Abstract
This study was aimed to analyze the association of chemerin/chemR23 with endothelial-mesenchymal transition (EndMT) in the kidney of rats during the progression of diabetic nephropathy (DN). Eighty male Sprague-Dawley rats were randomly distributed to age-matched control and diabetic nephropathy model groups. Diabetes was induced by intraperitoneal injection of streptozotocin (STZ). Morphological changes in the renal tissues were observed after periodic acid-Schiff staining. Chemerin and chemR23 expression was determined by immunohistochemistry. Co-expression of α-smooth muscle actin (α-SMA) and CD31 was determined by immunofluorescence double-labeling. Expression of all these markers was analyzed by Western blotting. Expression of chemerin, chemR23, and transforming growth factor-β1 (TGF-β1) genes at mRNA level was determined by qRT-PCR. Mild glomerular hypertrophy was observed at 4 weeks and mild glomerular basement membrane thickening and mesangial matrix proliferation at 12 weeks. Immunohistochemistry showed much higher levels of chemerin and chemR23 in diabetic rats compared with the control group. Expression of chemerin, chemR23, and α-SMA proteins increased with disease progression, while CD31 protein expression decreased. Expression of chemerin, chemR23, and TGF-β1 mRNAs also increased. α-SMA and CD31 co-expression increased with disease progression from 4 weeks. Spearman correlation analysis showed that chemerin mRNA levels correlated positively with Chem R23, TGF-β1, α-SMA expression, and CD31 and α-SMA double-positive cell numbers, but negatively with CD31 protein expression. In conclusion, chemerin expression correlates with EndMT in DN model rats.
Keywords: Chemerin, diabetic nephropathy, endothelial-mesenchymal transition
Introduction
Diabetic nephropathy (DN) is a common and morbid microvascular complication of diabetes mellitus (DM), which is traditionally defined as diabetes with progressive rise in urine albumin, increase in blood pressure, and impaired glomerular filtration rate [1,2]. This tends to induce end-stage renal disease (ESRD), and would improve health outcomes of persons with diabetes and places a significant social burden on healthcare systems worldwide. According to US Renal Data System, DN accounts for nearly half of all incident cases of ESRD in the US, and the relative medicare reached $26.8 billion in 2008 [3]. Besides DM, the development of DN is associated with other risk factors, such as age at diagnosis, systemic or glomerular hypertension, poor glycemic control, and dietary composition [4]. The development of DN has been described as a 5-stage process [4], but current treatment strategies still cannot arrest progression toward ESRD. Recently, a potential pathogenetic mechanism of DN is that glomerular endothelial cells (GEnCs) are injured, and converted into mesenchyme-like cells process, namely endothelial-to-mesenchymal transition (EndMT) [5]. That is to say that EndMT might be a critical point in the generation of myofibroblasts in DN [6]. EndMT contributes to the pathogenesis of many diseases through adversely changing the plasticity and integrity of endothelial cells and increasing characteristics of mesenchymal cells [7]. It was also reported that EndMT was related to the development of interstitial fibrosis and vascular angiogenesis in DN [8]. So it is essential to clarify the role of EndMT in DN, and this may provide reasonable and efficient therapy for DN.
Chemerin, a novel adipocyte-derived factor which a variety of functions mediated via its main receptor, chemR23 [9], plays a role in regulating inflammation [10]. Chemerin also plays a crucial role in DN [11]. Serum chemerin levels are closely associated with renal function [12], with significantly elevated levels reported in type 2 diabetic patients with macroalbuminuria, chronic kidney disease, as well as in patients receiving HD and after renal transplantation. This suggested that the decrease in renal function might affect the serum chemerin level [13]. Due to its function as a proinflammatory adipocytokine, chemerin is closely related to transdifferentiation stimulating factors, such as transforming growth factor-β1 (TGF-β1), connective tissue growth factor (CTGF) and TNF-α [14]. Under the influence of such factors, endothelial cells (ECs) acquire a mesenchymal phenotype characterized by the loss of the specific endothelial marker, CD31, and by the gain of mesenchymal markers, such as fibroblast-specific protein 1 (FSP-1) and α-smooth muscle actin (α-SMA) and transformation into fibroblasts.
To date, there are little articles about the relationship between chemerin and EndMT in DN. In this study, we investigated chemerin/chemR23 expression in the kidneys of diabetic rats during the progression of DN to determine associations with EndMT.
Materials and methods
Animals
Female SD rats (aged 16-18 weeks; 160-180 g) were used in this study. The animals were bred in the Laboratory Animal Center of Zhengzhou University under specific-pathogen free conditions with a 12 h day/night cycle and free access to food and water. Eighty SD rats allocated randomly to the following groups: (1) 5 STZ-treated diabetic (5×8). (2) 5 control (n = 5×8) with control rats. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted. This study was approved by the Ethics Committee of Zhengzhou University, China.
Experimental protocols
Diabetes was induced by a caudal vein injection of streptozotocin (STZ) at 75 mg/kg body weight in 0.01 M citrate buffer (pH 4-4.5). Control group rats received an equal volume of vehicle (0.01 M citrate buffer, pH 4.5). Blood glucose was monitored using a glucose meter (Roche, USA). After 72 h, rats with blood glucose levels > 16.7 mmoI/L for 3 days were considered to be diabetic. The rats in each group were sacrificed in 1, 2, 3, 4, and 12 weeks after induction of the DM model.
Biochemical analysis
Urine samples were collected at 7, 14, 21, 28, 84 days (8 pm) and cardiac blood samples were collected when the rats were sacrificed by cervical dislocation. Blood creatinine, urinary microalbumin, and urinary creatinine were measured using an automatic biochemical analyzer (Beckman Coulter, USA). The urinary albumin-to-creatinine ratio (UACR) was calculated as urinary microalbumin/urinary creatinine.
Periodic acid-Schiff staining
Formalin-fixed renal cortex tissues were embedded in paraffin and sections (thickness, 2 μm) prepared for periodic acid-Schiff (PAS) staining. Under examination by light microscopy, the extent of renal injury was estimated by the expansion of the mesangial matrix and the enlargement of glomeruli. We analyzed 10 randomly selected non-overlapping PAS-stained glomeruli from each rat as described previously [6].
Immunohistochemical analysis
Immunohistochemistry was performed on paraffin sections using a microwave-based antigen-retrieval technique. The following primary detection antibodies were used in this study: goat anti-chemerin (1:200, Santa Cruz Biotechnology, USA) and rabbit anti-chemR23 (1:200, Biotechnology, China). Secondary detection antibodies (rabbit immunoglobulin G, goat immunoglobulin G) and a streptavidin peroxidase detection system (ZSGB-BIO, China) were used according to the manufacturer’s protocols. Subsequently, the sections were stained with diaminobenzidine and counterstained with hematoxylin. Semi-quantitative immunohistochemical analysis of 10 randomly selected glomeruli (40× magnification) was scored as follows: no staining (-), weak staining (+), moderate staining (++), and strong staining (+++) [2]. The score for each rat was expressed as the mean value of all the scores obtained.
Immunofluorescence
Frozen tissue sections (thickness, 10 μm) were incubated with primary antibodies [goat anti-CD31 (Santa Cruz Biotechnology) and rabbit anti-α-SMA (Biotechnology)] at 4°C overnight. Donkey anti-rabbit IgG (H+L)-Dylight 488 and mouse anti-goat IgG (H+L)-TRITC secondary antibodies (Biotechnology). The nuclei were counterstained with DAPI (Biotechnology). Staining was analyzed by two investigators independently using a Zeiss LSM 510 Meta scanning confocal microscope. Ten visual fields per kidney were analyzed for co-localization of endothelial and fibroblast markers. For the evaluation of α-SMA-positive fibroblasts, only single cells that were not associated with larger vessels were considered.
Quantitative real-time PCR
Total RNA was isolated from renal tissue using the Trizol reagent (Sangon Biotech, Beijing, China). Complementary DNA was synthesized using the PrimeScript RT Reagent Kit (GeneCopoeia, Rockville, MD, USA). Relative levels of chemerin, chemR23 and TGF-β1 mRNAs were determined by real-time PCR with specific primers purchased from GeneCopoeia using the ABI Prism 7500 sequence detection system (Applied Biosystems, USA). To control for variations in the amount of DNA available for PCR in the different samples, the expression levels of target genes were normalized to that of GAPDH. Relative expression of mRNA was evaluated using the 2-ΔΔCT method. All reactions were carried out in triplicate.
Western blot analysis
Tissue proteins were obtained from each group using RIPA lysis buffer (Dingguo Changsheng Biotech). Proteins (15-40 μg) were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) using 10% or 12% gels and were transferred onto polyvinylidene fluoride (PVDF) membranes (Roche, Mannheim, Germany). Non-specific binding was blocked using 5% non-fat milk diluted in TBS (13.7 mM NaCl, 0.27 mM KCl, 2.5 mM Tris) containing 0.05% Tween 20 (TBST) for 1 h. Membranes were incubated with primary antibodies (goat anti-CD31 (Santa Cruz Biotechnology) and rabbit anti-α-SMA (Biotechnology), β-actin (Shanghai Kang Cheng Biological Engineering, China) overnight at 4°C. After washing with TBST three times, the membranes were incubated with horseradish peroxidase-labeled mouse anti-rabbit and donkey anti-goat secondary antibodies (Dingguo Changsheng Biotech) at 37°C for 2 h. Images were captured on X-ray film. Immunoreactive bands were quantified by densitometry using Image J software (National Institutes of Health, USA).
Statistical analysis
All statistical analyses were performed using SPSS version 19.0 software. Results are expressed as means ± SD. Differences among the groups were analyzed by one-way ANOVA to compare the difference among the groups. The differences in expression of chemerin, chemR23, CD31, α-SMA, and TGF-β1 and the number of CD31 and α-SMA double-positive cells between the four groups were compared using one-way ANOVA or Wilcoxon rank-sum tests. The Spearman coefficient was calculated to assess the correlations between chemerin expression levels and ChemR23, CD31, α-SMA, and TGF-β1 expression, and the number of CD31 and α-SMA double-positive cells. P values less than 0.05 were considered to indicate statistical significance.
Results
Baseline and morphological characteristics of the STZ-induced diabetic rats
After STZ injection, there were significant differences in the baseline characteristics between the investigated groups (Figure 1). Compared with the age-matched control groups, the blood glucose level was markedly elevated in the STZ-induced diabetic group at each time-point throughout the study (Figure 1A). The UACRs at 4 and 12 weeks post-injection were also significantly increased (Figure 1B). In accordance with the changes in this ratio, the kidney-to-body weight ratios at 4 and 12 weeks post-injection were significantly higher than those of the age-matched control rats (Figure 1C). These changes are consistent with the early stages of DN. In addition, the serum creatinine levels were significantly increased at 12 weeks post-injection (Figure 1D). Significant enlargement of the mesangial matrix and thickening of the glomerular basement membrane were observed at 12 weeks after STZ injection (Figure 1E).
Figure 1.
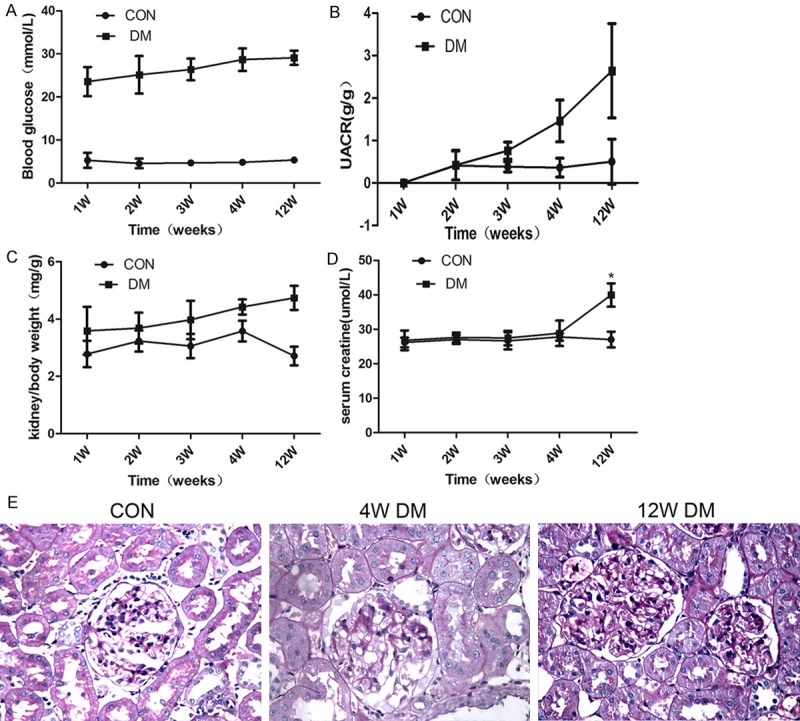
The baseline and morphological characteristics of the STZ-induced diabetic rats. A. Blood glucose; B. Urine albumin-to-creatinine ratio; C. Kidney-to-body weight ratio; D. Serum creatinine; E. PAS staining of kidney sections at different time-points. *P < 0.05; **P < 0.01.
Detection of chemerin and chemR23 expression in kidney tissue detection by immunohistochemistry
No expression of chemerin or chemR23 was detected in normal kidney tissue, whereas the expression of both proteins was detected in DN model rat kidneys at both 4 and 12 weeks after STZ injection. Chemerin was mainly expressed in the glomerulus, tubular epithelial cells and interstitium, while chemR23 was mainly expressed in tubular epithelial cells and interstitium (Figure 2A and 2B).
Figure 2.
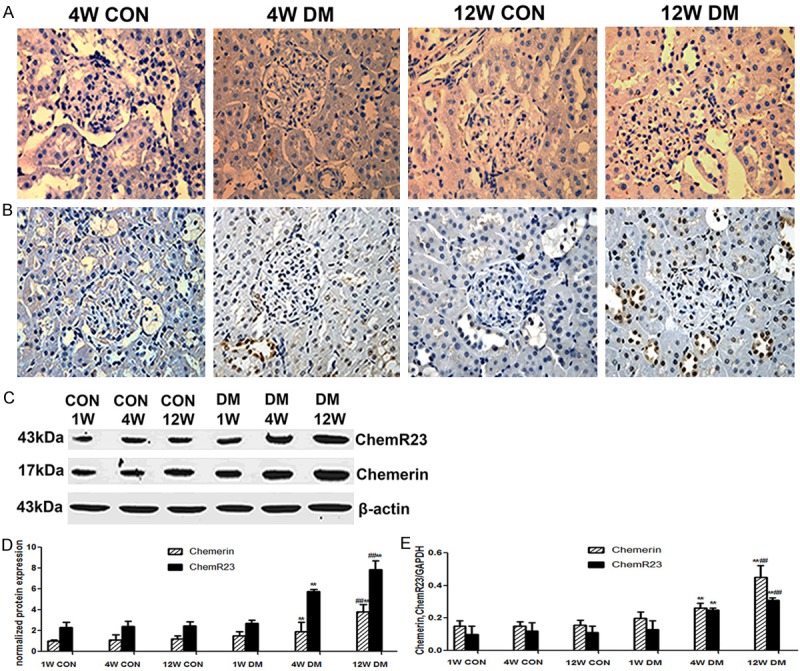
Upregulated renal chemerin and chemR23 expression in the STZ-induced diabetic rats. A. Immunohistochemical staining of chemerin in the rats at 4 and 12 weeks (magnification, ×400); B. Immunohistochemical staining of chemR23 in the rats at 4 and 12 weeks (magnification, ×400); C, D. Western blot analysis of chemerin and chemR23 protein levels in renal tissue of age-matched control rats and diabetic rats at 1, 4 and 12 weeks post-STZ injection. E. qRT-PCR analysis of chemerin and ChemR23 mRNA expression in the renal tissue of age-matched control rats and diabetic rats at 1, 4 and 12 weeks post-STZ injection. **P < 0.001 versus 4 weeks control; ##P < 0.001 versus 12 weeks control.
Western blot analysis of chemerin and chemR23 protein expression
Expression of chemerin and chemR23 proteins was gradually upregulated with time after the induction of diabetes in model rats, whereas there was no significant difference in the levels detected at each time-point in the control rats. Chemerin and chemR23 protein expression was significantly elevated in the model rats compared with that in the age-matched control rats at 4 and 12 weeks (P < 0.01), with significantly higher expression in model rats at 12 weeks compared with that at 4 weeks (P<0.01) (Figure 2C and 2D).
qRT-PCR analysis of chemerin and chemR23 mRNA expression
The levels of chemerin and chemR23 mRNA were consistent with the levels of the corresponding proteins. Chemerin and chemR23 mRNA levels were significantly elevated in the model rats compared with those in the age-matched control rats at 4 and 12 weeks (P < 0.01), with significantly higher expression in model rats at 12 weeks compared with that at 4 weeks (P < 0.01) (Figure 2E).
Double-labeled immunofluorescence detection of the expression and localization of CD31 and α-SMA in kidney
Expression of CD31 in the glomerular and interstitial blood vessels and α-SMA in the interstitial blood vessels was detected in the control rats, although co-expression was not detected. However, in the model rats, CD31 and α-SMA co-expression was detected in the interstitial blood vessels at 4 and 12 weeks, with greater co-expression detected at week 12 than at week 4. The co-expression of CD31 and α-SMA indicated the transformation of interstitial microvascular endothelial cells (Figure 3A).
Figure 3.
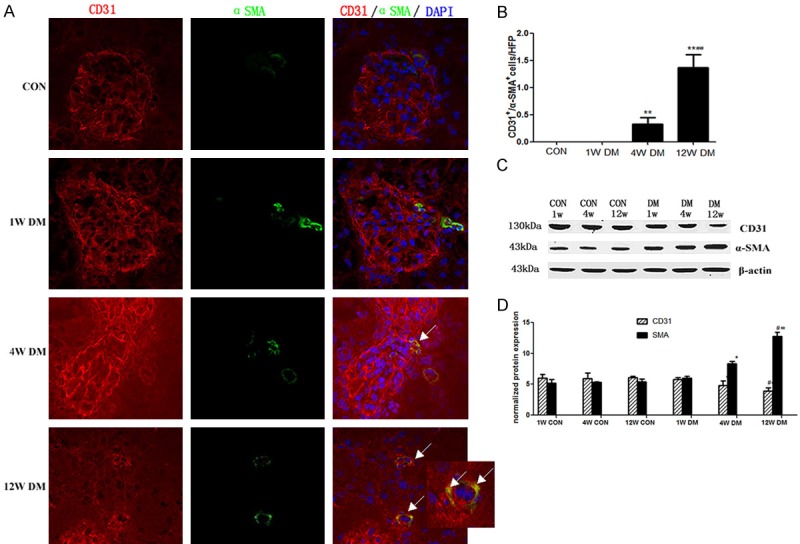
Endothelial-mesenchymal transition in the STZ-induced mouse model of diabetic nephropathy. A. α-SMA and CD31 double-labeling. Frozen kidney sections were double-stained with antibodies to α-SMA (green) and CD31 (red); nuclei were stained with DAPI (blue). Yellow color in the merged panel indicates α-SMA and CD31 co-expression. The arrows in the merged panel indicate CD31+ and α-SMA+ cells. B. Quantification of fibroblasts. The bar graphs summarize average numbers of CD31+ and α-SMA+ cells per visual field in both control and diabetic kidneys (magnification, ×200). C, D. Western blot analysis of CD31 and α-SMA protein levels in the renal tissue of age-matched control rats and diabetic rats at 1, 4 and 12 weeks post-STZ injection.
Confocal microscopic enumeration of CD31 and α-SMA double-positive cells
The number of CD31 and α-SMA co-expressing cells was significantly increased in model rats at 4 and 12 weeks compared with the numbers detected in age-matched control rats (P < 0.01); moreover the number of double-positive cells was significantly increased in model rats at 12 weeks compared with the numbers detected at 4 weeks (P < 0.01) (Figure 3B).
Western blot analysis of CD31 and α-SMA protein expression in kidney tissue
The expression of CD31 protein decreased gradually with time after diabetes-induction in model rats, whereas, α-SMA protein expression increased gradually; there were significant differences between model rats and age-matched control rats at 4 and 12 weeks (P < 0.05). Furthermore, there were significant differences between the levels detected in model rats at 4 weeks after diabetes-induction compared with those detected at 1 week, as well as the levels detected in model rats at 12 weeks compared with those detected at 4 weeks (P < 0.05) (Figure 3C and 3D).
Association analysis
Spearman correlation analysis revealed that the chemerin mRNA expression correlated positively with α-SMA protein expression and the number of CD31 and α-SMA co-expressing cells (P < 0.05), while a negatively association with CD31 protein expression was identified (Table 1).
Table 1.
Spearman correlation analysis of the association of chemerin mRNA with theother variables
| R-value | P-value | |
|---|---|---|
| ChemR23 | 0.766 | 0.012 |
| TGF-β1 | 0.837 | 0.003 |
| CD31 | -0.358 | 0.023 |
| α-SMA | 0.793 | 0.027 |
| EndMT cell number | 0.773 | 0.039 |
Discussion
Chemerin, which is also known as retinoic acid receptor responder 2 (RARRES2) and tazarotene-induced gene 2 (TIG2), was first discovered in 1997 by Napal et al. in psoriasis skin lesion using a differential display method [15]CCN2. Recently, it was considered as a regulator of adipocyte differentiation, and could modulate the expression of adipocyte genes associated with glucose and lipid homeostasis, such as glucose transporter-4, adiponectin, and leptin [16]. The highest expression of chemerin was detected in liver and white adipose tissue, with moderate expression in lung and brown adipose tissue, and relatively low expression in heart, kidney, and ovary [17]. Chemerin levels were related to components of the metabolic syndrome, such as elevated levels of body mass index and plasma triglycerides [16,18]. Also, Bozaoglu et al. (2007) reported that compared to lean and normoglycemic Psammomys obesus, the chemerin expression experienced a marked increase in adipose tissue of obese and type 2 diabetic Psammomys obesus [19]. Meanwhile, in our previous studies, it was suggested that chemerin expression was associated with multiple kinds disorders of kidney with detection in the renal tubular cells and lymphatic endothelial cells of patients with severe lupus nephritis [19]. Serum levels of chemerin in patients undergoing hemodialysis were two-fold higher than those in control patients with glomerular filtration rates (GFR) > 50 ml/min. In addition, serum levels of chemerin in hemodialysis patients and control cohorts were negatively associated with GFR, glucose- and lipid metabolism-related parameters and inflammatory parameters, indicating an association between circulating chemerin levels and renal function [20].
This association has also been reported in the other studies in relation to diabetic nephropathy. In the current study, the expression of chemerin and chemR23 was significantly increased in the kidney of DN rats. Furthermore, chemerin is a chemo-attractant protein which is able to attract immune cells to the site of inflammation, intendifying their adhesion [9]. Chemerin mRNA levels were positively associated with inflammatory and fibrosis factors, such as TGF-β1, TNF-α, CTGF and ICAM-1, indicating that chemerin is closely associated with renal inflammation and fibrosis. Significantly elevated chemerin expression has also been reported in type 2 diabetes compared with the levels in diabetic patients with slight proteinuria or without proteinuria [12]. In the current study, chemerin and chemR23 expression in the kidney of model rats was gradually upregulated with the progression of DN and was mainly detected in the glomerulus, tubular epithelial cells, and stromal vascular endothelial cells (Figure 2A and 2B). This pattern of chemerin and chemR23 expression was also observed in qRT-PCR and Western blot analyses, indicating that the expression of chemerin and chemR23 is closely associated with the pathogenesis and progression of DN (Figure 2C-E). These findings are consistent with those of previous studies [12].
Accumulating evidence indicates that EndMT in the early stages of DN facilitates kidney fibrosis. During EndMT, endothelial cells lose their adhesion, and apical-basal polarity to form highly invasive, migratory, spingdle-shaped, elongated mesenchymal cells [14]. The most important biochemical alterations associated with EndMT are the decreased expression of endothelial cell surface makers, such as vascular endothelial adhesion molecule-1 and CD31, and the increased expression of interstitial cell surface markers, such as FSP-1 and α-SMA [6]. These were consistent with our results that in the kidney of model rats CD31 protein expression was gradually down-regulated with the progression of DN and α-SMA protein expression experienced a upward trend (Figure 3C and 3D). This indicated that the expression of CD31 and α-SMA is closely associated with the pathogenesis and progression of DN which is consistent with previous study [6]. These findings are indicating that EndMT in the diabetic kidney occurs in the phase of microalbuminuria, and is mainly found in the renal interstitium further analysis indicated that the number of CD31+ and α-SMA+ cells increases with the progression of DN in the model rats.
Several factors and pathways are known to regulate EndMT, but TGF-β is considered as the most potent inducer capable of initiating and completing the entire process [21]. TGF-β promoted EndMT both in vivo an in vitro, thereby facilitating the fibrosis of organs. TGF-β was reported to induce EndMT in lung, heart, and kidney tissues [22]. Moreover, TGF-β induces α-SMA and type I collagen expression in glomerular mesangial mast cells, and aggravates kidney fibrosis by inducing transformation of renal tubular epithelial cells and by increasing fibroblast EndMT [7]. Other studies had shown that serum chemerin level was also significantly increased in patients with chronic pancreatitis, and positively associated with TGF-β1. These observations indicate that pancreatic fibrosis is induced by increased chemerin levels, which in turn facilitate macrophage infiltration and TGF-β production [23].
In the current study, chemerin levels were shown to be positively associated with the number of CD31 and α-SMA double-positive cells, while a negative association with CD31 protein expression was identified (Table 1), indicating that chemerin levels had a relationship with EndMT in DN. TGF-β1 expression was also found to be increased with the progression of DN and positively associated with chemerin levels. Therefore, it can be speculated that chemerin induces chemR23-expressing inflammatory cells to produce TGF-β1, subsequently facilitating EndMT and kidney fibrosis.
In summary, according to the current study of the STZ-induced diabetic rat model of DN, the expression of chemerin and chemR23 in kidney tissue gradually increased with the progression of DN and was accompanied by an increased number of endothelial-transformed fibroblasts. Thus, the results of this study indicated that chemerin/chemR23 expression was associated with EndMT in diabetic nephropathy.
Acknowledgements
This study was supported by Excellent Youth Foundation of Henan Scientific Committee (Grant No.: 154100510017).
Disclosure of conflict of interest
None.
References
- 1.de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2011;305:2532–2539. doi: 10.1001/jama.2011.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ng KP, Jain P, Gill PS, Heer G, Townend JN, Freemantle N, Greenfield S, McManus RJ. Results and lessons from the spironolactone to prevent cardiovascular events in early stage chronic kidney disease (STOP-CKD) randomised controlled trial. BMJ Open. 2016;6:e010519. doi: 10.1136/bmjopen-2015-010519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.USRDS N. Annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States. Bethesda, MD: National Institutes of Health; 2010. [Google Scholar]
- 4.Aghadavod E, Khodadadi S, Baradaran A, Nasri P, Bahmani M, Rafieian-Kopaei M. Role of oxidative stress and inflammatory factors in diabetic kidney disease. Iran J Kidney Dis. 2016;10:337–343. [PubMed] [Google Scholar]
- 5.Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 6.Li J, Qu X, Bertram JF. Endothelial-myofibroblast transition contributes to the early development of diabetic renal interstitial fibrosis in streptozotocin-induced diabetic mice. Am J Pathol. 2009;175:1380–1388. doi: 10.2353/ajpath.2009.090096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeisberg EM, Potenta SE, Sugimoto H, Zeisberg M, Kalluri R. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J Am Soc Nephrol. 2008;19:2282–2287. doi: 10.1681/ASN.2008050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanasaki K, Shi S, Kanasaki M, He J, Nagai T, Nakamura Y, Ishigaki Y, Kitada M, Srivastava SP, Koya D. Linagliptin-mediated DPP-4 inhibition ameliorates kidney fibrosis in streptozotocin-induced diabetic mice by inhibiting endothelial-to-mesenchymal transition in a therapeutic regimen. Diabetes. 2014;63:2120–2131. doi: 10.2337/db13-1029. [DOI] [PubMed] [Google Scholar]
- 9.Wittamer V, Franssen JD, Vulcano M, Mirjolet JF, Le Poul E, Migeotte I, Brezillon S, Tyldesley R, Blanpain C, Detheux M, Mantovani A, Sozzani S, Vassart G, Parmentier M, Communi D. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J Exp Med. 2003;198:977–985. doi: 10.1084/jem.20030382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin S, Teng J, Li J, Sun F, Yuan D, Chang J. Association of chemerin and vascular endothelial growth factor (VEGF) with diabetic nephropathy. Med Sci Monit. 2016;22:3209–3214. doi: 10.12659/MSM.896781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu QX, Zhang H, Xu WH, Hao F, Liu SL, Bai MM, Mu JW, Zhang HJ. Effect of irbesartan on chemerin in the renal tissues of diabetic rats. Kidney Blood Press Res. 2015;40:467–477. doi: 10.1159/000368523. [DOI] [PubMed] [Google Scholar]
- 12.Hu W, Feng P. Elevated serum chemerin concentrations are associated with renal dysfunction in type 2 diabetic patients. Diabetes Res Clin Pract. 2011;91:159–163. doi: 10.1016/j.diabres.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 13.Salama FE, Anass QA, Abdelrahman AA, Saeed EB. Chemerin: a biomarker for cardiovascular disease in diabetic chronic kidney disease patients. Saudi J Kidney Dis Transpl. 2016;27:977–984. doi: 10.4103/1319-2442.190867. [DOI] [PubMed] [Google Scholar]
- 14.He J, Xu Y, Koya D, Kanasaki K. Role of the endothelial-to-mesenchymal transition in renal fibrosis of chronic kidney disease. Clin Exp Nephrol. 2013;17:488–497. doi: 10.1007/s10157-013-0781-0. [DOI] [PubMed] [Google Scholar]
- 15.Phanish MK, Winn SK, Dockrell ME. Connective tissue growth factor-(CTGF, CCN2)-a marker, mediator and therapeutic target for renal fibrosis. Nephron Exp Nephrol. 2010;114:e83–92. doi: 10.1159/000262316. [DOI] [PubMed] [Google Scholar]
- 16.Goralski KB, McCarthy TC, Hanniman EA, Zabel BA, Butcher EC, Parlee SD, Muruganandan S, Sinal CJ. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J Biol Chem. 2007;282:28175–28188. doi: 10.1074/jbc.M700793200. [DOI] [PubMed] [Google Scholar]
- 17.Nagpal S, Patel S, Jacobe H, DiSepio D, Ghosn C, Malhotra M, Teng M, Duvic M, Chandraratna RA. Tazarotene-induced gene 2 (TIG2), a novel retinoid-responsive gene in skin. J Invest Dermatol. 1997;109:91–95. doi: 10.1111/1523-1747.ep12276660. [DOI] [PubMed] [Google Scholar]
- 18.Bozaoglu K, Segal D, Shields KA, Cummings N, Curran JE, Comuzzie AG, Mahaney MC, Rainwater DL, VandeBerg JL, MacCluer JW, Collier G, Blangero J, Walder K, Jowett JB. Chemerin is associated with metabolic syndrome phenotypes in a Mexican-American population. J Clin Endocrinol Metab. 2009;94:3085–3088. doi: 10.1210/jc.2008-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bozaoglu K, Bolton K, McMillan J, Zimmet P, Jowett J, Collier G, Walder K, Segal D. Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology. 2007;148:4687–4694. doi: 10.1210/en.2007-0175. [DOI] [PubMed] [Google Scholar]
- 20.De Palma G, Castellano G, Del Prete A, Sozzani S, Fiore N, Loverre A, Parmentier M, Gesualdo L, Grandaliano G, Schena FP. The possible role of ChemR23/Chemerin axis in the recruitment of dendritic cells in lupus nephritis. Kidney Int. 2011;79:1228–1235. doi: 10.1038/ki.2011.32. [DOI] [PubMed] [Google Scholar]
- 21.Li J, Bertram JF. Review: Endothelial-myofibroblast transition, a new player in diabetic renal fibrosis. Nephrology (Carlton) 2010;15:507–512. doi: 10.1111/j.1440-1797.2010.01319.x. [DOI] [PubMed] [Google Scholar]
- 22.Pfau D, Bachmann A, Lossner U, Kratzsch J, Bluher M, Stumvoll M, Fasshauer M. Serum levels of the adipokine chemerin in relation to renal function. Diabetes Care. 2010;33:171–173. doi: 10.2337/dc09-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Willis BC, duBois RM, Borok Z. Epithelial origin of myofibroblasts during fibrosis in the lung. Proc Am Thorac Soc. 2006;3:377–382. doi: 10.1513/pats.200601-004TK. [DOI] [PMC free article] [PubMed] [Google Scholar]


