (Bayer, Whippany, NJ). Past use of an oral contraceptive pill was not associated with a change in AN. Over the years, she was treated with metformin (up to 2000 mg), isotretinoin, and various topical regimens, including 40% urea, 0.025% tretinoin cream, and 4% hydroquinone cream, all without improvement. There was no family history of AN or other genetic conditions.
Physical examination showed that the patient was a nondysmorphic female with a height of 170 cm and body mass index of 32 kg/m2. She was Fitzpatrick skin type II. An examination of the skin showed symmetric, extensive, papillomatous, hyperpigmented velvety plaques on the neck and axillae (Fig 1). Additionally, she had hyperpigmented velvety plaques on the lateral eyes, perioral region, inframammary folds, antecubital fossae, volar wrists, and inguinal folds. There was no evidence of masculinization, lymphadenopathy, cushingoid features, or organomegaly.
Fig 1.
Extensive, papillomatous, hyperpigmented velvety plaques on (A) the left axilla and (B) the posterior aspect of the neck.
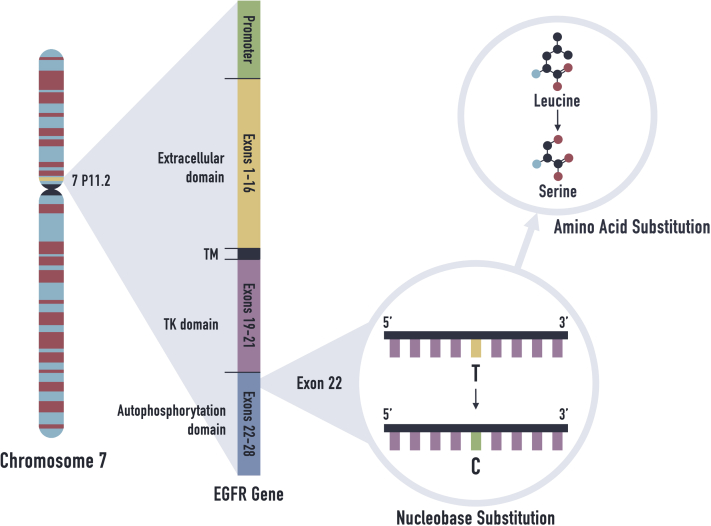
A biopsies of the left side of the neck and left side of the upper back were performed. Histology results were consistent with AN. Immunohistologic staining for human papillomavirus was negative. An extensive endocrinology evaluation excluded diabetes mellitus, hyperinsulinemia, hyperandrogenemia, lipodystrophy, and thyroid function abnormalities (Table I). Abdominal computed tomography scan showed no abnormalities. Whole-exome sequencing, a genetic test that provides sequencing for all of the protein-coding regions of genes known as exons, was obtained. It showed a single, heterozygous variant of uncertain significance (c.2648T>C, p.Leu883Ser) in the EGFR gene (Fig 2). Sanger sequencing confirmed the finding.
Table I.
Laboratory evaluation results
| Laboratory evaluation (serum) | Value | Normal range |
|---|---|---|
| Hemoglobin A1c, % | 5.0 | <5.7 |
| Insulin, μIU/ml | 16.8 | 3-25 |
| Nonfasting glucose, mg/dl | 86 | 70-100 |
| IGF-1, ng/ml | 185 | 107-367 |
| IGF-II | Normal | |
| C-peptide, ng/ml | 5.07 | 0.9-7.1 |
| Antipancreatic islet cells | Negative | |
| GAD-65 antibody, IU/ml | <5 | <5 |
| ICA-512, U/ml | <1 | <1 |
| Testosterone total, ng/dl | 23 | 14-76 |
| Sex hormone binding globulin, nmol/L | 43 | 18-144 |
| TSH, μIU/ml | 1.393 | 0.350-5.5 |
| Leptin, ng/ml | 15.5 | 4.7-23.7 |
| Adiponectin, μg/ml | 93 | 4-22 |
GAD, Glutamic acid decarboxylase; IGF, insulin-like growth factor; ICA, islet cell antibody; TSH, thyroid-stimulating hormone.
Fig 2.
The EGFR mutation at nucleotide c.2648 was detected with whole-genome sequencing. EGFR, Epidermal growth factor receptor; TK, tyrosine kinase; TM, transmembrane.
Discussion
Most theories of the pathogenesis of AN have focused on insulin-like growth factor (IGF), tyrosine kinase receptors, EGFR, and fibroblast growth factor receptor (FGFR), all of which stimulate keratinocyte and fibroblast growth and proliferation.
Similar to the disease in adults, the vast majority of AN in early childhood occurs in the setting of obesity and insulin resistance (IR).2 This includes genetic defects in insulin-signaling pathways (type A IR) and blocking autoantibodies against the insulin receptor (type B IR). High concentrations of insulin cause keratinocyte proliferation by binding to IGF-1R. Hence, AN has been reported at skin sites where insulin has been injected.3
Our patient's Homeostatic Model Assessment of Insulin Resistance score was 1, indicating insulin sensitivity and an overall low metabolic risk. Normal glucose, insulin, and IGF-1 levels excluded other causes of IR because IR requires significant abnormalities of glucose metabolism.4 Although this patient had a reported history of hyperinsulinemia secondary to corticosteroid use, medication-associated AN is unlikely because, in that situation, the lesions regress after discontinuation of the offending medication, and they did not in this patient. In addition, the onset occurred years after exposure, and subsequent testing showed no evidence of hyperinsulinemia. The patient's normal height and anatomy, lack of significant family history, and absence of an FGFR mutation excluded familial AN. This form of AN is exceedingly rare and manifests early in life in the absence of obesity and endocrinopathies, the clinical presentation seen in this case. Familial cases of Beare-Stevenson syndrome, Crouzon syndrome, and severe achondroplasia with developmental delay and acanthosis nigricans, which have mutations in FGFR, have also been associated with AN. All reported cases of familial AN, with the exception of a de novo case from China, are inherited in an autosomal dominant manner.5 In addition, FGFR defects and/or skeletal abnormalities are associated with familial AN.5
Paraneoplastic AN was excluded in this patient by the lack of laboratory evidence of cancer and by the long duration of its presence. The onset of paraneoplastic AN is sudden and rapidly progressive. It most commonly occurs with adenocarcinoma, especially adenocarcinoma of the stomach, lung, and breast.6 It is exceedingly rare in children.7 Gastric tumors with AN have been reported to secrete high levels of transforming growth factor alpha, leading to the amplification of EGFR.8 Also, biopsy specimens of paraneoplastic AN exhibited an enhanced expression pattern of EGFR that included most of the suprabasal keratinocytes.9
Our patient fit none of the known causes of AN. For this reason, we performed whole-exome sequencing and observed that she had a mutation in EGFR. To our knowledge, this is the first report of an EGFR mutation associated with AN. EGFR is a transmembrane protein that is a member of the receptor tyrosine kinase family of signal transduction molecules. It is expressed mainly in the basal layer of normal epidermis, and its role in modulating keratinocyte proliferation, migration, and survival is well established.1
Conclusion
Despite a thorough medical evaluation, the cause of this severe case of AN was unknown and, for this reason, whole-exome sequencing was performed. Because the clinical findings were identical to those of other mutations that cause overactivation of the receptor, we speculate that this mutation was an activating mutation that enhances EGFR signaling by its known ligands or even causes activation of the signal transduction pathway without the requirement for its ligands. Profiling EGFR activity may provide additional answers as to how this mutation is involved in the pathogenesis of AN. Furthermore, whole-exome sequencing of other patients with AN, for which the cause is unknown, may generate additional information about the pathogenesis of this disease. It also provides an excellent example of how, in the future, whole-exome and genome sequencing may be used for personalized medicine.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
References
- 1.Jost M., Kari C., Rodeck U. The EGF receptor—an essential regulator of multiple epidermal functions. Eur J Dermatol. 2000;10(7):505–510. [PubMed] [Google Scholar]
- 2.Otto D.E., Wang X., Tijerina S.L., Reyna M.E., Farooqi M.I., Shelton M.L. A comparison of blood pressure, body mass index, and acanthosis nigricans in school-age children. J Sch Nurs. 2010;26(3):223–229. doi: 10.1177/1059840510365154. [DOI] [PubMed] [Google Scholar]
- 3.Buzasi K., Sapi Z., Jermendy G. Acanthosis nigricans as a local cutaneous side effect of repeated human insulin injections. Diabetes Res Clin Pract. 2011;94(2):e34–e36. doi: 10.1016/j.diabres.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 4.Rogers D.L. Acanthosis nigricans. Semin Dermatol. 1991;10(3):160–163. [PubMed] [Google Scholar]
- 5.Fu J., Zhao Y., Wang T., Zhang Q., Xiao X. Acanthosis nigricans in a Chinese girl with FGFR3 K650 T mutation: a case report and literature review. BMC Med Genet. 2019;20(1):8. doi: 10.1186/s12881-019-0748-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rigel D.S., Jacobs M.I. Malignant acanthosis nigricans: a review. J Dermatol Surg Oncol. 1980;6(11):923–927. doi: 10.1111/j.1524-4725.1980.tb01003.x. [DOI] [PubMed] [Google Scholar]
- 7.Isaacoff E., Dimitriadi F.F., Barrows F., Pawel B., Mattei P., Mostoufi-Moab X. Acanthosis nigricans associated with an adrenocortical tumor in a pediatric patient. Case Rep Endocrinol. 2013;2013 doi: 10.1155/2013/174593. 174593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koyama S., Ikeda M., Sato M. Transforming growth factor-alpha (TGF alpha)-producing gastric carcinoma with acanthosis nigricans: an endocrine effect of TGF alpha in the pathogenesis of cutaneous paraneoplastic syndrome and epithelial hyperplasia of the esophagus. J Gastroenterol. 1997;32(1):71–77. doi: 10.1007/BF01213299. [DOI] [PubMed] [Google Scholar]
- 9.Haase I., Hunzelmann N. Activation of epidermal growth factor receptor/ERK signaling correlates with suppressed differentiation in malignant acanthosis nigricans. J Invest Dermatol. 2002;118(5):891–893. doi: 10.1046/j.1523-1747.2002.17631.x. [DOI] [PubMed] [Google Scholar]