Abstract
Viral myocarditis (VMC) is a common disease causing heart failure (HF) for which no specific treatments are available. As apoptosis of cardiomyoctes is a hallmark of VMC and HF, strategies targeting apoptosis are an effective way of prevention and treatment of HF. Recent studies found endoplasmic reticulum stress (ERS) reaction is a new signal transduction pathway mediating apoptosis. Calumenin protein (CP) is located within the endoplasmic reticulum Ca2+ binding proteins, and is important in ER-initiated apoptosis. The aim of this study was to investigate whether the function of CP was influenced in cardiomyocytes infected by coxsackievirus B3. The expression of CP was down-regulated in cardiomyocytes infected by coxsackievirus B3. TUNEL studies showed that apoptosis was increased in CP-deficient and ΔCP-mutant cardiomyocytes infected by coxsackievirus B3. Additionally, ERS-associated proteins (GRP78, p-PERK, p-eIF2α, ATF4 and CHOP) were up-regulated in coxsackievirus B3-infected CP-deficient and ΔCP-mutant cardiomyocytes compared to wild type control cells. These results suggested ER-initiated apoptosis was induced by coxsackievirus B3-infected cardiomyocytes and caused apoptosis through ER stress. CP can relieve ERS-initiated apoptosis in viral myocarditis.
Keywords: Endoplasmic reticulum stress, calumenin protein, ERS-initiated apoptosis, heart failure (HF), coxsackievirus B3
Introduction
Viral myocarditis (VMC) is an important cause of heart failure (HF) among adolescents and young adults [1], and is mainly characterized by non-specific myocardial interstitial inflammatory lesions [2]. VMC is caused most commonly in developed countries by viral infections such as coxsackie virus, and echovirus [3]. Coxsackievirus B3 (CVB3) is the most common virus that causes VMC [4]. Over recent years, the incidence of viral myocarditis shows an increasing trend in developing countries, especially China. Evidence shows that patients with persistent viral infections in the myocardium are likely to develop dilated cardiomyopathy and congestive heart failure (CHF) [5]. Unfortunately, HF caused by viral myocarditis lacks effective and specific treatments. This underscores the importance of understanding the mechanisms of HF caused by viral myocarditis.
HF is a complex clinical syndrome that results from the dysfunction of myocardial systolic and diastolic systems. HF is an epidemic in developed countries [6]. Apoptosis has been reported to be related with HF [7]. Apoptosis is activated in the myocardium after ischemic insult [8,9]. Imaging studies have made the visualization of the phenomenon of apoptosis possible in vivo using radiolabeled Annexin V [10]. Abbate et al. found apoptotic rate related to the development of early onset CHF by pathological examination of ventricles of patients who died within 2 months of myocardial infarction [11]. Therefore, intervention of apoptosis may be an effective way of controlling HF by experimental research and clinical trials [12]. The mechanisms of apoptosis are complex, and endoplasmic reticulum (ER) stress signaling has recently been identified as a new transduction pathway involved in apoptosis [13]. ER was first described by Porter et al. in 1945 [14]. ER is an important intracellular organelle supporting many functions including protein synthesis, translocation across the membrane and integration into the membrane [15]. ER is abundant in the myocardium, and is sensitive to changes of intracellular homeostasis. The inhibition of protein glycosylation, oxidative stress and accumulation of unfolded proteins in the ER lumen may disrupt normal ER function and trigger the unfolded protein response named ER stress (ERS) [16]. ERS is a condition that is accelerated by the accumulation of unfolded or misfolded proteins after a disturbance in the ER quality control system because of various pathological and physiological occurrences [17]. When ERS occurs, the chaperone proteins GRP78 and GRP94 are upregulated in response to unfolded proteins to enhance the ability of ER to regulate its intracellular Ca2+ levels [18,19]. ERS induced apoptosis in further development. Joo et al. used a recombinant adenovirus system to over-express calumenin protein (CP) and found that CP played an important role in ER-initiated apoptosis [20].
Calumenin, belonging to the CREC protein family, is a multiple EF-hand Ca2+-binding protein, and has been found to have unique C-terminal SR retention signal HDEF [21]. CP can combine with ryanodine receptor to active sarcoplasmic reticulum Ca2+ ATPase (SERCA2a) to regulate the release of intracellular calcium, intake, and storage of calcium in order to maintain homeostasis of calcium cycling [22]. CP can also relieve ERS of myocardial cells and suppress ERS-mediated apoptosis [20], but the relationship between CP and myocardial cells infected with CVB3 has not been reported. In the present study, we first investigated the expression of CP in myocardial cells infected with CVB3, and studied the potential mechanisms and signaling pathways involved in this effect. Our results showed that expression of CP was down-regulated in myocardial cells infected with CVB3 and activated ER-initiated cellular apoptosis. Our study provides a theoretical foundation of targeting apoptosis in the pathogenesis of viral myocarditis.
Materials and methods
Isolation and cultivation of neonatal rat cardiomyocytes
All experiments in this study were performed in accordance with the Guidelines of Animal Experiments from the Committee of Medical Ethics, National Health Department of China. Neonatal rat cardiomyocytes were isolated from 1-3 day old neonatal Sprague-Dawley rats. After washing with PBS, cardiomyocytes were cut into 1-3 mm pieces. The pieces then were digested by collagenase type II and trypsin mixture. Finally the isolated cells were plated in culture dishes. Cells were cultured in DMEM (high glucose) with 10% fetal bovine serum (FBS), and 1% antibiotics.
SiRNA design and transfection
The siRNA oligonucleotides (5’ggatggagacctaattgcc3’) were purchased from Wanlei Life Sciences (Shenyang) Co. Ltd, and incorporated into pGCSIL-GFP vectors (Vendor, City) for knocking down calumenin gene. For siRNA transfection, cardiomyocytes were cultured in culture plates overnight and transfected with control and calumenin siRNA vectors. The medium was changed with fresh culture medium after 24 h, and cells were used for subsequent experiments after 72 h of siRNA transfection.
Virus infection in neonatal rat cardiomyocytes
The coxsackievirus B3 was provided by Jilin University. After generation of three passages, the virus was collected to infect neonatal rat cardiomyocytes. Cardiomyocytes were seeded in six-well plates and cultured for 24 hours in 5% CO2 incubator at 37°C. The monolayers were then infected (MOI = 100) for 24 hour. After 24 hours, the infection media was removed; the cells were rinsed twice with PBS, and then incubated with fresh complete medium.
TUNEL assay
TUNEL kit was used to detect the apoptotic cells following the manufacturer’s instruction. Cardiomyocytes were cultured on cover slips for 24 h and infected with virus as described above. Three days post-viral infection, the cells were fixed using 4% paraformaldehyde solution for 30 min at room temperature, and incubated with 0.3% H2O2 for 30 min at room temperature to block endogenous peroxidase activity. Then the cells were incubated with terminal deoxynucleotidl transferase (TdT) reaction mix for 1 hour at 37°C. After washing, the cells were incubated with streptavidin-biotin-peroxidase for 30 min, stained with 3,3’-diaminobenzidine tetrahydrochloride, and counterstained with hematoxylin and cells observed under microscope (Vendor, City).
Western blot analysis
Western blot technique was used to analyze the expression of calumenin, GRP78, PERK, p-PERK, p-eIF2α, ATF4 and CHOP. Cells were washed in cold PBS and lysed in RIPA buffer with protease inhibitor mixture (Vendor, City). Protein concentration was measured by Bradford assay kit (Vendor, City). Cell lysates were solubilized in 2 × SDS loading buffer and were separated by electrophoresis on 10% gels and transferred to nitrocellulose (NC) membranes. The membranes were incubated with 5 % skim milk in TBST for 2 h at room temperature. Then the membranes were incubated with primary antibodies overnight at 4°C. The following antibodies were used: calumenin, GRP78, PERK, p-PERK, p-eIF2α, ATF4 and CHOP obtained from XYZ and used at XYZ dilution. After primary antibody incubation, membranes were washed with TBST and further incubated with appropriate peroxidase-conjugated secondary antibodies (Vendor, City).
Statistical analysis
Results were carried out by Student’s t test. All values were expressed as mean ± SEM. P values less than or equal to 0.05 was considered significant.
Results
Down-regulation of calmunenin expression upon coxsackievirus B3 (CVB3) infection
In order to analyze whether calumenin was related with viral myocarditis, we repressed the calumenin expression by RNA interference. Rat neonatal cardiomyocytes were infected with CVB3 and the expression levels of calumenin were assessed by Western blotting. As shown in (Figure 1), compared to control group (uninfected cells), the expression of calumenin was down-regulated in VMC-infected group and siRNA transfection model group. This data suggested that calumenin expression was regulated by viral myocarditis caused by CVB3 infection.
Figure 1.
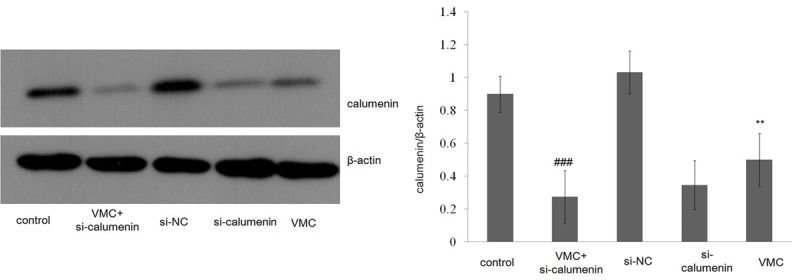
Expression of rat myocardial cell of calumenin in rat myocardial cell (NC) or siRNA transfection (RNAi). Western blot analyzed the expression of Calumenin. All data are shown as mean ± SEM. **P<0.01, n = 3; ###P<0.001, n = 3.
Apoptosis was increased by calumenin down-regulation in CVB3-infection
In order to examine whether CP influenced cell survival, terminal deoxynucleotidyltransferase-mediated dUTP Nick-End Labeling (TUNEL) assay was performed (Figure 2). TUNEL assay results revealed an increase in the number of TUNEL-positive cells by 10.02% in the CVB3-infected group compared to control group. However in the CVB3-infected calcumenin-mutant group, which had the least expression of CP, 33.97% TUNEL-positive cells were indentified. This finding indicates that the apoptosis of cardiomyocytes may be affected by viral myocarditis caused by CVB3 infection.
Figure 2.
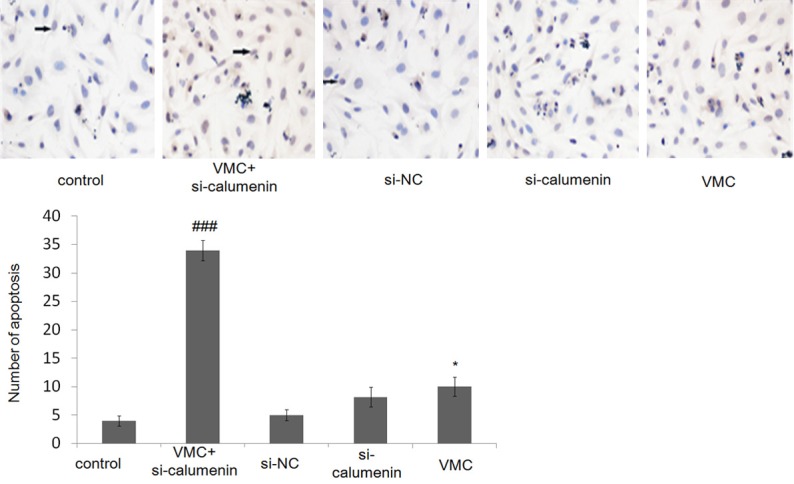
Apoptosis of mice myocardial cells detected by TUNEL assays. Scale bar = 50 µm. All data are shown as mean ± SEM. *P<0.05, n = 6; ###P<0.001, n = 6.
Calumenin reduced ERS-associated apoptosis in CVB3-infection
To determine whether calumenin was related with ERS, expression levels of ER stress chaperone protein GRP78 and ERS-related protein (PERK, P-PERK, p-eIF2α, ATF4) were evaluated by Western blot. Our results showed that down-regulation of CP in CVB3-infection resulted in an increase in GRP78, phosphorylation of PERK, p-eIF2α, and ATF4 expression as compared to the control group (Figure 3). The above results suggest that down-regulation of CP in VWC caused by CVB3 infection could cause ERS by disruption of ER function in rat myocardial cells.
Figure 3.
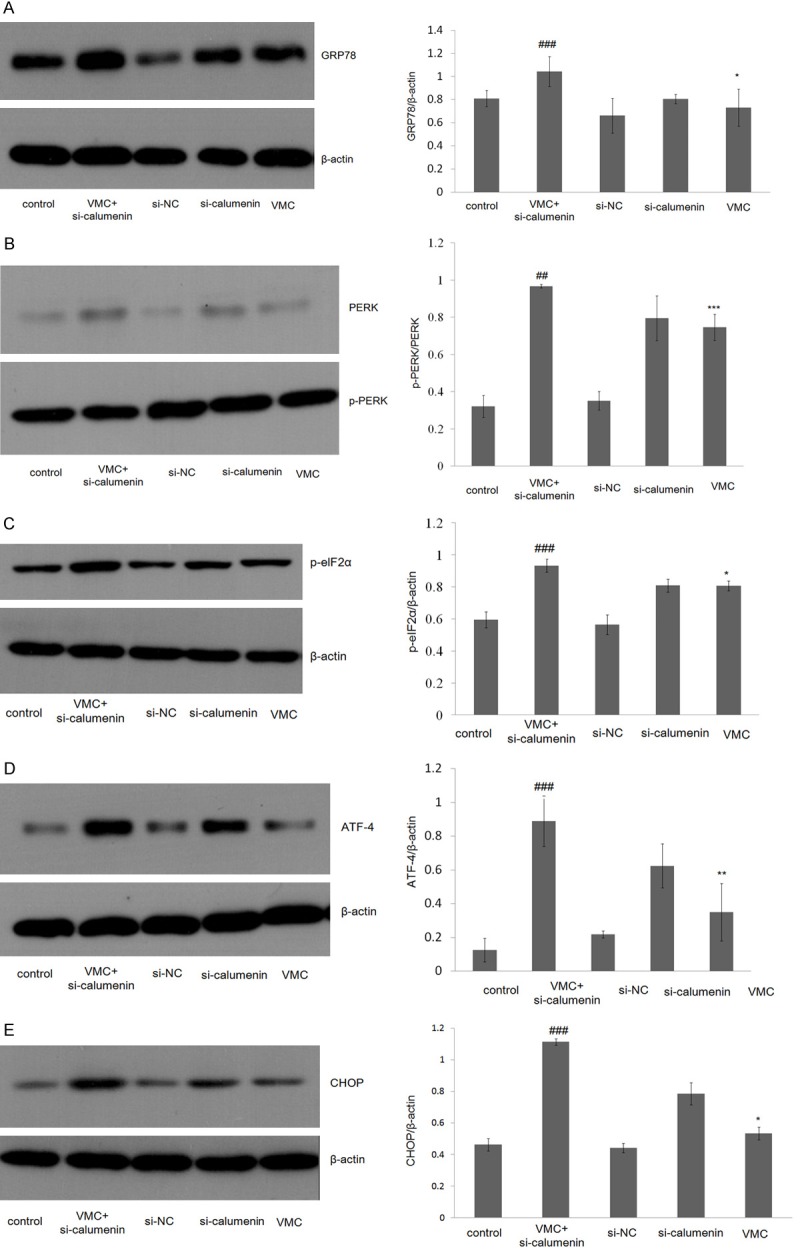
Expression of rat myocardial cells of endoplasmic reticulum stress signal pathway GRP78 (A), PERK/p-PERK (B), p-eIF2α (C), ATF4 (D), CHOP (E). All data are shown as mean ± SEM. *P<0.05, n = 3; **P<0.01, ##P<0.01, n = 3; ***P<0.001, ###P<0.001, n = 3.
CHOP is known as an important factor that mediates ERS-induced apoptosis [23]. To investigate whether ERS is involved in CP-inhibited apoptosis, the expression level of CHOP was measured. We found that CHOP significantly increased in cardiomyocytes or CP-mutant cardiomyocytes infected by CVB3 (Figure 3E). These results suggest that calumenin was related with VMC from undergoing ER-initiated apoptosis.
Discussion
As the family of picornaviridae, coxsackieviruses are non-enveloped, single-stranded RNA enteroviruses and the group B coxsackieviruses (CVB) are associated with the development of myocarditis in humans in particular serotype B3 [24]. Viral myocarditis caused by coxsackievirus B3 is a major cause of HF worldwide [25]. Despite the increasing knowledge of viral myocarditis, it remains challenging to manage patients with viral myocarditis due to lack of effective therapeutic strategies [26]. Therefore understanding pathogenic mechanisms of viral myocarditis is vital.
In our study, we examined a possible mechanism of viral myocarditis. The key findings contain: calumenin protein was down-regulated in CVB3-infection viral myocarditis model; down-regulation the expression of CP could increase ERS as evidenced by the up-regulation of GPR78; down-regulation of CP could reduce the viability of ER-initiated apoptotic cells; and finally, myocardial cells infected with CVB3 caused ER-initiated apoptosis through CP down-regulation.
Quality control is one of main mechanisms that maintain protein biosynthesis in the ER [27]. Some molecular chaperones such as GRP78 play an important role in this process. GRP78 has the ability to recognize exposed hydrophobic regions, a common feature of nascent misfolded proteins, thus assisting protein folding and assembly [28]. In our study CP was down-regulated in CVB3-infection viral myocarditis in vitro model. The molecular chaperone GRP78 was up-regulated, suggesting that ER-initiated apoptosis was activated through down-regulated expression of CP on viral myocarditis.
In our research, we reduced the expression of CP by RNAi to investigate the effects of down-regulated calumenin on ERS-mediated signaling cascades. We detected the apoptotic cells using TUNEL assay and found that the viability of cardiomyocytes was affected by CVB3-infection. PERK pathway is one of pathway related with ER-initiated apoptosis. PERK is type I transmembrane sensor protein. An increase in unfolded proteins accumulation in the ER induces the dissociation of the GRP78/PERK complex and activation by auto-phosphorylation [29]. Next, elF2α is activated by its phosphorylation [30]. ATF4 has been shown to be activated by p-elF2α [31]. In our study, p-PERK, p-eIF2α, and ATF4 were significantly up-regulated in CVB3-infected group and virus transfection model group. As an apoptosis marker, CHOP was also up-regulated in CVB3-infected group and virus transfection model group. These results suggest the down-regulation of calumenin in cardiomyocytes or Δcalumenin-mutant infected by coxsackievirus B3 can result in increased amount of unfolded proteins in the ER. Therefore calumenin protein affects ER-initiated apoptosis through PERK pathway (Figure 4).
Figure 4.
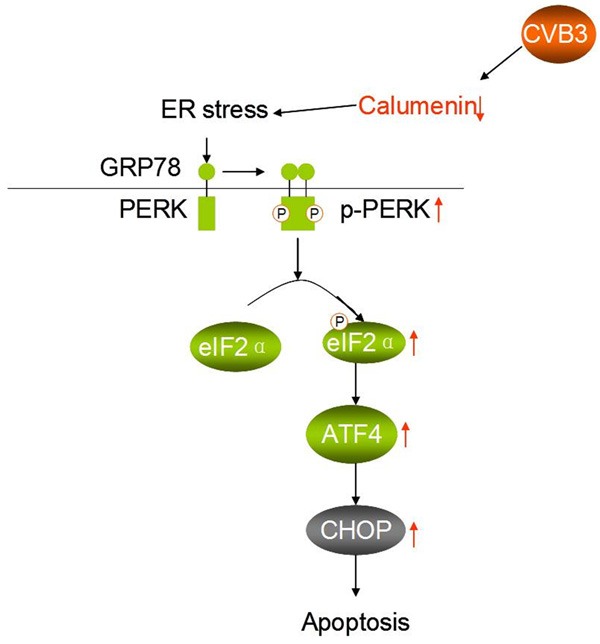
Schematic diagram shows CVB3 in ER-initiated apoptosis by down-regulation of calumenin.
In summary, we demonstrate in this study that ER-initiated apoptosis was induced by coxsackievirus B3-infected cardiomyocytes and caused myocardial apoptosis through PERK pathway. Calumenin protein can relieve ER-initiated apoptosis in viral myocarditis. These results provide a theoretical basis for drug therapy and pathogenesis of viral myocarditis.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81360587), the natural science foundation of Inner Mongolia (No. 2016BS0806), Dr. Science research Startup funds of Inner Mongolia University for Nationalities (No. BS345) and the Mongolian medicine systems biology science and technology innovation team plan of Inner Mongolia.
Disclosure of conflict of interest
None.
References
- 1.Eckart RE, Scoville SL, Campbell CL, Shry EA, Stajduhar KC, Potter RN, Pearse LA, Virmani R. Sudden death in young adults: a 25-year review of autopsies in military recruits. Ann Intern Med. 2004;141:829–834. doi: 10.7326/0003-4819-141-11-200412070-00005. [DOI] [PubMed] [Google Scholar]
- 2.Lv S, Rong J, Ren S, Wu M, Li M, Zhu Y, Zhang J. Epidemiology and diagnosis of viral myocarditis. Hellenic J Cardiol. 2013;54:382–391. [PubMed] [Google Scholar]
- 3.Bedard KM, Semler BL. Regulation of picornavirus gene expression. Microbes Infect. 2004;6:702–713. doi: 10.1016/j.micinf.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Wei J, Gao DF, Wang H, Yan R, Liu ZQ, Yuan ZY, Liu J, Chen MX. Impairment of myocardial mitochondria in viral myocardial disease and its reflective window in peripheral cells. PLoS One. 2014;9:e116239. doi: 10.1371/journal.pone.0116239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kishimoto C, Takamatsu N, Ochiai H, Kuribayashi K. Nucleotide differences of coxsackievirus B3 and chronic myocarditis. Heart Vessels. 2015;30:126–135. doi: 10.1007/s00380-014-0478-7. [DOI] [PubMed] [Google Scholar]
- 6.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2013 update: a report from the American heart association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boon RA, Dimmeler S. MicroRNAs in myocardial infarction. Nat Rev Cardiol. 2015;12:135–142. doi: 10.1038/nrcardio.2014.207. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Wang YL, Huang X, Yang Y, Zhao YJ, Wei CX, Zhao M. Ibutilide protects against cardiomyocytes injury via inhibiting endoplasmic reticulum and mitochondrial stress pathways. Heart Vessels. 2017;32:208–215. doi: 10.1007/s00380-016-0891-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.James TN. The variable morphological coexistence of apoptosis and necrosis in human myocardial infarction: significance for understanding its pathogenesis, clinical course, diagnosis and prognosis. Coron Artery Dis. 1998;9:291–307. doi: 10.1097/00019501-199809050-00007. [DOI] [PubMed] [Google Scholar]
- 10.Yamada T, Matsumori A, Wang WZ, Ohashi N, Shiota K, Sasayama S. Apoptosis in congestive heart failure induced by viral myocarditis in mice. Heart Vessels. 1999;14:29–37. doi: 10.1007/BF02481740. [DOI] [PubMed] [Google Scholar]
- 11.Abbate A, Biondi-Zoccai GG, Bussani R, Dobrina A, Camilot D, Feroce F, Rossiello R, Baldi F, Silvestri F, Biasucci LM, Baldi A. Increased myocardial apoptosis in patients with unfavorable left ventricular remodeling and early symptomatic post-infarction heart failure. J Am Coll Cardiol. 2003;41:753–760. doi: 10.1016/s0735-1097(02)02959-5. [DOI] [PubMed] [Google Scholar]
- 12.Ganguly S, Mitra A, Sarkar S. Role of α-crystallin B in regulation of stress induced cardiomyocyte apoptosis. Cardiovasc Hematol Agents Med Chem. 2014;12:60–65. doi: 10.2174/1871525713666150123151731. [DOI] [PubMed] [Google Scholar]
- 13.Xin W, Li X, Lu X, Niu K, Cai J. Involvement of endoplasmic reticulum stress-associated apoptosis in a heart failure model induced by chronic myocardial ischemia. Int J Mol Med. 2011;27:503–509. doi: 10.3892/ijmm.2011.612. [DOI] [PubMed] [Google Scholar]
- 14.Porter KR, Claude A, Fullam EF. A study of tissue culture cells by electron miscroscopy: methods and preliminary observations. J Exp Med. 1945;81:233–246. doi: 10.1084/jem.81.3.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anelli T, Sitia R. Protein quality control in the early secretory pathway. EMBO J. 2008;27:315–327. doi: 10.1038/sj.emboj.7601974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 17.Ellgaard L, Helenius A. Quality control in the endoplasmic reticulum. Nat Rev Mol Cell Biol. 2003;4:181–191. doi: 10.1038/nrm1052. [DOI] [PubMed] [Google Scholar]
- 18.Groenendyk J, Sreenivasaiah PK, Kim DH, Agellon LB, Michalak M. Biology of endoplasmic reticulum stress in the heart. Circ Res. 2010;107:1185–1197. doi: 10.1161/CIRCRESAHA.110.227033. [DOI] [PubMed] [Google Scholar]
- 19.Groenendyk J, Agellon LB, Michalak M. Coping with endoplasmic reticulum stress in the cardiovascular system. Annu Rev Physiol. 2013;75:49–67. doi: 10.1146/annurev-physiol-030212-183707. [DOI] [PubMed] [Google Scholar]
- 20.Lee JH, Kwon EJ, Kim do H. Calumenin has a role in the alleviation of ER stress in neonatal rat cardiomyocytes. Biochem Biophys Res Commun. 2013;27:327–332. doi: 10.1016/j.bbrc.2013.08.087. [DOI] [PubMed] [Google Scholar]
- 21.Jung DH, Kim DH. Characterization of isoforms and genomic organization of mouse calumenin. Gene. 2004;327:185–194. doi: 10.1016/j.gene.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 22.Sahoo SK, Kim T, Kang GB, Lee JG, Eom SH, Kim DH. Characterization of calumenin-SERCA2 interaction in mouse cardiac sarcoplasmic reticulum. J Biol Chem. 2009;284:31109–31121. doi: 10.1074/jbc.M109.031989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen M, Wang L, Yang G, Gao L, Wang B, Guo X, Zeng C, Xu Y, Shen L, Cheng K, Xia Y, Li X, Wang H, Fan L, Wang X. Baicalin protects the cardiomyocytes from ER stress-induced apoptosis: inhibition of CHOP through induction of endothelial nitric oxide synthase. PLoS One. 2014;9:e88389. doi: 10.1371/journal.pone.0088389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esfandiarei M, McManus BM. Molecular biology and pathogenesis of viral myocarditis. Annu Rev Pathol. 2008;3:127–155. doi: 10.1146/annurev.pathmechdis.3.121806.151534. [DOI] [PubMed] [Google Scholar]
- 25.Cheng Z, Li-Sha G, Jing-Lin Z, Wen-Wu Z, Xue-Si C, Xing-Xing C, Yue-Chun L. Protective role of the cholinergic anti-inflammatory pathway in a mouse model of viral myocarditis. PLoS One. 2014;9:e112719. doi: 10.1371/journal.pone.0112719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guglin M, Nallamshetty L. Myocarditis diagnosis and treatment. Curr Treat Options Cardiovasc Med. 2012;14:637–651. doi: 10.1007/s11936-012-0204-7. [DOI] [PubMed] [Google Scholar]
- 27.Trombetta ES, Parodi AJ. Quality control and protein folding in the secretory pathway. Annu Rev Cell Dev Biol. 2003;19:649–676. doi: 10.1146/annurev.cellbio.19.110701.153949. [DOI] [PubMed] [Google Scholar]
- 28.Otero JH, Lizák B, Hendershot LM. Life and death of a BiP substrate. Semin Cell Dev Biol. 2010;21:472–478. doi: 10.1016/j.semcdb.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2:326–332. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- 30.Raven JF, Baltzis D, Wang S, Mounir Z, Papadakis AI, Gao HQ, Koromilas AE. PKR and PKRlike endoplasmic reticulum kinase induce the proteasome-dependent degradation of cyclin D1 via a mechanism requiring eukaryotic initiation factor 2alpha phosphorylation. J Biol Chem. 2008;283:3097–3108. doi: 10.1074/jbc.M709677200. [DOI] [PubMed] [Google Scholar]
- 31.Li X, Wang Y, Wang H, Huang C, Huang Y, Li J. Endoplasmic reticulum stress is the crossroads of autophagy, inflammation, and apoptosis signaling pathways and participates in liver fibrosis. Inflamm Res. 2015;64:1–7. doi: 10.1007/s00011-014-0772-y. [DOI] [PubMed] [Google Scholar]


