Abstract
Synovial sarcoma (SS) is an aggressive soft tissue tumor, which occurs predominantly in young adults. The SS can arise in almost any part of the body, especially in the lower extremity. The SS of head and neck accounts for 1.9-3.5% of all SS, however, the mass appears very extremely rare in the larynx. We report a case of a biphasic SS in the larynx. A 14-year-old boy appeared without apparent inducement with hoarseness, dyspnea, fever, cough, hemoptysis, pharyngeal foreign body sensation, dysphagia, odynophagia and other symptoms since nearly one month ago. The patient underwent partial laryngectomy and performed a wide surgical excision of the tumor with a free margin. Pathological examinations of the tumor specimen revealed an encapsulated and firm tumor lesion. The grayish-white to dark-brown mass was 8×6×4 cm, arising from the left of aryepiglottic fold. Histological examination showed the characteristic histomorphological features such as biphasic pattern of short spindle cells and epithelioid cells, including glandular differentiation. Immunohistochemically, staining for vimentin, bcl-2 and calponin were typically positive in the spindle tumor cells. Staining for CK was typically positive in the epithelioidtumor cells. Staining for EMA, CD99 and TLE-1 were typically positive in both the epithelioid and spindle tumor cells. The presence of SYT-SSX fusion gene from chromosomal translocation was detected by FISH in this case. Two months postoperatively, the patient received local radiotherapy. Combined treatment may be effective and the patient is alive without tumor recurrence in radiological and clinical examination after 18 months of follow-up.
Keywords: Synovial sarcoma, larynx, biphasic, immunohistochemistry (IHC), fluorescence in situ hybridization (FISH)
Introduction
Synovial sarcoma (SS) is a rare and aggressive soft tissue tumor, which accounts for 7-8% of all human malignant sarcomas. WHO classification of SS is as a type of mesenchymal tissue spindle cell tumor that exhibits variable epithelioid differentiation including glandular differentiation [1], with the description of the distinctive chromosomal translocation specific t(X;18)(p11;q11) lead to the incorporation of this particular genetic aberration [2,3]. Synovial sarcoma occurs mainly in young adults and teenagers, although they also occur in older adults and preteen children [4]. The synovial sarcomas can arise in almost any part of the body, especially in the lower extremity, often around knee joint and tendon sheath around [5-7]. The synovial sarcomas of head and neck accounts for 1.9-3.5% of all synovial sarcoma, however, the mass appears very extremely rare in the larynx [8,9], and 23 cases in the literature arising from the larynx (Table 1).
Table 1.
Summary of English-Literature review of synovial sarcomas of larynx
| Group | Total | Reference |
|---|---|---|
| Age (year) | ||
| 10-20 | 7 | [8,10,13,21,25,26, The current case] |
| 21-40 | 10 | [9,11,12,16,19,20,23,27-29] |
| 41-60 | 3 | [15,18,22] |
| >60 | 3 | [14,24,30] |
| Location | ||
| Supraglottic | 11 | [8,10,12,14-16,18-20,26,27] |
| Aryepiglottic fold | 3 | [17,23, The current case] |
| Cricoids cartilage | 1 | [30] |
| Arytenoid | 2 | [21,29] |
| Subglottic | 1 | [24] |
| Pathological diagnosis method | ||
| IHC | 14 | [8,9,11-15,18-21,23,24, The current case] |
| FISH | 5 | [10,11,14,18, The current case] |
| Cytogenetics | 2 | [10,20] |
| RT-PCR | 2 | [13,18] |
| Karyotyping | 1 | [14] |
| Histology grouping | ||
| Biphasic | 10 | [8-11,14,19-21,24, The current case] |
| Monophasic | 5 | [12,13,15,18,23] |
| Treatment | ||
| Surgery | 8 | [14,18,20,24,25,28-30] |
| Surgery + radiotherapy | 8 | [8,9,11,21-23,27, The current case] |
| Surgery + chemotherapy + radiotherapy | 4 | [10,12,13,19] |
| CO2 laser surgery | 3 | [15-17] |
| CO2 laser surgery + radiotherapy | 1 | [26] |
| Follow-up | ||
| 0-1 y NED | 4 | [14,20,21,25] |
| 1-5 y NED | 12 | [8-10,13,15,17-19,23,24,26, The current case] |
| 5-10 y NED | 1 | [11] |
| >10 y NED | 2 | [27,29] |
| LNM | 1 | [11] |
| Distant metastasis | 4 | [12,19,22,28] |
| Died | 2 | [12,28] |
Abbreviation: Immunohistochemistry (IHC); Reverse transcriptase-polymerase chain reaction (RT-PCR); No evidence of disease (NED); Lymph node metastasis (LNM).
Histological examination of these tumors has biphasic and monophasic variants, the latter are more rare and difficult to determine. When synovial sarcomas exist in such unusual sites, diagnosis only according to the histological features might be problematic. Immunohistochemistry and fluorescence in situ hybridization (FISH) play crucial roles in the diagnosis. In immunohistochemistry, spindle cells were positive staining for vimentin, bcl-2 and calponin. Immunoreactivity for cytokeratin (CK) and epithelial membrane antigen (EMA) were demonstrated in all epithelioid cells of tumor tissue. The both types of tumor cells were positive expression of CD99 and TLE-1 protein [31-33]. The SYT-SSX fusion gene from chromosomal translocation was detected by FISH in this case [34,35].
Case report
A 14-year-old boy appeared without apparent inducement with hoarseness, dyspnea, fever, cough, hemoptysis, pharyngeal foreign body sensation, dysphagia, odynophagia and other symptoms since nearly one month ago. The patient thought he had caught a bad cold, after taking the drug did not take seriously. During the study in boarding school, the patient’s condition was not improved. As for further treatment, they went to the local central hospital for the sake of the cure. The diagnosis of pulmonary tuberculosis, the patient was given anti infection treatment. No significant improvement in disease, the patient made a chest X-ray examination and found no tuberculosis, but computed tomography (CT) examination revealed a huge tumor in the larynx (Figure 1A). The patient had a laryngoscopy on the following day, which saw a large new tissue mass in the laryngeal cavity, took a small amount of tissue for pathological biopsy. The pathologist of the local central hospital considered as squamous cell carcinoma. The patient came to the Department of Otolaryngology, the Second Xiangya Hospital of Central South University for the differential diagnosis and therapy of tumor after a fortnight. Laryngoscopic view showed a huge, lobulated soft tissue mass in the left aryepiglottic fold (Figure 1B). Re-biopsy was performed; the lesion was diagnosed histologically as SS. The patient admitted to the Department of otolaryngology and underwent partial laryngectomy and performed a wide surgical excision of the tumor with a free margin. Two months postoperatively, radiotherapy was added. He has been followed up for 18 months and has remained free of recurrence or metastases in radiological and clinical examination. The effect of comprehensive treatment is satisfactory.
Figure 1.
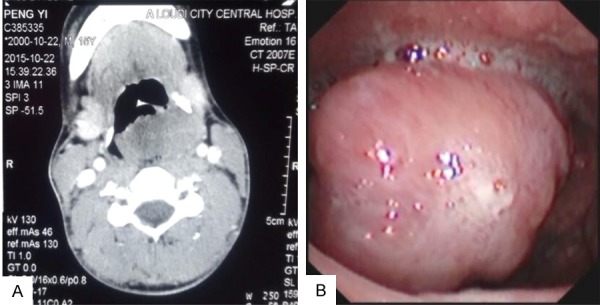
A: Enhanced axial CT showed the tumor mass across the laryngopharynx, and the left side of the piriform fossa was destroyed. B: Laryngoscopic view showed a huge, lobulated soft tissue mass in the left of aryepiglottic fold.
Pathological findings
After surgical resection of the tumor, pathological examinations of the tumor specimen revealed an encapsulated and firm tumor lesion. The grayish-white to dark-brown mass was 8×6×4 cm, arising from the left of aryepiglottic fold (Figure 2A). Histological examination of the tumor photomicrograph by H&E staining showed the characteristic histomorphological logical features such as biphasic pattern of short spindle cells and epithelioid cells, including glandular differentiation (Figure 2B-D). The tumor cells had infiltrated the muscular layer but had not infiltrated the epiglottis and thyroid cartilage. Immunohistochemically, staining for vimentin, bcl-2 and calponin were typically positive in the spindle tumor cells (original magnification 200×, IHC, DAB staining) (Figure 3A-C). Immunohistochemically, staining for cytokeratin (CK) was typically positive in the epithelioidtumor cells (original magnification 200×, IHC, DAB staining) (Figure 3D). However, immunohistochemically, staining for epithelial membrane antigen (EMA), CD99 and TLE-1 were typically positive in both the epithelioid and spindle tumor cells (original magnification 200×, IHC, DAB staining) (Figure 3E-G). The typically positive staining for p53 was confirmed only in the spindle tumor cells (original magnification 200×, IHC, DAB staining) (Figure 3H). The proliferative index (Ki-67) was found in approximately 30% of tumor cells (Figure 3I).The presence of SYT-SSX fusion gene was unique to cytogenetic abnormalities, indicating that some of the major causes of synovial sarcoma. FISH was performed using the locus specific identifier (LSI) SYT (18q11.2) LSI SS18 (18q11.2) Dual Color Break-Apart Rearrangement Probe (Vysis, Abbott Laboratories Inc), which consisted of a mixture of 2 FISH DNA probes. The first probe was an about 650-kb probe labeled in Spectrum Orange, which extended distally from the SYT gene. The second was approximately an about 1040-kb in length and labeled in Spectrum Green, lied 3’ or proximal to the SYT gene [14,36]. These signal separation meant the rearrangement of the SYT gene (Figure 4). These results supported the diagnosis of synovial sarcoma.
Figure 2.
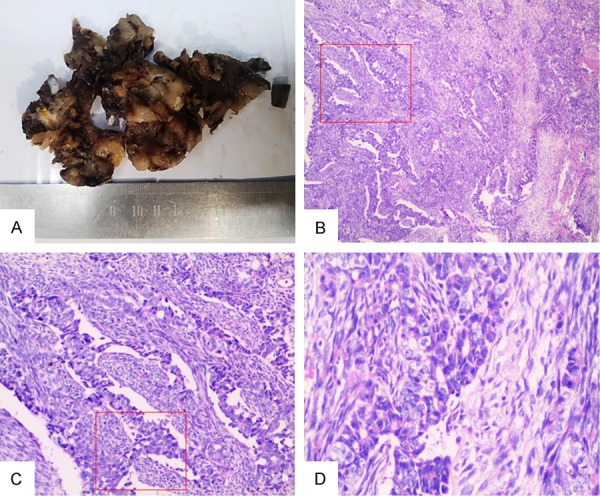
A: After surgical resection of the tumor, pathological examinations of the tumor specimen revealed an encapsulated and firm tumor lesion, looking grayish-white to dark-brown and arising from the left of aryepiglottic fold. B-D: The Photomicrograph showed the characteristic morph histological features such as biphasic pattern of short spindle cells and epithelioid cells, including glandular differentiation (red frame represent the typical photomicrograph of tumor showing biphasic pattern under the different magnification) (original magnification 100×, 200×, 400×, H&E staining).
Figure 3.
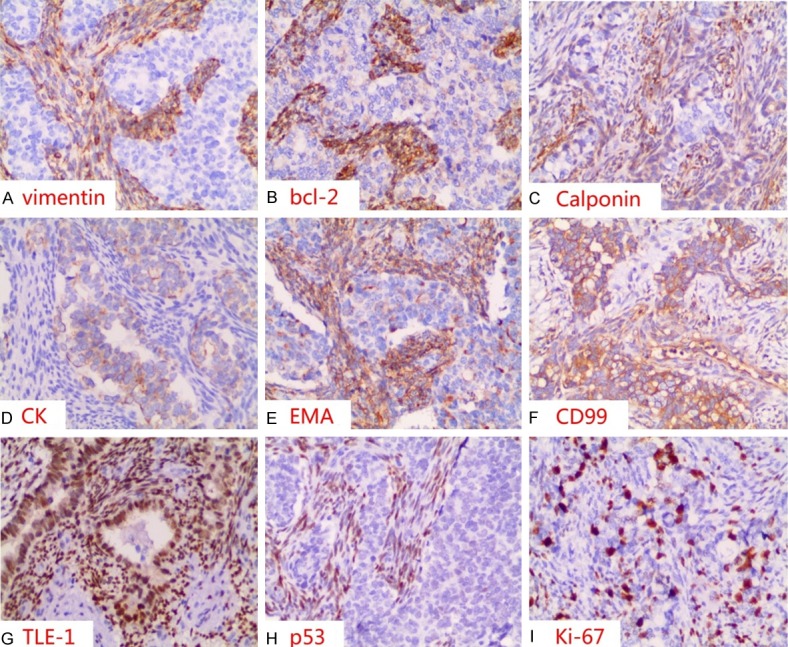
Immunohistochemically, staining for vimentin (A), bcl-2 (B) and calponin (C) was typically positive in the spindle tumor cells; staining for CK (D) was typically positive in the epithelioid tumor cells. However, immunohistochemically, staining for EMA (E), CD99 (F) and TLE-1 (G) was typically positive in both the epithelioid and spindle tumor cells; the typically positive staining for p53 (H) was only in the spindle tumor cells. The proliferative index (Ki-67) (I) was found in approximately 30% of tumor cells (original magnification 200×, IHC, DAB staining).
Figure 4.
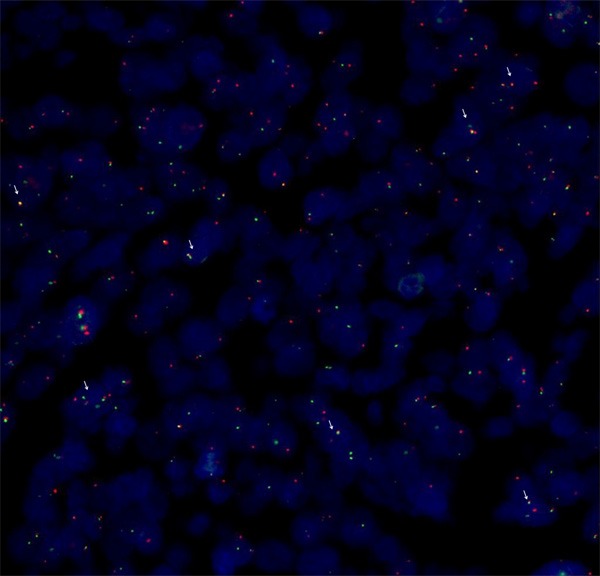
Disruption of SYT gene was proved by FISH using a break apart rearrangement probe. In normal cells the two signals are close and overlap of these shows yellow signals. The separation of the two color signals reveals the translocation of the SYT gene. The abnormal cell shows a one fusion, one orange, and one green signal pattern identifying t(18q11.2). (white arrow represent the positive signal).
Discussion
In the past, SS was considered to be a malignant tumor derived from synoviocytes. However, SS is ultrastructurally and immunophenotypically distinct from normal synovium, only rarely arising in articular cavities, and generally occurs in association with para-articular regions of the extremities, in which absent synovial tissue. In view of SS with epithelioid characteristics, it was suggested that it should be renamed as soft tissue sarcoma or spindle cell carcinoma [37].
T(X;18) translocation involved genes: SS18 gene on chromosome 18 (encoding SYT or SSXT protein) and SSX1, SSX2, SSX4 genes on the X chromosome [2,38-41]. The data suggested that t(X;18) chromosomal translocation only occurred in synovial sarcoma. Other soft tissue sarcomas have specific genetic abnormalities. The characteristics of Ewing family of tumors are frequent occurrence of t(11;22)(q24;q12) chromosomal translocation [42]. T(12;16)(q13;p11) is a translocation specific for the myxoid liposarcoma and round cell liposarcoma [43].
The patient had been diagnosed as pulmonary tuberculosis with fever and bloody sputum symptoms in the local central hospital. It was because they had ignored the patient had pharyngeal foreign body sensation, dysphagia symptoms. Primary malignant tumors of the larynx are predominantly classified as squamous cell carcinoma, which usually occur in older people. Pathologist of the local central hospital diagnosed as poorly differentiated squamous cell carcinoma because of epithelioid cell components of tumor, while ignoring the gland components and spindle shaped stromal cell. A 14 years old patient diagnosed as squamous cell carcinoma should be suspected by pathologists. Immunohistochemistry and FISH can improve the accuracy of diagnosis, so as to promote the proper treatment plan. P53 gene mutation is a poor prognostic factor [44,45]. In this case, the p53 immunohistochemically reaction was positive in spindle cells and Ki-67 was found in approximately 30% of tumor cells, indicating that the prognosis of the patient might be not good.
Because of the rare SS of the larynx and lack of consistent prognostic markers, there is no clear consensus to treat these patients. The optimal treatment for synovial sarcoma is multimodal and has not yet been established, and treatment is often based on accumulative reports (Table 1). Radical surgical excision is generally accepted as the primary of therapy, combined with adjuvant chemotherapy and radiotherapy. In the head and neck region the anatomical complexity of the surgery, complete resection with adequate margins is very difficult or impossible in many cases. Therefore, the treatment effect is not the same for each patient.
Since there are few cases of laryngeal synovial sarcoma, each new case will bring some new information about diagnosis and treatment. Each case of laryngeal SS should be published, because it is very important to understand the new aspects and treatment strategies of suffering from this rare tumor.
Acknowledgements
This work was supported by the National Natural Science Foundations of China (No: 81472773).
Disclosure of conflict of interest
None.
References
- 1.Antonescu CR, Kawai A, Leung DH, Lonardo F, Woodruff JM, Healey JH, Ladanyi M. Strong association of SYT-SSX fusion type and morphologic epithelial differentiation in synovial sarcoma. Diagn Mol Pathol. 2000;9:1–8. doi: 10.1097/00019606-200003000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Clark J, Rocques PJ, Crew AJ, Gill S, Shipley J, Chan AM, Gusterson BA, Cooper CS. Identification of novel genes, SYT and SSX, involved in the t(X;18)(p11.2;q11.2) translocation found in human synovial sarcoma. Nat Genet. 1994;7:502–508. doi: 10.1038/ng0894-502. [DOI] [PubMed] [Google Scholar]
- 3.Panagopoulos I, Mertens F, Isaksson M, Limon J, Gustafson P, Skytting B, Akerman M, Sciot R, Dal Cin P, Samson I, Iliszko M, Ryoe J, Dêbiec-Rychter M, Szadowska A, Brosjö O, Larsson O, Rydholm A, Mandahl N. Clinical impact of molecular and cytogenetic findings in synovial sarcoma. Genes Chromosomes Cancer. 2001;31:362–372. doi: 10.1002/gcc.1155. [DOI] [PubMed] [Google Scholar]
- 4.Mansuy L, Bernier V, Ranchère-Vince D, Mainard L, Orbach D, Corradini N. Synovial sarcoma in children and adolescents. Bull Cancer. 2016;103:210–218. doi: 10.1016/j.bulcan.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Salcedo-Hernández RA, Lino-Silva LS, Luna-Ortiz K. Synovial sarcomas of the head and neck: comparative analysis with synovial sarcoma of the extremities. Auris Nasus Larynx. 2013;40:476–480. doi: 10.1016/j.anl.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 6.Fisher C. Synovial sarcoma. Ann Diagn Pathol. 1998;2:401–421. doi: 10.1016/s1092-9134(98)80042-7. [DOI] [PubMed] [Google Scholar]
- 7.Thway K, Fisher C. Synovial sarcoma: defining features and diagnostic evolution. Ann Diagn Pathol. 2014;18:369–380. doi: 10.1016/j.anndiagpath.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Javed N, Iqbal J. Synovial sarcoma of the larynx. J Ayub Med Coll Abbottabad. 2015;27:729–730. [PubMed] [Google Scholar]
- 9.Mohammadi G, Khansarinia A. Synovial sarcoma-a rare tumor of the larynx. Iran J Otorhinolaryngol. 2016;28:233–236. [PMC free article] [PubMed] [Google Scholar]
- 10.Saxby C, Bova R, Edwards M. Laryngeal synovial sarcoma: a rare clinical entity. Case Rep Otolaryngol. 2013;2013:578606. doi: 10.1155/2013/578606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luna-Ortiz K, Cano-Valdez AM, da Cunha IW, Mosqueda-Taylor A. Synovial sarcoma of the larynx treated by supraglottic laryngectomy: case report and literature review. Ear Nose Throat J. 2013;92:E20–26. doi: 10.1177/014556131309200717. [DOI] [PubMed] [Google Scholar]
- 12.Bao YY, Wang QY, Zhou SH, Zhao K, Ruan LX, Yao HT. Poor outcome of comprehensive therapy in a case of laryngeal synovial sarcoma. Radiol Oncol. 2013;47:111–118. doi: 10.2478/raon-2013-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sato T, Hasegawa H, Sugasawa M, Yasuda M, Morita K, Nakahira M, Nakatsuka T. Free jejunal transfer for a 15-year-old girl with synovial sarcoma of the hypopharynx. J Plast Reconstr Aesthet Surg. 2011;64:1100–1103. doi: 10.1016/j.bjps.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Mhawech-Fauceglia P, Ramzy P, Bshara W, Sait S, Rigual N. Synovial sarcoma of the larynx in a 79-year-old woman, confirmed by karyotyping and fluorescence in situ hybridization analysis. Ann Diagn Pathol. 2007;11:223–227. doi: 10.1016/j.anndiagpath.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Capelli M, Bertino G, Morbini P, Proh M, Falco CE, Benazzo M. CO2 laser in the treatment of laryngeal synovial sarcoma: a clinical case. Tumori. 2007;93:296–299. doi: 10.1177/030089160709300313. [DOI] [PubMed] [Google Scholar]
- 16.Abou Zeid HA, Arab SA, Al-Ghamdi AM, Al-Qurain AA, Mokhazy KM. Airway management of a rare huge-size supraglottic mass. Saudi Med J. 2006;27:711–713. [PubMed] [Google Scholar]
- 17.Boniver V, Moreau P, Lefebvre P. Synovial sarcoma of the larynx: case report and literature review. B-ENT. 2005;1:47–51. [PubMed] [Google Scholar]
- 18.Szuhai K, Knijnenburg J, Ijszenga M, Tanke HJ, Baatenburg de Jong RJ, Bas Douwes Dekker P, Rosenberg C, Hogendoorn PC. Multicolor fluorescence in situ hybridization analysis of a synovial sarcoma of the larynx with a t(X;18) (p11.2;q11.2) and trisomies 2 and 8. Cancer Genet Cytogenet. 2004;153:48–52. doi: 10.1016/j.cancergencyto.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 19.Bilgic B, Mete O, Oztürk SA, Demiryont M, Keles N, Basaran M. Synovial sarcoma: a rare tumor of larynx. Pathol Oncol Res. 2003;9:242–245. doi: 10.1007/BF02893385. [DOI] [PubMed] [Google Scholar]
- 20.Dei Tos AP, Dal Cin P, Sciot R, Furlanetto A, Da Mosto MC, Giannini C, Rinaldo A, Ferlito A. Synovial sarcoma of the larynx and hypopharynx. Ann Otol Rhinol Laryngol. 1998;107:1080–1085. doi: 10.1177/000348949810701215. [DOI] [PubMed] [Google Scholar]
- 21.Morland B, Cox G, Randall C, Ramsay A, Radford M. Synovial sarcoma of the larynx in a child: case report and histological appearances. Med Pediatr Oncol. 1994;23:64–68. doi: 10.1002/mpo.2950230112. [DOI] [PubMed] [Google Scholar]
- 22.Danninger R, Humer U, Stammberger H. [Synovial sarcoma, a rare tumor of the larynx. Case report and differential diagnostic considerations] . Laryngorhinootologie. 1994;73:442–444. doi: 10.1055/s-2007-997169. [DOI] [PubMed] [Google Scholar]
- 23.Pruszczynski M, Manni JJ, Smedts F. Endolaryngeal synovial sarcoma: case report with immunohistochemical studies. Head Neck. 1989;11:76–80. doi: 10.1002/hed.2880110113. [DOI] [PubMed] [Google Scholar]
- 24.Quinn HJ Jr. Synovial sarcoma of the larynx treated by partial laryngectomy. Laryngoscope. 1984;94:1158–1161. doi: 10.1288/00005537-198409000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Mohr W, Pirsig W. [Synovial sarcoma of the larynx. Case report and brief review of the literature] . LaryngolRhinolOtol (Stuttg) 1984;63:453–456. [PubMed] [Google Scholar]
- 26.Papaspyrou S, Kyriakides G, Tapis M. Endoscopic CO2 laser surgery for large synovial sarcoma of the larynx. Otolaryngol Head Neck Surg. 2003;129:630–631. doi: 10.1016/S0194-59980301385-8. [DOI] [PubMed] [Google Scholar]
- 27.Ferlito A, Caruso G. Endolaryngeal synovial sarcoma. An update on diagnosis and treatment. ORL J Otorhinolaryngol Relat Spec. 1991;53:116–119. doi: 10.1159/000276200. [DOI] [PubMed] [Google Scholar]
- 28.Gatti WM, Strom CG, Orfei E. Synovial sarcoma of the laryngopharynx. Arch Otolaryngol. 1975;101:633–636. doi: 10.1001/archotol.1975.00780390047013. [DOI] [PubMed] [Google Scholar]
- 29.Miller LH, Santaella-Latimer L, Miller T. Synovial sarcoma of the larynx. Trans Sect Otolaryngol Am Acad Ophthalmol Otolaryngol. 1975;80:448–451. [PubMed] [Google Scholar]
- 30.Taylor SM, Ha D, Elluru R, El-Mofty S, Haughey B, Wallace M. Synovial sarcoma of the pericricoidal soft tissue. Otolaryngol Head Neck Surg. 2002;126:428–429. doi: 10.1067/mhn.2002.123833. [DOI] [PubMed] [Google Scholar]
- 31.Folpe AL, Lloyd RV, Bacchi CE, Rosai J. Spindle epithelial tumor with thymus-like differentiation: a morphologic, immunohistochemical, and molecular genetic study of 11 cases. Am J Surg Pathol. 2009;33:1179–1186. doi: 10.1097/PAS.0b013e31819e61c8. [DOI] [PubMed] [Google Scholar]
- 32.Fisher C, Montgomery E, Healy V. Calponin and h-caldesmon expression in synovial sarcoma; the use of calponin in diagnosis. Histopathology. 2003;42:588–593. doi: 10.1046/j.1365-2559.2003.01652.x. [DOI] [PubMed] [Google Scholar]
- 33.Chuang HC, Hsu SC, Huang CG, Hsueh S, Ng KF, Chen TC. Reappraisal of TLE-1 immunohistochemical staining and molecular detection of SS18-SSX fusion transcripts for synovial sarcoma. PatholInt. 2013;63:573–580. doi: 10.1111/pin.12113. [DOI] [PubMed] [Google Scholar]
- 34.Fricke A, Ullrich PV, Cimniak AF, Follo M, Nestel S, Heimrich B, Nazarenko I, Stark GB, Bannasch H, Braig D, Eisenhardt SU. Synovial sarcoma microvesicles harbor the SYT-SSX fusion gene transcript: comparison of different methods of detection and implications in biomarker research. Stem Cells Int. 2016;2016:6146047. doi: 10.1155/2016/6146047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kato K, Tanaka M, Toyoda Y, Kigasawa H, Ohama Y, Nishi T, Okuzumi S, Kurosawa K, Aida N, Nagahara N, Tanaka Y. A novel fluorescence in situ hybridization assay for synovial sarcoma. Pathol Res Pract. 2013;209:309–313. doi: 10.1016/j.prp.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 36.Kawata R, Terada T, Takenaka H, Kurisu Y, Tsuji M. Primary synovial sarcoma arising in the parotid region diagnosed by fluorescence in situ hybridization. Auris Nasus Larynx. 2008;35:583–586. doi: 10.1016/j.anl.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 37.Miettinen M, Virtanen I. Synovial sarcoma--a misnomer. Am J Pathol. 1984;117:18–25. [PMC free article] [PubMed] [Google Scholar]
- 38.Crew AJ, Clark J, Fisher C, Gill S, Grimer R, Chand A, Shipley J, Gusterson BA, Cooper CS. Fusion of SYT to two genes, SSX1 and SSX2, encoding proteins with homology to the Kruppel-associated box in human synovial sarcoma. EMBO J. 1995;14:2333–2340. doi: 10.1002/j.1460-2075.1995.tb07228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Leeuw B, Balemans M, Olde Weghuis D, Geurts van Kessel A. Identification of two alternative fusion genes, SYT-SSX1 and SYT-SSX2, in t(X;18)(p11.2;q11.2)-positive synovial sarcomas. Hum Mol Genet. 1995;4:1097–1099. doi: 10.1093/hmg/4.6.1097. [DOI] [PubMed] [Google Scholar]
- 40.de Leeuw B, Balemans M, Weghuis DO, Seruca R, Janz M, Geraghty MT, Gilgenkrantz S, Ropers HH, Geurts van Kessel A. Molecular cloning of the synovial sarcoma-specific translocation (X;18)(p11.2;q11.2) breakpoint. Hum Mol Genet. 1994;3:745–749. doi: 10.1093/hmg/3.5.745. [DOI] [PubMed] [Google Scholar]
- 41.Agus V, Tamborini E, Mezzelani A, Pierotti MA, Pilotti S. Re: a novel fusion gene, SYT-SSX4, in synovial sarcoma. J Natl Cancer Inst. 2001;93:1347–1349. doi: 10.1093/jnci/93.17.1347. [DOI] [PubMed] [Google Scholar]
- 42.Trancău IO. Chromosomal translocations highlighted in Primitive Neuroectodermal Tumors (PNET) and Ewing sarcoma. J Med Life. 2014;7:44–50. [PMC free article] [PubMed] [Google Scholar]
- 43.Knight JC, Renwick PJ, Dal Cin P, Van den Berghe H, Fletcher CD. Translocation t(12;16) (q13;p11) in myxoid liposarcoma and round cell liposarcoma: molecular and cytogenetic analysis. Cancer Res. 1995;55:24–27. [PubMed] [Google Scholar]
- 44.Antonescu CR, Leung DH, Dudas M, Ladanyi M, Brennan M, Woodruff JM, Cordon-Cardo C. Alterations of cell cycle regulators in localized synovial sarcoma: a multifactorial study with prognostic implications. Am J Pathol. 2000;156:977–983. doi: 10.1016/S0002-9440(10)64965-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schneider-Stock R, Onnasch D, Haeckel C, Mellin W, Franke DS, Roessner A. Prognostic significance of p53 gene mutations and p53 protein expression in synovial sarcomas. Virchows Arch. 1999;435:407–412. doi: 10.1007/s004280050418. [DOI] [PubMed] [Google Scholar]


