Abstract
Heat shock protein 10 (Hsp10), located in mitochondria, is a co-chaperone involved in the protein folding and aggregation with Hsp60. Besides, a wide range of other extramitochondrial and extracellular activities, such a mammalian mitochondrial chaperonin including modulation of apoptosis, inflammation, and carcinogenesis, have been reported. Expression of Hsp10 protein in oral squamous cell carcinomas (OSCC) and the non-cancerous squamous epithelium was detected using immunohistochemistry we retrospectively evaluated the correlations between Hsp10 expression and the clinicopathological characteristics of OSCC. Our results showed that percentage of high expression of Hsp10 in the OSCC was statistically higher than that in the non-cancerous squamous epithelium (P = 0.006). What is more, high Hsp10 expression was significantly associated with shorter overall survival in patients with OSCC (P<0.001). In addition, our results identified that the high expression of Hsp10 was significantly correlated with OSCC patients age, the history of chewing betel nut, pathological grade, lymph node metastasis and radiotherapy after operation (P = 0.008, P = 0.021, P = 0.026, P = 0.008, P = 0.049 respectively). Multivariate Cox regression analysis further identified that high expression of Hsp10 protein was an independent poor prognostic factor for OSCC (P<0.001). High Hsp10 expression might play important roles in the progression of OSCC, and it might act as a novel valuable independent biomarker to predict poor prognosis in patients with OSCC.
Keywords: Oral squamous cell carcinoma, heat shock protein 10 (Hsp10), lymph node metastasis, biomarker, prognosis
Introduction
About 90% of oral cancer is oral squamous cell carcinoma (OSCC) [1], which is the sixth most common cancer in the world [2]. The clarified predisposing factors of OSCC mainly include tobacco consumption, abusing of alcohol, and chewing betel nut [3,5]. Other important risk factors also cannot be ignored, including infection with human papilloma virus (HPV) [6] or human immunodeficiency virus (HIV) [6,7] and so on. So far, the mainstream treatment for OSCC patients is surgery, combining with radiotherapy or chemotherapy. In spite of the increase of alternative approaches for the treatment of OSCC, the overall 5-year survival rate for the patients with OSCC is still disappointed [8]. In this situation, further elucidation of the molecular mechanism underlying OSCC is vital for the development of new effective therapeutic agents and novel prognostic markers.
Heat shock proteins (Hsps), widely expressed and highly conserved in prokaryotes and eukaryotes, are molecular chaperones playing an important role in various routine biological processes, such as transcription, translation and posttranslational modifications, protein folding, and aggregation and disaggregation of proteins [9]. However, most Hsps are related to carcinogenesis. As to the relationship between Hsps and tumor progression, some Hsps can serve as biomarkers [10]. Hsp10, located in mitochondria, is a co-chaperone involved in the protein folding and aggregation with Hsp60. Besides, a wide range of other extramitochondrial and extracellular activities, such a mammalian mitochondrial chaperonin including modulation of apoptosis, inflammation, and carcinogenesis, have been reported [11]. The overexpression of Hsp10 correlated with carcinogenesis and tumor malignancy such as uterine, large bowel and ovarian cancer has already been reported [12,13]. However, up to now there is not any literature about whether high Hsp10 expression of associated with clinicopathological characteristics and had prognostic implications in OSCC. In this study, we detected the expression of Hsp10 protein by immunohistochemistry (IHC) in 110 cases of OSCC and 32 cases of the non-cancerous control squamous epithelium tissues. Also, we retrospectively evaluated the correlations between Hsp10 expression and the clinicopathological characteristics of OSCC by univariate and multivariate analyses.
Materials and methods
Tissue samples and clinical data
All patients with OSCC were submitted to surgical treatment at the Department of Stomatology, the Second Xiangya Hospital of Central South University (Changsha, China) from April 2007 to March 2012. All OSCC samples and non-cancerous squamous epithelium were obtained from Department of Pathology, the Second Xiangya Hospital of Central South University. This study protocol, specimen usage, and data retrieval were approved by the Ethics Review Board of the Second Xiangya Hospital of Central South University (Scientific and Research Ethics Committee). Written informed consent was obtained from all patients. If the patients were minors/children, written informed consent was obtained from the next of kin, caretakers, or guardians on the behalf of the minors/children participants involved in your study. Complete clinical record and follow-up data were available for all patients. Also, written informed consent was obtained from all patients.
These patients had a confirmed histological diagnosis of OSCC according to WHO histological classification of oral tumors. 110 patients (90 males, 20 females), had not been previously treated with chemotherapy and radiotherapy at the time of original operation. Complete clinical record and follow-up data were available for all patients. Overall survival time was calculate from the date of diagnosis to the date of death or the date of last follow-up if the patient was still surviving. At the time of analysis, 80 patients (72.7%) were still alive with a median follow-up of 33.2 months (range, 10 to 60 months).
Tissue microarrays (TMA) construction
In this study, we used the TMA technology to construct high-throughput OSCC TMAs according to protocol previously described [14]. Representative areas of OSCC and non-cancerous squamous epithelium were marked on each hematoxylin and eosin (H&E) stained slide and tissue paraffin block. The marked areas of tissue paraffin blocks were sampled for the TMAs. Two 0.6-mm-diameter tissue cores were taken from each paraffin block of donor OSCC samples.
IHC and scores
The IHC staining of Hsp10 protein in tissue array of OSCC was carried out using ready-to-use Envision TM + Dual Link System-HRP methods (Dako; Carpintrria, CA). The staining of Hsp10 protein was performed according to the manufacturer’s instructions and the staining conditions of antibody were adjusted according to our laboratory experience. Heat based antigen retrieval was accomplished by incubating TMAs with 0.01 M citrate buffer (pH 7.0) in a pressure cooker for 7 minutes, then the samples were immersed into 0.3% H2O2 to inactivate endogenous peroxidase at 37°C for 30 minutes. To eliminate nonspecific staining, the slides were incubated with appropriate preimmune serum for 30 minutes at room temperature. After incubation with a 1:1000 dilution of primary antibody to Hsp10 protein (Mouse monoclonal antibody, Catalog: sc-376313, Santa Cruz Biotechnology, Inc.) at 4°C overnight, slides washed with physiological phosphate buffered saline (PBS) three times for 10 minutes each and second antibody conjugated with a labeled polymer-HRP was added in accordance with the manufacturer’s instructions and incubated at 37°C for 30 minutes. Staining was developed using 3,3’-diaminobenzidine tetrachloride (DAB) as a chromogen substrate. All slides were counterstained with Mayer’s hematoxylin after rinsing with distilled H2O.
Immunohistochemically staining of TMA sections of OSCC was evaluated independently by JF and SF, who were blinded to the clinicopathological data, at 200× magnification light microscopy. The positive staining of Hsp10 was located in the cytoplasm of cancer cells. Representative micrographs in the Figures 1 and 2 showed that strong positive staining of Hsp10 was found only in OSCC, while only moderate and weak positive staining of Hsp10 presented in the non-cancerous control squamous epithelium tissues.
Figure 1.
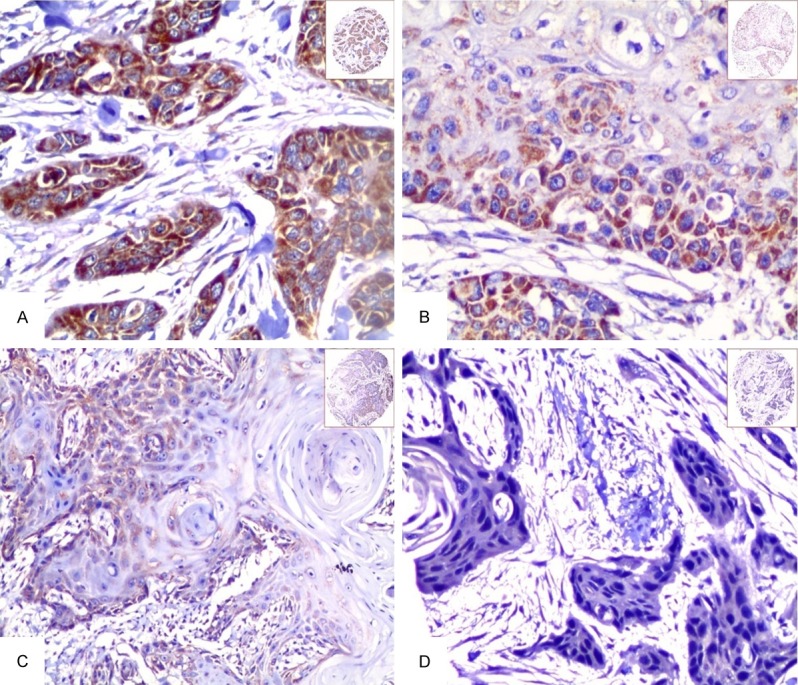
Expression of Hsp10 in OSCC tissue was detected by IHC. The expression of Hsp10 was detected by IHC using specific antibodies as described in the section of materials and methods. Strong positive staining of Hsp10 was identified in the cytoplasm of OSCC (A, 20×, IHC, DAB staining). Moderate positive staining of Hsp10 was showed in the cytoplasm of OSCC cells (B, 20×, IHC, DAB staining). Weak positive staining of Hsp10 was localized in the cytoplasm of OSCC cells (C, 20×, IHC, DAB staining). Negative staining of Hsp10 was in the OSCC cells (D, 20×, IHC, DAB staining).
Figure 2.
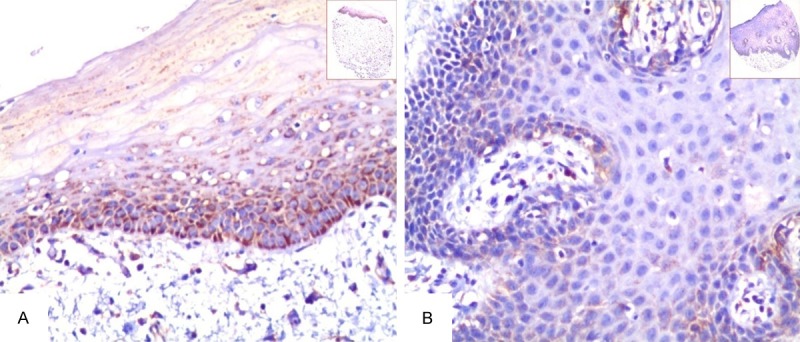
Expression of Hsp10 in the non-cancerous squamous epithelium was detected by IHC. The expression of Hsp10 was detected by IHC using specific antibodies as described in the section of materials and methods. Moderate positive staining of Hsp10 was showed in the cytoplasm of the non-cancerous squamous epithelium (A, 20×, IHC, DAB staining). Weak positive staining of Hsp10 was displayed in the cytoplasm of the non-cancerous squamous epithelium (B, 20×, IHC, DAB staining).
A semiquantitative evaluation of Hsp10 was performed using a method described in the literature [15] as follows: staining intensity for Hsp10 was scored as 0 (negative), 1 (weak), 2 (moderate), and 3 (strong). Staining extent was scored as 0 (0%), 1 (1-25%), 2 (26-50%), 3 (51-75%) and 4 (76-100%), depending on the percentage of positive-stained cells. Staining positivity was determined by the formula: overall score = positive percentage score × intensity score. The total score ranged from 0 to 12, scores of 0, 1, 2, 3, 4 and 6 (0-6) were considered low expression and scores of 8, 9 and 12 (8-12) were defined as high expression. Agreement between the two evaluators was 98%, and all scoring discrepancies were resolved through discussion between the two evaluators.
Statistical analyses
All statistical analyses were performed using SPSS 18.0. The chi-square test was used to analyze the relationship between the expression of Hsp10 protein and clinicopathological characteristics of OSCC. The Spearman’s rank correlation coefficient was hired to assess the significance of the expression of Hsp10 in OSCC. Overall survival (OS) rates according to Hsp10 expression status was analyzed using Kaplan-Meier survival analysis, and statistical significance was estimated with the log-rank test. The Cox proportional hazard regression model was performed to identify whether high expression of Hsp10 protein was an independent prognostic factor of overall survival for OSCC, All P-values were based on the two-sided statistical analysis and P-value less than 0.05 was considered to be statistically significant.
Results
Association between expression of Hsp10 and clinicopathological features of OSCC
The positive expression and cellular location of Hsp10 in OSCC and in the non-cancerous squamous epithelium were detected by IHC. Positive staining of Hsp10 was identified in the cytoplasm of OSCC and non-cancerous squamous epithelium (Figures 1, 2). Strong and moderate positive staining of Hsp10 was found in the OSCC (Figure 1A, 1B). However, strong positive staining of Hsp10 was not found in the non-cancerous squamous epithelium (Figure 2A, 2B). In the 110 cases of OSCC, the percentage of high expression of Hsp10 was 60.0% (66/110) and low expression was 40.0% (44/110). In the 32 cases of the non-cancerous squamous epithelium, the percentage of high expression was 31.3% (10/32) and low expression of Hsp10 was 68.7% (22/32). There was an observably higher percentage of high expression of Hsp10 in OSCC than the non-cancerous squamous epithelium (P = 0.006).
Furthermore, we investigated the associations between the expression of Hsp10 and clinicopathological characteristics of OSCC, containing patient age, gender, status of chewing betel nut, clinical stages, pathological grades, lymph node metastasis, radiotherapy after oral cancer operation and survival status, all data were analyzed by univariate chi-square test. Data in Table 1 showed that the level of Hsp10 protein expression in OSCC was significantly correlated with patient age, history of chewing betel nut, pathological grades, lymph node metastasis status, radiotherapy of operation and survival status (P = 0.008, P = 0.021, P = 0.026, P = 0.008, P = 0.049, P<0.001 respectively). However, no statistically difference was found in relation to gender and clinical stage (P>0.05, for all).
Table 1.
Association between expression of Hsp10 and OSCC clinical pathological feature (n = 110)
| Clinical pathological characteristics (n) | Expression of Hsp10 | P-value | |
|---|---|---|---|
|
| |||
| High | Low | ||
| Age (yr) | |||
| <45 (n = 27) | 20 (74.1) | 7 (25.9) | 0.008* |
| ≥45 (n = 83) | 37 (44.6) | 46 (55.4) | |
| Gender | |||
| Female (n = 20) | 7 (35.0) | 13 (65.0) | 0.096 |
| Male (n = 90) | 50 (55.6) | 40 (44.4) | |
| Chewing betel nut | |||
| Yes (n = 74) | 44 (59.5) | 30 (40.5) | 0.021* |
| No (n = 36) | 13 (36.1) | 23 (63.9) | |
| Clinical stages | |||
| Stages I and II (n = 71) | 35 (49.3) | 36 (50.7) | 0.475 |
| Stages III and IV (n = 39) | 22 (56.4) | 17 (43.6) | |
| Pathological grades | |||
| Well (n = 55) | 24 (43.6) | 31 (56.4) | 0.026* |
| Moderate (n = 43) | 29 (67.4) | 14 (32.6) | |
| Poor (n = 12) | 4 (33.3) | 8 (66.7) | |
| LNM status | |||
| LNM (n = 41) | 28 (68.3) | 13 (31.7) | 0.008* |
| No LNM (n = 69) | 29 (42.0) | 40 (58.0) | |
| Radiotherapy after operation | |||
| Yes (n = 48) | 30 (62.5) | 18 (37.5) | 0.049* |
| No (n = 62) | 27 (43.5) | 35 (56.5) | |
| Survival status | |||
| Alive (n = 80) | 31 (38.8) | 49 (61.3) | 0.000* |
| Dead (n = 30) | 26 (86.7) | 4 (13.3) | |
Abbreviation: LNM: lymph node metastasis.
The data were tested using the two-tailed chi-square test, statistically significant difference (P<0.05).
Impact of high expression of Hsp10 protein on overall survival of patients with OSCC
Since the high expression of Hsp10 was significantly correlated with the survival status of OSCC in univariate such as chi-square tests, Hsp10 expression levels and the clinical follow-up information in all 110 patients with OSCC were further evaluated by Kaplan-Meier analysis and log-rank test. Figure 3 illustrated the Kaplan-Meier survival plots for OSCC patients with expression of Hsp10 (Figure 3A). Univariate survival (long-rank test) analysis showed that the overall survival rate of OSCC patients with high expression of Hsp10 was significantly lower than those with low expression of Hsp10 (P<0.001). We also plotted the survival curves for OSCC patients with conventional prognosis parameters including pathological grades, clinical stages and status of lymph node metastasis. As shown in Figure 3B-D, the patients with advanced-stage OSCC (clinical stage III and IV), lymph node metastasis and moderately and poor differentiation had a lower overall survival rate than patients with early stage OSCC (clinical stage I and II) without lymph node metastasis and with well differentiated tumors (P = 0.004, Figure 3C, and P = 0.033, Figure 3D, and P = 0.043, Figure 3B, respectively).
Figure 3.
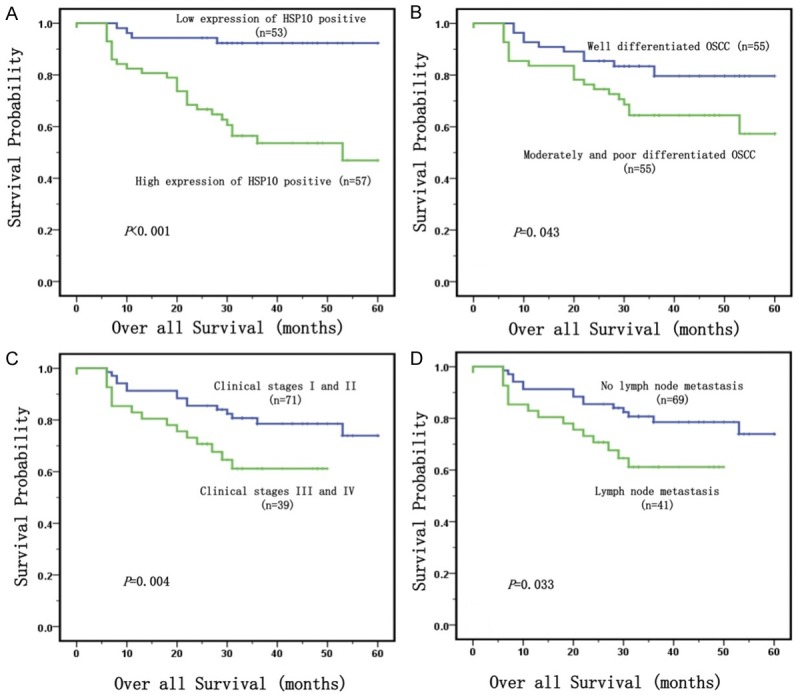
Kaplan-Meier curves for overall survival of OSCC were assessed using the log-rank test. A: High expression of Hsp10 had been significantly related to short survival times of OSCC patients (P<0.001, two-sided). B: OSCC patients with well differentiation were significantly related to higher overall survival rates compared to patients with moderate and poor differentiation (P = 0.043, two-sided). C: OSCC patients with clinical stage III and IV were significantly related to lower overall survival rates compared to those patients with clinical stage I and II (P = 0.004, two-sided). D: OSCC patients with lymph node metastasis were significantly related to poor overall survival rates compared to those patients without lymph node metastasis (P = 0.033, two-sided).
Besides univariate analysis, multivariate Cox proportional hazard regression analysis was also carried out to further investigate whether high expression of Hsp10 protein was an independent prognostic factor for OSCC, and these results were revealed in Table 2. In multivariate analysis of the features of OSCC patients, which included age, gender, history of chewing betel nut, clinical stages, pathological grades, status of lymph node metastasis, treatment strategy after operation and expression of Hsp10 protein, results showed that high expression of Hsp10 protein was identified as an independent poor prognostic factor for OSCC (P<0.001), so did clinical stages (P = 0.007).
Table 2.
Summary of multivariate analysis of Cox proportional hazard regression for overall survival in 110 cases of OSCC patients
| Parameter | Wald | Sig. | Exp (B) | 95.0% CI for Exp (B) | |
|---|---|---|---|---|---|
|
| |||||
| Lower | Upper | ||||
| Gender | 0.369 | 0.543 | 0.683 | 0.155 | 2.668 |
| Age | 0.360 | 0.549 | 1.302 | 0.549 | 3.086 |
| Chewing betel nut | 0.354 | 0.552 | 0.704 | 0.221 | 2.239 |
| Clinical stages | 7.231 | 0.007* | 3.448 | 1.399 | 8.501 |
| Pathological grades | 1.386 | 0.239 | 1.439 | 0.785 | 2.638 |
| Lymph node metastasis | 0.935 | 0.334 | 0.565 | 0.178 | 1.797 |
| Radiotherapy after operation | 1.415 | 0.234 | 1.778 | 0.689 | 4.587 |
| Expression of Hsp10 | 14.539 | 0.000* | 8.710 | 2.863 | 26.498 |
95% CI: 95% confidence interval;
P<0.05.
Discussion
In the past, TNM-staging system is a mainly determined factor for the prognosis in OSCC [16]. However, more and more biomarkers can not only play an important role in contributing to establish an accurate diagnosis [17,18], but also provide occurrence, progression and prognosis data [18] for OSCC. This is a kind of trend that propels people to discover new biomarkers for the development of therapeutic strategies to improve outcome and survival for patients with OSCC.
The levels of Hsp10 beyond the normal range could have a pathogenic significance. A past study had shown that Hsp10, single stranded DNA-binding protein (SSBP1) and peptidyl-prolyl cis-trans isomerase A, which was deemed as increasing in the tumor samples. In this study, Hsp10 was emphasized to be closely correlated with protein folding and translation [19]. In addition, the increase of Hsp10 expression in cancers could be involved in regulation of apoptosis of tumor or dysplastic cells [20]. It had been proved that there was a higher expression of Hsp10 in large bowel cancer, and it may be closely related to the presence of lymph node metastases [21]. Furthermore, Hsp10 was overexpressed early in prostate carcinogenesis [22]. In our present study, the expression of Hsp10 in OSCC was significantly higher than that in the non-cancerous squamous epithelium. And high expression of Hsp10 in the poorly differentiated OSCC was significantly higher than that in the well differentiated OSCC. There was significantly negative association between high expression of Hsp10 and overall survival rates of patients with OSCC. The most interesting result in the study is that the expression of Hsp10 in OSCC patients who received radiation therapy of after oral cancer operation was significantly higher than that of OSCC patients who did not receive radiation therapy. And the expression of Hsp10 in OSCC patients with lymph node metastasis was significantly higher than the patient without lymph node metastasis. It is well known that lymph node metastasis status is a classic predictor of poor prognosis of patients with advanced tumors. Because of these findings, we definitely consider that Hsp10 is a potential novel biomarker for prognosis and represents a therapeutic target for the treatment of OSCC patients.
Moreover, we provided the evidence that high expression of Hsp10 protein might play a major pathogenic role in the development of OSCC. Hobbies of tobacco smoking, alcohol consumption and chewing betel nut were known risk factors for OSCC [3-5]. We found that OSCC patients with high Hsp10 expression whom had the longer history of chewing betel nut than other groups. In additional, expression of Hsp10 in the younger patients was significantly higher than that in the older ones. The hobby of chewing betel nut in young people whether increased the risk of the high expression of Hsp10 in OSCC? The molecular mechanism of Hsp10 about pathogenic factor remained unclear. Further studies are required for us to elucidate the precise mechanism underlying the role of Hsp10 in OSCC.
In summary, this study demonstrated that high expression of Hsp10 was significantly correlated with worse prognosis of patients with OSCC. High expression of Hsp10 protein might act as a valuable independent biomarker to predict poor prognosis of patient with OSCC.
Acknowledgements
The work was supported by grants from the National Natural Sciences Foundations of China (No: 81472773).
Disclosure of conflict of interest
None.
References
- 1.Arya S, Rane P, Deshmukh A. Oral cavity squamous cell carcinoma: role of pretreatment imaging and its influence on management. Clin Radiol. 2014;69:916–930. doi: 10.1016/j.crad.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 2.Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45:309–316. doi: 10.1016/j.oraloncology.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Kimple AJ, Welch CM, Zevallos JP, Patel SN. Oral cavity squamous cell carcinoma--an overview. Oral Health Dent Manag. 2014;13:877–882. [PubMed] [Google Scholar]
- 4.Lee CH, Ko YC, Huang HL, Chao YY, Tsai CC, Shieh TY, Lin LM. The precancer risk of betel quid chewing, tobacco use and alcohol consumption in oral leukoplakia and oral submucous fibrosis in southern Taiwan. Br J Cancer. 2003;88:366–372. doi: 10.1038/sj.bjc.6600727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muttagi SS, Chaturvedi P, Gaikwad R, Singh B, Pawar P. Head and neck squamous cell carcinoma in chronic areca nut chewing Indian women: case series and review of literature. Indian J Med Paediatr Oncol. 2012;33:32–35. doi: 10.4103/0971-5851.96966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li G, Huang Z, Chen X, Wei Q. Role of human papillomavirus and cell cycle-related variants in squamous cell carcinoma of the oropharynx. J Biomed Res. 2010;24:339–346. doi: 10.1016/S1674-8301(10)60047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baumgarth N, Szubin R, Dolganov GM, Watnik MR, Greenspan D, Da Costa M, Palefsky JM, Jordan R, Roederer M, Greenspan JS. Highly tissue substructure-specific effects of human papilloma virus in mucosa of HIV-infected patients revealed by laser-dissection microscopyassisted gene expression profiling. Am J Pathol. 2004;165:707–718. doi: 10.1016/S0002-9440(10)63334-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Featherston T, Yu HH, Dunne JC, Chibnall AM, Brasch HD, Davis PF, Tan ST, Itinteang T. Cancer stem cells in moderately differentiated buccal mucosal squamous cell carcinoma express components of the renin-angiotensin system. Front Surg. 2016;3:52. doi: 10.3389/fsurg.2016.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tiwari S, Thakur R, Shankar J. Role of heatshock proteins in cellular function and in the biology of fungi. Biotechnol Res Int. 2015;2015:132635. doi: 10.1155/2015/132635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rappa F, Pitruzzella A, Marino Gammazza A, Barone R, Mocciaro E, Tomasello G, Carini F, Farina F, Zummo G, Conway de Macario E, Macario AJ, Cappello F. Quantitative patterns of Hsps in tubular adenoma compared with normal and tumor tissues reveal the value of Hsp10 and Hsp60 in early diagnosis of large bowel cancer. Cell Stress Chaperones. 2016;21:927–933. doi: 10.1007/s12192-016-0721-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nisemblat S, Yaniv O, Parnas A, Frolow F, Azem A. Crystal structure of the human mitochondrial chaperonin symmetrical football complex. Proc Natl Acad Sci U S A. 2015;112:6044–6049. doi: 10.1073/pnas.1411718112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cappello F, Bellafiore M, David S, Anzalone R, Zummo G. Ten kilodalton heat shock protein (HSP10) is overexpressed during carcinogenesis of large bowel and uterine exocervix. Cancer Lett. 2003;196:35–41. doi: 10.1016/s0304-3835(03)00212-x. [DOI] [PubMed] [Google Scholar]
- 13.Akyol S, Gercel-Taylor C, Reynolds LC, Taylor DD. HSP-10 in ovarian cancer: expression and suppression of T-cell signaling. Gynecol Oncol. 2006;101:481–486. doi: 10.1016/j.ygyno.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 14.Fan SQ, Ma J, Zhou J, Xiong W, Xiao BY, Zhang WL, Tan C, Li XL, Shen SR, Zhou M, Zhang QH, Ou YJ, Zhuo HD, Fan S, Zhou YH, Li GY. Differential expression of Epstein-Barr virus-encoded RNA and several tumor-related genes in various types of nasopharyngeal epithelial lesions and nasopharyngeal carcinoma using tissue microarray analysis. Hum Pathol. 2006;37:593–605. doi: 10.1016/j.humpath.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 15.Hu J, Wang N, Wang YJ. XRCC3 and RAD51 expression are associated with clinical factors in breast cancer. PLoS One. 2013;8:e72104. doi: 10.1371/journal.pone.0072104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mao L, Hong WK, Papadimitrakopoulou VA. Focus on head and neck cancer. Cancer Cell. 2004;5:311–316. doi: 10.1016/s1535-6108(04)00090-x. [DOI] [PubMed] [Google Scholar]
- 17.Sethi S, Ali S, Philip PA, Sarkar FH. Clinical advances in molecular biomarkers for cancer diagnosis and therapy. Int J Mol Sci. 2013;14:14771–14784. doi: 10.3390/ijms140714771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ciocca DR, Calderwood SK. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones. 2005;10:86–103. doi: 10.1379/CSC-99r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ye Y, Huang A, Huang C, Liu J, Wang B, Lin K, Chen Q, Zeng Y, Chen H, Tao X, Wei G, Wu Y. Comparative mitochondrial proteomic analysis of hepatocellular carcinoma from patients. Protemics Clin Appl. 2013;7:403–415. doi: 10.1002/prca.201100103. [DOI] [PubMed] [Google Scholar]
- 20.Corrao S, Anzalone R, Lo Iacono M, Corsello T, Di Stefano A, D’Anna SE, Balbi B, Carone M, Sala A, Corona D, Timperio AM, Zolla L, Farina F, de Macario EC, Macario AJ, Cappello F, La Rocca G. Hsp10 nuclear localization and changes in lung cells response to cigarette smoke suggest novel roles for this chaperonin. Open Biol. 2014;4 doi: 10.1098/rsob.140125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cappello F, David S, Rappa F, Bucchieri F, Marasà L, Bartolotta TE, Farina F, Zummo G. The expression of HSP60 and HSP10 in large bowel carcinomas with lymph node metastase. BMC Cancer. 2005;5:139. doi: 10.1186/1471-2407-5-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cappello F, Rappa F, David S, Anzalone R, Zummo G. Immunohistochemical evaluation of PCNA, p53, HSP60, HSP10 and MUC-2 presence and expression in prostate carcinogenesis. Anticancer Res. 2003;23:1325–1331. [PubMed] [Google Scholar]


