Abstract
Bone morphogenetic proteins (BMP) are members of the transforming growth factor β superfamily (TGF-β). BMPs are involved in tumourigenesis and disease progression of certain malignancies. To date, the role played by BMPs in gastric cancer (GC) remains largely unknown. In the present study, we systematically analysed the expression and clinical significance of BMP and BMP receptors (BMPR) in TCGA gastric cancer database and GEO database and explored the possible mechanism of action. BMP5 is reduced in gastric cancer tissues, while ACVRL1, ACVR1, TGFBR1, and BMPR2 were significantly increased in the gastric tumours. BMP3, ACVR1, TGFBR1, BMPR1B (also known as ALK6), TGFBR2 and BMPR2 were significantly associated with poorer overall survival of GC patients. A negative correlation was seen between BMP/BMPR and proliferation markers which was supported by their correlation with the cell cycle promoters and inhibitors. More interestingly, further analyses showed that BMPs and their receptors are positively correlated with matrix metalloproteinases (MMPs), epithelial mesenchymal transition (EMT) markers and stemness in GC. Furthermore, positive correlations were also frequently seen between BMP receptors and markers/regulators of angiogenesis and lymphangiogenesis in the gastric tumours. Taken together, these findings suggest that BMPs play dual roles in GC. They may inhibit proliferation of GC cells. On the other hand, they can also promote disease progression through a promotion of invasion, EMT and stemness. The elevated expression of BMP receptors in GC were also highly associated with tumour associated angiogenesis and lymphangiogenesis which facilitate tumour growth, expansion and spread.
Keywords: Bone morphogenetic protein, Gastric cancer, Prognosis, Proliferation, Epithelial mesenchymal transition, Angiogenesis and lymphangiogenesis
1. Introduction
Gastric cancer (GC) is one of the most common gastrointestinal malignancies and the second leading cause of cancer-related death worldwide [1]. To date, patients with GC have poor prognosis, with a 5-year survival rate of between 25 and 30% [2]. Little improvement has been made for the patients’ long-term survival though certain advances have been made in chemotherapeutics in recent years [3]. Target therapies such as EGFR (Epidermal Growth Factor Receptor) inhibitors, MET inhibitors, and mTOR (Mammalian Target of Rapamycin) inhibitors have been evaluated in clinical studies/trials but no obvious benefit has been seen [4]. Therefore, it remains essential and vital to understand the molecular mechanisms of gastric cancer in order to identify new biomarkers and target for personalised disease management.
Bone morphogenetic proteins (BMPs) belong to the transforming growth factor β (TGF-β) superfamily. BMP ligands signal through transmembrane receptors including BMPR-I (ACVRL1, ACVR1, BMPR1A, ACVR1B, TGFBR1, BMPR1B, ACVR1C, also known as ALK1-7) and BMPRII (TGFBR2, TGFBR3, BMPR2, ANCR2A and ANCR2B) to induce differentiation of mesenchymal cells and form cartilage and bone tissue. BMPs are essential and indispensable for development of diverse tissues and organs such as bone, cartilage, heart and other organs. It has been shown that BMPs are involved in the development of many tumours. There are two pathways mediating signalling for BMPs: Smad-dependent pathway and Smad-independent pathways [5]. Upon binding to pre-formed BMP receptors complex which comprises both Type 1 and Type 2 receptors, activated BMPR-II phosphorylates the GS region of BMPR-I leading to activation and translocation of R-Smads into nucleus and regulation of BMP responsive genes [6]. Upon binding to the BMP ligands [7], the Type 1 receptor recruits Type 2 receptor which form BMP-induced receptor complex. The Type I receptor then activates MAPK pathway via an adaptor protein, XIAP [8].
Aberrant expression of BMPs has been shown in a number of solid tumours, including bone tumours, odontogenic tumours, and maxillofacial tumours [9], [10], [11], [12], [13], and they are also related to the development and metastasis of tumours [14], [15], [16], [17]. For example, overexpression of BMP6 in prostate cancer is associated with skeletal metastasis [14]. It has been shown that BMP4 promotes the migration and invasion of breast cancer cells through an up-regulation of MMP1 and CXCR4 [15]. In contrast to these positive effects in the disease progression, certain BMPs exhibit inhibitory effects on tumour cells. For example, BMP10 inhibits the growth of prostate cancer cells largely due to induced apoptosis via Smad-independent signalling in which XIAP and ERK1/2 are involved. It can also suppress migration and invasion of prostate cancer cells [16].
To date, the role played by BMPs in gastric cancer remains largely unknown. An increased expression of BMP4 has been shown in gastric adenocarcinomas in comparison with normal gastric mucosae which is inversely related to the prevalence of lymph node metastasis and tumour invasiveness [18]. BMP2 was more frequently observed in intestinal-type cases than diffuse-type cases in gastric cancers which is associated with the status of differentiation and lymph node metastasis [19]. BMP2 promotes metastases in gastric cancer through regulation of NF-κB and MMP9 activity via PI3K/Akt and MAPK pathways [19].
In the current study, we aim to evaluate the role played by BMPs (with a focus on BMP2 to BMP7) and BMP receptors in gastric cancer by analysing gene array data and RNA sequencing data of gastric cancers.
2. Materials and methods
2.1. Database and online analysis
Download the RNA sequencing data in the Stomach adenocarcinoma (STAD) dataset of The Cancer Genome Atlas (TCGA). The normalised gene expression level of the dataset was analysed to evaluate the expression of BMPs and BMP receptors in gastric cancer tissues (n = 274) compared to normal gastric tissues (n = 33). The gene expression profiles (GSE33335 and GSE27342) were downloaded from the Gene Expression Omnibus database and normalized using Limma package in R software. We verified the above results in GSE33335 (n = 50, GPL5175) and GSE27342 database (n = 160, GPL5175), which comprise 25 and 80 pairs of tumours and corresponding adjacent normal gastric tissues, respectively.
To evaluate the association with prognosis, we performed survival analyses for the BMPs (BMP2-7) and BMP receptors in TCGA-STAD (n = 375) using the LinkedOmics [20]. The Kaplan Meier plotter (http://kmplot.com/), which is based on an online database and is capable of assessing the association of genes on survival in four types of cancer samples including breast cancer, ovarian cancer, lung cancer and gastric cancer, was also performed to evaluate the association of BMPs and BMP receptors with prognosis of GC patients (n = 1065) [21]. Auto-selected cut-off values were employed.
After identifying the genes with significantly altered expression levels in gastric cancer tissues and the genes associated with prognosis of gastric cancer patients, we focussed our investigation on the genes which fell into both of these categories.
TCGA-STAD (treated as discovery cohort) and GSE 84433 database (n = 357, employed for validation) were analysed to evaluate the correlation between BMPs/BMP receptors and key genes relevant to the hallmarks of cancer including proliferation, cell cycle, invasion, epithelial mesenchymal transition (EMT), stemness, angiogenesis and lymphangiogenesis. The results were presented with heatmaps and scatter plots.
Overall correlation between BMPs/BMPR mRNA expression and some hallmarks of cancer (TCGA-STAD) was plotted using Circos (available from http://qplot.cn/) [22].
2.2. Statistical analysis
Following a normality check, t-tests were employed for normally distributed data whilst non-normally distributed data was analysed using Mann-Whitney tests. The Spearman test was used to analyse the correlation between different genes. Association with patients’ survival was evaluated using Kaplan-Meier survival analysis. Differences were considered to be statistically significant at p < 0.05.
3. Results
3.1. Aberrant expression of BMPs and receptors in human gastric cancer tissues
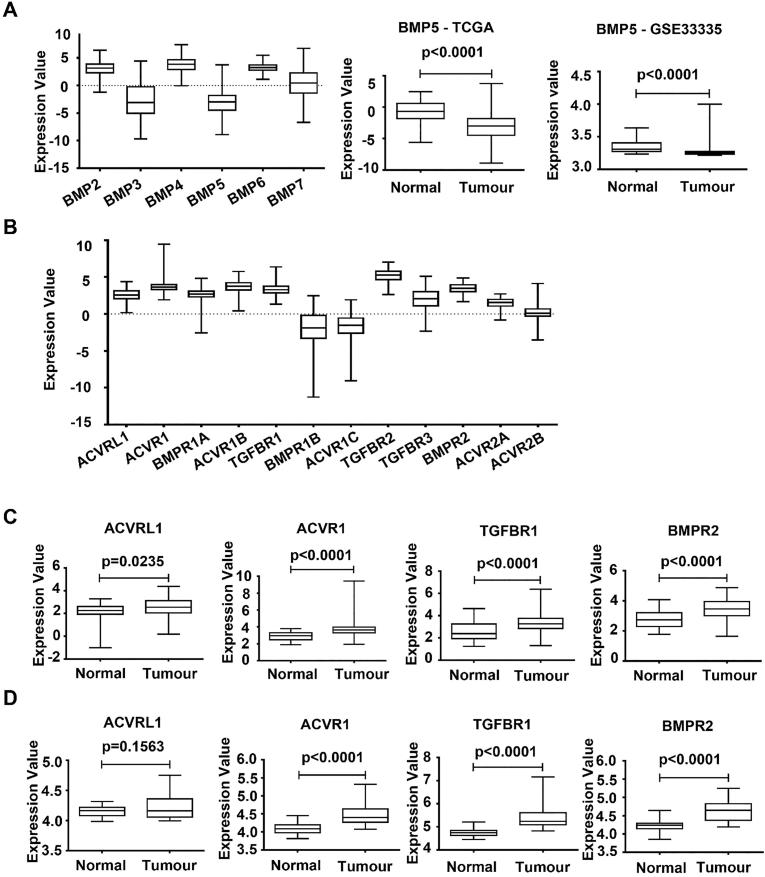
Expression of the BMPs (BMP2-7) in GC was first analysed using the RNA sequencing data of the TCGA-STAD cohort. BMP2, BMP4 and BMP6 are expressed at relatively higher levels, while the expression levels of BMP3 and BMP5 were relatively lower among the BMPs (Fig. 1A). The expression of these BMPs was then evaluated against their expression in normal gastric tissues. The expression of BMP4 in gastric cancer tissues was significantly increased compared with normal tissue, while the expression of BMP3 and BMP5 were significantly decreased (Fig. 1B) (Supplementary Table 1). The reduced expression of BMP5 in gastric cancer was also evident in both GSE33335 (Fig. 1C) (n = 50, GPL5175) and GSE27342 database (n = 160, GPL5175), which comprises 25 and 80 tumours paired with adjacent normal tissues, respectively (Supplementary Table 2).
Fig. 1.
Aberrant expression of BMPs and BMP receptors in gastric cancer. (A) Expression of BMPs in gastric tumour tissues was analysed using the TCGA database (n = 274, TCGA-STAD cohort). (B) Expression of BMP5 in gastric tumour tissues (n = 274) compared with normal tissues (n = 33) at mRNA levels in the TCGA database. (C) The mRNA expression of BMP5 in gastric tumours and paired adjacent normal gastric tissues (n = 50, GSE33335). (D) Expression of BMP receptors was analysed in the TCGA-STAD cohort (n = 274). (E) Expression of ACVRL1, ACVR1, TGFBR1 and BMPR2 in gastric tumour tissues (n = 274) compared with normal tissues (n = 33) in the TCGA-STAD. (F) The mRNA expression of ACVRL1, ACVR1, TGFBR1 and BMPR2 in the gastric tumours in comparison with paired adjacent normal gastric tissues (n = 50, GSE33335).
The expression of BMP receptors in GC was also evaluated. TGFBR2, ACVR1B, ACVR1, BMPR2, and TGFBR1are expressed at higher levels compared with BMPR1B and BMPR1B (Fig. 1D). In comparison to the expression in normal gastric tissues, ACVRL1, ACVR1, ACVR1B, TGFBR1 and BMPR2 exhibit increased expression in the gastric cancers, while the expression of TGFBR3 was significantly reduced in GC (Fig. 1E) (Supplementary Table 1). The increased expression of ACVRL1, ACVR1, TGFBR1 and BMPR2 were also evident in both GSE33335 (Fig. 1F) (n = 50, GPL5175) and GSE27342 database (n = 160, GPL5175) (Supplementary Table 2).
3.2. The aberrant expression of BMP/BMP receptors is involved in the disease progression of GC
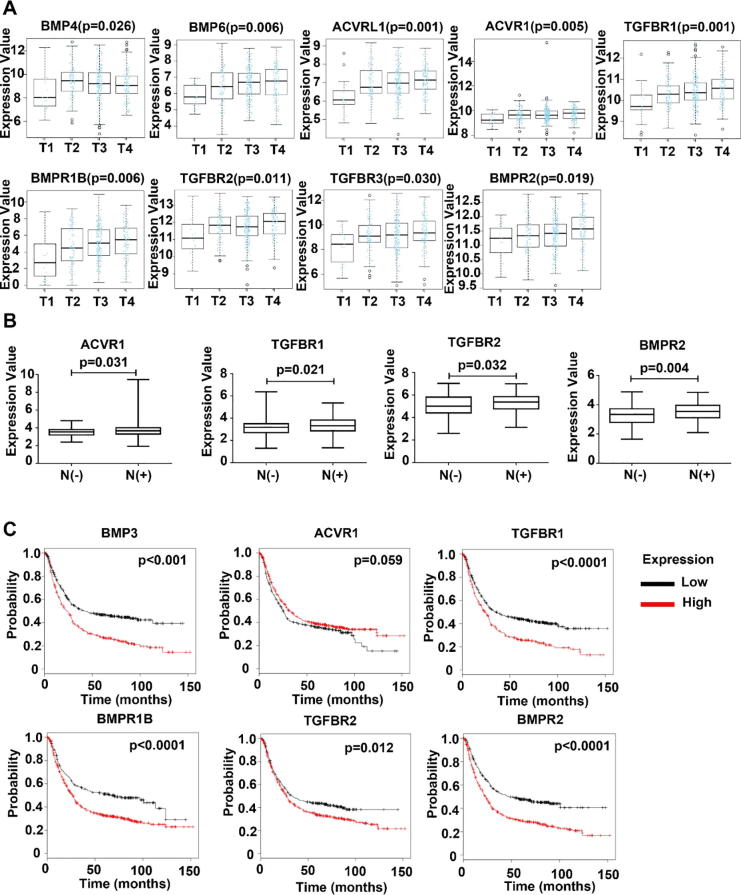
The correlation between the expression BMP/BMPRs and clinic pathological parameters was analysed in the TCGA-STAD cohort. Certain BMPs and BMP receptors were significantly correlated with the T stage of GCs including BMP4, BMP6, ACVRL1 (ACVRL1), ACVR1, TGFBR1, BMPR1B, TGFBR2, TGFBR3 and BMPR2 (Fig. 2A). In addition, elevated expression of ACVR1, TGFBR1, TGFBR2 and BMPR2 was associated with lymph node metastases (Fig. 2B). No obvious change was seen in the expression of these BMPs and BMP receptors when the gastric tumours developed distant metastases (data not shown).
Fig. 2.
Aberrant expression of BMPs and BMP receptors correlates with the disease progression and prognosis of gastric cancer. (A) Correlation between BMPs/BMPRs mRNA expression and T stage, N stage of gastric cancer in the TCGA cohort (n = 274). (B) Kaplan-Meier survival analyses showed correlations between BMPs/BMPRs expression and overall survival of GC patients (n = 876) using the online Kaplan-Meier survival analysis (http://kmplot.com).
We further evaluated the prognostic value of BMPs and BMPRs in GC using TCGA-STAD dataset and an online analysis tool LinkedOmics. Higher expression of BMP3, ACVR1, TGFBR1, BMPR1B, TGFBR2 and BMPR2 in the primary tumours are associated poorer overall survival (Table 1 and Supplementary Fig. 1). This is further supported by the Kaplan-Meier analyses using the KMplot. Patients had much shorter survival if their tumour presented higher expression of BMP3, ACVR1, TGFBR1, BMPR1B, TGFBR2 and BMPR2 (Fig. 2C).
Table 1.
BMPs/BMPRs expression and overall survival of GC patients (the KMplot cohort).
| Gene | Median | Cut off | Median OS (months) |
p | |
|---|---|---|---|---|---|
| Low expression (n, %) | High expression (n, %) | ||||
| BMP2 | 644 | 429 | 26 (257, 29.3%) | 29.4 (619, 70.7%) | 0.055 |
| BMP3 | 17 | 23 | 42.07 (476, 54.3%) | 23.9 (400, 45.7%) | <0.001 |
| BMP4 | 111 | 201 | 34.1 (605, 69.1%) | 22.5 (271, 30.9%) | <0.001 |
| BMP5 | 28 | 56 | 29.8 (648, 74.0%) | 22.83 (228, 26.0%) | <0.01 |
| BMP6 | 122 | 88 | 70.2 (285, 32.5%) | 25.9 (591, 67.5%) | <0.001 |
| BMP7 | 116 | 157 | 39.8 (534, 61.0%) | 2.3 (342, 39.0%) | <0.001 |
| ACVRL1 | 111 | 127 | 30.4 (527, 60.2%) | 27.47 (349, 39.8%) | 0.099 |
| ACVR1 | 1178 | 1038 | 25.17 (313, 35.7%) | 31.33 (563, 64.3%) | 0.059 |
| BMPR1A | 404 | 394 | 25.9 (420, 47.9%) | 34.1 (456, 52.1%) | 0.065 |
| ACVR1B | 259 | 365 | 30.7 (625, 71.3%) | 26.7 (251, 28.7%) | <0.05 |
| TGFBR1 | 70 | 101 | 35.77 (556, 63.5%) | 23.6 (320, 36.5%) | <0.001 |
| BMPR1B | 18 | 7 | 70.2 (238, 27.2%) | 26.7 (638, 72.8%) | <0.001 |
| ACVR1C | 160 | 80 | 45 (419, 66.4%) | 57.13 (212, 33.6%) | 0.104 |
| TGFBR2 | 59 | 47 | 33.27 (353, 40.3%) | 27.4 (523, 59.7%) | <0.05 |
| TGFBR3 | 471 | 440 | 32.6 (403, 46.0%) | 27.47 (473, 54.0%) | <0.05 |
| BMPR2 | 155 | 143 | 47.7 (389, 44.4%) | 23.4 (487, 55.6%) | <0.001 |
| ACVR2A | 406 | 427 | 35.77 (489, 55.8%) | 25.2 (387, 44.2%) | <0.01 |
| ACVR2B | 22 | 30 | 63.9 (387, 61.3%) | 32.1 (244, 38.7%) | <0.05 |
3.3. BMP and tumour growth in GC
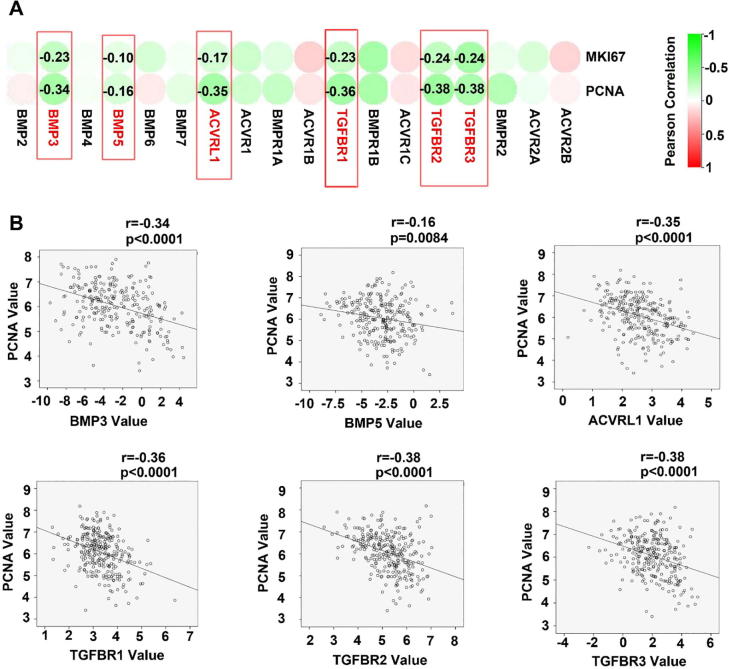
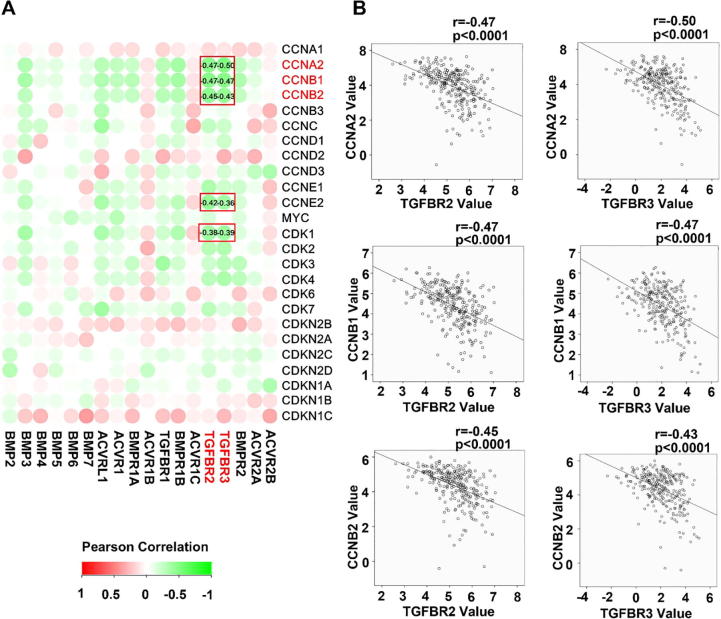
To explore the molecular machinery underlying the implication of BMP/BMPRs in disease progression of GC, correlation between BMP/BMPR and cell proliferation index MKI67 and PCNA was determined. An inverse correlation was frequently seen for most of the BMPs and BMP receptors we examined (Fig. 3A and Supplementary Fig. 2A), BMP3, BMP5, ACVRL1, TGFBR1, TGFBR2 and TGFBR3 exhibited significant inverse correlation with both MKI67 and PNCA in the gastric tumours (Fig. 3B and Supplementary Fig. 2B). In line with the inverse correlation between the expression of BMP/BMPRs and proliferation markers, correlations were also present for cell cycle regulators in the gastric tumours. Certain BMPs and BMPRs were inversely correlated with cell cycle promoting factors such as CCNA2, CCNB1, CCNB2, CCNE2, CDK1 and CDK3, while a positive correlation was evident for cell cycle inhibitors such as CDKN2B and CDKN1C (Fig. 4A), especially for ACVRL1, TGFBR1, BMPR1B, TGFBR2, TGFBR3 and BMPR2 (Fig. 4B). These were also observed in the GSE84433 cohort (data not shown).
Fig. 3.
BMPs/BMPR and tumour growth in GC. Correlation between BMPs/BMPRs mRNA expression and PCNA were analysed using Spearman tests, results shown as a heatmap (A) and scatter plots (B). BMPs/BMPRs are highlighted with red boxes when they exhibit significantly correlation and are labelled with corresponding correlation co-efficient. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 4.
Correlation between BMPs/BMPRs mRNA expression and cell cycle regulators were shown as heatmap (C) and scatter plots (D). BMPs/BMPRs are highlighted with red boxes when they exhibit significantly correlation and are labelled with corresponding correlation co-efficient. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.4. BMP and regulators of invasion/migration in GC
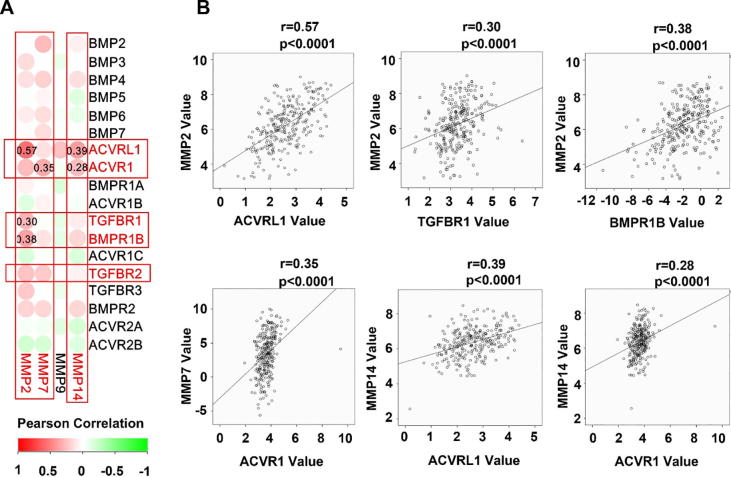
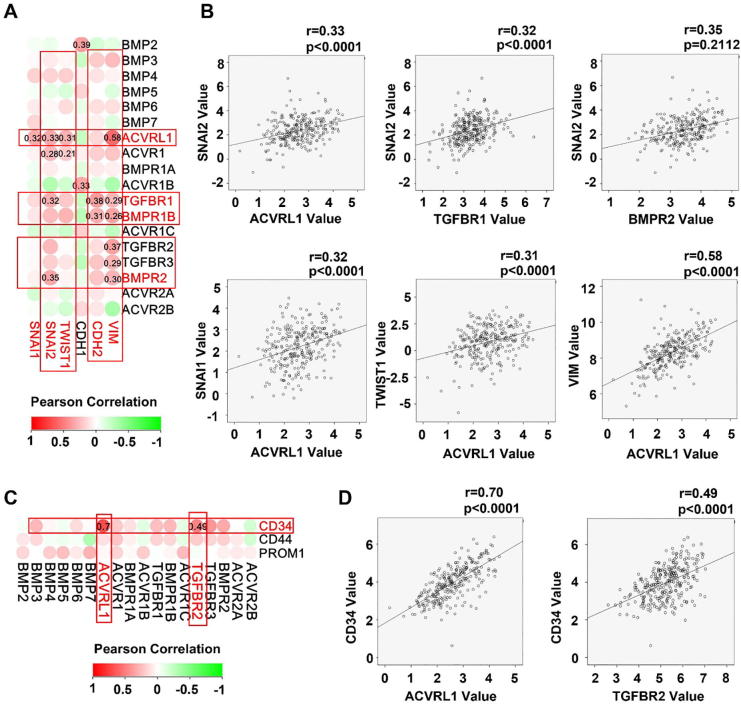
Poor differentiation, stemness and invasiveness are hallmarks of cancer cells. We analysed the correlation between the expression of BMP/BMPRs and some important molecules including EMT related molecules (SNAI1, SNAI2, and TWIST1), matrix metalloproteinases (MMP2, MMP7, MMP9 and MMP14) and stemness markers (CD34, CD44 and CD133). ACVRL1, ACVR1, TGFBR1, BMPR1B, TGFBR2, TGFBR3 and BMPR2 were positively correlated with the MMPs (MMP2, MMP7 and MMP14) (Fig. 5), EMT markers (Fig. 6A and B), and stem cell markers (Fig. 6C and D). The positive correlation between these molecules and EMT-related molecules, MMPs and stem cell markers were also observed in the GSE84433 (data not shown).
Fig. 5.
Correlation between BMPs/BMPRs mRNA expression and MMPs are shown as a heatmap (A) and scatter plots for BMP/BMP receptors with significant correlation (D.
Fig. 6.
Aberrant expression of BMPs/BMPR correlates with the EMT and stemness in GC. Shown are correlations between BMPs/BMPRs mRNA expression and EMT markers as heatmap (A) and scatter plots (B). Correlation between BMPs/BMPRs mRNA expression and stem cell markers are shown as heatmap (C) and scatter plots (D).
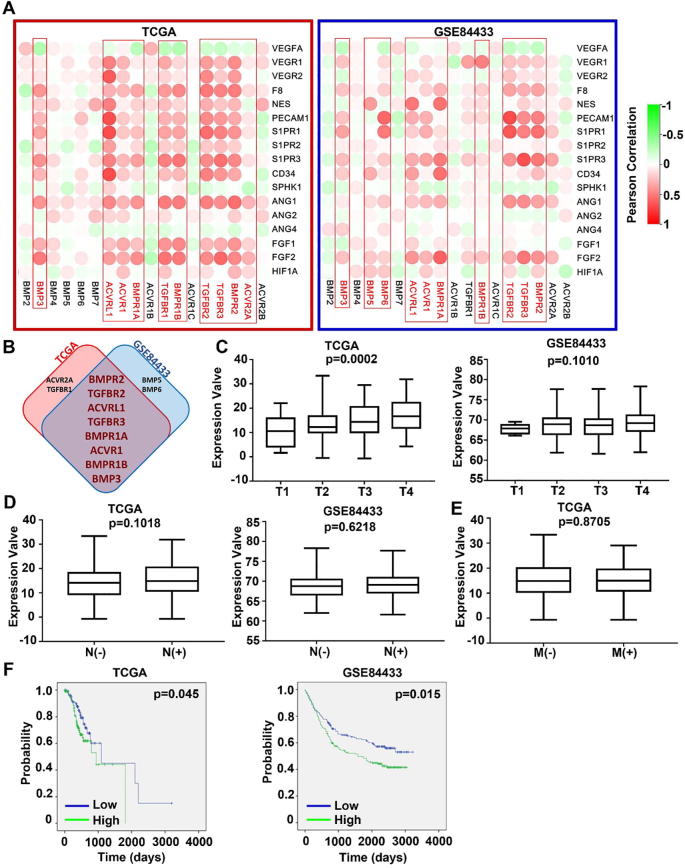
3.5. BMP/BMPRs and tumour associated angiogenesis in GC
Angiogenesis is associated with disease progression and prognosis of tumours in the digestive system including colorectal cancers [23], gastric cancers [24], oesophageal cancers [25], and pancreatic cancers [26], [27]. In the correlation analyses between the expression levels of BMP/BMPR and angiogenesis-related factors, it has been revealed that ACVRL1, ACVR1, TGFBR1, BMPR1B, TGFBR2, TGFBR3 BMPR2 and BMP3 are positively correlated with angiogenic markers in both the discovery cohort (TCGA-STAD) and the validation cohort (GSE84433) (Fig. 7A and B). Clinical implication was then analysed for overall expression of the top 8 correlated BMPs/BMP receptors including BMPR2, TGFBR2, ACVRL1, TGFBR3, BMPR1A, ACVR1, BMPR1B and BMP3. A significant association with T staging was seen in the TCGA-STAD cohort but not in the validation cohort (GSE84433) (Fig. 7C). No difference was observed for the overall expression in tumours regarding their lymph node metastasis and distant metastases (Fig. 7D). Kaplan-Meier survival analyses showed that higher levels of the overall expression were significantly associated with poorer overall survival in both cohorts (Fig. 7F).
Fig. 7.
Association between BMPs/BMPRs and angiogenesis in GC. (A) Correlations between BMPs/BMPRs mRNA expression and angiogenesis markers in both TCGA-STAD (cohort for discovery) and GSE84433 (cohort for validation) cohorts are shown as heatmaps. (B) Shown are the overlapping BMPs/BMPRs that are more closely associated with angiogenic markers in both GC cohorts. (C) Correlation between overall expression (BMPR2, TGFBR2 and ACVRL1) and T stage, N stage, M stage and prognosis of gastric cancer were analysed in both TCGA-STAD and GSE84433 cohorts. Overall expression was calculated using an equation: overall expression of A/B/C = Log (10^(Expression value of A))×(10^(Expression value of B))×(10^(Expression value of C)).
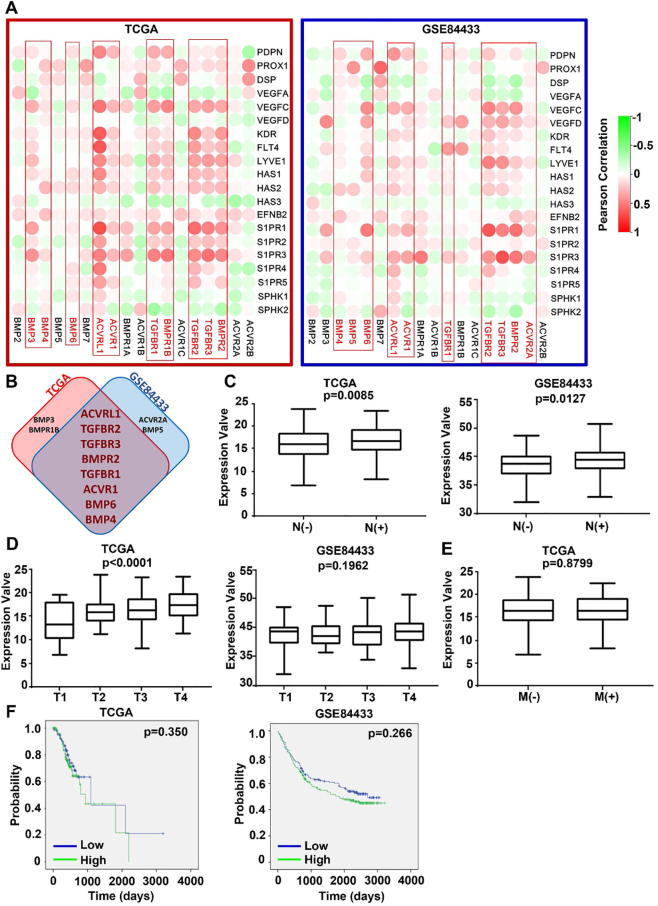
3.6. BMP and lymphangiogenesis/lymph node metastasis in GC
Lymphangiogenesis plays an important role in cancer metastasis, especially for dissemination of cancer cells through lymphatic vessels [28]. Therefore, we analysed the correlation between the BMP/BMPRs and lymphangiogenesis factors in both TCGA-STAD and GSE84433 cohorts (Fig. 8A). ACVRL1, TGFBR2, TGFBR3, BMPR2, TGFBR1, ACVR1, BMP6 and BMP4 present positive correlation with most of the lymphangiogenesis factors in both discovery and validation cohorts (Fig. 8B). Overall expression of the top 5 (ACVRL1, TGFBR2, TGFBR3, BMPR2 and TGFBR1) identified from both cohorts exhibited significant correlation with lymph node metastasis in both TCGA and GSE84433 cohorts (Fig. 8C) and also the T stage of tumours in the TCGA cohort, but not for the T stage in the GSE84433 cohort and distant metastasis (Fig. 8D). The overall expression of these five BMP receptors had no association with overall survival of patients in both TCGA and GSE84433 cohorts (Fig. 8F).
Fig. 8.
BMPs/BMPR and lymphangiogenesis/lymphatic metastasis in GC. (A) Shown are heatmaps of correlations between BMPs/BMPRs mRNA expression and lymphangiogenesis markers in both TCGA-STAD and GSE84433 cohorts for discovery and validation. (B) Shown are BMPs/BMPRs highly associated lymphangiogenesis that are most commonly seen in both GC cohorts. (C) Correlation between overall expression (ACVRL1, TGFBR2, TGFBR3, BMPR2 and TGFBR1) and T stage, N stage, M stage and prognosis of gastric cancer were analysed in both TCGA-STAD and GSE84433 cohorts. The overall expression was calculated following the same formula shown in the Fig. 5.
4. Discussion
BMPs play a vital role in embryo formation, development and differentiation. Dysregulated BMP pathways may lead to embryonic death or abnormalities of tissues and organs including bone, cartilage, heart and lung [29]. In recent years, more and more studies have shown that BMPs are also involved in the development and progression of many kinds of solid tumours [14], [15], [16], [17], particularly in the disease-specific bone metastasis. Many studies suggest that BMPs are involved in the bone metastasis of breast cancer. Decreased expression of BMP-7 has been indicated in primary tumours in association with bone metastases. BMP-7 inhibits the growth of breast cancer tumours at primary sites and in bone in vivo [30]. In addition, BMPs are extensively involved in the regulation of cellular functions of breast cancer cells, including cell proliferation, apoptosis, migration, invasion, epithelial mesenchymal transition (EMT). BMP-2 and BMP-6 could inhibit the proliferation of breast cancer cells [31], [32]. BMP-2 promotes the aggressiveness of breast cancer cell (MCF-7), in vitro and in vivo [33]. In addition, some studies in other tumour types have also shown that BMPs are also associated with tumour associated angiogenesis. Current knowledge regarding the role of BMPs on angiogenesis is mainly from studies in prostate cancer. Experimental studies showed that BMPs promoted angiogenesis directly and indirectly through upregulation of the expression of VEGF in osteoblasts [34], [35].
To date, little is known about the role played by BMPs in gastric cancer. Recent bioinformatical analyses focused on the discovery of novel biomarkers/ therapeutic targets, such as the analysis of an integrated datasets of gastric cancer by the online platform of KMplot which highlighted 29 markers for poor prognosis of the disease [21]. The present study was an attempt to dissect the implication of certain BMPs in gastric cancer by analysing publicly available data in a relatively comprehensive fashion but more specifically focusing on the BMPs and corresponding molecular machineries instead of examining the whole transcriptome. We analysed the expression of BMPs (BMP2-7) and their receptors in gastric cancer using the TCGA gastric cancer database, and a further validation was performed in two GEO databases (GSE33335 and GSE27342) which have both gastric tumours and paired adjacent normal tissues. After the verification, it was found that the expression level of BMP5 in gastric cancer tissues was significantly decreased compared with normal tissues, while the expression levels of ACVRL1, ACVR1, TGFBR1 and BMPR2 were significantly increased. BMPR2 mediates inhibitory effect on cell proliferation. Reduced expression of BMPR2 has been evident in some solid tumours, such as prostate cancer, breast cancer and bladder cancer [36]. We also analysed the expression of these five molecules in other tumours, especially other digestive tract tumours (oesophageal cancer, colon cancer, rectal cancer, pancreatic cancer, liver cancer, cholangiocarcinoma) using the TCGA database. The expression of these genes in oesophageal cancer, liver cancer and cholangiocarcinoma appeared to be similar as that was seen in gastric cancer, but not in pancreatic cancer. In pancreatic cancer, the expression level of BMP5 is higher than that in normal tissues, while the expression of ACVRL1, TGFBR1 and BMPR2 are reduced (data not shown). We speculate that the role played by BMP5, ACVRL1, ACVR1, TGFBR1, and BMPR2 in gastric cancer, oesophageal cancer, and hepatobiliary carcinoma may be different from those in pancreatic cancer.
It has been well-demonstrated that most BMPs elicit inhibitory effect on the growth of non-transformed epithelial, endothelial and haematopoietic cells, and also primary fibroblasts of embryonic origin [37]. The inhibition of growth was often executed by BMP/BMPR/Smad induced cell-cycle inhibitors CDKN2B, CDKN1A and CDKN1C leading to an arrest at G1 phase [38]. BMPs could regulate the proliferation of breast cancer cells in some studies, and the nature of cell response is influenced by the individual BMP, with some BMPs having an inhibitory effect on proliferation of breast cancer cells, while others show a reverse effect. For example, BMP-2 inhibits the proliferation of breast cancer cells via up-regulation of cyclin kinase inhibitor CDKN1A [39], but BMP-4 has a synergetic effect on the proliferation of breast cancer cells induced by fibroblast growth factor (FGF), epidermal growth factor (EGF) and hepatocyte growth factor (HGF) [40]. At present, only a few studies have explored the relationship between BMP/BMPR and proliferation of gastric cancer cells. For example, several studies showed that BMP2 could inhibit the growth of gastric cancer cells through CDKN1A/WAF1/CIP1 [41], [42], [43]. BMP2 and BMP4 may function as potent tumour suppressors in diffuse-type gastric carcinoma, and the inhibitory effect of BMP-4 on the growth is mediated in part by an induction of CDKN1A [44]. The present study showed that most of BMP/BMPRs were negatively correlated with cell proliferation related marker PCNA and MKI67, especially for BMP3, ACVRL1, TGFBR1, BMPR1B, TGFBR2, TGFBR3 and BMPR2. Furthermore, they were also significantly and negatively correlated with cell cycle promoting factors such as CCNA2, CCNB1, CCNB2, CCNE2, CDK1 and CDK3, while positively correlated with cell cycle inhibitors such as CDKN2B and CDKN1C. This suggests that BMP3, ACVRL1, TGFBR1, BMPR1B, TGFBR2, TGFBR3 and BMPR2 act as inhibitory factors for the proliferation of gastric cancer by a regulation of the cell cycle.
Invasiveness is the essential characteristic for cancer cells to spread. The possible role of BMPs in the regulation of cancer cell invasiveness remains unclear. Previous studies showed that BMP2 and BMP7 may promote the invasion and migration of gastric cancer cells via the induction of tenascin-W in the tumour surrounding stroma. [33], [45], [46], However, some BMPs have been reported to inhibit the aggressiveness of breast cancer cells [47]. MMPs are indispensable for cancer cells during their local invasion and distant metastasis. To date, little is known about the potential of BMP signalling in the regulation of MMPs which has just been highlighted by the current study. BMP2 has been shown to suppress the expression of MMP13 in breast cancer cells [48]. The present study revealed BMP receptors including ACVRL1, ACVR1, TGFBR1, BMPR1B and TGFBR2 are positively correlated with the expression MMP2, MMP7 and MMP14.
In addition to the positive correlation between BMP receptors and MMPs, we also analysed the correlation between the BMP/BMPRs and EMT in GC. EMT is also very important during the development and progression of cancer. It not only causes a disruption of epithelial homeostasis which may lead to carcinogenesis, it can also transform the indolent tumour cells into a more aggressive colony, leading to metastasis. BMP-4 can subvert the ability of mammary epithelial cells to form polarized lumen-containing structures and also endows them with invasive properties [49]. BMP-7 has been shown to induce EMT with classical changes in morphology and promote both motility and invasiveness in prostate cancer cells [50]. In contrast, some BMPs are able to reverse EMT and reduce the aggressive properties of tumour cells. For example, BMP-6 restores E-cadherin-mediated cell-to-cell adhesion and prevents breast cancer metastasis through the downregulation of ẟEF1 [51]. It was not a surprise that a positive correlation was seen between the EMT markers (SNAI1, SNAI2 (also known as slug), TWIST1, VIM, CDH1 and CDH2) and BMP receptors as they are induced by the BMP/Smad signalling.
Tumour stem cells are a group of tumour cells with self-renewal ability and multi-directional potential regarding differentiation. They play a key role in the occurrence, development, invasion and metastasis of tumours. Some recent studies suggested that cancer stem cells might form the basis of cancer metastasis [34], [52]. The current analyses showed a markedly positive correlation between CD34 and two BMP receptors (ACVRL1 and TGFBR2) in GC though this may also have implications in tumour associated angiogenesis. Taken together, it was shown that ACVRL1, ACVR1, TGFBR1, BMPR1B, TGFBR2, TGFBR3 and BMPR2 were all significant positively with EMT markers, MMPs and stem cell markers. It suggests that these molecules may contribute to the enhanced invasiveness of gastric cancer cells leading to spread and metastasis.
Angiogenesis is an important event during the development and progression of tumours. Current knowledge regarding the role of BMPs and angiogenesis is mainly from studies in prostate cancer. It has been demonstrated that BMPs, including BMP2, 4, 6, 7 and GDF5 can induce angiogenesis through upregulation of VEGF in prostate cancer [34]. In this study, most of the BMP/BMPRs are significantly positively correlated with angiogenesis markers, especially for BMPR2, TGFBR2, ACVRL1, TGFBR3, BMPR1A, ACVR1, BMPR1B, BMP3, and the higher expression of ACVRL1, ACVR1, BMPR1B, TGFBR2, TGFBR3, BMPR2 were indeed significantly correlated with higher T stage of GC. It suggests that BMP signalling plays a profound role in the tumour growth and expansion through their regulation of tumour associated angiogenesis. Therapeutic potential of targeting BMP receptors to prevent tumour associated angiogenesis in GC warrants further investigation.
Lymph node metastasis is of great significance for the staging and prognosis of many tumours, and lymphangiogenesis is a necessary condition for lymph node metastasis. However, current knowledge about the role of BMP/BMPR in lymphangiogenesis remains poor. BMP9 has been shown to inhibit lymphatic vessel formation via ACVRL1 during development and cancer progression [53]. In the current study, we found that there were marked and positive correlations between BMP/BMPRs and lymphangiogenesis factors, especially for ACVRL1, TGFBR2, TGFBR3, BMPR2, TGFBR1, ACVR1, BMP6 and BMP4. Furthermore, higher expression of ACVR1, TGFBR1, TGFBR2, and BMPR2 were indeed significantly correlated with lymphatic metastases in GC.
Furthermore, a retrospective study analysed 2000 GC patients in 22 Italian hospitals diagnosed over a period from 1998 to 2011. 208 (10%) had bone metastases, either presented at the diagnosis of GC (59/208) or developed the metastases after the diagnosis (149/208) [54]. Osteolytic lesions (52%) appeared to be more common than the osteoblastic lesions (23%) and mixed ones (25%). The implications in bone metastasis could not be analysed due to lack of relevant information in the data of the present study. However, this highlights a potential area of future study.
5. Conclusion
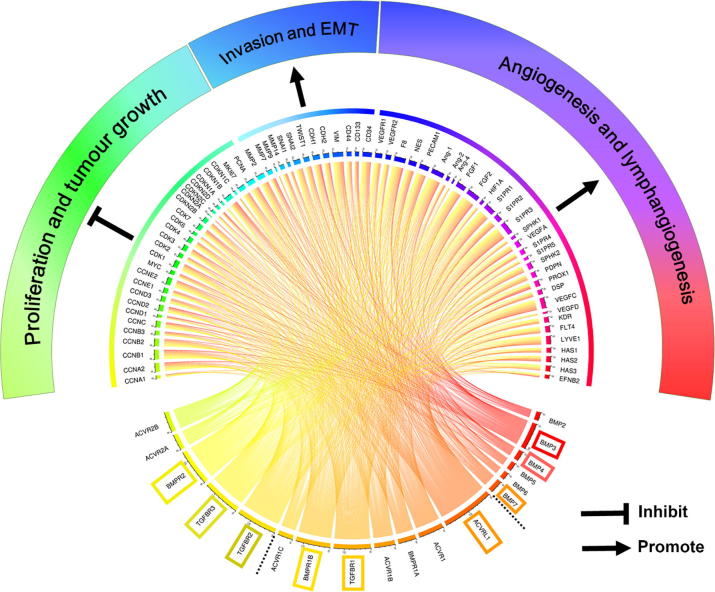
The study showed that the expression level of BMP5 in gastric cancer tissues was significantly lower than the levels found in normal tissues, and the expression levels of ACVRL1, ACVR1, TGFBR1, and BMPR2 were significantly increased. In addition, the survival analysis in TCGA and GEO databases showed that patients with higher expression of BMP3, ACVR1, TGFBR1, BMPR1B, TGFBR2 and BMPR2 had much shorter survival times than the patients with low expression tumours. These results are important for the diagnosis and prognosis assessment of gastric cancer patients. Further analyses showed that BMP/BMPR may elicit inhibitory an effect on proliferation in gastric tumours but promote gastric cancer invasion as they exhibit a positive correlation with certain MMPs, EMT markers and stemness markers. BMP/BMPR also has a significant positive correlation with angiogenesis and lymphangiogenesis, mainly concentrated in BMP3, ACVRL1, TGFBR1, BMPR1B, TGFBR2, TGFBR3 and BMPR2 (Fig. 9). Further in vivo and in vitro investigations will shed light on their multiple roles in the tumourigenesis and disease progression of GC, and also their diagnostic, prognostic and therapeutic potential.
Fig. 9.
Overall correlation between BMPs/BMPR mRNA expression and some hallmarks of cancer (TCGA-STAD) was plotted using Circos (available from http://qplot.cn/) [22]. The three highly associated BMPs, type 1 receptors and type receptors are highlighted, respectively. Results shown are from the analyses of gene expression in the TCGA-STAD cohort using Spearman correlation test.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
Dr Zhiwei Sun is a recipient of China Scholarship from Cardiff University.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.csbj.2019.12.014.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Khazaei S. Global incidence and mortality rates of stomach cancer and the human development index: an ecological study. Asian Pac J Cancer Prev. 2016;17(4):1701–1704. doi: 10.7314/apjcp.2016.17.4.1701. [DOI] [PubMed] [Google Scholar]
- 2.Garattini S.K. Molecular classifications of gastric cancers: novel insights and possible future applications. World J Gastrointest Oncol. 2017;9(5):194–208. doi: 10.4251/wjgo.v9.i5.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bang Y.J. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 4.Tirino G. What's new in gastric cancer: the therapeutic implications of molecular classifications and future perspectives. Int J Mol Sci. 2018;19(9) doi: 10.3390/ijms19092659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Canalis E., Economides A.N., Gazzerro E. Bone morphogenetic proteins, their antagonists, and the skeleton. Endocr Rev. 2003;24(2):218–235. doi: 10.1210/er.2002-0023. [DOI] [PubMed] [Google Scholar]
- 6.Maeda S. Endogenous TGF-beta signaling suppresses maturation of osteoblastic mesenchymal cells. EMBO J. 2004;23(3):552–563. doi: 10.1038/sj.emboj.7600067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hassel S. Initiation of Smad-dependent and Smad-independent signaling via distinct bmp-receptor complexes. J Bone Joint Surg Am. 2003;85:44–51. doi: 10.2106/00004623-200300003-00009. [DOI] [PubMed] [Google Scholar]
- 8.Jeong J. Bone morphogenetic protein signaling: implications in urology. Korean J Urol. 2010;51(8):511–517. doi: 10.4111/kju.2010.51.8.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hardwick J.C. Bone morphogenetic protein signalling in colorectal cancer. Nat Rev Cancer. 2008;8(10):806–812. doi: 10.1038/nrc2467. [DOI] [PubMed] [Google Scholar]
- 10.Raval P. Expression of bone morphogenetic proteins by osteoinductive and non-osteoinductive human osteosarcoma cells. J Dent Res. 1996;75(7):1518–1523. doi: 10.1177/00220345960750071301. [DOI] [PubMed] [Google Scholar]
- 11.Guo W. Expression of bone morphogenetic proteins and receptors in sarcomas. Clin Orthop Relat Res. 1999;365:175–183. doi: 10.1097/00003086-199908000-00023. [DOI] [PubMed] [Google Scholar]
- 12.Gao Y.H., Yang L.Y. In situ hybridization and immunohistochemical detection of bone morphogenetic protein genes in ameloblastomas. Zhonghua Yi Xue Za Zhi. 1994;74(10):621–623. 647. [PubMed] [Google Scholar]
- 13.Kusafuka K. Immunohistochemical evaluation of cartilage-derived morphogenic protein-1 and -2 in normal human salivary glands and pleomorphic adenomas. Virchows Arch. 2003;442(5):482–490. doi: 10.1007/s00428-003-0761-y. [DOI] [PubMed] [Google Scholar]
- 14.Tamada H. Epigenetic regulation of human bone morphogenetic protein 6 gene expression in prostate cancer. J Bone Miner Res. 2001;16(3):487–496. doi: 10.1359/jbmr.2001.16.3.487. [DOI] [PubMed] [Google Scholar]
- 15.Guo D., Huang J., Gong J. Bone morphogenetic protein 4 (BMP4) is required for migration and invasion of breast cancer. Mol Cell Biochem. 2012;363(1–2):179–190. doi: 10.1007/s11010-011-1170-1. [DOI] [PubMed] [Google Scholar]
- 16.Ye L., Bokobza S.M., Jiang W.G. Bone morphogenetic proteins in development and progression of breast cancer and therapeutic potential (review) Int J Mol Med. 2009;24(5):591–597. doi: 10.3892/ijmm_00000269. [DOI] [PubMed] [Google Scholar]
- 17.Cao Y. BMP4 inhibits breast cancer metastasis by blocking myeloid-derived suppressor cell activity. Cancer Res. 2014;74(18):5091–5102. doi: 10.1158/0008-5472.CAN-13-3171. [DOI] [PubMed] [Google Scholar]
- 18.Kim S.G. Expression of bone morphogenic protein-4 is inversely related to prevalence of lymph node metastasis in gastric adenocarcinoma. Surg Today. 2011;41(5):688–692. doi: 10.1007/s00595-010-4320-2. [DOI] [PubMed] [Google Scholar]
- 19.Kang M.H. Metastatic function of BMP-2 in gastric cancer cells: the role of PI3K/AKT, MAPK, the NF-kappaB pathway, and MMP-9 expression. Exp Cell Res. 2011;317(12):1746–1762. doi: 10.1016/j.yexcr.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Vasaikar S.V. LinkedOmics: analyzing multi-omics data within and across 32 cancer types. Nucleic Acids Res. 2018;46(D1):D956–D963. doi: 10.1093/nar/gkx1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szasz A.M. Cross-validation of survival associated biomarkers in gastric cancer using transcriptomic data of 1,065 patients. Oncotarget. 2016;7(31):49322–49333. doi: 10.18632/oncotarget.10337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krzywinski M. Circos: an information aesthetic for comparative genomics. Genome Res. 2009;19(9):1639–1645. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papamichael D. Prognostic role of angiogenesis in colorectal cancer. Anticancer Res. 2001;21(6B):4349–4353. [PubMed] [Google Scholar]
- 24.Saito H., Tsujitani S. Angiogenesis, angiogenic factor expression and prognosis of gastric carcinoma. Anticancer Res. 2001;21(6B):4365–4372. [PubMed] [Google Scholar]
- 25.Nakagawa S. Tumor angiogenesis as an independent prognostic factor after extended radical esophagectomy for invasive squamous cell carcinoma of the esophagus. Surgery. 2001;129(3):302–308. doi: 10.1067/msy.2001.111122. [DOI] [PubMed] [Google Scholar]
- 26.Ikeda N. Prognostic significance of angiogenesis in human pancreatic cancer. Br J Cancer. 1999;79(9–10):1553–1563. doi: 10.1038/sj.bjc.6690248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuwahara K. Expressions of angiogenic factors in pancreatic ductal carcinoma: a correlative study with clinicopathologic parameters and patient survival. Pancreas. 2003;26(4):344–349. doi: 10.1097/00006676-200305000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Al-Rawi M.A., Jiang W.G. Lymphangiogenesis and cancer metastasis. Front Biosci (Landmark Ed) 2011;16:723–739. doi: 10.2741/3715. [DOI] [PubMed] [Google Scholar]
- 29.Wu X., Shi W., Cao X. Multiplicity of BMP signaling in skeletal development. Ann N Y Acad Sci. 2007;1116:29–49. doi: 10.1196/annals.1402.053. [DOI] [PubMed] [Google Scholar]
- 30.Buijs J.T. Bone morphogenetic protein 7 in the development and treatment of bone metastases from breast cancer. Cancer Res. 2007;67(18):8742–8751. doi: 10.1158/0008-5472.CAN-06-2490. [DOI] [PubMed] [Google Scholar]
- 31.Du J. Bone morphogenetic protein 6 inhibit stress-induced breast cancer cells apoptosis via both Smad and p38 pathways. J Cell Biochem. 2008;103(5):1584–1597. doi: 10.1002/jcb.21547. [DOI] [PubMed] [Google Scholar]
- 32.Arnold S.F., Tims E., McGrath B.E. Identification of bone morphogenetic proteins and their receptors in human breast cancer cell lines: importance of BMP2. Cytokine. 1999;11(12):1031–1037. doi: 10.1006/cyto.1999.0508. [DOI] [PubMed] [Google Scholar]
- 33.Clement J.H. Bone morphogenetic protein 2 (BMP-2) induces in vitro invasion and in vivo hormone independent growth of breast carcinoma cells. Int J Oncol. 2005;27(2):401–407. [PubMed] [Google Scholar]
- 34.Dai J. Vascular endothelial growth factor contributes to the prostate cancer-induced osteoblast differentiation mediated by bone morphogenetic protein. Cancer Res. 2004;64(3):994–999. doi: 10.1158/0008-5472.can-03-1382. [DOI] [PubMed] [Google Scholar]
- 35.Deckers M.M.L. Bone morphogenetic proteins stimulate angiogenesis through osteoblast-derived vascular endothelial growth factor A. Endocrinology. 2002;143(4):1545–1553. doi: 10.1210/endo.143.4.8719. [DOI] [PubMed] [Google Scholar]
- 36.Jiao G. BMPR2 inhibition induced apoptosis and autophagy via destabilization of XIAP in human chondrosarcoma cells. Cell Death Dis. 2014;5 doi: 10.1038/cddis.2014.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ten Dijke P. Regulation of cell proliferation by Smad proteins. J Cell Physiol. 2002;191(1):1–16. doi: 10.1002/jcp.10066. [DOI] [PubMed] [Google Scholar]
- 38.Massague J. G1 cell-cycle control and cancer. Nature. 2004;432(7015):298–306. doi: 10.1038/nature03094. [DOI] [PubMed] [Google Scholar]
- 39.Ghosh-Choudhury N. Bone morphogenetic protein-2 induces cyclin kinase inhibitor p21 and hypophosphorylation of retinoblastoma protein in estradiol-treated MCF-7 human breast cancer cells. Biochim Biophys Acta. 2000;1497(2):186–196. doi: 10.1016/s0167-4889(00)00060-4. [DOI] [PubMed] [Google Scholar]
- 40.Montesano R., Sarkozi R., Schramek H. Bone morphogenetic protein-4 strongly potentiates growth factor-induced proliferation of mammary epithelial cells. Biochem Biophys Res Commun. 2008;374(1):164–168. doi: 10.1016/j.bbrc.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 41.Lee K.B. Recombinant human bone morphogenetic protein-2 inhibits gastric cancer cell proliferation by inactivating Wnt signaling pathway via c-Myc with aurora kinases. Oncotarget. 2016;7(45):73473–73485. doi: 10.18632/oncotarget.11969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang J. Effect of bone morphogenetic protein-2 on proliferation and apoptosis of gastric cancer cells. Int J Med Sci. 2012;9(2):184–192. doi: 10.7150/ijms.3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wen X.Z. BMP-2 modulates the proliferation and differentiation of normal and cancerous gastric cells. Biochem Biophys Res Commun. 2004;316(1):100–106. doi: 10.1016/j.bbrc.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 44.Shirai Y.T. Bone morphogenetic protein-2 and -4 play tumor suppressive roles in human diffuse-type gastric carcinoma. Am J Pathol. 2011;179(6):2920–2930. doi: 10.1016/j.ajpath.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alarmo E.L. BMP7 influences proliferation, migration, and invasion of breast cancer cells. Cancer Lett. 2009;275(1):35–43. doi: 10.1016/j.canlet.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 46.Scherberich A. Tenascin-W is found in malignant mammary tumors, promotes alpha8 integrin-dependent motility and requires p38(MAPK) activity for BMP-2 and TNF-alpha induced expression in vitro. Oncogene. 2005;24(9):1525–1532. doi: 10.1038/sj.onc.1208342. [DOI] [PubMed] [Google Scholar]
- 47.Hanavadi S. The role of growth differentiation factor-9 (GDF-9) and its analog, GDF-9b/BMP-15, in human breast cancer. Ann Surg Oncol. 2007;14(7):2159–2166. doi: 10.1245/s10434-007-9397-5. [DOI] [PubMed] [Google Scholar]
- 48.Johansson N. Collagenase-3 (MMP-13) is expressed by hypertrophic chondrocytes, periosteal cells, and osteoblasts during human fetal bone development. Dev Dyn. 1997;208(3):387–397. doi: 10.1002/(SICI)1097-0177(199703)208:3<387::AID-AJA9>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 49.Montesano R. Bone morphogenetic protein-4 abrogates lumen formation by mammary epithelial cells and promotes invasive growth. Biochem Biophys Res Commun. 2007;353(3):817–822. doi: 10.1016/j.bbrc.2006.12.109. [DOI] [PubMed] [Google Scholar]
- 50.Yang S. Diverse biological effect and Smad signaling of bone morphogenetic protein 7 in prostate tumor cells. Cancer Res. 2005;65(13):5769–5777. doi: 10.1158/0008-5472.CAN-05-0289. [DOI] [PubMed] [Google Scholar]
- 51.Yang S. BMP-6 promotes E-cadherin expression through repressing delta EFI in breast cancer cells. BMC Cancer. 2007:7. doi: 10.1186/1471-2407-7-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mani S.A. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoshimatsu Y. Bone morphogenetic protein-9 inhibits lymphatic vessel formation via activin receptor-like kinase 1 during development and cancer progression. Proc Natl Acad Sci U S A. 2013;110(47):18940–18945. doi: 10.1073/pnas.1310479110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Silvestris N. Natural history of malignant bone disease in gastric cancer: final results of a multicenter bone metastasis survey. PLoS ONE. 2013;8(10) doi: 10.1371/journal.pone.0074402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.