Abstract
Purpose: Myeloid cell leukaemia-1 (Mcl-1) is a valuable target in tumour treatments. However, several reports have suggested that Mcl-1 may play a role in tuberculosis infection. Therefore, we investigated the function of Mcl-1 in tuberculosis infection and the underlying regulatory mechanism. Methods: Mcl-1-shRNA was used to down-regulate Mcl-1 expression in BCG-, H37Ra-, H37Rv- and XJ-MTB-infected mouse peritoneal macrophages. TUNEL staining detected macrophage apoptosis. The colony-forming units (CFUs) were determined to assess the Mycobacterium tuberculosis (MTB) clearance after down-regulating Mcl-1. Immunohistochemical analysis of Mcl-1 expression in mouse peritoneal macrophages was performed. Haematoxylin and eosin staining detected pathologic damage of the liver, spleen, lung, and kidney in mice. Real-time PCR and Western blotting determined the expression of cytochrome-c in Mcl-1-shRNA-treated mouse peritoneal macrophages infected with MTB strains that differ in virulence. Results: Mcl-1-shRNA significantly promoted host macrophage apoptosis and cytochrome-c induction, and the apoptotic induction of the XJ-MTB and H37Rv strains was stronger than the H37Ra and BCG strains (P<0.05). Apoptotic protein cytochrome-c levels continued to increase in mouse peritoneal macrophages infected MTB before and after treatment, Caspase-8 levels only slightly increased after Mcl-1-shRNA-treated (P<0.05), but the increase of Cytochrome-c have no significant differences compared with Caspase-8 levels (P>0.05). Conclusion: Mcl-1-shRNA intervention effectively down-regulated Mcl-1 expression, significantly increased host macrophage apoptosis, and induced cytochrome-c expression in mouse peritoneal macrophages infected with MTB strains of different virulence, and these changes were influenced by the virulence of the MTB strains. The mitochondria-mediated intrinsic apoptotic pathway play an important role before Mcl-1-shRNA-trated, and then with the extrinsic apoptotic pathway co-regulate host macrophage apoptosis.
Keywords: Mcl-1, MTB, cytochrome-c, macrophage apoptosis
Introduction
In recent years, the morbidity of tuberculosis (TB) infection was reported to be decreasing; however, the incidence and prevalence of multidrug-resistant TB (MDR-TB) have increased [1]. Mycobacterium tuberculosis (MTB) infection affects almost one-third of the total worldwide population. In China, TB has affected more than 5 million patients (80% in rural areas), and thus, China is currently ranked second worldwide for the quantity of TB-infected patients [2]. In Xinjiang, where the TB epidemic is more serious and complicated, prevention and control of TB infection is particularly urgent [3]. Nonetheless, our current tools to combat MDR-TB are unsatisfactory. Therefore, proposing new strategies to fight TB infection is imperative.
MTB is a typical intracellular pathogen that can evade the immune system and is cleared by a variety of mechanisms, thus affecting the regulation of its host cells (monocyte-macrophage cells). It also alters macrophage functions to inhibit activation of apoptotic pathways, while the pathogen replicates, reproduces, and lives within the macrophages [4]. Furthermore, MTB strains with differential virulence regulate host macrophage apoptosis to evade macrophage killing [5]. Host macrophages that can activate the normal apoptotic process can eliminate TB infection and prevent latency and spread of TB in the body. The anti-apoptotic protein myeloid cell leukaemia-1 (Mcl-1) is closely related to this host macrophage apoptotic process [6].
Mcl-1 belongs to the Bcl-2 family, and its major role is to inhibit cell apoptosis. Increasing evidence indicates that Mcl-1 could be a potential target in latent TB. A previous study demonstrated that during long-term survival of MTB in macrophages, the Mcl-1 gene was highly expressed, and at the same time, the macrophage apoptosis rate declined [6]. In 2016, Wang et al. found that down-regulated Mcl-1 expression increased MTB-infected macrophage apoptosis [7,8]. Therefore, an in-depth analysis of Mcl-1 down-regulation-induced apoptosis of MTB-infected host macrophages is needed.
Mitochondria are central organelles in the molecular events involved in energy production, cell survival, and cell apoptosis. Analysing the mitochondrial responses to MTB infection may help elucidate the mechanisms underlying mycobacterial pathogenesis and the host-pathogen relationships. Studies have shown that H37Rv induces cytochrome-c (cyt-c) release from the mitochondrial intermembrane space to the cytosol, suggesting that cyt-c plays a role in the MTB and host cell interactions, and that a virulent strain of MTB could regulate cyt-c translocation [9]. However, the primary function of Mcl-1 is stabilising the mitochondrial membrane and inhibiting the release of cyt-c, thereby promoting cell survival and preventing apoptosis [10]. This study will focus on analysing the time course of changes in cyt-c expression, the effect of inhibition of Mcl-1 expression and the regulation of MTB-infected host macrophage apoptosis to explore the regulatory mechanisms of Mcl-1 in different MTB strains infection.
Materials and methods
Animals
Two-month-old female BALB/c mice (weighing 18 ± 2 g) were purchased from the Experimental Animal Centre of Shihezi University (Xinjiang, China). The mice were maintained under a 12 h light/dark cycle, and food and water were provided ad libitum. All studies were implemented according to protocols approved by the Institutional Animal Ethics Committee (IAEC) prior to experimentation.
Bacteria
The BCG strains H37Ra and H37Rv were obtained from Chinese Medicine Biological Products (Beijing, China). A strongly virulent MTB isolate from the Xinjiang region (XJ-MTB) was identified previously and stored by our laboratory. A mixture of Sutong culture and normal saline (0.5 ml; volume ratio of 3:1) was used to dilute the bacteria, and 100 μl of the bacterial dilution was spread on modified Roche medium. After 23 weeks, we selected the bacteria that grew well on Roche medium and resuspended them in a small amount of normal saline solution containing 0.05% Tween-80. The bacterial suspension was adjusted to the McFarland standard corresponding to 1 × 107 colony-forming units (CFU)/ml.
Plasmid
We previously screened plasmids for successful silencing of the Mcl-1 gene and selected a specific shRNA eukaryotic expression plasmid, Mcl-1-shRNA [7]. Mcl-1-shRNA was provided by the Shanghai Ji Kai gene company.
MTB infection mouse model construction
At room temperature, previously untreated groups of mice (n = 10/group) were infected intraperitoneally (i.p.) with 0.3 ml (0.3 × 107 CFU) of the bacterial suspension, and uninfected control mice were administered saline on the same schedule [11]. The experimental group was administered Mcl-1-shRNA [7] using a previously described method [12]. The control and MTB-infected mice were treated i.p. with 75 μg of Mcl-1-shRNA 3 days after infection (time 0). Mice were sacrificed every 24 h, and the peritoneal macrophages were then collected and cultured from each group (Figure 1).
Figure 1.
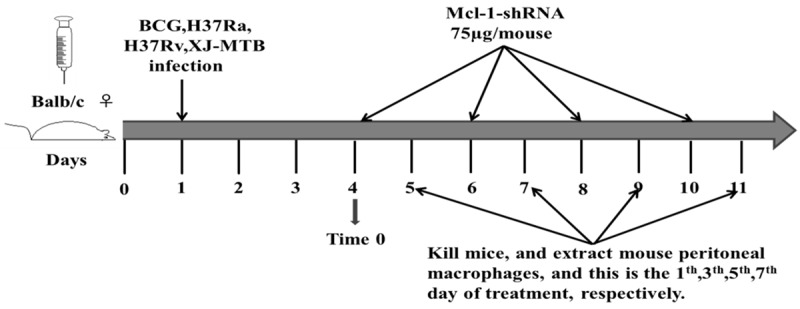
The schematic of the grouping and treatment. A schematic diagram of the mouse peritoneal macrophage treatments after BCG, H37Ra, H37Rv and XJ-MTB infection.
Mouse peritoneal macrophage collection and culture
On the 1st, 3rd, 5th, and 7th days after infection, the mice were decapitated, and their abdominal skin was disinfected with 75% ethanol under ultraclean Taichung. Then, the abdominal skin was lifted with forceps, a small opening was cut to tear the skin, and the peritoneum was completely exposed. Using forceps, the peritoneum was filled with a 5-ml injection of 1640 medium (HyClone, USA). The abdomen was massaged repeatedly for 10 min, avoiding the intestines and fat. The abdomen was massaged several times for 10 min, avoiding the intestine and fat. The peritoneal fluid was repeatedly withdrawn and rinsed in different directions several times. The tissues were rinsed several times until there was no more peritoneal fluid. The cell suspension was centrifugated at 1200 r/min for 5 min, the supernatant was decanted, and phosphate-buffered saline (PBS) was used to wash the samples, with DMEM (HyClone, USA) containing 10% foetal bovine serum for the suspension culture. Cells were added to 6 wells in the cell culture plate and incubated at 37°C in a 5% CO2 incubator in 6 h for adherent mouse peritoneal macrophages [13,14].
Determination of CFUs
The mouse peritoneal macrophages were lysed 3 days after infection to release the XJ-MTB strain. Serial 10-fold dilutions of the samples were plated on 7H9 agar, and colonies were counted after incubation for 3 weeks of incubation at 37°C to determine the bacterial CFUs.
Detection of apoptosis: TUNEL assay
Mouse peritoneal macrophages were harvested after treatment, cell suspensions (0.4 × 105 cells in 0.5 ml) were generated, and macrophages were grown on 4-well slides. The cells were resuspended with DMEM containing 10% foetal bovine serum, and the cell suspension was added to six wells. The macrophages were washed once in PBS before being fixed in 4% paraformaldehyde (pH 7.4) for 1 h at room temperature. After an additional wash, cells were permeabilised by adding a permeabilisation solution (0.1% Triton X-100, 0.1% sodium citrate in H2O) for 8 min on ice and washed with PBS. DNA strand breaks were labelled by addition of the terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) reaction mixture, according to the manufacturer’s instructions (In Situ Cell Death Detection Kit, Roche Applied Science). The number of TUNEL-positive cells was reported as the percent of total number of cells counted.
Immunohistochemical detection of the anti-apoptotic molecule Mcl-1
For immunohistochemical analysis, the mouse peritoneal macrophage concentration was adjusted, and smears were prepared and fixed in 4% paraformaldehyde overnight at 4°C. The smears were washed twice with PBS and pre-blocked for 30 min in 10% foetal bovine serum (Thermo, USA). After the PBS washes, the samples were incubated overnight at 4°C with 1:1000 diluted polyclonal rabbit anti-mouse Mcl-1 antibody (Bioss, Beijing, China) or with diluted normal rabbit serum as a control. Slides were rinsed three times with PBS before adding 1:200 diluted biotinylated goat anti-rabbit immunoglobulin G (ZSGB-BIO, Beijing, China). This was followed by incubation with streptavidin-peroxidase complex (ZSGB-BIO, Beijing, China) for 30 min at room temperature. After 30 min incubation, slides were washed with PBS. Colour development was achieved by application of 3’-diaminobenzidine for 3-5 min in the dark. The slides were then washed and counterstained with haematoxylin. Two pathologists performed the immunohistochemical analysis, and the subcellular localization of Mcl-1 (nuclear, cytoplasmic) was recorded at 400× magnification. Positive- and negative-stained macrophages were counted, and data are expressed as the percentage of Mcl-1-positive macrophages, as described previously. The staining pattern was classified as follows: (0) no staining or fewer than 5% positive cells; (1) weak staining, 6-20% positive cells; (2) moderate staining, 21-50% positive cells; and (3) strong staining, more than 51% positive cells.
Histopathological analysis
After the mice were sacrificed, the lung, liver, spleen, and kidney were extracted immediately, fixed in 10% formalin and embedded in paraffin. After the samples were processed in 95% ethanol for anhydrous ethanol dehydration processing, they were treated with xylene for 30 minutes. Then, they were paraffin embedded, sectioned, dewaxed, and treated with 70% ethanol, 80% ethanol, 90% ethanol, 95% ethanol, and anhydrous ethanol dehydration for 5 min. Finally, they were stained with haematoxylin and eosin (H&E) and photographed using an Olympus CKX41 microscope (Olympus) fitted with an Olympus DP20 camera connected to a computer. The Image-Pro Plus programme (Media Cybernetics) was utilised to objectively assess the level of inflammation present in each image. The inflammatory areas stained a more intense purple than the non-inflammatory areas. Because of the different components, the cytoplasm showed different colours. Cells or tissue components and lesions of the general morphology were observed.
Quantitative RT-PCR
Total RNA from host macrophages was extracted using TRIzol reagent (Invitrogen). Reverse transcription was performed with a SuperRT cDNA Kit (TianGen) according to the manufacturer’s instructions. Q-PCR primers were designed and synthesised by Sangon Biotech. The PCR reaction conditions were as follows: 95°C for 5 min, 95°C for 10 s, and 57°C for 30 s, for a total of 40 cycles. The primers sequences are shown in Table 1. Samples were amplified according to the QuantiFast SYBR Green PCR Kit manufacturer’s instruction. The β-actin constitutive control was included to standardise the data, and the relative levels of Bcl-2, Bax, Caspase-1, 3, and 8, and cyt-c mRNA were calculated using the 2-ΔΔCt method.
Table 1.
Primes used for real-time PCR
| Gene | Sequence (5’-3’) |
|---|---|
| β-actin | Forward: AATTCCATCATGAAGTGTGA |
| Reverse: ACTCCTGCTTGCTGATCCAC | |
| Mcl-1 | Forward: TATTTCTTTCGGTGCCTTTGTG |
| Reverse: AGTCCCGTTTCGTCCTTACA | |
| Bcl-2 | Forward: CGACTTCTTCAGCATCAGGA |
| Reverse: TGAGCCACAGGGAGGTTCT | |
| Bax | Forward: TGACTGGAAAGCCGAAACTC |
| Reverse: GCAAGCCATCTCCTCATCA | |
| Caspase-3 | Forward: TGACTGGAAAGCCGAAACTC |
| Reverse: GCAAGCCATCTCCTCATCA | |
| Caspase-8 | Forward: GCCCTCAAGTTCCTGTGCT |
| Reverse: GATTGCCTTCCTCCAACATC | |
| Caspase-1 | Forward: AATTCCATCATGAAGTGTGA |
| Reverse: CCTCCAGCAGCAACTTCATT | |
| Cytochrome-c | Forward: CACGCTTTACCCTTCGTTCT |
| Reverse: CACTCATTTCCCTGCCATTC |
Immunoblotting analysis
Macrophages were harvested after treatment, washed with ice-cold PBS, and lysed in RIPA lysis buffer (Solarbio Biotechnology). Protein concentrations were determined by the bicinchoninic acid (BCA) assay. The total amount of protein was quantified, and equal amounts of proteins were resolved in 12% and 15% SDS-polyacrylamide gels and transferred to polyvinylidene difluoride membranes (Millipore, Billerica, MA). Membranes were blocked with 5% nonfat dried milk in Tris-buffered saline-Tween-20 (TBST) and probed with a primary antibody overnight at 4°C. After the membranes were washed with TBST, they were probed with target-specific primary antibodies purchased from Cell Signaling Technology (CST). Western blot was performed using the following antibodies: rabbit anti-mouse Mcl-1 and Bcl-2 (dilution: 1:10,000), Bax, Caspase-3 and cyt-c (dilution 1:20,000), Caspase-8 (dilution 1:5000), and Caspase-1. Mouse anti-mouse β-actin (dilution 1:1000) was purchased from ZSGB-BIO. The membranes were then stained by horseradish peroxidase-conjugated secondary antibody (Jackson Immunologicals, West Grove, PA). Blots were developed with an enhanced chemiluminescence (ECL) detection system (Perkin Elmer Bioscience, Waltham, MA) in accordance with the manufacturer’s protocol.
Statistical analyses
Data are presented as the means ± SDs. Data comparisons were performed using a one-way ANOVA followed by LSD test for multiple comparisons. These analyses were implemented using SPSS 17.0. Two-tailed unpaired Student’s t-tests were used to evaluate the differences between the control and treated groups. (GraphPad Software, Inc., San Diego, CA). P<0.05 was considered significant.
Results
Mcl-1-shRNA treatment increased apoptosis of mouse peritoneal macrophages infected with MTB strains that differ in virulence
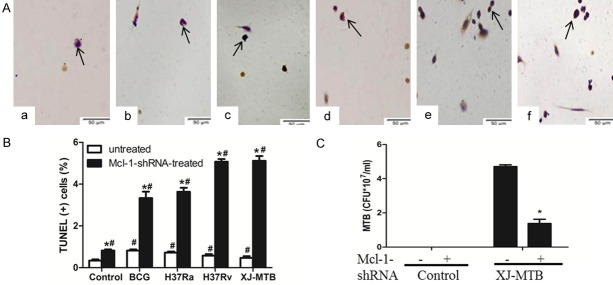
RNA interference (RNAi) is the most direct and common method used to down-regulate gene expression [15]. The Mcl-1-shRNA eukaryotic expression plasmid was previously screened and shown to silence Mcl-1 expression. And we found that Mcl-1 expression peaked on the fifth day of MTB infection of host macrophages. Therefore, we selected the fifth day for Mcl-1-shRNA treatment of mouse peritoneal macrophage infected with different variants of MTB for analysis. After Mcl-1-shRNA inhibited Mcl-1 expression, apoptosis rates showed slight increases compared with those in the untreated group of mouse peritoneal macrophages. Similar results were observed for the infection only group (Figure 2B; Table 2) (P<0.05). However, the apoptosis rate had a significant increase in the Mcl-1-shRNA-treated MTB-infected mouse peritoneal macrophages compared with the infected group (Figure 2A, 2B; Table 2) (P<0.05). Moreover, the apoptosis induction of the Mcl-1-shRNA-treated H37Rv- and XJ-MTB-infected groups was significantly stronger than the BCG- and H37Ra-infected groups, and the differences were statistically significant (Figure 2A, 2B; Table 2) (P<0.05). However, the H37Ra-infected treatment group had no significant differences compared with the XJ-MTB-infected group (Table 2) (P>0.05).
Figure 2.
Mcl-1-shRNA treatment increased apoptosis of mouse peritoneal macrophages infected with BCG, H37Ra, H37Rv and XJ-MTB (×400) and reduced the intracellular growth of MTB. A and B: Apoptosis of Mcl-1-shRNA-treated mouse peritoneal macrophages infected with different strains. TUNEL assays of mouse peritoneal macrophages Apoptotic cells were observed to be dark brown under light microscopy, while normal cells were not stained. a: Control group; b: Mcl-1-shRNA treatment group; c: Mcl-1-shRNA+BCG; d: Mcl-1-shRNA+H37Ra; e: Mcl-1-shRNA+H37Rv; f: Mcl-1-shRNA+XJ-MTB. Percentages of cells undergoing apoptosis. Data represent the mean ± SD of three independent assays in each experiment. *P<0.05 for Mcl-1-shRNA-treated group compared with untreated group. #P<0.05 for infected group compared with uninfected group. Values are the mean ± SD. C: CFUs were recovered from 107 untreated host cells or from Mcl-1-shRNA-treated host cells that were infected with XJ-MTB. Data represent the mean ± SD of three independent assays plated in triplicate. *P<0.05 for XJ-MTB-infected host cells compared with Mcl-1-shRNA-treated XJ-MTB-infected cells.
Table 2.
Mcl-1 expression and apoptosis rate in Mcl-1 inhibitors-treated mouse peritoneal macrophages infected with XJ-MTB
| Groups (n = 10) | Mcl-1 expression | Positive rate (%) | Apoptosis rate (%) | |||
|---|---|---|---|---|---|---|
|
| ||||||
| - | + | ++ | +++ | |||
| Control | 10 | 0 | 0 | 0 | 0 | 0.03 ± 0.01b,c,d,e,f |
| Mcl-1-shRNA | 9 | 1 | 0 | 0 | 10 | 0.44 ± 0.06a,c,d,e,f |
| BCG+Mcl-1-shRNA | 6 | 1 | 2 | 1 | 40 | 3.37 ± 0.03a,b,d,e,f |
| H37Ra+Mcl-1-shRNA | 7 | 1 | 2 | 0 | 30 | 3.7 ± 0.03a,b,c,e,f |
| H37Rv+Mcl-1-shRNA | 9 | 1 | 0 | 0 | 10 | 5.2 ± 0.02a,b,c,d |
| XJ-MTB+Mcl-1-shRNA | 9 | 1 | 0 | 0 | 10 | 5.31 ± 0.03a,b,c,d |
P<0.05 vs. control group.
P<0.05 vs. Mcl-1-shRNA.
P<0.05 vs. BCG+Mcl-1-shRNA.
P<0.05 vs. H37Ra+Mcl-1-shRNA.
P<0.05 vs. H37Rv+Mcl-1-shRNA.
P<0.05 vs. XJ-MTB+Mcl-1-shRNA.
Knockdown of Mcl-1 expression by Mcl-1-shRNA reduced the intracellular survival of XJ-MTB
To determine whether Mcl-1-shRNA inhibition of Mcl-1 expression correlated with the intracellular growth of MTB, considering that more higher apoptosis rate of XJ-MTB, so we assessed the survival of XJ-MTB in infected peritoneal macrophages that were untreated or treated with Mcl-1-shRNA (as agent only). There were significant decreases in the number of CFUs recovered from the Mcl-1-shRNA-treated group compared with the untreated XJ-MTB-infected group (Figure 2C) (P<0.05). The survival rate of XJ-MTB in Mcl-1-shRNA-treated mouse peritoneal macrophages decreased from 4.7 to 1.4 (by 3.4-fold).
Mcl-1-shRNA intervention reduced MTB in host macrophages
Immunostaining of Mcl-1 protein in mouse peritoneal macrophages revealed that it was localised predominantly to the cytoplasm and nucleus (Figure 3). Our results showed strong staining of Mcl-1 in mouse peritoneal macrophages infected with MTB (data not shown), while diffuse staining of Mcl-1 was observed in uninfected host macrophages (Figure 3A). Mcl-1 staining was weakened following treatment with Mcl-1-shRNA in mouse peritoneal macrophages (Figure 3B). The expression of Mcl-1 was decreased significantly in Mcl-1-shRNA-treated mouse peritoneal macrophages, especially the XJ-MTB and H37Rv treatment group. In addition, the effects of the intervention in the XJ-MTB and H37Rv strain treatment groups were better than those in the BCG and H37Ra infection groups (Figure 3) (P<0.05).
Figure 3.
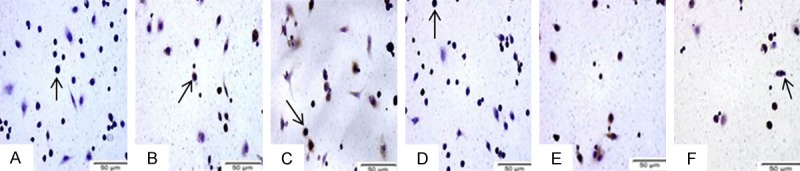
Typical Mcl-1 immunohistochemistry results for mouse peritoneal macrophages (×400). A. Control cells: Fewer than 5% of macrophages had detectable cytoplasmic or nuclear expression of Mcl-1 protein. B. Control cells treated with Mcl-1-shRNA: Mcl-1 expression was absent. C. BCG-infected host cells treated with Mcl-1-shRNA. D. H37Ra-infected host cells treated with Mcl-1-shRNA. E. H37Rv-infected host cells treated with Mcl-1-shRNA. F. XJ-MTB-infected host cells treated with Mcl-1-shRNA. Mcl-1-shRNA-treated cells showed weak staining for Mcl-1. Arrows indicate the observed host cells.
Mcl-1-shRNA treatment relieved pathological damage of mouse tissues
To determine whether down-regulated Mcl-1 affects MTB infection in mouse macrophage apoptosis, we observed the pathological damage of the lung, liver, spleen, and kidney before and after the Mcl-1-shRNA intervention in mice. After MTB infection, multifocal epithelioid cells in the lung tissue, lymphocyte infiltration of nodular lesions, alveolar capillary expansion and congestion, and interlobular vein expansion were observed. Furthermore, liver cell oedema, cavitation and degeneration around the central vein cavity and major central vein blood clots were observed. At the same time, liver antrum narrowing, regional bleeding, and interstitial lymphocytic infiltrates were noted. Splenic sinus congestion and expansion were observed with increased mononuclear macrophages. In addition, glomerular and renal interstitium blood clot, focal haemorrhage, tubular epithelial cells and oedema increased significantly (Figure 4A-D).
Figure 4.
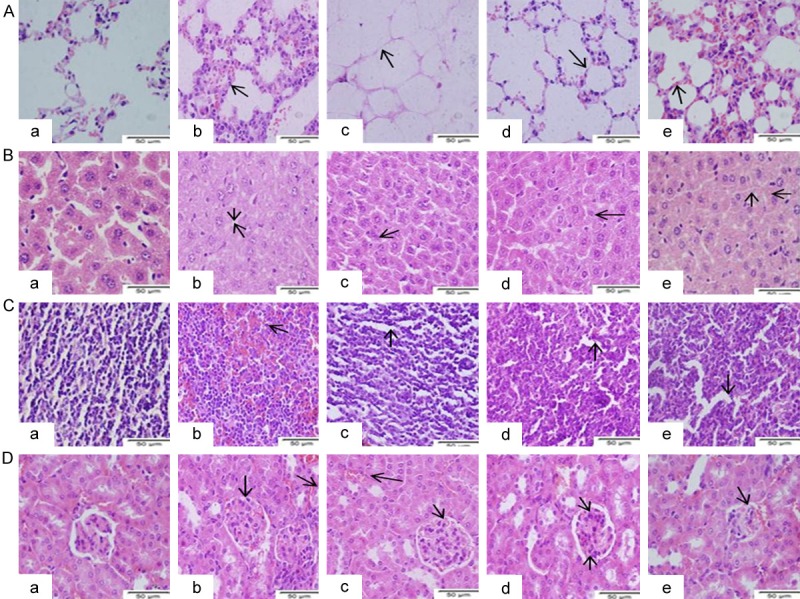
Histological changes in BCG-, H37Ra-, H37Rv- and XJ-MTB-infected mice treated with Mcl-1-shRNA. A-D. are H&E staining of normal and Mcl-1-shRNA-treated MTB-infected lung, liver, spleen, and kidney tissues in mice. A. Normal lung tissue and Mcl-1-shRNA-treated MTB-infected lung tissues. B. Normal liver tissue and Mcl-1-shRNA-treated MTB-infected liver tissues. C. Normal spleen tissue and Mcl-1-shRNA-treated MTB-infected spleen tissues. D. Normal kidney tissue and Mcl-1-shRNA-treated MTB-infected kidney tissues. a. Control group: normal mouse tissues. b. Mcl-1-shRNA-treated BCG-infected mouse tissues. c. Mcl-1-shRNA-treated H37Ra-infected mouse tissues. d. Mcl-1-shRNA-treated H37Rv-infected mouse tissues. e. Mcl-1-shRNA-treated XJ-MTB-infected mouse tissues. Arrows indicate pathological changes of lung tissue.
The pathological damages of the Mcl-1-shRNA-treated BCG infection group showed no significant improvement: mouse lung and spleen tissues still showed widespread blood clots as well as glomerular and renal interstitial capillary and venule expansion and ecchymosis. Only the central vein blood clot was significantly reduced, and the portal area lymphocyte infiltration decreased significantly in liver tissue (Figure 4A-D). The Mcl-1-shRNA-treated H37Ra infection group showed ameliorated congestion and expansion of the viscera compared with the Mcl-1-shRNA-treated BCG infection group (Figure 4A-D).
Mcl-1-shRNA intervention significantly relieved the mouse organ/tissue pathological damage caused by H37Rv and XJ-MTB infection. In the Mcl-1-shRNA-treated H37Rv infection group, pulmonary capillary and central venous liver congestion and renal interstitial blood vessel expansion were not obvious; splenic red pulp congestion and haemorrhage were reduced significantly (Figure 4A-D). Similarly, the Mcl-1-shRNA-treated H37Rv infection group mouse tissues showed a significant improvement. The degree of congestion of the organs/tissues was significantly reduced, lymphocyte effusion decreased significantly, and kidney epithelial cells did not show oedema (Figure 4A-D).
Mcl-1-shRNA treatment increased cyt-c mRNA expression to different extents in mouse macrophages infected with different strains
As shown in the previous study, Mcl-1-shRNA significantly increased the apoptosis rates of mouse peritoneal macrophages infected with MTB strains with different levels of virulence. Therefore, we detected cyt-c expression to explore the mechanism of Mcl-1-shRNA-induced apoptosis of macrophages infected with different strains. With prolonged infection, the expression of cyt-c mRNA increased gradually, and the fifth day showed a peak of expression after Mcl-1-shRNA treatment (i.e., the ninth day after infection). This difference was statistically significant (P<0.05). Compared to control infected cells, there was a small increase of cyt-c mRNA expression (1.07-fold, 1.1-fold, 1.23-fold, and 1.30-fold, respectively) in mouse peritoneal macrophages infected with BCG, H37Ra, H37Rv, and XJ-MTB (Figure 5) (P<0.05). However, cyt-c mRNA had a 2.03-fold, 2.13-fold, 2.41-fold, and 2.6-fold increase in Mcl-1-shRNA-treated mouse peritoneal macrophages infected with different strains of MTB compared with the untreated group (Figure 5) (P<0.05).
Figure 5.
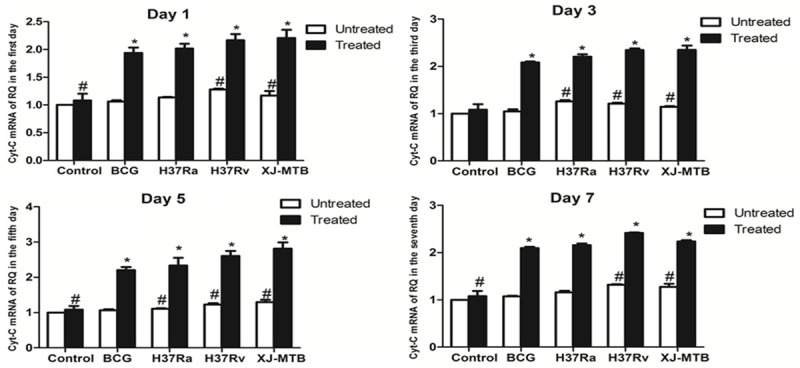
Mcl-1-shRNA treatment significantly increased cyt-c mRNA expression in MTB-infected mouse peritoneal macrophages. After MTB infection of Mcl-1-shRNA-treated mice at 1, 3, 5, and 7 days, total RNA was obtained from mouse peritoneal macrophages. For all groups (uninfected, Mcl-1-shRNA-treated, Mcl-1-shRNA-treated BCG-infected, Mcl-1-shRNA-treated H37Ra-infected, Mcl-1-shRNA-treated H37Rv-infected, Mcl-1-shRNA-treated Xinjiang-infected), quantitative PCR was performed on the cDNA. Cyt-c mRNA expression was measured by real-time PCR. *P<0.05 for Mcl-1-shRNA-treated group compared with untreated group. #P<0.05 for infected group compared with the control group. Values are the mean ± SD.
XJ-MTB induced increased cyt-c protein expression in Mcl-1-shRNA-treated mouse peritoneal macrophages
Cyt-c mRNA expression was significantly increased in Mcl-1-shRNA-treated different virulence M. tuberculosis-infected mouse peritoneal macrophages, and we examined whether the cyt-c protein level was consistent with gene expression. As shown in the Figure 6, the increased trend of cyt-c protein level was the same as the change in mRNA, and cyt-c protein level peaked on the fifth day after intervention. In addition, densitometry of ECL-developed bands showed a 1.8-fold, 1.93-fold, 1.8-fold, and 2.9-fold increase in cyt-c protein, as evaluated by Western blotting, in the BCG-, H37Ra-, H37Rv-, and XJ-MTB-infected mouse peritoneal macrophages compared with uninfected control cells (Figure 6) (P<0.05). Mcl-1-shRNA intervention significantly increased the cyt-c protein level, and there was a 2.9-fold, 3.3-fold, 4.0-fold, and 4.8-fold increase in BCG, H37Ra, H37Rv, and XJ-MTB-infected mouse peritoneal macrophages compared with the untreated group (Figure 6) (P<0.05).
Figure 6.
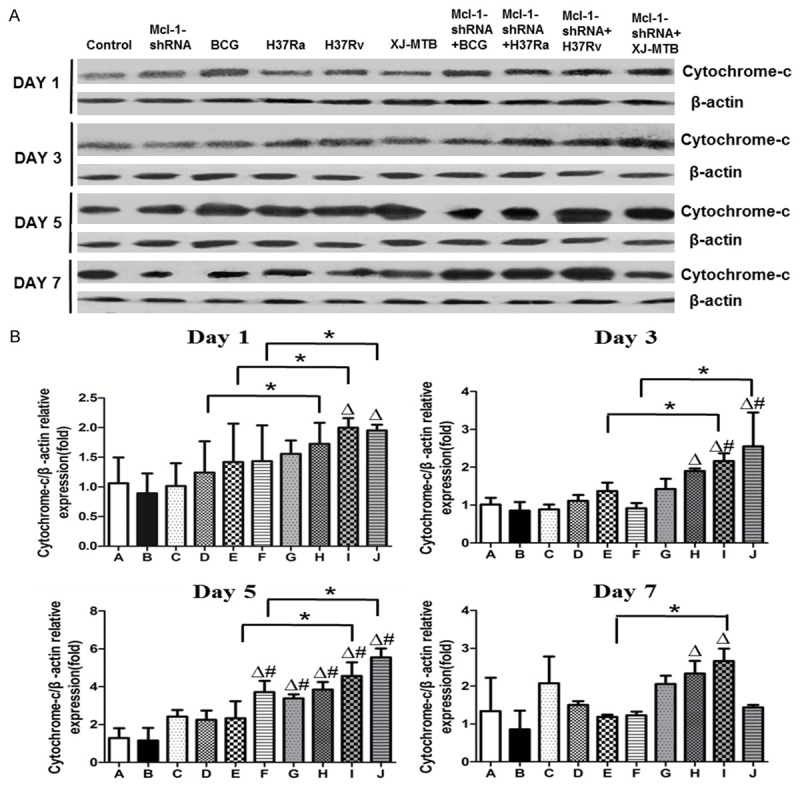
Mcl-1-shRNA treatment significantly increased cyt-c protein expression in MTB-infected mouse peritoneal macrophages. After MTB infection of Mcl-1-shRNA-treated mice at 1, 3, 5, and 7 days, Western blot detected cyt-c protein expression. ECL was obtained from mouse peritoneal macrophages from all groups (uninfected, Mcl-1-shRNA3-treated, Mcl-1-shRNA3-treated BCG-infected, Mcl-1-shRNA3-treated H37Ra-infected, Mcl-1-shRNA3-treated H37Rv-infected, Mcl-1-shRNA3-treated Xinjiang-infected). A: ECL results of cyt-c protein expression of Mcl-1-shRNA-treated MTB-infected mouse peritoneal macrophages at 1, 3, 5, and 7 days. B: Cyt-c protein expression of Mcl-1-shRNA-treated MTB-infected mouse peritoneal macrophages at 1, 3, 5, and 7 days. *P<0.05 for Mcl-1-shRNA-treated groups compared with untreated groups. #P<0.05 for infected groups compared with uninfected groups. ΔP<0.05 for Mcl-1-shRNA-treated groups compared with uninfected group groups. Values are the mean ± SD.
The mitochondrial apoptotic pathway may play an important role in the Mcl-1 regulatory mechanism
As we all know, cell apoptosis is mediated can’t without the extrinsic pathway and the mitochondrial apoptotic pathway [16,17]. Thus, we hypothesised that the extrinsic pathway and the mitochondrial apoptotic pathway may be the major regulatory pathways of Mcl-1 expression in MTB infected-macrophages. Therefore, we measured the mRNA and protein levels of the apoptosis-associated genes Mcl-1, Bcl-2, Bax, and Caspase-1, 3, and 8 and comprehensively analysed of the changes in their expression. After H37Rv infection of mouse peritoneal macrophages, Mcl-1 and Bcl-2 protein levels increased significantly compared with those of the control group (Figure 7) (P<0.05). At the same time, the cyt-c protein levels also increased significantly (Figure 7) (P<0.05), while Caspase-8 protein levels only increased slightly. After Mcl-1-shRNA treatment of mouse peritoneal macrophages infected with H37Rv, the Mcl-1 protein levels decreased sharply compared with those of the H37Rv group (Figure 7) (P<0.05). However, the cyt-c and Caspase-8 protein levels increased significantly, but there was no significant difference between these two proteins (Figure 7) (P>0.05). In addition, cyt-c protein levels showed increases in all processes, and higher than all proteins levels (Figure 7) (P<0.05).
Figure 7.
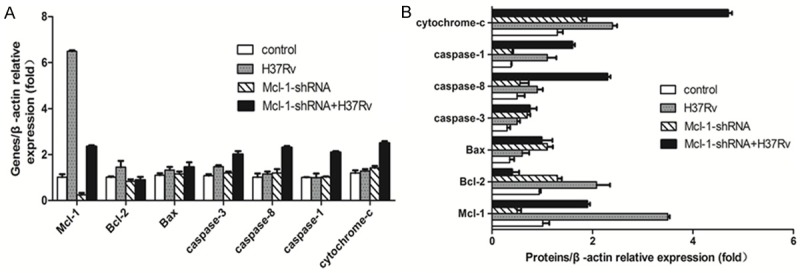
Comparison of the expression of apoptosis-related genes and proteins. Real-time PCR and Western blot detected the expression of apoptosis-related genes and proteins, including Bcl-2, Bax, Caspase-1, 3, and 8, and Cyt-c, after the Mcl-1-shRNA-treated mouse peritoneal macrophages were infected with H37Rv. A. Real-time PCR detected the relative expression of Bcl-2, Bax, Caspase-1, 3, and 8, and Cyt-c mRNA. B. Western blot detected the relative expression levels of Bcl-2, Bax, Caspase-1, 3, and 8, and Cyt-c protein. Data shown are the mean ± SD of three independent experiments.
Discussion
In recent years, research on the Mcl-1 gene has predominantly focused on tumour-related diseases [18]; thus, application of this gene to control TB will be a novel function. In the current study, TUNEL analyses showed that the application of Mcl-1-shRNA to down-regulate Mcl-1 resulted in increased apoptosis to varying degrees of the mouse peritoneal macrophages infected with different strains (Figure 2). The results indicated that Mcl-1 introduction to TB is feasible, and it may be a promising strategy for prevention and control of latent TB infection. However, macrophage apoptosis levels were closely associated with the virulence of the MTB strain: the weaker the virulence of the MTB strains, the higher the apoptosis rate. The result is consistent with a previous study showing that MTB invasion of host macrophages caused apoptosis; MTB strains with different virulence levels induced different levels of macrophage apoptosis, and the level of host cell apoptosis showed variation with infection by different MTB strains [19,20]. The results indicated that the virulence of MTB strains is the key to the regulatory effect of Mcl-1. Therefore, we hypothesized that make use of down-regulation of Mcl-1 altering the effect of MTB strains with different levels of virulence on host macrophage apoptosis and may be targeted to overcome the different types of TB infection.
Currently, RNAi is the most commonly used method to silence gene expression [15,21]. At home and abroad [22-24], studies have found that shRNA silencing of Mcl-1 in different tumour cell lines sensitised cancer cells to chemotherapy and molecular targeted therapy, increased the cancer cell apoptosis rate, and showed no damage to normal cells. In this experiment, immunostaining results showed that Mcl-1-shRNA intervention significantly weakened the expression of Mcl-1, especially in Mcl-1-shRNA-treated H37Rv- and XJ-MTB-infected mouse peritoneal macrophages (Figure 2). The results confirmed that Mcl-1-shRNA intervention is an effective method of down-regulation of Mcl-1 in TB infection.
Treating TB infection is generally judged by host macrophage intracellular clearance and the effects on organs/tissues. In our experiments, CFU count results showed that Mcl-1-shRNA treatment significantly reduced the number of CFUs recovered from host macrophages, with a decrease of 3.4 times (Figure 2). Moreover, Mcl-1-shRNA intervention also relieved the pathological damage of the lung, liver, spleen, and kidney in mice, especially in the XJ-MTB infection group (Figure 4). The results indicate the important role of Mcl-1 in latent TB infection and suggest it may have a role in the prevention and control of TB.
To further study the regulatory effect of Mcl-1 in TB infection, we explored its apoptosis regulatory mechanism. Given the important role of cyt-c in the mitochondrial signalling pathway and Mcl-1 expression [8,9], we focused on analysing the changes of cyt-c expression in Mcl-1-shRNA-treated MTB strain-infected mouse peritoneal macrophages. Real-time PCR and Western blot results showed that Mcl-1-shRNA intervention can induce more cyt-c mRNA and protein expression in mouse peritoneal macrophages infected with strains with different virulence levels (Figures 5 and 6), and with time, the expression of cyt-c gradually increased and reached a peak at the fifth day after Mcl-1-shRNA treatment. Thus, down-regulated Mcl-1 promoted the release of cyt-c, leading to increased mouse peritoneal macrophage apoptosis. These results may be due to activation of the mitochondrial apoptotic pathway.
However, numerous studies have shown that cell apoptosis is related not only to the intrinsic apoptotic pathway, but also to the extrinsic apoptotic pathway. The mitochondrial pathway predominantly involves the release of cyt-c, and the extrinsic pathway involves the activation of Caspase-8, and both eventually lead to the activation of effector caspases (Caspase-3) to execute cell death [16,17]. Mycobacteria interfere with host cell apoptosis by modifying Bcl-2 protein expression, and virulent MTB has been shown to repress apoptosis by up-regulation of Bcl-2 [25] and Mcl-1 [6] and by deactivation of Bad [26] and Bax [27]. After comprehensive analysis of the expression of these proteins, we found that cyt-c, a key member of the intrinsic apoptotic pathway, was always increased (Figure 7). These results indicate that the intrinsic apoptotic pathway plays an important regulatory role in MTB infection. After Mcl-1-shRNA treatment of mouse peritoneal macrophages, Caspase-8 protein levels increased significantly, and there were no differences compared with cyt-c. These findings indicate that Mcl-1-shRNA treatment activated the extrinsic and the intrinsic apoptotic pathways to co-regulate macrophage apoptosis.
In conclusion, Mcl-1-shRNA treatment down-regulated the expression of Mcl-1, significantly increased host macrophage apoptosis, and induced cyt-c expression in mouse peritoneal macrophages infected with MTB strains that differ in virulence. Furthermore, host macrophage apoptosis levels were associated with the virulence of MTB, and virulence factors are key to the induction of Mcl-1. The mitochondria-mediated apoptotic pathways and the extrinsic apoptotic pathways likely co-regulate the apoptosis of macrophages. Given the key role of the host macrophage apoptosis in the pathogenesis of TB and adaptive immune response, this study may provide a theoretical basis for the emergence of new vaccines and new experimental treatments for TB.
Acknowledgements
The Department of Pathophysiology, Key Laboratory of Xinjiang Endemic and Ethnic Diseases Cooperated by Education Ministry with Xinjiang Province, Shihezi University supported this study. We thank Le Zhang and my colleagues for their technical assistance. This work was supported by the National Natural Science Foundation of China (No. 81660330, No. 81260241).
Disclosure of conflict of interest
None.
Authors’ contribution
Conceived and designed the experiments: Zhang L, Wang C, and Wang FY. Performed the experiments: Wang XF, Wang FY, Zhang YQ, and Lu Y. Analyzed the data: Wang XF, Wang XM, Wang FY, and Zhang YQ. Contributed reagents/materials/analysis tools: Zhang L. Wrote the manuscript: Wang XF and Wang XM.
Abbreviations
- TB
Tuberculosis
- Cyt-c
Cytochrome-c
- MTB
Mycobacterium tuberculosis
- BCG
Bacillus Calmette-Guerin
- H37Ra
International standard Mycobacterium tuberculosis attenuated strain
- H37Rv
International standard Mycobacterium tuberculosis virulent strain
- XJ-MTB
common Xinjiang region clinical Mycobacterium tuberculosis isolate
- MDR-TB
multidrug-resistant TB
- Mcl-1
Myeloid leukaemia cell-1
- Bcl-2
B Cell Lymphoma-2
- RNAi
RNA interference
- shRNA
Short hairpin RNA
- Real-time-PCR
Quantitative real-time polymerase chain reaction
- TUNEL
TdT-mediated dUTP nick-end labeling
- HE staining
Haematoxylin-eosin staining
- CFU
Colony-forming units
Ethics statement
The Experimental Animal Centre at Shihezi University provided healthy BALB/c mice. The Institutional Review Board of the First Affiliated Hospital of Medical College, Shihezi University approved this study (No. A2016-063). All studies were conducted following the principles stipulated by the Institutional Animal Ethics Committee (IAEC).
References
- 1.Zumla A, George A, Sharma V, Herbert RH Baroness Masham of Ilton. Oxley A, Oliver M. The WHO 2014 global tuberculosis report--further to go. Lancet Glob Health. 2015;3:e10–2. doi: 10.1016/S2214-109X(14)70361-4. [DOI] [PubMed] [Google Scholar]
- 2.Young DB, Perkins MD, Duncan K, Barry CE. Confronting the scientific obstacles to global control of tuberculosis. Clin Invest. 2008;118:1255–65. doi: 10.1172/JCI34614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qi YC, Ma MJ, Li DJ, Chen MJ, Lu QB, Li XJ, Li JL, Liu W, Cao WC. Multidrug-resistant and extensively drug-resistant tuberculosis in multi-ethnic region, Xinjiang Uygur Autonomous Region, China. PLoS One. 2012;7:e32103. doi: 10.1371/journal.pone.0032103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oddo M, Renno T, Attinger A, Bakker T, MacDonald HR, Meylan PR. Fas ligand-induced apoptosis of infected human macrophages reduces the viability of intracellular Mycobacterium tuberculosis. J Immunol. 1998;160:5448–54. [PubMed] [Google Scholar]
- 5.Danelishvili L, McGarvey J, Li YJ, Bermudez LE. Mycobacterium tuberculosis infection causes different levels of apoptosis and necrosis in human macrophages and alveolar epithelial cells. Cell Microbiol. 2003;5:649–60. doi: 10.1046/j.1462-5822.2003.00312.x. [DOI] [PubMed] [Google Scholar]
- 6.Sly LM, Hingley-Wilson SM, Reiner NE, McMaster WR. Survival of mycobacterium tuberculosis in host macrophages involves resistance to apoptosis dependent upon induction of antiapoptotic Bcl-2 family member Mcl-1. J Immunol. 2003;170:430–437. doi: 10.4049/jimmunol.170.1.430. [DOI] [PubMed] [Google Scholar]
- 7.Wang FY, Zhang YQ, Wang XM, Wang C, Wang XF, Wu JD, Wu F, Zhang WJ, Zhang L. A small hairpin RNA targeting myeloid cell leukemia-1 enhances apoptosis in host macrophages infected with mycobacterium tuberculosis. J Microbiol. 2016;54:330–7. doi: 10.1007/s12275-016-5627-5. [DOI] [PubMed] [Google Scholar]
- 8.Wang FY, Wang XM, Wang C, Wang XF, Zhang YQ, Wu JD, Wu F, Zhang WJ, Zhang L. Suppression of Mcl-1 induces apoptosis in mouse peritoneal macrophages infected with mycobacterium tuberculosis. Microbiol Immunol. 2016;60:215–27. doi: 10.1111/1348-0421.12368. [DOI] [PubMed] [Google Scholar]
- 9.Abarca-Rojano E, Rosas-Medina P, Zamudio-Cortéz P, Mondragón-Flores R, Sánchez-García FJ. Mycobacterium tuberculosis virulence correlates with mitochondrial cytochrome-c release in infected macrophages. Scand J Immunol. 2003;58:419–427. doi: 10.1046/j.1365-3083.2003.01318.x. [DOI] [PubMed] [Google Scholar]
- 10.Michels J, Johnson PW, Packham G. Mcl-1. Int J Biochem Cell Biol. 2005;37:267–71. doi: 10.1016/j.biocel.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Thiefes A, Wolter S, Mushinski JF, Hoffmann E, Dittrich-Breiholz O, Graue N, Dörrie A, Schneider H, Wirth D, Luckow B, Resch K, Kracht M. Stmultaneous blockade of NF-kappaB, JNK, and p38 MAPK by a kinase-inactive mutant of the protein kinase TAK1 sensitizes cells to apoptosis and affects a distinct spectrum of tumor necrosis factor [corrected] target genes. J Biol Chem. 2005;280:27728–41. doi: 10.1074/jbc.M411657200. [DOI] [PubMed] [Google Scholar]
- 12.McCaffrey AP, Meuse L, Pham TT, Conklin DS, Hannon GJ, Kay MA. Kay: RNA interference in adult mice. Nature. 2002;418:38–39. doi: 10.1038/418038a. [DOI] [PubMed] [Google Scholar]
- 13.Yamashita M, Shinohara T, Tsuji S, Myrvik QN, Nishiyama A, Henriksen RA, Shibata Y. Catalytically inactive cyclooxygenase 2 and absence of prostaglandin E2 biosynthesis in murine peritoneal macrophages following in vivo phagocytosis of heat-killed mycobacterium bovis bacillus Calmette-Guérin. J Immunol. 2007;79:7072–7078. doi: 10.4049/jimmunol.179.10.7072. [DOI] [PubMed] [Google Scholar]
- 14.Rahman A, Sobia P, Gupta N, Kaer LV, Das G. Mycobacterium tuberculosis subverts the TLR-2-MyD88 pathway to facilitate its translocation into the cytosol. PLoS One. 2014;9:e86886. doi: 10.1371/journal.pone.0086886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park DH, Jung BK, Lee YS, Jang JY, Kim MK, Lee JK, Park H, Seo J, Kim CW. Evaluation of in vivo antitumor effects of ANT2 shRNA delivered using PEI and ultrasound with microbubbles. Gene Ther. 2015;22:325–32. doi: 10.1038/gt.2014.120. [DOI] [PubMed] [Google Scholar]
- 16.Maushagen R, Reers S, Pfannerstill AC, Hahlbrock A, Stauber R, Rahmanzadeh R, Rades D, Pries R, Wollenberg B. Effects of paclitaxel on permanent head and neck squamous cell carcinoma cell lines and identification of antiapoptotic caspase 9b. J Cancer Res Clin Oncol. 2016;142:1261–71. doi: 10.1007/s00432-016-2150-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasan Z, Ashraf M, Tayyebi A, Hussain R. M. leprae inhibits apoptosis in THP-1 cells by downregulation of bad and Bak and upregulation of Mcl-1 gene expression. BMC Microbiol. 2006;6:78. doi: 10.1186/1471-2180-6-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pfannenstiel LW, Gastman BR. Mcl-1 and tumor cell persistence. Oncotarget. 2015;6:5–6. doi: 10.18632/oncotarget.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mc Garvey JA, Wagner D, Bermudez LE. Differential gene expression in mononuclear phagocytes infected with pathogenic and non-pathogenic mycobacteria. Clin Exp Immunol. 2004;136:490–500. doi: 10.1111/j.1365-2249.2004.02490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rengarajan J, Bloom BR, Rubin EJ. Genomewide requirements for Mycobacterium tuberculosis adaptation and survival in macrophages. Proc Natl Acad Sci U S A. 2005;102:8327–8332. doi: 10.1073/pnas.0503272102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dutta TK, Banakar P, Rao U. The status of RNAi-based transgenic research in plant nematology. Front Microbiol. 2015;12:760. doi: 10.3389/fmicb.2014.00760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou W, Hu J, Tang H, Wang D, Huang X, He C, Zhu H. Small interfering RNA targeting mcl-1 enhances proteasome inhibitor-induced apoptosis in various solid malignant tumors. BMC Cancer. 2011;11:485. doi: 10.1186/1471-2407-11-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee WS, Park YL, Kim N, Oh HH, Son DJ, Kim MY, Oak CY, Chung CY, Park HC, Kim JS, Myung DS, Cho SB, Joo YE. Myeloid cell leukemia-1 regulates the cell growth and predicts prognosis in gastric cancer. Int J Oncol. 2015;46:2154–62. doi: 10.3892/ijo.2015.2890. [DOI] [PubMed] [Google Scholar]
- 24.Karami H, Baradaran B, Esfahani A, Sakhinia M, Sakhinia E. Therapeutic effects of myeloid cell leukemia-1 siRNA on human acute myeloid leukemia cells. Adv Pharm Bull. 2014;4:243–8. doi: 10.5681/apb.2014.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang J, Jiang R, Takayama H, Tanaka Y. Survival of virulent Mycobacterium tuberculosis involves preventing apoptosis induced by Bcl-2 upregulation and release resulting from necrosis in J774 macrophages. Microbial Immunol. 2005;49:845–852. doi: 10.1111/j.1348-0421.2005.tb03673.x. [DOI] [PubMed] [Google Scholar]
- 26.Danelishvili L, McGarvey J, Li Y, Bermudez LE. Mycobacterium tuberculosis infection causes different levels of apoptosis and necrosis in human macrophages and alveolar epithelial cells. Cell Microbiol. 2003;5:649–660. doi: 10.1046/j.1462-5822.2003.00312.x. [DOI] [PubMed] [Google Scholar]
- 27.Perskvist N, Long M, Stendahl O, Zheng L. Mycobacterium tuberculosis promotes apoptosis in human neutrophils by activating caspase-3 and altering expression of Bax/Bcl-xL via an oxygen-dependent pathway. J Immunol. 2002;168:6358–6365. doi: 10.4049/jimmunol.168.12.6358. [DOI] [PubMed] [Google Scholar]