Abstract
Previous study found that higher Capn4 mRNA level is observed in patients with more advanced pathological stage of ccRCC and is also associated with decreased overall survival of patients with ccRCC. However, the mechanism by which Capn4 promotes progression of RCC is not understood. In the present study, we found that over-expression of Capn4 in RCC cells enhances tumor cell growth and down-regulation of Capn4 in RCC cells decreases tumor cell growth in vitro. Interestingly, Capn4 was found to increase phosphorylation of specific tyrosine residues of FAK and subsequent activate NF-κB p65 phosphorylation. Furthermore, Capn4-mediated cell proliferation of RCC cells required up-regulation of NF-κB p65 phosphorylation through activation of FAK signaling pathway. Taken together, our data showed that Capn4 can contribute to RCC growth via activation of the FAK and the downstream signaling pathways leading to the activation of NF-κB.
Keywords: Renal cell carcinoma (RCC), calpain small subunit 1 (Capn4), focal adhesion kinase (FAK), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), proliferation
Introduction
Renal cell carcinoma (RCC) is a universal tumor of the urological system, ranking second in urological cancer mortality and representing approximately 4% of all adult malignances [1]. In the recent decades, the incidence of RCC has been steadily rising by 2-4% each year [2]. The incidence of RCC in Asia is lower than in US and Europe, while the mortality-to-incidence ratio is much higher in Asia than in the developed nations [3,4]. In China, limited studies showed there is obvious increment of the RCC morbidity in recent years with the increasing of early diagnosed cases [5,6]. The five years’ survival rate of the latter group is under 10%, even though the FDA recently approved new drugs targeting specific pathways (tyrosine-kinase inhibitors/mTOR-inhibitors), which is available for RCC [3]. The underlying mechanisms for RCC progression are still not fully understood and no molecular strategies are currently recommended for routine clinical use to improve risk stratification of patients with RCC. Clear cell renal cell carcinoma (ccRCC) is the most common subtype characterized by high metastatic potential and resistance to traditional radiotherapy and chemotherapy [7-9]. Therefore, it is essential to explore the underlying mechanisms for localized ccRCC to offer possible personalized therapy.
The calpains (Capns) are a family of proteases that are widely expressed in lots of organisms and have both ubiquitous and tissue-specific isoforms [10,11]. Calpain-mediated proteolysis represents a major post-translational modification pathway that influences various aspects of cell physiology, including cell migration or invasion, cell proliferation and apoptosis [12,13]. Emerging evidence suggests that Capns may be critical factors in tumor development, progression and metastasis. Several calpain members, such as Capn2 [14-17], Capn4 [18-21], and Capn6 [22,23] and Capn10 [24], have been associated with oncogenesis in various types of cancer. The results of our previous study indicated that higher Capn4 mRNA levels are observed in patients with more advanced pathological stage of ccRCC and were also associated with decreased overall survival of patients with ccRCC [25]. However, expression of other isoforms of Capns was not found to be associated with the invasive abilities of ccRCC cells. Capn4 acts as a binding partner to form a heterodimer with the large catalytic subunit and is crucial for maintaining the activity of calpain [26]. Disruption of murine Capn4 eliminates the function of Capns [27]. It is also reported that fibroblasts from the Capn4-/- mice show a decreased migration rate and number of focal adhesions, suggestive of a defect in adhesion maturation and/or turnover [28]. Together, these results strongly suggest a significant role for Capn4 in tumor progression of human cancer. However, the role of Capn4 in tumor growth and the mechanism by which Capn4 promotes progression of ccRCC are currently unknown. In this study, we studied the effect of changes in the expression level of Capn4 through RNA interference or over-expression mediated by lentiviral vectors on the growth and metastasis of RCC in vitro. We also explored the signaling pathway in which Capn4 involves in the progression of RCC.
Materials and methods
Cell culture, treatment and transfection
Human (clear cell) renal cell carcinoma cell lines (Caki-1, 786-O) were obtained from the American Type Culture Collection (ATCC). Cell lines were maintained in high glucose RPMI 1640 (786-O) or McCoy’s 5a (Caki-1) (all from Gibco), supplemented with 10% fetal bovine serum (FBS, Gibco), 2 mML-glutamine (Life Technologies). Other reagents used in the study were as follows: recombinant human IL-6 (Peprotech), dimethyl sulfoxide (Sigma-Aldrich), 5-aza-2-deoxycytidine (Sigma-Aldrich), Stattic (Selleck), MK-2206 (Selleck) and U0126 (Medchem Express).
Cell proliferation
Cell proliferation was analyzed by CCK8 assay. In brief, cells were seeded in 96 well plates at a density of 1000 cells per well and cultured for 24 h (as day 0), and then subjected to various treatments in each four replicate wells. At the indicated time points, each well was added with 10 μL CCK8 reagent solution (Donjido), and incubated for 1 h. Optical density was determined by a microplate reader at the absorbance of 450 nm. The CCK-8 experiments were repeated four times.
Wound healing assay
5×10^5 cells are seeded in 6-well plates respectively. A small wound area was made in the 90% confluent monolayer by using a 200 μl pipette tip in a lengthwise stripe. Cells were then washed twice with PBS and incubated in serum-free DMEM medium at 37°C in a 5% CO2 incubator for 24 hours. Photographs were taken at the indicated time points.
Transwell invasion assay
The co-culture system was used to evaluate the regulation of invasiveness in cells as described in the previous study. In brief, the upper portion of Transwell® inserts with an 8 μm pore size and a 6.5 mm diameter was coated with 20 μl matrigel diluted 1:3 in serum-free DMEM and incubated at 37°C for 4 hours. The coated inserts were placed in the well of 24-well plate with 600 μl DMEM medium containing 10% FBS in the bottom chamber. After 12 hours of serum starvation the trypsinized cells respectively, were harvested and diluted to a 1×10^6/ml cell suspension with serum-free DMEM medium. Each cell suspension (200 μl) was added to the upper chambers. After incubation at 37°C for 24 hours in a 5% CO2 atmosphere the non-invading cells and gel were removed from the upper chamber with cotton tipped swabs. The cells were rinsed with PBS and cells on the filters were fixed with methanol for 30 minutes and stained with crystal violet solution (Sigma). The number of invading cells on the filters was counted in 5 random fields per filter at 100× magnification in triplicate wells of each group.
RNA isolation and qPCR
Total RNA was isolated using the RNeasy kit (Qiagen), according to the manufacturer’s instructions and was reverse-transcribed to cDNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystem). cDNA was synthesized by reverse transcription using a fixed volume of RNA (2 μL) and the TaqMan Reverse Transcription kit (Life Technologies), according to the manufacturer’s instructions. Quantitative PCR reactions were performed in triplicate on a 7900 HT fast real time PCR system (Applied Biosystems), according to the following program: 10 minutes at 95°C, 40 cycles of 15 seconds at 95°C and 60 seconds at 60°C. Values were normalized to cel-miR-39, analyzed by the comparative method of Ct (2-ΔΔCt) and expressed in log10.
Western blotting
The total protein of cells was extracted using RIPA buffer supplemented with protease inhibitor PMSF and phosphatase inhibitors NaF and Na3VO4 (Roche). Western blotting assay was performed as previously described [25]. The intensity level of each protein band was quantified using Quantity One. The primary antibodies were as follows: anti-Capn4 (1:1000), anti-Talin (1:1000), anti-FAK (1:2000), anti-total-FAK (1:1000) and anti-phospho-FAK (Tyr397, 1:2000) were from Abcam; anti-total-NF-κB (1:1000) and anti-phospho-NF-κB (Ser536, 1:2000) were from Cell signaling technology. All protein expression experiments were repeated three times.
Results
Capn4 promotes proliferation, migration, and invasion of RCC cells
Our previous study has found that higher Capn4 mRNA levels is observed in patients with more advanced pathological stage of RCC and were also associated with decreased overall survival of patients with RCC. Using western blotting, we also found that Capn4 expression was high in 780-O, and low in Caki1 cells, which coincided with the invasiveness of these cells (data not shown). To investigate the role of Capn4 in RCC, up-regulation of Capn4 in Caki1 cells (Figure 1A) caused significant increasing of cell proliferation (Figure 1B). Similarly, down-regulation of 780-O cells by small hairpin RNA (Figure 1A) caused markedly decreasing of cell proliferation (Figure 1C). In vitro, migration assays showed that the distance of migrated Caki1-Capn4 cells was significantly higher than the distance of migrated Caki1-Mock cells (P<0.05; Figure 1D). Similarly, the distance of migrated 780-O-shCapn4 cells was significantly less than those of 780-O -Mock cells (P<0.05; Figure 1D). The results of the cell invasive assays revealed that there was an apparent change in migration and invasive ability of Caki1 and 780-O cells transfected with Capn4 or Capn4 shRNA (Figure 1E). These results suggest that Capn4 could contribute to proliferation, migration and invasion of HCC cells.
Figure 1.
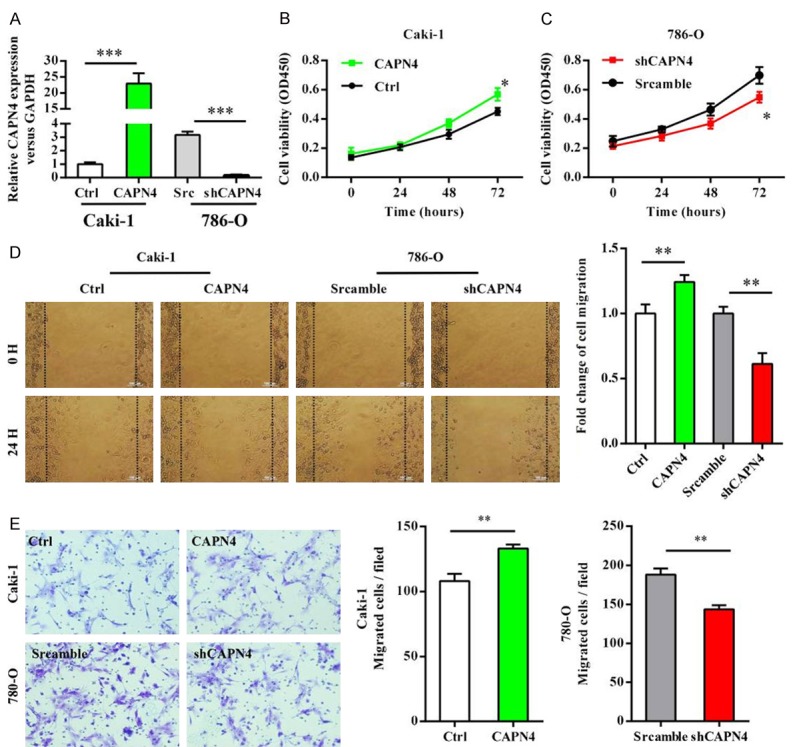
Capn4 promotes proliferation, migration, and invasion of RCC cells. A. Stable up- or down-regulation of Capn4 expression in transfected RCC cell lines was confirmed by qRT-PCR. B, C. In the CCK8 assay, cell proliferation was significantly increased after Capn4 over-expression (P<0.01) and significantly decreased after Capn4 shRNA transfection (P<0.05). D. Wound-healing migration assays and the quantification of the percentage of open area are shown. E. The invasion of cancer cells was measured by transwell Matrigel invasion assays; data are shown are mean ± SEM from three independent experiments. Statistical significance was assessed by Student’s t-test; *P<0.05, **P<0.01, ***P<0.001; scale bar =100 μm.
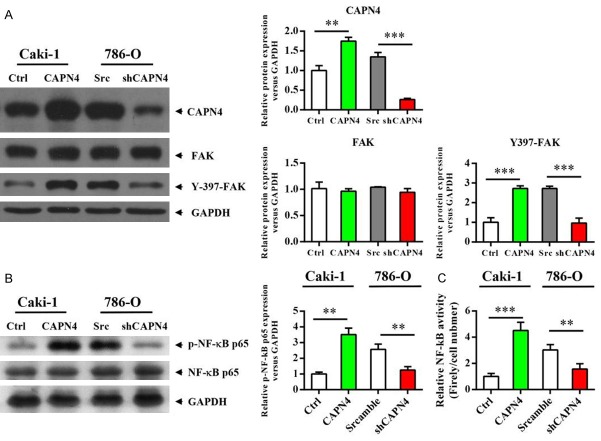
Capn4 activates FAK and NF-κB signaling pathways in RCC cells
It is reported that Capn4 contributes to tumor growth and metastasis of hepatocellular carcinoma by activation of the FAK signaling pathway [21]. Meanwhile, FAK is reported to activate NF-κB signaling pathway [29,30]. To explore the molecular mechanism by which Capn4 contributes to RCC progression and metastasis, we evaluated the changes in the protein levels of phosphorylated FAK and NF-κB in transfected RCC cells using western blotting. Impressively, the level of Tyr387-phosphorylated FAK (Figure 2A) and Ser536-phosphorylated NF-κB (Figure 2B) were significantly up-regulated in Cak1-Capn4 cells, while down-regulated in 780-O-shCapn4 cells compared to respective controls, which coincided with Capn4 expression and the invasiveness of these cells. Furthermore, we tested the activity of NF-κB using a NF-κB reporter and validated that the activity of NF-κB signaling pathway was also up-regulated in Cak1-Capn4 cells, while down-regulated in 780-O-shCapn4 cells compared to respective controls. These results suggested that FAK and NF-κB signaling pathways might play critical roles in Capn4-enhanced RCC cells proliferation and progression.
Figure 2.
Capn4 activates FAK and NF-κB signaling pathways in RCC cells. A. Western blot analysis revealed that the phosphorylation level of FAK is up-regulated in the Caki1-Capn4 group or down-regulated in the 780-O-shCapn4 group compared to the respective control cells. B. Western blot analysis revealed that the phosphorylation level of NF-κB is up-regulated in the Caki1-Capn4 group or down-regulated in the 780-O-shCapn4 group compared to the respective control cells. C. Caki1 and 780-O cells were transfected with an NF-κB-dependent reporter plasmid (pBVIx-Luc) and NF-κB activation was determined by measuring relative luciferase activity 48 hours after treatment. Luciferase activity is reported as arbitrary relative light units. Data are shown are mean ± SEM from three independent experiments. Statistical significance was assessed by Student’s t-test; **P<0.01, ***P<0.001.
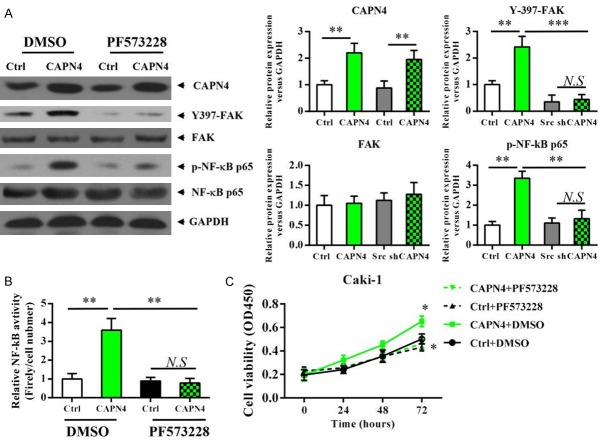
FAK phosphorylation is crucial to the Capn4 induced NF-κB activation and enhanced RCC cells proliferation
To determine the association of FAK phosphorylation and Capn4-induced NF-κB activation, we tested whether FAK pathway was essential for NF-κB activation. Inhibition of FAK phosphorylation using a FAK inhibitor (PF573228) in Caki1 cell did no change on the expression level of Capn4, while significantly reduced the phosphorylation of FAK (Figure 3A) and NF-κB (Figure 3B). We also found that inhibition of FAK phosphorylation in Caki1-Capn4 cells resulted in elevated activity of NF-κB signaling pathway and abolished the increase in proliferative potential produced by Capn4 over-expression (Figure 3C). These results demonstrated that Capn4 activates NF-κB signaling pathway and stimulate RCC proliferation through activating the FAK signaling pathway.
Figure 3.
FAK phosphorylation is crucial to the Capn4 induced NF-κB activation and enhanced RCC cells proliferation. A. Western blot analysis revealed that the up-regulated phosphorylation levels of FAK and NF-κB in the Caki1-Capn4 group are completely abolished with PF573228 treatment (2.5 μM for 12 h) compared to the respective control cells. B. Caki1 was transfected with an NF-κB-dependent reporter plasmid (pBVIx-Luc) and NF-κB activation was determined by measuring relative luciferase activity 48 hours after treatment. Luciferase activity is reported as arbitrary relative light units. C. Inhibition of FAK phosphorylation using a PF573228 (2.5 μM for 12 h) in Caki1 cells abolished the increase in cancer cell growth by Capn4 over-expression; data are shown are mean ± SEM from three independent experiments. Statistical significance was assessed by Student’s t-test; P<0.05, **P<0.01, ***P<0.001.
NF-κB activation is critical to the Capn4 enhanced RCC cells proliferation
We investigated whether the NF-κB pathway play critical roles in the promotion of growth of RCC cells mediated by Capn4. Inhibition of NF-κB using a specific inhibitor (QNZ) in Cak1 cell did no change on the expression levels of Capn4 and the phosphorylation of FAK, while completely abolished the Capn4 induced phosphorylation of NF-κB (Figure 4A). Similarly, Inhibition of NF-κB by QNZ in 780-O cell did no change on the expression levels of Capn4 and the phosphorylation of FAK, while further inhibited the level of phosphorylated NF-κB (Figure 4B). Meanwhile, inhibition of NF-κB phosphorylation completely abolished the Capn4 enhanced cell proliferation in Caki1 cell and the Capn4 down-regulation decreased cell proliferation in 780-O cell (Figure 4C). Collectively, the results suggested that Capn4 activates FAK phosphorylation and subsequently activates NF-κB signaling pathway to promote the cell proliferation of RCC cells.
Figure 4.
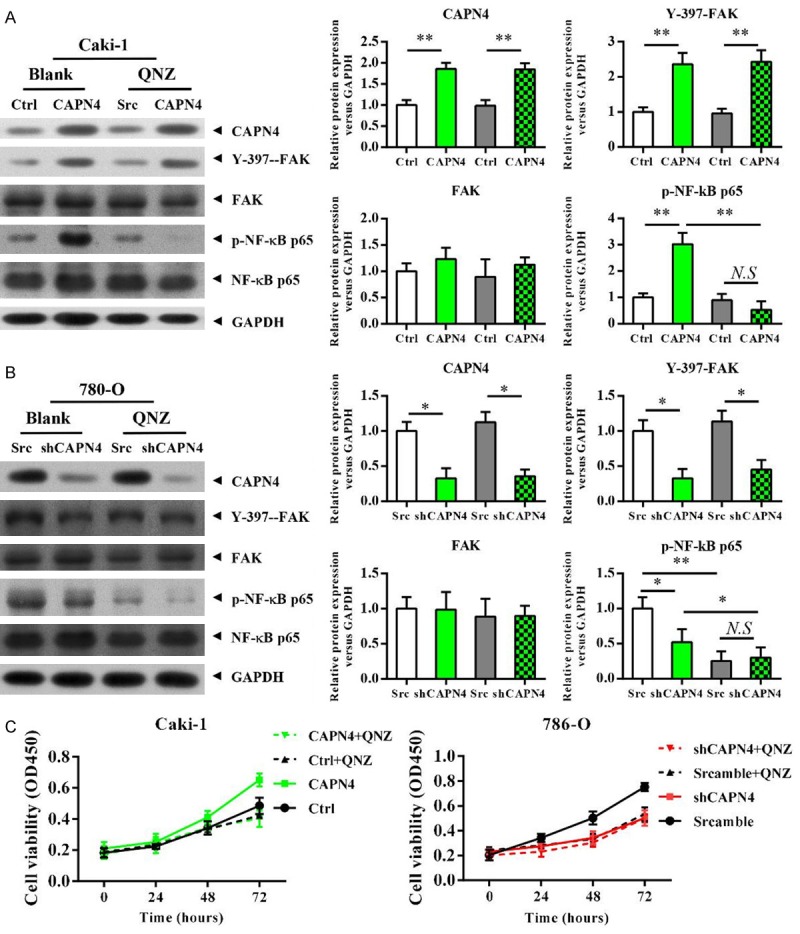
NF-κB activation is critical to the Capn4 enhanced RCC cells proliferation. A. Western blot analysis revealed that the up-regulated phosphorylation levels of FAK and NF-κB in the Caki1-Capn4 group, while only the phosphorylation of NF-κB is completely abolished with QNZ treatment (3 μM for 24 h) compared to the respective control cells. B. Western blot analysis revealed that the down-regulated phosphorylation levels of FAK and NF-κB in the 780-O-shCapn4 group, while the phosphorylation level of NF-κB is further decreased with QNZ treatment (3 μM for 24 h) compared to the respective control cells. C. Inhibition of NF-κB phosphorylation using a QNZ (3 μM for 24 h) in Caki1 cells abolished the increase in cancer cell growth induced by Capn4 over-expression, and NF-κB inhibitor alone or combined with Capn4 shRNA led to a similar decrease in 780-O cell growth with Capn4 shRNA alone; data are shown are mean ± SEM from three independent experiments. Statistical significance was assessed by Student’s t-test; P<0.05, **P<0.01, ***P<0.001.
Discussion
ccRCC is the most aggressive subtype of common RCC and it is lethal when metastatic [6-8]. Capn4 was identified to be a promising biomarker for metastatic RCC [25], however, the possible mechanism of Capn4 regulating RCC progression is largely unknown. Furthermore, it is important to develop prognostic markers for localized ccRCC. Capn4 has been suggested to be a valuable prognostic marker for metastatic and localized ccRCC in our previous study [25]. The underlined mechanism by which Cpan4 involves in localized ccRCC is required to be validated. In the present investigation, we identified a novel mechanism that Capn4 controls RCC progression by promoting RCC cell proliferation via activation of FAK and NF-κB pathway. Although numerous descriptive studies suggested that Capn4 expression correlates with tumor aggressiveness in a variety of malignancies, the role of Capn4 in modulating tumor cellular functions was poorly understood. To our knowledge so far there was no report on mechanisms underlying Capn4 regulation of RCC progression. Our data showed that Capn4 promotes RCC cell proliferation, which was consistent with previous findings of Capn4 in RCC [25]. Intriguingly, we identified that Capn4 activates FAK signaling pathway and Capn4 promotes RCC cell proliferation through FAK signaling pathway. FAK is a non-receptor tyrosine kinase found at focal adhesions, which are sites of integrin clustering at the cell-extracellular matrix interface, where it provides both signaling and scaffolding functions. FAK is activated in a range of tumor cells, and its increased activity correlates with the malignancy and invasiveness of human RCC and other tumors. The observation that there is a direct interaction between Capn4 and FAK may provide an explanation for the role of Capn4 in RCC progression.
Another important finding in this study is the discovery of a crucial role of NF-κB in the FAK signaling pathway mediating RCC progression. FAK phosphorylation activates NF-κB pathway in RCC cells and Capn4 promotes RCC cell migration through NF-κB pathway. The NF-κB family consists of five members: RelA (p65), RelB, c-Rel, NF-κB1 (p105/p50) and NF-κB2 (p100/p52) [31]. The NF-κB pathway was reported to be involved in almost all important aspects of RCC progression including angiogenesis, invasion, metastasis and multi-drug resistance [32]. By immunostaining of p50 and p65, there was a study suggesting a correlation between invasion and metastasis of RCC and the expression and activation of NF-κB [33]. However, the relationship between Capn4 and NF-κB was not established. By inhibition of NF-κB using QNZ which completely inhibited the phosphorylation of p65, we demonstrated for the first time that the NF-κB signaling pathway was indeed upregulated by Capn4 and was crucial to the Capn4 enhanced RCC cells proliferation.
In summary, the present study demonstrated that Capn4 contributes to RCC progression by activation of FAK and subsequent activation of NF-κB signaling pathway. These features of Capn4 make it a potential therapeutic target in RCC treatment.
Acknowledgements
This work was supported by grants from the Natural Science Foundation of Jiangsu (BK20150251).
Disclosure of conflict of interest
None.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Patel AR, Prasad SM, Shih YC, Eggener SE. The association of the human development index with global kidney cancer incidence and mortality. J Urol. 2012;187:1978–1983. doi: 10.1016/j.juro.2012.01.121. [DOI] [PubMed] [Google Scholar]
- 3.Cairns P. Renal cell carcinoma. Cancer Biomark. 2010;9:461–473. doi: 10.3233/CBM-2011-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsieh JJ, Cheng EH. The panoramic view of clear cell renal cell carcinoma metabolism: values of integrated global cancer metabolomics. Transl Androl Urol. 2016;5:984–986. doi: 10.21037/tau.2016.11.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen K, Zhang J, Guo Z, Ma Q, Xu Z, Zhou Y, Xu Z, Li Z, Liu Y, Ye X, Li X, Yuan B, Ke Y, He C, Zhou L, Liu J, Ci W. Loss of 5-hydroxymethylcytosine is linked to gene body hypermethylation in kidney cancer. Cell Res. 2016;26:103–118. doi: 10.1038/cr.2015.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qin S, Bi F, Jin J, Cheng Y, Guo J, Ren X, Huang Y, Tarazi J, Tang J, Chen C, Kim S, Ye D. Axitinib versus sorafenib as a second-line therapy in Asian patients with metastatic renal cell carcinoma: results from a randomized registrational study. Onco Targets Ther. 2015;8:1363–1373. doi: 10.2147/OTT.S83302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Z, Shao Y, Yao H, Zhuang Q, Wang K, Xing Z, Xu X, He X, Xu R. Preoperative albumin to globulin ratio predicts survival in clear cell renal cell carcinoma patients. Oncotarget. 2017 doi: 10.18632/oncotarget.15162. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benavides-Huerto MA, Chavez-Valencia V, Lagunas-Rangel FA. Synchronous renal neoplasm: clear cell renal cell carcinoma and papillary urothelial carcinoma in the same kidney. Urol Case Rep. 2017;11:60–62. doi: 10.1016/j.eucr.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conceicao AL, Da Silva CT, Badial RM, Valsechi MC, Stuqui B, Goncalves JD, Jasiulionis MG, De Freitas Calmon M, Rahal P. Downregulation of OCLN and GAS1 in clear cell renal cell carcinoma. Oncol Rep. 2017;37:1487–1496. doi: 10.3892/or.2017.5414. [DOI] [PubMed] [Google Scholar]
- 10.Ono Y, Saido TC, Sorimachi H. Calpain research for drug discovery: challenges and potential. Nat Rev Drug Discov. 2016;15:854–876. doi: 10.1038/nrd.2016.212. [DOI] [PubMed] [Google Scholar]
- 11.Potz BA, Abid MR, Sellke FW. Role of calpain in pathogenesis of human disease processes. J Nat Sci. 2016:2. [PMC free article] [PubMed] [Google Scholar]
- 12.Kerstein PC, Patel KM, Gomez TM. Calpainmediated proteolysis of Talin and FAK regulates adhesion dynamics necessary for axon guidance. J Neurosci. 2017;37:1568–1580. doi: 10.1523/JNEUROSCI.2769-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu SM, Lin WY, Shen CC, Pan HC, Keh-Bin W, Chen YC, Jan YJ, Lai DW, Tang SC, Tien HR, Chiu CS, Tsai TC, Lai YL, Sheu ML. Melatonin set out to ER stress signaling thwarts epithelial mesenchymal transition and peritoneal dissemination via calpain-mediated C/EBPbeta and NFkappaB cleavage. J Pineal Res. 2016;60:142–154. doi: 10.1111/jpi.12295. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez-Fernandez L, Ferrer-Vicens I, Garcia C, Oltra SS, Zaragoza R, Vina JR, Garcia-Trevijano ER. Isoform-specific function of calpains in cell adhesion disruption: studies in postlactational mammary gland and breast cancer. Biochem J. 2016;473:2893–2909. doi: 10.1042/BCJ20160198. [DOI] [PubMed] [Google Scholar]
- 15.Starska K, Forma E, Jozwiak P, Lewy-Trenda I, Danilewicz M, Stasikowska-Kanicka O, Skora M, Kolary K, Miazga J, Krzeslak A, Brys M. Gene/protein expression of CAPN1/2-CAST system members is associated with ERK1/2 kinases activity as well as progression and clinical outcome in human laryngeal cancer. Tumour Biol. 2016;37:13185–13203. doi: 10.1007/s13277-016-5178-8. [DOI] [PubMed] [Google Scholar]
- 16.Raimondi M, Marcassa E, Cataldo F, Arnandis T, Mendoza-Maldonado R, Bestagno M, Schneider C, Demarchi F. Calpain restrains the stem cells compartment in breast cancer. Cell Cycle. 2016;15:106–116. doi: 10.1080/15384101.2015.1121325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Storr SJ, Safuan S, Woolston CM, Abdel-Fatah T, Deen S, Chan SY, Martin SG. Calpain-2 expression is associated with response to platinum based chemotherapy, progression-free and overall survival in ovarian cancer. J Cell Mol Med. 2012;16:2422–2428. doi: 10.1111/j.1582-4934.2012.01559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang E, Wang D, Li B, Ma H, Wang C, Guan L, Zhang H, Yi L, Li S. Capn4 promotes epithelialmesenchymal transition in human melanoma cells through activation of the Wnt/betacatenin pathway. Oncol Rep. 2017;37:379–387. doi: 10.3892/or.2016.5247. [DOI] [PubMed] [Google Scholar]
- 19.Cai JJ, Qi ZX, Chen LC, Yao Y, Gong Y, Mao Y. miR-124 suppresses the migration and invasion of glioma cells in vitro via Capn4. Oncol Rep. 2016;35:284–290. doi: 10.3892/or.2015.4355. [DOI] [PubMed] [Google Scholar]
- 20.Gu J, Xu FK, Zhao GY, Lu CL, Lin ZW, Ding JY, Ge D. Capn4 promotes non-small cell lung cancer progression via upregulation of matrix metalloproteinase 2. Med Oncol. 2015;32:51. doi: 10.1007/s12032-015-0500-7. [DOI] [PubMed] [Google Scholar]
- 21.Dai Z, Zhou SL, Zhou ZJ, Bai DS, Xu XY, Fu XT, Chen Q, Zhao YM, Zhu K, Yu L, Yang GH, Wang Z, Wu WZ, Zhou J, Fan J. Capn4 contributes to tumour growth and metastasis of hepatocellular carcinoma by activation of the FAK-Src signalling pathways. J Pathol. 2014;234:316–328. doi: 10.1002/path.4395. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Wang Y, Sun X, Mei C, Wang L, Li Z, Zha X. miR-449a promotes liver cancer cell apoptosis by downregulation of Calpain 6 and POU2F1. Oncotarget. 2016;7:13491–13501. doi: 10.18632/oncotarget.4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y, Mei C, Sun L, Li X, Liu M, Wang L, Li Z, Yin P, Zhao C, Shi Y, Qiu S, Fan J, Zha X. The PI3KAkt pathway regulates calpain 6 expression, proliferation, and apoptosis. Cell Signal. 2011;23:827–836. doi: 10.1016/j.cellsig.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 24.Chan D, Tsoi MY, Liu CD, Chan SH, Law SY, Chan KW, Chan YP, Gopalan V, Lam AK, Tang JC. Oncogene GAEC1 regulates CAPN10 expression which predicts survival in esophageal squamous cell carcinoma. World J Gastroenterol. 2013;19:2772–2780. doi: 10.3748/wjg.v19.i18.2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhuang Q, Qian X, Cao Y, Fan M, Xu X, He X. Capn4 mRNA level is correlated with tumour progression and clinical outcome in clear cell renal cell carcinoma. J Int Med Res. 2014;42:282–291. doi: 10.1177/0300060513505524. [DOI] [PubMed] [Google Scholar]
- 26.Arthur JS, Greer PA, Elce JS. Structure of the mouse calpain small subunit gene. Biochim Biophys Acta. 1998;1388:247–252. doi: 10.1016/s0167-4838(98)00166-6. [DOI] [PubMed] [Google Scholar]
- 27.Arthur JS, Elce JS, Hegadorn C, Williams K, Greer PA. Disruption of the murine calpain small subunit gene, Capn4: calpain is essential for embryonic development but not for cell growth and division. Mol Cell Biol. 2000;20:4474–4481. doi: 10.1128/mcb.20.12.4474-4481.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dourdin N, Bhatt AK, Dutt P, Greer PA, Arthur JS, Elce JS, uttenlocher A. Reduced cell migration and disruption of the actin cytoskeleton in calpain-deficient embryonic fibroblasts. J Biol Chem. 2001;276:48382–48388. doi: 10.1074/jbc.M108893200. [DOI] [PubMed] [Google Scholar]
- 29.Yurdagul A Jr, Sulzmaier FJ, Chen XL, Pattillo CB, Schlaepfer DD, Orr AW. Oxidized LDL induces FAK-dependent RSK signaling to drive NF-kappaB activation and VCAM-1 expression. J Cell Sci. 2016;129:1580–1591. doi: 10.1242/jcs.182097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dwyer SF, Gao L, Gelman IH. Identification of novel focal adhesion kinase substrates: role for FAK in NFkappaB signaling. Int J Biol Sci. 2015;11:404–410. doi: 10.7150/ijbs.10273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khan MT, Mischiati C, Ather A, Ohyama T, Dedachi K, Borgatti M, Kurita N, Gambari R. Structure-based analysis of the molecular recognitions between HIV-1 TAR-RNA and transcription factor nuclear factor-kappaB (NFkB) Curr Top Med Chem. 2012;12:814–827. doi: 10.2174/156802612800166800. [DOI] [PubMed] [Google Scholar]
- 32.Morais C, Gobe G, Johnson DW, Healy H. The emerging role of nuclear factor kappa B in renal cell carcinoma. Int J Biochem Cell Biol. 2011;43:1537–1549. doi: 10.1016/j.biocel.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 33.Oya M, Takayanagi A, Horiguchi A, Mizuno R, Ohtsubo M, Marumo K, Shimizu N, Murai M. Increased nuclear factor-kappa B activation is related to the tumor development of renal cell carcinoma. Carcinogenesis. 2003;24:377–384. doi: 10.1093/carcin/24.3.377. [DOI] [PubMed] [Google Scholar]