Abstract
The objective was to determine the regulatory dynamic of Nrf2 during liver regeneration and the administration of EtOH and/or the G. schiedeanum extract. Male Wistar rats weighing 200-230 g were subjected to a 70% partial hepatectomy; they were then divided into three groups (groups 1-3). During the experiment, animals in Group 1 drank only water. The other two groups (2-3) received an intragastric dose of ethanol (1.5 g/kg BW, solution at 40% in isotonic saline solution). Additionally, rats in group 3 received a geranium extract daily at a dose of 300 mg/kg BW i.g. EtOh and/or Geranium schiedeanum was administered to rats with regenerating livers for 7 days. At the end of treatment, the activity was determined of the antioxidant enzymes, DNA concentration, TBARS, and TAC, in addition to the expression of Nrf-2, Cyclin D1, and Nqo1. EtOH increased ROS and Nrf-2, which activated the antioxidant defenses and delayed liver proliferation. On the other hand, Geranium schiedeanum exerted an antioxidant effect, diminishing ROS, but Nrf-2 expression increased, favoring liver proliferation through the increase of DNA concentration and the overexpression of Cyclin D1, however it did not activate the antioxidant defenses. In sum, it can be concluded that Nrf-2 possesses a regulatory dynamic that is evident in the presence of a toxic agent (EtOH) and/or a phytochemical agent with antioxidant capacity (Geranium schiedeanum) during liver regeneration.
Keywords: Liver regeneration, Nrf-2, free radicals, geranium schiedeanum, ethanol
Introduction
Ethanol (EtOH) is one of the toxic substances with the highest consumption in Mexico and worldwide. Chronic EtOH consumption causes damage to diverse organs of the body, among these the liver, giving rise to alcohol liver disease [1]. Among EtOH-associated mechanisms of liver damage, we found the damage per se of the EtOH molecule and the formation of Free Radicals (FR) derived from its metabolism [2]. On the other hand, it is known that the liver possesses the capacity to regenerate itself in compensatory fashion as a response to damage produced by toxic substances, such as Acetaminophen, Thioacetamine (TAA), and EtOH [3-5]. There are distinct models for studying liver proliferation, among which the most utilized model is the surgically induced liver regeneration model by means of the surgical process known as Partial hepatectomy (PH) [6]. In particular, this surgical model is employed to study the mechanisms of damage to which these toxic substances give rise in the liver and the alteration of its regeneration [7]. Diverse factors regulate liver regeneration, such as mitogenic factors, co-mitogenic factors, and the cell environment itself, including the cell’s redox state [8,9]. There are reports in which it has been proposed that equilibrium in the redox state participates in liver regeneration [10].
A cytoprotection regulator is the Nrf2 transcription factor, which regulates a great number of genes, both antioxidant proteins as well as detoxificant enzymes. Under normal conditions, Nrf2 binds to the KEAP1 protein in the cytoplasm; the increase in Reactive Oxygen Species (ROS) comprises the stimulus for the release of Nrf2 from the KEAP1 protein and this translocates to the nucleus to activate Antioxidant Response Elements (ARE), favoring mRNA synthesis of the antioxidant defenses (Catalase [CAT], Superoxide dismutase [SOD], Glutathione reductase [GR], Glutathione peroxidase [GPx]), and Noq1) to protect the cell from FR damage [11,12]. Recently, Fan et al., [5] reported that Nrf2 possesses the capacity to activate, in orderly fashion, the diverse genes that regulate in the liver, on causing acetaminophen-related damage; at an initial stage (48 h), Nrf2 activates the antioxidant defenses, probably to limit oxidative and genotoxic damage, and later, to limit the damage caused by acetaminophen, once the compensatory liver-regeneration process of this damage is initiated.
On the other hand, it has been demonstrated that the redox state participates in cell cycle regulation, the latter demonstrated because the expression of cell-cycle expression levels are conditioned by the cellular redox state [13]. Cyclin D participles in the regulation of phase-G1 of the cell cycle. It has been reported that, on increasing the generation of the Superoxide (O2•-), cyclin D levels diminish and SOD activity increases, giving rise to the arrest of the G1-phase cell cycle [14]. Likewise, high pro-oxidant (H2O2) levels in the cell diminishes the expression of cyclins D1 and D3 and transitory detention of the cell cycle in phases, G1, S, and G2, probably for it to defend itself against damage caused by Reactive Oxygen Species (ROS) [15]. Moderate levels of pro-oxidants (H2O2) favor the accumulation of cyclins D1 and D2 in fibroblasts due to the inhibition of the cyclin degradation process, probably because of transitory inhibition of ubiquitination and/or the proteasome [16]. It has been reported that various proteins contain motifs that can be temporarily regulated by oscillation of the intracellular redox environment, and that these proteins participate in the progression of the cell cycle [17].
On the other hand, the use of herbal treatments is increasingly being employed to treat diverse pathologies, including hepatopathies. There are reports of the hepatoprotective effects of diverse plants and natural extracts against agents that induce FR production. The bioactivity of these extracts has been shown as directly related with the sequestering capacity of FR [18-25], a property situating them as an excellent antioxidant. Prior reports of our group have found that the extract of Geranium schiedeanum (Gs) is a good hepatoprotective agent [26-28]. Vargas-Mendoza et al., [29] reported a hepatoprotective effect of Gs against damage caused by a sublethal dose of TAA, increasing the levels of antioxidant enzymes CAT, SOD, Glutathione peroxidase (GPx), and Glutathione reductase (GR), and diminishing the serum levels of Aspartate aminotransferase (AST) and Alanine aminotransferase (ALT) 24 and 48 h after TAA administration. On the other hand, Madrigal-Santillán et al., [26], on utilizing the surgically induced liver regeneration model, administered G. schiedeanum extract in conjunction with EtOH for 7 days, finding inhibition of the damage caused by this toxin on liver proliferation.
In sum, it is known that EtOH is a liver regeneration inhibitor by means of the formation of FR [30]; on the other hand, the extract of G. schiedeanum has been reported to be a hepatoprotective agent through its antioxidant activity [26,27,29]. Thus, we assume that the effect of EtOH damage and/or the antioxidant effect of the G. schiedeanum extract during liver regeneration can regulate factor Nrf2 in order to coordinate the repair process and liver protection during the surgically induced proliferation process and liver repair.
For the above, the objective was to determine the regulatory dynamic of Nrf2 during liver regeneration and the administration of EtOH and/or the G. schiedeanum extract.
Materials and methods
Reagents and antibodies
All electrophoresis reagents, Molecular Weights (MW) standard, Fuji x-ray films, and polyvinylidene difluoride membranes (PVDF) were obtained from Bio-Rad (Hercules, CA, USA). Anti-Nrf2, anti-NQO1, anti-Cyclin D1, and anti-β-actin antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Compatible secondary antibodies for Western blotting conjugated with Horseradish Peroxidase (HRP) and the Western blotting chemiluminescence Luminol reagent were obtained from Santa Cruz Biotechnology, Inc., and were employed according to the instructions of the manufacturer. All other chemical reagents were obtained from Merck (Merck de México, S.A.) and were of the best quality available.
Animals
We utilized male Wistar rats with an initial Body Weight (BW) of 200-230 g, which were obtained from the Escuela Superior de Medicina (ESM) Bioterium of the Instituto Politécnico Nacional (IPN) in Mexico City. The rats were housed in cages at the Bioterium (ESM). They were maintained at a temperature of 22°C with 12-h/12-h light-dark cycles and received standard rat-pellet food (Purina de México, S.A.) and water ad libitum prior to the treatments. After 14 days of adaptation, the procedure was initiated. The protocol and the experimental procedures were conducted according to the Mexican Official Norm for the Use and Care of Laboratory Animals (NOM-062-ZOO-1999, México) [31].
Obtaining the extract Geranium schiedeanum
We obtained the extract as previously described [24]. In brief, 1 kg of the dried and ground aerial parts of Geranium schiedeanum were extracted by maceration over 7 days with 20 L acetone-water (at a ratio of 7:3) and were concentrated by reduced pressure until obtaining a volume of 3 L, which was extracted with CHCl3, yielding 12.75 g of F-CHCl3 and 105 of F-Ac. Twenty grams of F-Ac was submitted to chromatography in a column with Sephadex LH-20, utilizing mixtures of H2O-MeOH (1:0; 9:1; 4:1; 7:3; 3:2; 1:1; 2:3; 3:7; 1:4; 1:9, and 0:1) with 300 mL in each (Gayosso-de-Lucio et al., [24]). The fractions were grouped based on their chromatographic profiles using Thin-Layer Chromatography (TLC), and subsequent chromatographies (silica gel and C-18) achieved identification of the following four majority components: ellagic acid; gallic acid; 3-O-a-L arabinofuranoside-7-O-a-L-ramnopyranoside of Kaempferol, and geranium acetonitrile. Notably, the latter represents approximately 40% of the F-Ac; thus, we suggest that it is the active compound [32].
Surgical procedures
Surgical removal of two thirds of the liver employing a technique known as Partial Hepatectomy (PH) was performed according to the procedure described by Higgins and Anderson [6]. The surgical procedures were performed between 08:00 and 10:00 am under light anesthesia with ethyl ether. As a surgical control, we utilized control rats on which we only carried out laparotomy, without removing the hepatic mass.
Experimental design
After the surgical procedure, the rats were housed individually. They were grouped (n = 5-6 for each experimental group) in the following manner: (1) Control group; (2) Group with PH; (3) Group with PH plus intragastric (i.g.) administration of EtOH (PH-EtOH); (4) Group with hepatectomy and receiving a Gs extract and EtOH (PH-Gs-EtOH), and (5) Group with PH and receiving the Gs extract (PH-Gs). The rats in all groups received food and water throughout the treatment period.
EtOH-treated animals received an i.g. dose of 1.5 g/kg BW (an EtOH solution at 40% in isotonic saline solution), equivalent to blood alcohol values between 75 and 150 mg/dL, which have been reported as capable of inhibiting the liver regenerative process, as reported previously [33,34]. The Geranium extract dose was 300 mg/kg BW i.g., as reported previously [24]. All treatments (EtOH solution and Geranium extract) were administered daily for 7 days.
Liver samples
On day 8, the animals were sacrificed by decapitation after being previously anesthetized with pentobarbital sodium (40 mg/kg BW). The liver was isolated, weighed, rapidly placed in cold Phosphate-Buffered Saline solution (PBS) solution with a phosphate tampon, pH 7.5, and washed for complete elimination of blood. The liver was placed in nine volumes of cold buffer (sucrose 0.25 M, TRIS 10 mM, and EGTA 0.3 mM, pH 7.4). The liver was homogenized employing a homogenizer with a piston-type driver with a Teflon tip. The homogenate was divided into aliquots and frozen at -70°C until later use. The total concentration of the protein of the homogenate was determined by the method of Lowry [35], utilizing BSA solution as standard.
Determination of DNA
After the rats were killed, the liver of each animal was resected and washed as previously described. The DNA concentration was determined in liver samples according to the technique of Labarca and Paigen [36] as modified by Ramírez-Farías et al., [37].
Total antioxidant capacity in liver
Total Antioxidant Capacity (TAC) was determined utilizing a BioAssay Systems DTAC-100 (BioAssay Systems, CA, USA), reporting the result in µmol/mg (Trolox).
Determination of thiobarbituric acid reactive substances
We determined Thiobarbituric Acid Reactive Substances (TBARS) using the DTBA-100 Assay Kit (BioAssay Systems), following the manufacturer’s instructions and reporting results in µmol/mg of protein.
Determination of enzyme antioxidants
The activities of the enzymes Superoxide Dismutase [SOD; Expansion Coefficient (EC) 1.15.1.1], Catalase (CAT, EC 1.11.1.6), and Glutathione Peroxidase (GPx, EC 1.11.1.9) were measured colorimetrically using diagnostic kits (BioAssay Systems), and Glutathione Reductase (GR, EC 1.6.4.2) employing diagnostic kits (Sigma Chemical Co., St. Louis, MO, USA), following the manufacturer’s instructions. The result is expressed as U/mg of protein.
Western blot analysis
Western blot analysis was performed as described in our previous report [38]. Briefly liver homogenates (100 µg) were separated by Polyacrylamide Gel Electrophoresis (PAGE) under reducing conditions. Proteins from the gels were electrophoretically transferred onto polyvinylidene difluoride membranes (PVDF). Primary antibodies used included Nrf2 (H-300) (sc-13032), cyclin D1 (H-295) (sc-753), NQO1 (H-90) (sc-25591), and β-actin (C-11) (sc-1615) (Santa Cruz Biotechnology). Protein bands were visualized using Horseradish Peroxidase (HRP)-conjugated secondary antibody (406401; BioLegend) and the enhanced chemiluminescence detection system utilized according to manufacturer’s instructions (Santa Cruz Biotechnology, Inc.). As control of the technique, we employed β-actin. Relative protein levels were quantified by scanning densitometry and analyzed by ImageJ ver. 1.49. The results are expressed in each group as a percent of control.
Statistical analysis
The results were analyzed using SigmaPlot ver. 12.3 statistical program software. The results are expressed as the mean ± SEM, as required. We carried out a statistical analysis using Student t test and/or Analysis of Variance (ANOVA). We considered differences among the groups to be statistically significant when P<0.05.
Results
Effect of Gs extract on liver regeneration
The PH-Gs group did not show differences compared with the PH group in terms of any study indicator, which demonstrated that the Gs extract does not exert a toxic effect, in agreement with the previously reported results [24,26].
Effect of ethanol and of the Gs extract on DNA concentrations in liver regeneration
DNA concentration is an indicator of liver proliferation. DNA concentrations in hepatic tissue for each study group are depicted in Figure 1. We observed that treatment with EtOH significantly diminished the DNA concentration compared with that of the PH group (6.20 mg DNA/g vs 9.20 mg DNA/g; P<0.05). In contrast, the PH-Gs-EtOH group exhibited values of 10.20 mg DNA/g.
Figure 1.
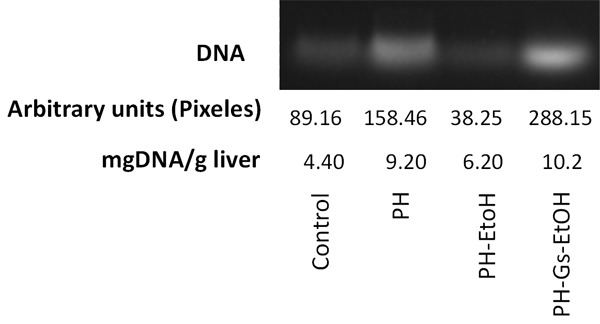
Representative-band DNA and DNA concentrations in each experimental group after 7 days of treatment with PH, EtOH, and the Geranium schiedeanum (Gs) extract. PH: Partial Hepatectomy; EtOH: Ethanol; Gs: Geranium schiedeanum.
Effect of treatment with the Gs extract on TAC and TBARS concentrations
In Table 1, Total Antioxidant Concentration (TAC) and Thiobarbituric Acid Reactive Substances (TBARS) levels are illustrated as determined in the liver, as indicators of Oxidative Stress (OS). The regenerative process increases TAC levels, as observed in the PH group compared with the control group as follows: 400 µmol/mg [Trolox] vs 225 µmol/mg [Trolox], respectively. Conversely, EtOH administration diminished TAC levels in the PH-EtOH group (360 µmol/mg [Trolox]) compared with the PH group. Finally, in the PH-Gs-EtOH group, we found values of 278 µmol/mg [Trolox].
Table 1.
TAC and TBARS Concentrations in the Diverse Study Groups
| Group | Total antioxidants capacity µmol/mg [Trolox] | TBARS µmol/mg |
|---|---|---|
| Control | 225.16 ± 10.5 | 50.12 ± 3.5 |
| PH | 400.12 ± 11.5a,c | 36.82 ± 2.3 |
| PH-EtOH | 360.38 ± 15.9a,b | 210.43 ± 4.3a,b,c |
| PH-Gs-EtOH | 278.56 ± 16.9a,b | 52.10 ± 3.92 |
Values are expressed as the mean ± Standard Error of the Mean (SEM) in each experimental group (n = 5-6).
P<0.05 vs the control group;
P<0.05 vs the PH group;
P<0.05 vs the PH-Gs-EtOH group.
PH: Partial Hepatectomy; EtOH: Ethanol; Gs: Geranium schiedeanum.
To evaluate the damage produced by ROS, we determined the TBARS concentration in the liver of animals treated with EtOH and with the Gs extract. TBARS concentrations in the different study groups are presented in Table 1. As observed in the table, there was an increase in TBARS in the PH-EtOH group of 210.43 µmol/mg, which was greater in comparison with those of the control group (50.12 µmol/mg) and the PH group (36.82 µmol/mg), respectively. On the other hand, the PH-Gs-EtOH group demonstrated a decrease in the hepatic concentration of TBARS (52.10 µmol/mg); this result was significant compared with the PH-EtOH group (vs 210.43 µmol/mg; P<0.05).
Activity of CAT, SOD, GPx, and GR in liver after treatment with Gs
The effect of the Gs extract was evaluated by means of determining the activity of enzymes CAT, SOD, GPx, and GR, which comprise the major endogenous antioxidant systems that reflect the cytoprotective ability of the cell.
Figure 2 depicts CAT (A) and SOD (B) activity in the diverse experimental groups. CAT activity increased 2.6 times in the HP group in comparison with the control group (242.71 U/mg protein vs 93.83 U/mg protein; P<0.05); ethanol administration in rats with HP induced a significant increase in CAT activity 7 days postsurgery in the control group (93.83 U/mg protein vs 413.86 U/mg protein; P<0.05), as well as in the HP group (242.71 U/mg protein vs 413.86 U/mg protein; P<0.05). In the group in which the G. schiedeanum extract was administered, CAT (231.69 U/mg protein) activity was found, similar to that found in the HP group (242.71 U/mg protein).
Figure 2.
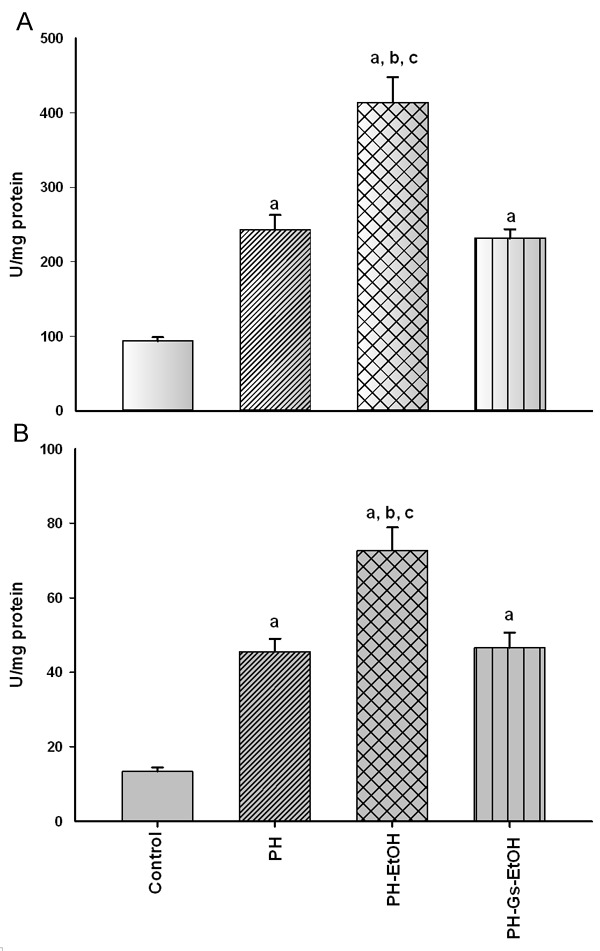
Catalase (CAT) (A) and Superoxide dismutase (SOD) (B) activity in the distinct study groups. Values are expressed as mean ± Standard Error of the Mean (SEM) in each experimental group (n = 5-6). aP<0.05 vs the control group; bP<0.05 vs the PH group; cP<0.05 vs the PH-Gs-EtOH group. PH: Partial Hepatectomy; EtOH: Ethanol; Gs: Geranium schiedeanum.
On the other hand, SOD activity in liver presented the following behavior (Figure 2B): the PH-EtOH group showed an increase in SOD activity compared with the control (5.40 times; P<0.05) and the PH (1.59 times; P<0.05) groups. In contrast, the PH-Gs-EtOH group (46.55 U/mg protein) presented levels similar to those in the HP group (45.54 U/mg protein).
Figure 3 illustrates GPx (A) and GR (B) activity in the experimental groups. In both cases, there was a significant increase in activity in the PH-EtOH group compared with the control and PH groups. In GPx (A), the activity of the enzyme in the control group was 111.18 U/mg protein, whereas a decrease was observed in the PH group (58.87 U/mg protein) and an increase, in the PH-EtOH group (984.25 U/mg protein; P<0.05). GR activity (B) in the control group was 5.42, with a significant difference in terms of the PH group (18.77 U/mg protein); conversely, the PH-EtOH group reported a significant increase compared with that of the two prior groups (33.22 U/mg protein; P<0.05).
Figure 3.
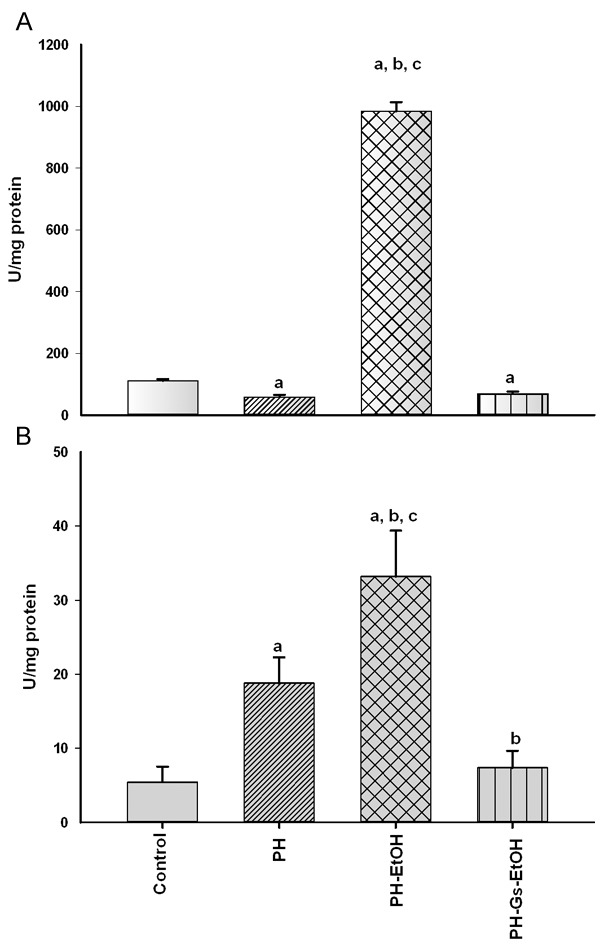
Gluthathione peroxidase (GPx) (A) and glutathione reductase (GR) (B) activity in the distinct study groups. Values are expressed as mean ± Standard Error of the Mean (SEM) in each experimental group; (n = 5-6). aP<0.05 vs the control group; bP<0.05 vs the PH group; cP<0.05 vs the PH-Gs-EtOH group. PH: Partial Hepatectomy; EtOH: Ethanol; Gs: Geranium schiedeanum.
In contrast, rats in the PH-Gs-EtOH group (68.43 U/mg protein) exhibited a significant decrease in GPx levels, reaching values comparable with those reported for the HP group (58.87 U/mg protein). When comparing the GR levels of this group with those of the EtOH-administered group, we obtained the following findings: PH-EtOH 33.22 U/mg protein vs the PH-Gs-EtOH group, 7.36 U/mg protein; P<0.05.
Nrf2, Cyclin D1, and NQO1 expression during liver regeneration and administration of ethanol and/or the G. schiedeanum extract
Nrf2 expression, as well as that of cyclin D1 and of Nqo1, was determined by means of Western blot analysis. No differences were found in Nrf2 levels in the control group in comparison with the HP group. In contrast, Nrf2 expression was significantly elevated in the HP-EtOH and HP-Gs-EtOH groups in > 50% (Figure 4). To determine the inhibitory mechanism of the proliferation by means of EtOH and the participation of Nrf2, the expression was measured of cyclin D1, a regulatory protein of the cell cycle that is responsible for the G1 phase-to-S phase transition [39]. As depicted in Figure 4, during liver regeneration, there is a significant increase of cyclin D1 in the HP (134%), which is diminished by the administration of EtOH in 40% in comparison with the HP group. This effect is reverted by the administration of the G. schiedeanum extract, as observed in the HP-Gs-EtOH group (131%). Enzyme Nqo1, an enzyme that participates in detoxification, as noted in Figure 4, does not present modifications in its expression in the PH (98%) and PH-Gs-EtOH groups (110%) in comparison with the control group (100%), but there is a significant rise in the HP-EtOH group of 142% in comparison with the previous groups.
Figure 4.
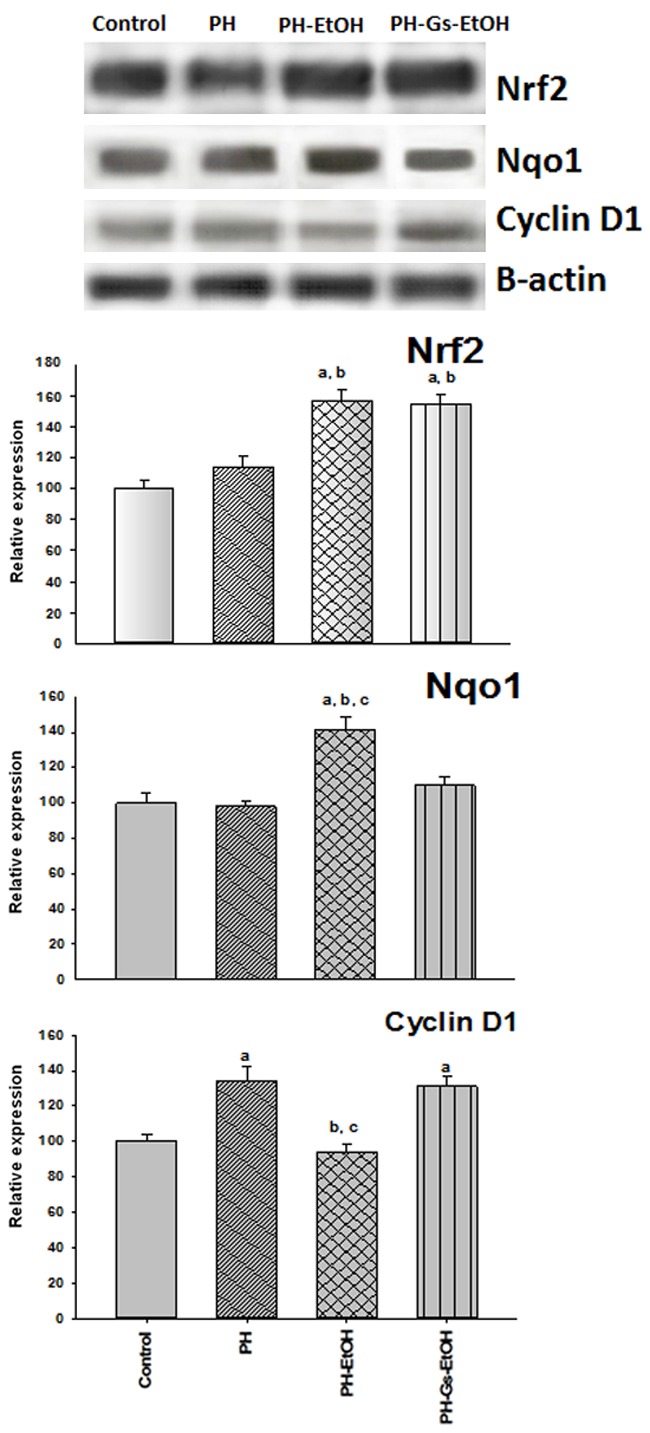
Representative-band protein of Nrf2, Nqo1, CD1, and β-actin tested by Western blotting (upper panel) and its quantification by scanning densitometry (lower panel). Values are expressed as the percentage ± Standard Error of the Mean (SEM) in each experimental group (n = 3). aP<0.05 vs the control group; bP<0.05 vs the PH group; cP<0.05 vs the PH-Gs-EtOH group. PH: Partial Hepatectomy; EtOH: Ethanol; Gs: Geranium schiedeanum.
Discussion
Liver regeneration is a highly controlled process in which intra- and extrahepatic factors participate [9]. Among these factors is found the cell’s redox state. There are reports that indicate that low levels of ROS are utilized as intracellular signals, but that high ROS levels give rise to cell damage [30]. On the other hand, it has been proposed that OS participates in liver regeneration. Aguilar-Delfín et al., [10]reported that lipid peroxidation levels in subcellular fractions of rats with PH or with acute administration of CC14 are qualitatively distinct among subcellular fractions, that this is probably a normal event in liver regeneration, and that controlled peroxidative modifications of membranes could be playing a role in the early steps of liver regeneration. On the other hand, Trejo-Solís et al., [40] found a diminution of PH-induced liver regeneration on administering pretreatment vitamin E, concluding that pretreatment with vitamin E probably could promote an expected termination of the preparative events leading to the replicative phase of PH-induced liver regeneration, and an enhanced but controlled lipid peroxidation seems to play a critical role during the early phases of liver regeneration. Ramírez-Farías et al., [37] reported that the administration of EtOH per day for seven days increases lipid peroxidation levels in rats submitted to PH, causing inhibition of liver regeneration, while the authors also reported that the administration of vitamin E diminished the lipid peroxidation levels that EtOH produces, favoring liver regeneration induced by PH. It is clear that OS plays an important role in liver regeneration and, depending on the experimental condition or on the environment, can exert an effect of favoring or inhibiting liver regeneration.
The results of this study demonstrated diminution in the DNA concentration and in cyclin D1 expression when EtOH was administered for 7 days, suggesting inhibition of liver regeneration (Figures 1 and 4). Additionally, an increase in OS, was noted through the increase of TBARS concentration and TAC diminution in the liver (Figures 2 and 3). Surprisingly, the activity of the antioxidant enzymes (CAT, SOD, GPx, and GR) and the expression of the detoxificant enzyme (Nqo1) were increased due to the administration of EtOH (Figures 2, 3 and 4). These data suggest that the constant production of ROS generated by daily EtOH administration favors the production of antioxidant and detoxificant defenses; thus, the liver regeneration process cannot be carried out by the constant presence of ROS in the liver. Various reports have described the importance of antioxidant enzymes in the regulation of the redox state and in the cell cycle. It has been reported that the increase in SOD activity gives rise to a diminution in ROS levels (superoxide), as well as cyclin B1 levels, causing a state of cell quiescence. Similarly, diminution in SOD activity causes elevation in ROS levels, favoring the increase of the expression of cyclins D1 and B1, which maintain proliferative growth. It is probable that SOD activity regulates a “ROS-switch”, this being of such great importance that loss of control of this “ROS-switch” would cause aberrant proliferation, such as cancer or cell aging [41]. CAT overexpression diminishes the concentration of hydrogen peroxide and inhibits smooth-muscle proliferation, with an increase of apoptosis. The latter suggests that the redox state is an important modulator of cell survival and proliferation [42]. On the other hand, overexpression of GPx diminishes peroxide levels, changing the cellular redox environment, which causes a delay in the G1 phase-to-S phase transition of the cell cycle. Therefore, GPx could comprise an important factor in cell growth [43]. These reports are in agreement with our results, in which we found that EtOH administration increases the activity of the antioxidant enzymes (Figures 2 and 3) and overexpression of Nqo1 (Figure 4), thus inhibition of liver regeneration; contrariwise, on the diminishing of antioxidant enzyme activity (SOD, CAT, GPx, and GR), liver proliferation was favored.
Fan et al., [5] on administering a dose of acetaminophen (400 mg/kg) to mice, found that at 48 h, expression of the genes of the antioxidant defenses (Nqo1, glutamate-cysteine ligase, and heme oxygenase-1) was favored for promoting the repair of the damage caused to the liver by the acetaminophen, with subsequent following of the compensatory liver regeneration observed with the increase of cyclin D1 and PCNA. On the other hand, Köhler et al., [30]generated transgenic mice that constitutively express Nrf2 (caNrf2) in the hepatocytes, reporting a delay in hepatocyte proliferation and an increase of apoptosis in these cells in transgenic mice (caNrf2). In that same study, on utilizing the liver damage model through the administration of carbon tetrachloride, Nrf2 activation in hepatocytes did not improve the compensatory liver regeneration that was expected to occur after damage by this toxin; however, the authors did find important regulation, by caNrf2 in the hepatocytes of transgenic mice, of the antioxidant defenses acting to limit the damage caused by the ROS. This probably is beneficial for hepatocytes found in the full OS process in order to delay proliferation, which allows for the more efficient elimination of DNA adducts and damaged cell proteins, diminishing the risk of genotoxicity or cell death and organ insufficiency [44]. In particular, it is known that transcriptional factor Nrf2 controls the expression of numerous genes that encode antioxidant proteins (CAT, SOD, GR, and GPx) and detoxificant enzymes (Nqo1) [11,12]. Given that Nrf2 is an enzyme modulator that regulates OS, the former surely participates in the liver proliferation process. We found that ethanol increases ROS levels 4-fold (quantified by TBARS), and the Gs returns these to levels similar to those of the controls (Table 1). Surprisingly, under both, contrary redox-state conditions, we found overexpression of transcriptional factor Nrf2 (Figure 4). What the latter demonstrates is that there is an Nrf2 regulatory dynamic in the genes that should activate according to the existing cellular conditions. In the former case, in which ROS are elevated, Nrf2 is overexpressed to confer protection from the damage that the EtOH is causing, in order to activate the antioxidant and detoxicant defenses (CAT, SOD, GPx, GR, and Nqo1) (Figures 2, 3 and 4) and liver proliferation is delayed (diminution of DNA concentration and the expression of cyclin D1) (Figures 1 and 4). In the latter case, the geranium in itself diminishes ROS levels (Table 1); on the other hand, Nrf2 overexpression favors liver proliferation through the increase of DNA concentration and cyclin D1 overexpression (Figures 1, 2, 3 and 4) and does not activate the antioxidant defenses. These data reinforce previous reports [5,30] on the regulatory modulation possessed by transcriptional factor Nrf2. Our results are the first to demonstrate that both redox-state conditions can exert an influence on Nrf2 regulation. This should be taken into account when there is pharmacological interest in activating or inhibiting Nrf2. In particular, it has been demonstrated that natural agents such as curcumin, resverastrol, or geranium exert an antioxidant and/or an activator effect on Nrf2 [12,26,29]. On the other hand, synthetic agents such as Bardoxolone methyl (a potent Nrf2 activator) has not exhibited the expected results [12].
Recent studies have demonstrated that phytochemical compounds activate Nrf2, favoring activation of the regulatory genes of the antioxidant enzymes [29,45]. Xiong et al., [45] reported a diminution of Nrf2, and in turn of the antioxidant defenses, on chronically administering EtOH to mice (2.4 g/kg/day for 6 weeks); on the other hand, on administering curcumin (150 mg/kg) together with EtOH, curcumin provided protection from the damage caused by the EtOH, increasing Nrf2 levels and those of the antioxidant defenses. Vargas-Mendoza et al., [29] when administering a dose of TAA (6.6 mmol/kg intraperitoneally [i.p.]), found at 24 and 48 h post-treatment, diminution of the antioxidant defenses (CAT, SOD, GPx, and GR) and an increase in the liver-damage markers (AST, ALT, and total bilirubin); however, when the authors administered a pretreatment with G. schiedeanum extract (100 mg/kg, i.g.) for 4 days and after this pretreatment administered TAA, antioxidant defenses increased and diminution was observed in the transaminases. Our results are in agreement with previous reports on demonstrating that the extract of G. schiedeanum possesses antioxidant and hepatoprotective capacity (Table 1; Figure 1).
In summary, it can be concluded that Nrf2 possesses a regulatory dynamic that is evident in the presence of a toxic agent (EtOH) and/or a phytochemical agent with antioxidant capacity (G. schiedeanum) during liver regeneration. But the complexity of the modulation of Nrf2 and its functions is even greater. For example, Fan et al., [5] observed an increase of antioxidant defenses at 48 h after treatment with acetaminophen; on the other hand, Vargas Mendoza et al., [29] reported a diminution of antioxidant defenses 48 h after a dose of TAA. The latter establishes that there are various factors that intervene in Nrf2 regulation, such as the following: the model (acute, chronic, or with genetic modifications); the organism (rat or mouse); the physiological condition (regenerating liver or complete liver); the damage-generating agent (EtOH, acetaminophen, or TAA); the EtOH dose (1.5 g/kg or 2.4 g/kg), and the administration route (i.g. or i.p,). The agreement in all of the reports lies in that Nrf2 possesses the capacity to identify the pathway to activate that is suitable for the survival of the cell.
Conclusions
Nrf2 possesses various cell functions, some contradictory (antiapoptotic and proliferative vs proapoptotic), in addition to reports that demonstrate that its beneficial effects in various diseases. Therefore, the biological importance of this study are based in demonstration that there is dynamic modulation of Nrf2 during liver regeneration in high as well as low concentrations of ROS, opening a therapeutic window to prevent alcohol-associated liver damage, in which the geranium has again demonstrating its being a hepatoprotective agent of damage caused by EtOH. Upcoming works should be focused on investigating the agents that regulate Nrf2 in order to indicate the route that should be activated. What are the specific stimuli that determine which elements should activate Nrf2. Responding to this query will be of utmost importance for utilizing it as a drug against various diseases. Several candidates can be Nrf2 regulators, such as molecules or proteins or redox motifs, or the cell’s microenvironment (redox state), or the Michael reaction center.
Acknowledgements
The authors thank Dr. José Gutiérrez-Salinas for their critical review of this manuscript. Supported by SIP Project No. 20171315 ESCOM-IPN and No. 20170786, ESM-IPN.
Disclosure of conflict of interest
None.
References
- 1.Morales-González JA. Un Enfoque Multidisciplinario. México: Universidad Autónoma del Estado de Hidalgo Pachuca; 2007. Alcohol, alcoholismo y cirrosis; p. 240. [Google Scholar]
- 2.Piña-Garza E, Gutiérrez-Salinas J, Morales-González JA, Zentella-de-Piña M. ¿Es tóxico el alcohol? In: Riveros Rosas H, Flores Herrera O, Sosa Peinado A, Vázquez Contreras E, editors. Temas Bioquímicos de vanguardia. México: Facultad de Medicina UNAM; 2003. pp. 121–146. [Google Scholar]
- 3.Bautista M, Gómez-del-Río MA, Benedí J, Sánchez-Reus MI, Morales-González JA, Téllez-López AM, López-Orozco M. Effect of dichloromethylene diphosphonate on liver regeneration following thioacetamide-induced necrosis in rats. World J Hepatol. 2013;5:379–386. doi: 10.4254/wjh.v5.i7.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morales-González JA, Gutiérrez-Salinas J, Yáñez L, Villagómez-Rico C, Badillo-Romero J, Hernández-Muñoz R. Morphological and biochemical effects of a low ethanol dose on rat liver regeneration: role of route and timing of administration. Dig Dis Sci. 1999;44:1963–1974. doi: 10.1023/a:1026601814082. [DOI] [PubMed] [Google Scholar]
- 5.Fan X, Chen P, Tan H, Zeng H, Jiang Y, Wang Y, Wang Y, Hou X, Bi H, Huang M. Dynamic and coordinated regulation of KEAP1-NRF2-ARE and p53/p21 signaling pathways is associated with acetaminophen injury responsive liver regeneration. Drug Metab Dispos. 2014;42:1532–1539. doi: 10.1124/dmd.114.059394. [DOI] [PubMed] [Google Scholar]
- 6.Higgins GM, Anderson RM. Experimental pathology of the liver I Restoration of the liver of the white rat following partial surgical removal. Arc Pathol. 1931;12:186–202. [Google Scholar]
- 7.Fausto N. Liver regeneration: from laboratory to clinic. Liver Transpl. 2001;7:835–844. doi: 10.1053/jlts.2001.27865. [DOI] [PubMed] [Google Scholar]
- 8.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. J Hepatol. 2012;57:692–694. doi: 10.1016/j.jhep.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 9.Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 10.Aguilar-Delfín I, López-Barrera F, Hernández-Muñoz R. Selective enhancement of lipid peroxidation in plasma membrane in two experimental models of liver regeneration: partial hepatectomy and acute administration. Hepatology. 1996;24:657–662. doi: 10.1002/hep.510240331. [DOI] [PubMed] [Google Scholar]
- 11.Morales-González JA, Madrigal-Santillan E, Morales-González A, Bautista M, Gayosso-Islas E, Sánchez-Moreno C. What is Known Regarding the Participation of Factor Nrf-2 in Liver Regeneration? Cells. 2015;4:169–177. doi: 10.3390/cells4020169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi BH, Kang KS, Kwak MK. Effect of redox modulating NRF2 activators on chronic kidney disease. Molecules. 2014;19:12727–12759. doi: 10.3390/molecules190812727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Menon SG, Goswani PC. A redox cycle within the cell cycle: ring in the old with the new. Oncogene. 2007;26:1101–1109. doi: 10.1038/sj.onc.1209895. [DOI] [PubMed] [Google Scholar]
- 14.Menon SG, Sarsour EH, Kalen AL, Venkataraman S, Hitchler MJ, Domann FE, Oberley LW, Goswami PC. Superoxide signaling mediates N-acetyl-L-cysteine-induced G1 arrest: regulatory role of cyclin D1 and manganese superoxide dismutase. Cancer Res. 2007;67:6392–6399. doi: 10.1158/0008-5472.CAN-07-0225. [DOI] [PubMed] [Google Scholar]
- 15.Barnouin K, Dubuisson ML, Child ES, Fernández S, Glassford J, Medema RH, Mann DJ, Lam E. H2O2 induces a transient multi-phase cell cycle arrest in mouse fibroblasts through modulating cyclin D and p21 Cip1 expression. J Biol Chem. 2002;277:13761–13770. doi: 10.1074/jbc.M111123200. [DOI] [PubMed] [Google Scholar]
- 16.Martínez-Muñoz C, Post JA, Verkleij AJ, Verrips CT, Boonstra J. The effect of hydrogen peroxide on the cyclin D expression in fibroblasts. Cell Mol Life Sci. 2001;58:990–996. doi: 10.1007/PL00013204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conour JE, Graham WV, Gaskins HR. A combined in vitro/bioinformatics investigation of redox regulatory mechanisms governing cell cycle progression. Physiol Genomics. 2004;18:196–205. doi: 10.1152/physiolgenomics.00058.2004. [DOI] [PubMed] [Google Scholar]
- 18.Saleem TS, Chetty CM, Ramkanth S, Rajan VS, Kumar KM, Gauthaman K. Hepatoprotective herbs-a review. Int J Res Pharm Sci. 2010;1:1–5. [Google Scholar]
- 19.Adewusi EA, Afolayan AJ. A review of natural products with hepatoprotective activity. J Med Plant Res. 2010;4:1318–1334. [Google Scholar]
- 20.Kono H, Arteel GE, Rusyn I, Sies H, Thurman RG. Ebselen prevents early alcohol-induced liver injury in rats. Free Radic Biol Med. 2001;30:403–411. doi: 10.1016/s0891-5849(00)00490-1. [DOI] [PubMed] [Google Scholar]
- 21.Wheeler MD, Nakagami M, Bradford BU, Uesugi T, Mason RP, Connor HD, Dikalova A, Kadiiska M, Thurman RG. Overexpression of manganese superoxide dismutase prevents alcohol-induced liver injury in the rat. J Biol Chem. 2001;276:36664–36672. doi: 10.1074/jbc.M105352200. [DOI] [PubMed] [Google Scholar]
- 22.Rosengren RJ. Catechins and the treatment of breast cancer: possible utility and mechanistic targets. IDrugs. 2003;6:1073–1078. [PubMed] [Google Scholar]
- 23.Vargas-Mendoza N, Madrigal-Santillan E, Morales-González A, Esquivel-Soto J, Esquivel-Chirino C, García-Luna Y, González-Rubio M, Gayosso-de-Lucio JA, Morales-González JA. Hepatoprotective effect of silymarin. World J Hepatol. 2014;6:144–149. doi: 10.4254/wjh.v6.i3.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gayosso-De-Lucio J, Bautista M, Velazquez-González C, De-la-O-Arciniega M, Morales-González JA, Benedí J. Chemical composition and hepatotoxic effect of Geranium schiedeanum in a thioacetamide-induced liver injury model. Pharmacogn Mag. 2014;10(Suppl 3):S574–S580. doi: 10.4103/0973-1296.139788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madrigal-Santillán E, Madrigal-Bujaidar E, Álvarez-González I, Sumaya-Martínez MT, Gutiérrez-Salinas J, Bautista M, Morales-González A, García-Luna Y, González-Rubio M, Aguilar-Faisal JL, Morales-González JA. Review of natural products with hepatoprotective effects. World J Gastroenterol. 2014;20:14787–14804. doi: 10.3748/wjg.v20.i40.14787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madrigal-Santillán E, Bautista M, Gayosso-de-Lucio JA, Reyes-Rosales Y, Posadas-Mondragon A, Morales-González A, Soriano-Ursúa MA, García-Machorro J, Madrigal-Bujaidar E, Álvarez-González I, Morales-González JA. Effect hepatoprotective of Geranium schiedeanum against the toxic action of ethanol during liver regeneration. World J Gastroenterol. 2015;21:7718–7729. doi: 10.3748/wjg.v21.i25.7718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gayosso-De-Lucio JA, Torres-Valencia J, Cerda-García-Rojas CM, Joseph-Nathan P. Ellagitannins from Geranium potentillaefolium and G. bellum. Nat Prod Commun. 2010;5:531–534. [PubMed] [Google Scholar]
- 28.Bautista M, Madrigal-Santillan E, Morales-González A, Gayosso-de-Lucio JA, Madrigal-Bujaidar E, Chamorro-Cevallos G, Álvarez-González I, Benedi J, Aguilar-Faisal JL, Morales-González JA. An alternative hepatoprotective and antioxidant agent: the geranium. Afr J Tradit Complement Altern Med. 2015;12:96–105. [Google Scholar]
- 29.Vargas Mendoza N. Tesis de Maestría. México: Universidad Autónoma del Estado de Hidalgo; 2012. Efecto hepatoprotector y antioxidante del extracto y los principios activos de Geranium shiedeanum . [Google Scholar]
- 30.Köhler UA, Kurinna S, Schwitter D, Marti A, Schäfer M, Hellerbrand C, Speicher T, Werner S. Activated Nrf2 impairs liver regeneration in mice by activation of genes involved in cellcycle control and apoptosis. Hepatology. 2014;60:670–678. doi: 10.1002/hep.26964. [DOI] [PubMed] [Google Scholar]
- 31.Norma Oficial mexicana NOM-062-ZOO-1999. Especificaciones técnicas para la producción, cuidado y uso de los animales de laboratorio. Available online: www.fmvz.unam.mx/fmvz/principal/archivos/062ZOO.PDF.
- 32.Bautista M, Gayosso-de-Lucio JA, Vargas-Mendoza N, Velázquez-González C, De-la-O Arciniega M, Almaguer-Vargas G. Oxidative stress and chronic degenerative diseases - A role for antioxidants. Primera edición.Croatia, Morales-Gonzalez JA; 2013. Geranium species as antioxidants; pp. 113–129. [Google Scholar]
- 33.Morales-González JA, Gutierrez-Salinas J, Hernández-Muñoz R. Pharmacokinetics of the ethanol bioavailability in the regenerating rat liver induced by partial hepatectomy. Alcohol Clin Exp Res. 1998;22:1557–1563. doi: 10.1111/j.1530-0277.1998.tb03949.x. [DOI] [PubMed] [Google Scholar]
- 34.Gill K, Frnace C, Amit Z. Voluntary ethanol consumption in rats: an examination of blood/brain ethanol levels and behavior. Alcohol Clin Exp Res. 1986;10:457–462. doi: 10.1111/j.1530-0277.1986.tb05124.x. [DOI] [PubMed] [Google Scholar]
- 35.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 36.Labarca C, Paigen KA. Simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980;102:344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- 37.Ramírez-Farías C, Madrigal-Santillan E, Gutiérrez-Salinas J, Rodríguez-Sánchez N, Martínez-Cruz M, Valle-Jones I, Gramlich-Martínez I, Hernández-Ceruelos A, Morales-Gonzaléz JA. Protective effect of some vitamins against the toxic action of ethanol on liver regeneration induced by partial hepatectomy in rats. World J Gastroenterol. 2008;14:899–907. doi: 10.3748/wjg.14.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anguiano-Robledo L, Reyes-Melchor P, Bobadilla-Lugo RA, Pérez-Alvarez VM, López-Sánchez P. Renal angiotensin-II receptors expression changes in a model of preeclampsia. Hypertens Pregnancy. 2007;26:151–161. doi: 10.1080/10641950701252827. [DOI] [PubMed] [Google Scholar]
- 39.Jiménez-García MN, Arellanes-Robledo J, Aparicio-Bautista DI, Rodríguez-Segura MA, Villa-Treviño S, Godina-Nava JJ. Anti-proliferative effect of extremely low frequency electromagnetic field on preneoplastic lesions formation in the rat liver. BMC Cancer. 2010;10:159. doi: 10.1186/1471-2407-10-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trejo-Solís C, Chagoya-de-Sánchez V, Aranda-Fraustro A, Sánchez-Sevilla L, Gómez-Ruíz C, Hernández-Muñoz R. Inhibitory effect of vitamin E administration on the progression of liver regeneration induced by partial hepatectomy in rats. Lab Investig. 2003;83:1669–1679. doi: 10.1097/01.lab.0000095688.89364.bf. [DOI] [PubMed] [Google Scholar]
- 41.Sarsour EH, Venkataram Kalem AL, Oberley LW, Goswami PC. Manganese superoxide dismutase activity regulates transitions between quiescent and proliferative growth. Aging cell. 2008;7:405–417. doi: 10.1111/j.1474-9726.2008.00384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown MR, Miller FJ Jr, Li WG, Ellingson AN, Mozena JD, Chatterjee P, Engelhardt JF, Zwacka RM, Oberley LW, Fang X, Spector AA, Weintraub NL. Overexpression of human catalase inhibits proliferation and promotes apoptosis in vascular smooth muscle cells. Circ Res. 1999;85:524–533. doi: 10.1161/01.res.85.6.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang HP, Schafer FQ, Goswami PC, Oberley LW, Buettner GR. Phospholipid hydroperoxide glutathione peroxidase induces a delay in G1 of the cell cycle. Free Radical Res. 2003;37:621–630. doi: 10.1080/1071576031000088283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Michalopoulos GK. NRF2, not always friendly but perhaps misunderstood. Hepatology. 2014;60:461–463. doi: 10.1002/hep.27090. [DOI] [PubMed] [Google Scholar]
- 45.Xiong ZE, Dong WG, Wang BY, Tong QY, Li ZY. Curcumin attenuates chronic ethanol-induced liver injury by inhibition of oxidative stress via mitogen-activated protein kinase/nuclear factor E2-related factor 2 pathway in mice. Pharmacogn Mag. 2015;11:707–715. doi: 10.4103/0973-1296.165556. [DOI] [PMC free article] [PubMed] [Google Scholar]


