Abstract
Rhegmatogenous retinal detachment (RRD) is one blinding disease, and has pathological features correlated with migration of retinal pigment epithelium (RPE) cells to viscous body. Matrix metalloproteinase-8 (MMP-8) participates in eye diseases including xerophthalmia and retinal disease. Its role in RRD, however, has not been illustrated with the functional mechanism. RPE cells from RRD model mice and normal mice were separated and cultured. MMP-8 expression plasmid was transfected into RPE cell in model group. Real time PCR and Western blot were employed to test expression level of MMP-8, whilst MTT method was used to test proliferation activity of RPE cells. Caspase 3 activity was quantified by test kit. Transwell migration assay was adopted to measure invasion ability of RPE cells. ELISA method was used to test expression level of inflammatory factors interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α). MMP-8 expression level was significantly decreased in RPE cells of RRD group, which also had enhanced cell proliferation and migration, accompanied with higher IL-1β and TNF-α levels (P<0.05 compared to control group). After MMP-8 transfection and over-expression, RPE cell proliferation and migration were inhibited, along with higher Caspase 3 activity, plus lower IL-1β and TNF-α expression (P<0.05 compared to model group). RRD caused decreased expression of MMP-8 in RPE cells. MMP-8 can facilitate RPE cells proliferation and migration via modulating cell apoptotic activity and secretion of inflammatory factor, thus participating in RRD pathogenesis and progression.
Keywords: Rhegmatogenous retinal detachment, retinal pigment epithelium, matrix metalloproteinase-8, cell apoptosis, inflammatory factor
Introduction
Rhegmatogenous retinal detachment (RRD) frequently occurs in middle and aged populations, especially in male patients. It normally develops sequentially in both eyes, and commonly happens in people with severe myopia refractive errors [1,2]. RRD is manifested as the detachment of neural layer from retinal pigment epithelium (RPE) layer of retina, and severely affects patient’s life quality as one blinding disease [3]. RRD is also featured as liquidation of viscous body following formation of cleft on retina, making the detachment between retina and RPE cells [4]. RPE is formed by single layers of epithelial cells with regular arrangement. Polygonal RPE cells can be divided into apical, soma and basal compartments [5]. There are over one million RPE cells in each human eye. However, due to the inability to regenerate, RPE cells cannot be replaced after death, but are occupied by adjacent cells via sliding [6,7]. Occurrence of RRD causes separation of retinal neural epithelium and RPE, depriving the nutrient supply of outer retina via choroid plexus, further aggravating post-macular area injury and retinal damage. Without timely treatment or relocation, detached retina may develop atrophy and denature, leading to vision damage [8]. RRD in macular region can cause sharply decreased vision function, and irreversible loss of vision without timely treatment [9,10].
RPE cells are activated after stimulus, followed by departure from original position, differentiation and migration into viscous body. Proliferation of RPE can secrete abundant extra cellular matrix (ECM), forming hyperplasia membrane with contractibility, stretching both sides of retina and viscous body, causing retinal detachment [11,12]. Matrix metalloproteinase (MMPs) can degrade effective component of ECM and basal membrane, and regulate cell adhesion, thus playing a critical role in ECM dynamic balance [13,14]. Previous study showed the involvement of MMPs in various diseases including tumor, rheumatoid arthritis and proliferative vitreoretinopathy [15,16]. The function and mechanism of MMP-8, which is one important member of MMPs family, in RRD, however, have not been elucidated.
Materials and methods
Experimental animals
Healthy male Wistar rats (2 months old, SPF grade, body weight 250±20 g) were purchased from laboratory animal center of Shandong University and were kept in an SPF grade facility with fixed temperature (21±1°C), fixed humidity (50-70%) and 12 h light/dark cycle. Rats were used for all experiments, and all procedures were approved by the Animal Ethics Committee of Provincial Hospital Affiliated to Shandong University.
Major materials and equipment
0.1% atropine eye dropping, 0.3% ofloxacin eye dropping and Mydrin-P were purchased from Merck (US). Pentobarbital sodium and lidocaine were purchased from Zhaohui Pharm (China). PVDF membrane was purchased from Pall Life Sciences (US). Western blotting reagents were purchased from Beyotime (China). ECL reagent was purchased from Amersham Biosciences (US). Rabbit anti-mouse MMP-8 monoclonal antibody and goat anti-rabbit horseradish peroxidase (HRP)-labelled IgG secondary antibody were purchased from Cell Signaling (US). Β-actin (MAB8929) was purchased from R&D (US). IL-β and TNF-α ELISA kits were purchased from R&D (US). Caspase 3 activity assay kit was purchased from Cell signaling (US). RNA extraction kit, pcDNA3.1 vector, and reverse transcription kit were purchased from Axygen (US). Transwell chamber was purchased from Corning (US). pcDNA3.1 empty plasmid and pcDNA3.1-MMP-8 plasmid were synthesized by Gimma (China). Surgical microscope was purchased from Suzhou Instrument (China). ABI 2000 fluorescent quantitative PCR cycler was purchased from ABI (US). Microplate reader was purchased from BD (US). Other common reagents were purchased from Sangon (China).
Animal grouping and treatment
Healthy male Wistar rats were randomly divided into two groups (N=20), including control and RRD model group.
RRD model preparation
Rats were anesthetized by 30 mg/kg pentobarbital sodium, and were fixed in a supine position. 0.1% atropine eye dropping, 0.3% ofloxacin eye droppings were applied 3 d before surgery. Mydrin-P was used to dilate the pupil. Focal anesthesia was performed by sub-conjunctival injection of 2% lidocaine and 0.75% levobupivacaine. Bulbar conjunctiva was opened, and an incision was made 3 mm posterior of corneoscleral junction to prepare channels for incision, perfusion, and optical fiber lighting. The scaffold of corneal contact lens was placed in, along with surgical microscope. Partial viscous body was removed under sterile condition. Retina was scratched gently to form 2~3 optic disc holes. Incisions of both cornea and bulbar conjunctiva were closed. 10000 U of gentamicin and dexamethasone were injected underneath the bulbar conjunctiva, along with post-op application of chloramphenicol eye dropping to prevent infection. Optical coherence tomography can observe interruption of retinal neural epithelium, in addition to significant uplift of highly-reflective pigmented epithelium to form detachment with certain angles. These features plus liquid dark region indicated successful generation of the model [17].
Culture of RPE cells
Control and model rats were anesthetized by 2% lidocaine via post-bulbar injection. Bilateral eyeballs were removed under sterile conditions. Attached fascia tissues were removed. The eyeball was rinsed in gentamicin-containing saline and was placed in DMEM culture medium. A circular incision was made 3 mm posterior of corneoscleral junction. Anterior segment and viscous body were removed, followed by 0.25% trypsin digestion for 10 min. Retinal neural epithelial layer was separated and removed. Remaining eyecup, which contained RPE cell layer, was cut radically. After 0.25% trypsin digestion for 30 min at 37°C, cells suspensions were then centrifuged at 800 rpm for 10 min. the supernatant was discarded and cultured medium containing 100 U/ml penicillin and 100 μg/ml streptomycin was added for incubation at 37°C with 5% CO2. Culture medium was changed every changed every other day. Cells were passed every 2-3 days. RPE cells at log-growth phase at 2nd to 8th generation were used for experiments. Model group was randomly divided into three groups: model group; empty transfection group, which was transfected by empty pcDNA3.1 plasmid; and MMP-8 group, which was transfected with pcDNA3.1-MMP8 plasmid.
Liposome transfection of MMP8 plasmid into RPE cells
pcDNA3.1 and MMP8 plasmid were transfected into RPE cells, which were cultured in 6-well plate until 70-80% confluence. Liposome reagent containing pcDNA3.1 and MMP8 plasmid were mixed well with 200 μl serum-free culture medium for 15 min room temperature incubation. Lipo2000 reagent was then mixed with pcDNA3.1 or pcDNA3.1-MMP8 plasmid dilutions for 30 min continuous incubation at room temperature. Serum was removed from cultured cells, which were then rinsed gently in PBS. 1.6 ml serum-free medium was added for 6 h incubation at 37°C with 5% CO2. Normal culture medium was then added for 48 h incubation in further experiments.
Real-time PCR for MMP8 mRNA expression in RPE cells
Trizol reagent was used to extract mRNA from all RPE cells. Following manual instruction of the test kit, reverse transcription kit was performed to synthesize DNA. Primers were designed by Primer 6.0 based on target gene sequence, and were synthesized by Invitrogen (China) as shown in Table 1. Real-time PCR was then performed to detect target gene expression under the following conditions: 56°C 1 min, followed by 35 cycles each containing 92°C 30 s, 58°C 45 s and 72°C 35 s; Data were collected by PCR cycler. CT value was measured for standard samples based on internal reference GAPDH gene for plotting standard curve. Quantitative analysis was performed by 2-ΔCt approach.
Table 1.
Primer sequence
| Target gene | Forward primer 5’-3’ | Reverse primer 5’-3’ |
|---|---|---|
| GAPDH | AGTACCAGTCTGTTGCTGG | TAATAGACCCGGATGTCTGGT |
| MMP8 | ACCCTTCCCTCTAGTGAATC | TAGATGGACCTCTGTTTAAT |
Western blot for MMP8 protein expression
Proteins of RPE cells were extracted by RIPA lysis buffer containing proteinase inhibitor. In brief, cells were mixed with lysis buffer for 15~30 min iced incubation. Using ultrasonic rupture (5 s, 4 times) and centrifugation (10000 g, 15 min at 4°C), proteins were quantified from the supernatant and were kept at -20°C for Western blotting. Proteins were separated in 10% SDS-PAGE, and were transferred to PVDF membrane by semi-dry method. Non-specific binding sites were blocked by 5% defatted milk powders for 2 hours. Anti-MMP8 monoclonal antibody (1:1000) or β-actin antibody (1:2000) was applied for 4°C overnight incubation. Goat anti-rabbit IgG (1:2000) was then added for 30-min incubation after PBST rinsing. After PBST washing and ECL development for 1 min, the membrane was exposed under X-ray. An imaging analyzing system and Quantity one software were then used to scan X-ray films and to detect the density of bands with repeated measures (N=4).
MTT assay for cell proliferation
RPE cells at log-phase were seeded into 96-well plate at 5000 cells per well containing DMEM medium with 10% FBS. After 24 h incubation, the supernatant was discarded, cells were then randomly divided into control, model, empty transfection, and MMP8 groups as abovementioned. After 48-hour incubation, 20 μl sterile MTT solution was then added into each test well in triplicates. With 4 h continuous culture, the supernatant was completely removed, with the addition of 150 μl DMSO for 10 min vortex until the complete resolving of crystal violet. Absorbance (A) values was measured at 570 nm in a microplate reader. The proliferation rate was calculated in each group. Each experiment was repeated in triplicates for statistical analysis.
Transwell chamber assay for cell migration
Following instruction of test kit, cells were changed from serum-free culture medium. After 24 h, 1:5 50 mg/L Matrigel dilution was used to coat bottom and upper membrane of the chamber, which was then air-dried at 4°C. 500 μl DMEM medium containing 10% FBS and 100 μl serum-free RPE cell suspensions were added into the inner and outer side of the chamber, respectively. Chambers were placed into 24-well plate in triplicates, in parallel with control group using Transwell chamber without Matrigel. After 48 h incubation, the lower chamber was rinsed by PBS. Cells on the membrane were removed, followed by cold ethanol fixation. The membrane was stained by crystal violet. Under the microscope, number of cells on the lower membrane was counted in triplicates.
Caspase 3 activity assay
Caspase 3 activity in cells was measured following manual instruction of test kit. In brief, cells were digested in trypsin, and were centrifuged at 600 g for 5 min under 4°C. The supernatant was discarded, followed by the addition of cell lysis buffer and iced incubation for 15 min. The mixture was then centrifuged at 20000 g for 5 min under 4°C, followed by the addition of 2 mM Ac-DECD-pNA. Optical density (OD) values at 400 nm wavelength were measured to calculate Caspase 3 activity.
ELISA for expression of inflammatory factor IL-1β and TNF-α
Cell culture supernatant was collected and tested following the manual instruction of ELISA kits. Absorbance (A) values at 450 nm wave length were measured in all wells using a microplate reader within 15 min of adding the quenching buffer. Linear regression model was then plotted based on the concentration of standard samples and respective A values. Sample concentration was further deduced based on A value and regression function.
Statistical analysis
All data were expressed as mean ± standard deviation (SD). Student t-test was used to compare means between two groups. SPSS 11.5 software was used to analyze all data. Analysis of variance (ANOVA) was used to compare means across groups. A statistical significance was defined when P<0.05.
Results
MMP-8 mRNA expression in RPE cells from RRD rats
Real time PCR was used to test mRNA expression of MMP-8 in RPE cells collected from RRD rat model and the effect of MMP-8 plasmid transfection on mRNA expression. Results showed significantly decreased MMP-8 expression in RRD group (P<0.05 compared to control group). The transfection of MMP-8 plasmid into RPE cells significantly facilitated MMP-8 mRNA expression in model cells (P<0.05 compared to model group). The transfection of empty plasmid into model RPE cells did not affect MMP-8 mRNA expression compared to model group (Figure 1).
Figure 1.
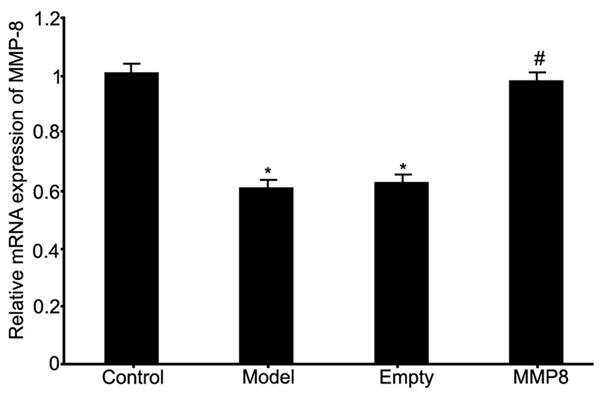
MMP-8 mRNA expression in RPE cells of RRD. *P<0.05 compared to control group. #P<0.05 compared to model group.
MMP-8 protein expression in RPE cells of RRD rats
Western blot was used to test protein expression of MMP-8 in RPE cells collected from RRD rat model and the effect of MMP-8 plasmid transfection on MMP-8 protein expression. Results showed similar results as those from mRNA levels, as shown by significantly decreased MMP-8 protein expression in RRD group (P<0.05 compared to control group). The transfection of MMP-8 plasmid into RPE cells significantly facilitated MMP-8 protein expression in model cells (P<0.05 compared to model group, Figure 2).
Figure 2.
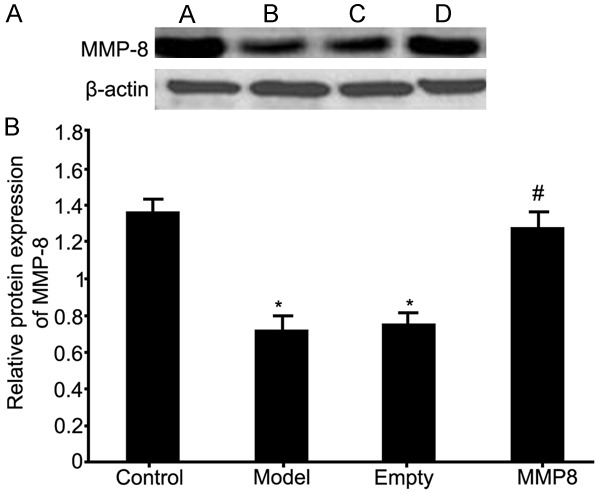
MMP-8 protein expression in RPE cells of RRD. A. MMP-8 protein expression in RPE cells from RRD model. A. Control group; B. Model group; C. Empty plasmid transfection group; D. MMP-8 group. B. Analysis of MMP-8 protein expression. *P<0.05 compared to control group; #P<0.05 compared to model group.
Proliferation of RPE cells by MMP-8 in RRD model
MTT assay was used to analyze the proliferation of RPE cells after MMP-8 plasmid transfection. Results showed significantly enhanced RPE cell proliferation in RRD group (P<0.05 compared to control group). The transfection of MMP-8 plasmid into RPE cells significantly facilitated MMP-8 expression and inhibited cell proliferation (P<0.05 compared to model group, Figure 3). These results suggested altered MMP-8 expression in RRD cells helped to regulate abnormal proliferation of RPE cells.
Figure 3.
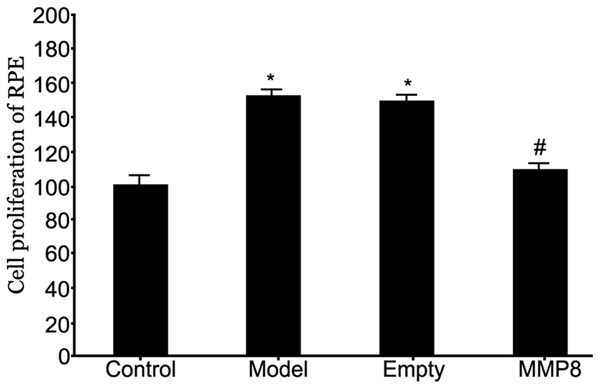
Effects on RPE cell proliferation by MMP-8 expression in RRD model. *P<0.05 compared to control group; #P<0.05 compared to model group.
Caspase 3 activity of RPE cells under regulating MMP-8 level in RRD model
Caspase 3 activity assay kit was used to analyze Caspase 3 activity of RPE cells in RRD model after MMP-8 plasmid transfection. Results showed significantly lower Caspase 3 activity in RPE cells of RRD group (P<0.05 compared to control group). The transfection of MMP-8 plasmid into RPE cells significantly facilitated Caspase 3 activity (P<0.05 compared to model group, Figure 4). These results suggested elevated MMP-8 expression in RPE cells of RRD model helped to enhance Caspase 3 activity and further RPE cell apoptosis.
Figure 4.
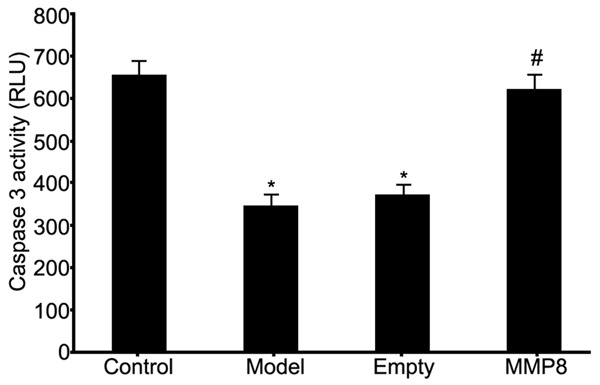
Effects on caspas3 activity of RPE cells by MMP-8 expression in RRD model. *P<0.05 compared to control group; #P<0.05 compared to model group.
RPE cell migration ability under MMP-8 over-expression
Transwell chamber assay kit was used to analyze the effect of MMP-8 on migration ability of RPE cells in RRD model. Results showed enhanced migration of RPE cells in RRD group (P<0.05 compared to control group). The transfection of MMP-8 plasmid into RPE cells significantly facilitated MMP-8 expression and inhibited cell migration ability (P<0.05 compared to model group, Figures 5 and 6). These results suggested elevated MMP-8 expression in RPE cells of RRD model enhanced migration ability of RPE cells.
Figure 5.
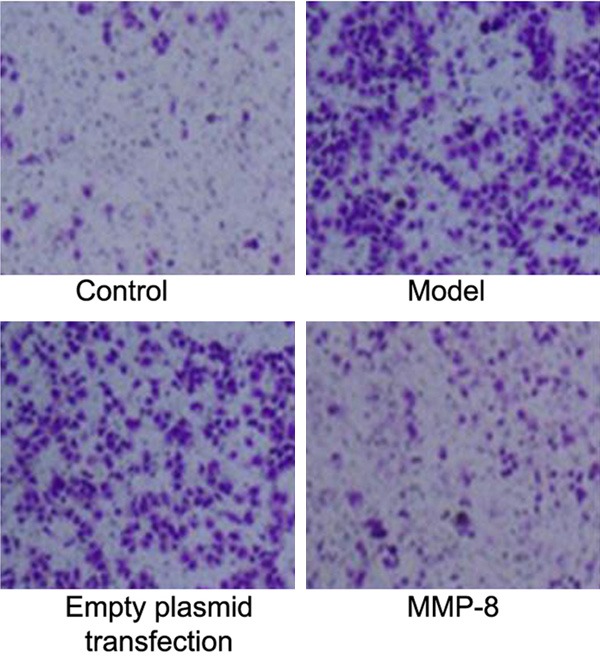
Effects of MMP-8 regulation on RPE cell migration of RRD group.
Figure 6.
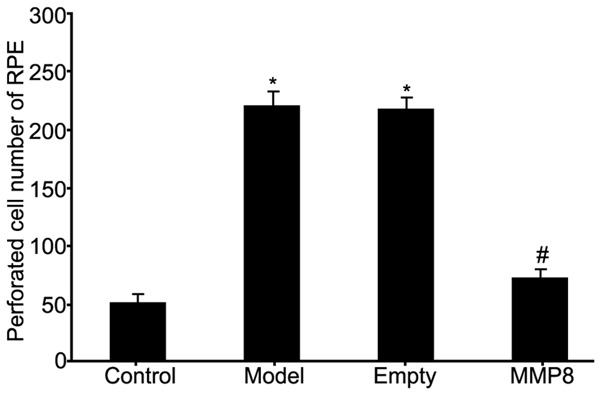
Analysis of the effects on RPE cell migration by MMP-8 expression in RRD model. *P<0.05 compared to control group; #P<0.05 compared to model group.
Expression of inflammatory factors in RPE cells by MMP-8 expression
ELISA was used to analyze the effect of MMP-8 on expression of inflammatory factors of RPE cells in RRD model. Results showed elevated IL-1β and TNF-α expressions in RPE cells of RRD group (P<0.05 compared to control group). The transfection of MMP-8 plasmid into RPE cells significantly facilitated MMP-8 expression and inhibited IL-1β and TNF-α expressions (P<0.05 compared to model group, Figure 7).
Figure 7.
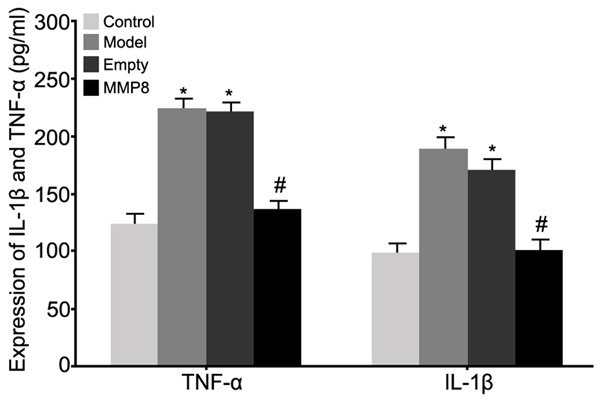
Effects on inflammatory factor expression in RPE cells by MMP-8 expression in RRD model. *P<0.05 compared to control group; #P<0.05 compared to model group.
Discussion
The early phase manifestation of RRD is the migration of RPE cells into viscous body, accompanied with abnormal hyperplasia of RPE cells. Further migration causes aggregation on the lower surface of retina or viscous-retina interface to secrete inflammatory factors, causing abundant secretion of ECM and its remodeling, eventually forming pathology precipitation, stretching retina and further detachment [18,19]. RRD is one inflammatory process and can affect ECM synthesis or degradation [20]. MMPs as the only proteinase that can hydrolyze fibrous collagen, can degrade ECM protein, maintain physiological renewal of ECM, and prevents ECM from over-precipitation [21]. MMPs can participate in various pathology-physiology processes including traumatic repair, tissue model regeneration and embryonic development, and regulate cell adhesion, plus regulation on extracellular components or other proteins [22]. Besides degrading ECM and maintaining homeostasis, MMPs can also participate in regulating various functions including cytokine secretion, cell surface protein and proteinase inhibitor [23].
As one important member of MMPs family, MMP-8 participates in pathogenesis of various diseases including corneal disease and sclera inflammation [24]. This study therefore established an RRD rat model whose RPE cells were separated and cultured to analyze the effect and mechanism of MMP-8 in RRD. Results showed significantly decreased MMP-8 expression in RPE cells of RRD model, plus elevated cell proliferation/migration potency, lower Caspase 3 activity, and elevated expression of inflammatory factors IL-1β and TNF-α. These results suggested that during pathogenesis of RRD, lower MMP-8 expression causes inhibition of RPE apoptosis, plus abnormal hyperplasia, further accelerating migration ability of RPE cells, and stimulating secretion of inflammatory factors. By transfection of MMP-8 plasmid into RPE cells of RRD model, the over-expression of MMP-8 facilitated RPE apoptosis, inhibited abnormal hyperplasia of RPE, and further suppressed RPE migration in addition to the inhibition of secretin of inflammatory factors. Such process might be correlated with ECM degradation by MMP-8, which further inhibited inflammatory stimulus and decrease RPE hyperplasia [25].
Conclusion
RRD causes decreased MMP-8 expression in RPE cells. MMP-8 can facilitate proliferation and migration of RPE cells via modulating cell apoptosis and secretion of inflammatory factors, thus participating in occurrence and progression of RRD.
Acknowledgements
This work was supported by Science-Technology Development Project of Shandong Province (2013G0021809); National Science Foundation for Young Scientists of China (81500698) and Promotive Research Fund for Excellent Young and Middle-Aged Scientisits of Shandong Province (BS2013YY044).
Disclosure of conflict of interest
None.
References
- 1.Huang C, Zhang T, Liu J, Ji Q, Tan R. Changes in axial length, central cornea thickness, and anterior chamber depth after rhegmatogenous retinal detachment repair. BMC Ophthalmol. 2016;16:121. doi: 10.1186/s12886-016-0296-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yumusak E, Ornek K, Ozkal F. Bilateral simultaneous rhegmatogenous retinal detachment following laser in situ keratomileusis. Case Rep Ophthalmol. 2016;7:341–5. doi: 10.1159/000446602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gotzaridis S, Liazos E, Petrou P, Georgalas I. 25-gauge vitrectomy and incomplete drainage of subretinal fluid for the treatment of primary rhegmatogenous retinal detachment. Ophthalmic Surg Lasers Imaging Retina. 2016;47:333–5. doi: 10.3928/23258160-20160324-05. [DOI] [PubMed] [Google Scholar]
- 4.Kominami A, Ueno S, Kominami T, Nakanishi A, Piao CH, Ra E, Yasuda S, Asami T, Terasaki H. Restoration of cone interdigitation zone associated with improvement of focal macular ERG after fovea-off rhegmatogenous retinal reattachment. Invest Ophthalmol Vis Sci. 2016;57:1604–11. doi: 10.1167/iovs.15-19030. [DOI] [PubMed] [Google Scholar]
- 5.Heriot WJ. Thermofusion of the retina with the RPE to seal tears during retinal detachment repair. Graefes Arch Clin Exp Ophthalmol. 2016;254:691–6. doi: 10.1007/s00417-016-3295-0. [DOI] [PubMed] [Google Scholar]
- 6.Koutsandrea C, Kanakis M, Papaconstantinou D, Brouzas D, Ladas I, Petrou P, Georgalas I. Scleral buckling versus vitrectomy for retinal detachment repair: comparison of visual fields and nerve fiber layer thickness. Ophthalmologica. 2016;235:10–7. doi: 10.1159/000439443. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi E, Fukushima A, Haga A, Inomata Y, Ito Y, Fukushima M, Tanihara H. Effects of mechanical stress and vitreous samples in retinal pigment epithelial cells. Biochem Biophys Res Commun. 2016;470:569–74. doi: 10.1016/j.bbrc.2016.01.104. [DOI] [PubMed] [Google Scholar]
- 8.Sukseree S, Chen YT, Laggner M, Gruber F, Petit V, Nagelreiter IM, Mlitz V, Rossiter H, Pollreisz A, Schmidt-Erfurth U, Larue L, Tschachler E, Eckhart L. Tyrosinase-cre-mediated deletion of the autophagy gene Atg7 leads to accumulation of the RPE65 variant M450 in the retinal pigment epithelium of C57BL/6 mice. PLoS One. 2016;11:e0161640. doi: 10.1371/journal.pone.0161640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knickelbein JE, Liu B, Arakelyan A, Zicari S, Hannes S, Chen P, Li Z, Grivel JC, Chaigne-Delalande B, Sen HN, Margolis L, Nussenblatt RB. Modulation of immune responses by extracellular vesicles from retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2016;57:4101–7. doi: 10.1167/iovs.15-18353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mones J, Leiva M, Pena T, Martinez G, Biarnes M, Garcia M, Serrano A, Fernandez E. A swine model of selective geographic atrophy of outer retinal layers mimicking atrophic AMD: a Phase I escalating dose of subretinal sodium iodate. Invest Ophthalmol Vis Sci. 2016;57:3974–83. doi: 10.1167/iovs.16-19355. [DOI] [PubMed] [Google Scholar]
- 11.Shaw PX, Stiles T, Douglas C, Ho D, Fan W, Du H, Xiao X. Oxidative stress, innate immunity, and age-related macular degeneration. AIMS Mol Sci. 2016;3:196–221. doi: 10.3934/molsci.2016.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moreira EF, Cai H, Tezel TH, Fields MA, Del Priore LV. Reengineering human Bruch’s Membrane increases rod outer segment phagocytosis by human retinal pigment epithelium. Transl Vis Sci Technol. 2015;4:10. doi: 10.1167/tvst.4.5.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandez-Godino R, Pierce EA, Garland DL. Extracellular matrix alterations and deposit formation in AMD. Adv Exp Med Biol. 2016;854:53–8. doi: 10.1007/978-3-319-17121-0_8. [DOI] [PubMed] [Google Scholar]
- 14.Fields MA, Cai H, Bowrey HE, Moreira EF, Beck Gooz M, Kunchithapautham K, Gong J, Vought E, Del Priore LV. Nitrite modification of extracellular matrix alters CD46 expression and VEGF release in human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2015;56:4231–8. doi: 10.1167/iovs.15-16438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kimura K, Orita T, Fujitsu Y, Liu Y, Wakuta M, Morishige N, Suzuki K, Sonoda KH. Inhibition by female sex hormones of collagen gel contraction mediated by retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2014;55:2621–30. doi: 10.1167/iovs.13-13501. [DOI] [PubMed] [Google Scholar]
- 16.Lee HS, Jun JH, Jung EH, Koo BA, Kim YS. Epigalloccatechin-3-gallate inhibits ocular neovascularization and vascular permeability in human retinal pigment epithelial and human retinal microvascular endothelial cells via suppression of MMP-9 and VEGF activation. Molecules. 2014;19:12150–72. doi: 10.3390/molecules190812150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cederlund M, Ghosh F, Arner K, Andreasson S, Akerstrom B. Vitreous levels of oxidative stress biomarkers and the radical-scavenger alpha1-microglobulin/A1M in human rhegmatogenous retinal detachment. Graefes Arch Clin Exp Ophthalmol. 2013;251:725–32. doi: 10.1007/s00417-012-2113-6. [DOI] [PubMed] [Google Scholar]
- 18.Hou X, Han QH, Hu D, Tian L, Guo CM, Du HJ, Zhang P, Wang YS, Hui YN. Mechanical force enhances MMP-2 activation via p38 signaling pathway in human retinal pigment epithelial cells. Graefes Arch Clin Exp Ophthalmol. 2009;247:1477–86. doi: 10.1007/s00417-009-1135-1. [DOI] [PubMed] [Google Scholar]
- 19.Coral K, Angayarkanni N, Madhavan J, Bharathselvi M, Ramakrishnan S, Nandi K, Rishi P, Kasinathan N, Krishnakumar S. Lysyl oxidase activity in the ocular tissues and the role of LOX in proliferative diabetic retinopathy and rhegmatogenous retinal detachment. Invest Ophthalmol Vis Sci. 2008;49:4746–52. doi: 10.1167/iovs.07-1550. [DOI] [PubMed] [Google Scholar]
- 20.Symeonidis C, Diza E, Papakonstantinou E, Souliou E, Dimitrakos SA, Karakiulakis G. Correlation of the extent and duration of rhegmatogenous retinal detachment with the expression of matrix metalloproteinases in the vitreous. Retina. 2007;27:1279–85. doi: 10.1097/IAE.0b013e3180592c00. [DOI] [PubMed] [Google Scholar]
- 21.Kim B, Abdel-Rahman MH, Wang T, Pouly S, Mahmoud AM, Cebulla CM. Retinal MMP-12, MMP-13, TIMP-1, and TIMP-2 expression in murine experimental retinal detachment. Invest Ophthalmol Vis Sci. 2014;55:2031–40. doi: 10.1167/iovs.13-13374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Du YH, Hirooka K, Miyamoto O, Bao YQ, Zhang B, An JB, Ma JX. Retinoic acid suppresses the adhesion and migration of human retinal pigment epithelial cells. Exp Eye Res. 2013;109:22–30. doi: 10.1016/j.exer.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 23.Kimura K, Orita T, Liu Y, Yang Y, Tokuda K, Kurakazu T, Noda T, Yanai R, Morishige N, Takeda A, Ishibashi T, Sonoda KH. Attenuation of EMT in RPE cells and subretinal fibrosis by an RAR-gamma agonist. J Mol Med (Berl) 2015;93:749–58. doi: 10.1007/s00109-015-1289-8. [DOI] [PubMed] [Google Scholar]
- 24.Bian F, Wang C, Tukler-Henriksson J, Pflugfelder SC, Camodeca C, Nuti E, Rossello A, Li DQ, de Paiva CS. MMP-8 is critical for dexamethasone therapy in alkali-burned corneas under dry eye conditions. J Cell Physiol. 2016;231:2506–16. doi: 10.1002/jcp.25364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bian F, Pelegrino FS, Henriksson JT, Pflugfelder SC, Volpe EA, Li DQ, de Paiva CS. Differential effects of dexamethasone and doxycycline on inflammation and MMP production in murine alkali-burned corneas associated with dry eye. Ocul Surf. 2016;14:242–54. doi: 10.1016/j.jtos.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


